Abstract
Microplastics (MPs) have become a global issue due to their potential adverse effects on sustainable marine resources and human health. In this study, MP pollution was investigated using natural mussels from all shelf regions of the SoM (Sea of Marmara), which is under the influence of many pollutant sources. A total of 322 mussels were collected along the entire coastline, and MP analyses were performed on these mussels. Mussel tissues were digested using a KOH solution to separate the MPs. Following extraction, the samples were filtered and the particles remaining on top were examined physically and chemically. In the study, the highest values were detected in samples taken both from locations under anthropogenic influence, especially from points close to where rivers flow. Across all the samples, the most predominant shape was fiber (61.08%), color was blue (57.87%) and size was (<0.5 mm) (62.55%). FTIR analysis shows that PE is the most common polymer type (44%). Calculated on the basis of 100 g of daily consumption, the annual ingestive exposures to MPs were found to be 1940, 342, 41 and 39 items for children, adolescents, female adults and male adults, respectively. As a result of a detailed risk assessment related to chronic daily intake (CDI) and microplastic carcinogenic risk (MPCR), it was determined that children are the most vulnerable group exposed to MPs and that these seafood products should be consumed with caution by children to prevent potential hazards. Additionally, it has been determined that the southern shelf and the Çanakkale Strait are the areas under the most intense pollution pressure according to the calculated MPCf and MPLI values. These findings are very relevant in terms of taking practical steps to take plans and actions to prevent contamination in the SoM and ensure the sustainability of food safety in the consumption of products obtained from the sea.
1. Introduction
The estimated 20 million metric tons of plastic garbage entering the environment annually is projected to increase significantly by 2040. This plastic pollution affects all environmental ecosystems, including all land, freshwater and marine environments. Plastic pollution has been reported as one of the causes of biodiversity loss and ecosystem degradation [1]. Authorities are concerned and attentive to the excessive plastics detected in various niches around the world [2].
Plastic particles with diameters ranging from 1 µm to 5 mm are called microplastics (MPs), and these MPs have become ubiquitous in all aquatic environments [3]. MPs are categorized in two ways. Primary MPs are found in micro-sized forms in the contents of daily use products such as scrubs, peeling gels and toothpaste, and are intentionally produced and released into the environment, particularly the ocean. Secondary MPs, on the other hand, are formed when macro-sized plastic waste reaches the oceans and, due to exposure to sunlight, UV rays, oxidation, waves, currents, etc., gradually breaks down into smaller pieces [4,5]. A recent study reported that the number of MPs on the surface of the seas is thought to be more than 170 trillion items [6]. They have physical properties such as a small size, slow decomposition and low density that make them difficult to remove from water, contributing to their dispersion in the environment and global distribution [7,8,9,10]. MPs, being highly persistent, are commonly found in the marine environment and oceans [7,11], and this feature may lead to the accidental ingestion of these particles by fish and shellfish, which are commercially important and widely edible species [12,13,14]. Information about the existence of plastics in the marine environment and their toxicity to biota has increased significantly over the last decade [15,16,17,18]. However, risk assessments of the direct human consumption of marine products contaminated with environmental MPs are very limited.
Mytilus galloprovincialis (known as Mediterranean mussel—M. galloprovincialis) is a widely distributed bioindicator species in the SoM and the Black Sea, indicating levels of environmental pollutants. Mussels, which are commonly used in studies related to marine pollution, are easily accessible, can tolerate different salinity levels and have a wide distribution area. In addition to being filter feeders, they easily accumulate all pollutants, including microplastics, in their bodies [19]. Therefore, most mussels have lately been successfully used to study the spatial distributions of microplastics in the marine environment [20,21,22,23,24,25].
The SoM is an inland body of water situated between the Black and Aegean Seas. Covering approximately 11,500 km2, with a maximum depth of about 1300 m, it serves as a vital international waterway of strategic significance, connecting the Straits of İstanbul and Çanakkale. Unfortunately, it faces pollution from touristic, domestic, industrial and agricultural wastes, which has been increasing in recent years and poses a major risk to the coastal region [26,27]. In addition to intensive activities in agriculture and industry in the locality, large quantities of pollutant-carrying wastes are discharged from point sources into the SoM. In addition to discharges, contaminants are transported by river to the SoM, with contaminants originating from Danube River also accounting for 50% of the pollution in the Black Sea [28].
The risk of MP ingestion by humans is greatly reduced by removing the internal organs of commercially consumed fish species and consuming only the muscle tissue. However, bivalve mollusks such as mussels, whose entire soft tissue is not subject to any processing other than removal from its shell and is entirely edible, are considered a direct exposure route for humans [29]. Since mussels are a commercial and frequently consumed species, humans are exposed to MPs through direct ingestion. MP studies in mussels have become widespread in Turkish Seas in recent years [22,24,30,31,32,33]. Gedik and Eryaşar [24] stated that the findings of their study on the coasts of Türkiye did not cover the entire the SoM, and in a separate study, they reported MPs in natural environment mussels surrounding the coasts of the SoM [22]. Tunçelli and Erkan [31] studied mussels at only two points at the entrance of the Çanakkale Strait, one of which was a site for mussel aquaculture.
However, since no sampling has been conducted along the Çanakkale Strait in previous studies, there are no data on MP pollution in Çanakkale mussels. Although the population density of Çanakkale province is not as high as that of Istanbul, the reporting of the Çanakkale Strait as a receiving environment for discharges from wastewater treatment plants [34] has made sampling from this region necessary to determine the extent of pollution. Therefore, this study has several unique contributions. The objectives of this study are as follows: (a) to present MP findings in mussels collected from SoM coasts, (b) to reveal the presence and abundance of MPs in mussels collected along the Dardanelles Strait, which has not been sampled previously, (c) to compare contamination levels regionally based on station locations, (d) to calculate the potential health risks to humans for different and various age groups in the event of consumption of the collected mussels. This study is the first of its kind in terms of evaluating risk estimates over various age groups, unlike risk estimates that have already been made in Türkiye for SoM mussels. This study also determines the degree of contamination according to stations using the microplastic contamination factor (MPCf) and the microplastic pollution load index (MPLI), to understand pollution load according to the regions where the stations are located, and microplastic carcinogenic risk (MPCR) to determine the carcinogenic risk associated with the consumption of mussels. In this way, various risk levels were also identified for MPs, which are pollutants to which risk groups with different physical characteristics are exposed.
2. Materials and Methods
2.1. Sampling Area
The SoM comprises a volume of 3378 km3 and an area of 11,500 km2. The waters originating from the Black Sea and the Aegean Sea constitute two separate layers in the straits and the SoM. The most important oceanographic feature of the SoM is that it has a continuous double-layer water regime in which the less salty (18 PSU—Practical Salinity Units) Black Sea water constitutes the upper layer and salty (38.5 PSU) Mediterranean water constitutes the lower layer, and they flow through the straits in opposite directions relative to each other [35,36,37]. In terms of pollution, it is known that the coastal regions of the SoM are home to Türkiye’s most densely populated and industrialized areas [38].
Natural mussels were sampled from 18 distinct stations along the SoM coast between 15 and 30 April 2024 (Figure 1). Additionally, the coordinates of the sampling points are provided in Table A1. The stations for the samplings were identified as sites in which mussels naturally settle and attach themselves (rocks, pier columns, hard ground, etc.). An average of 30 to 50 mussel samples of different sizes were obtained from each station in this area. Mussels were carried to the Institute of Marine Sciences and the Management Laboratory of Marine Chemistry under cold-chain conditions. They were stored at −20 °C until extraction [39].
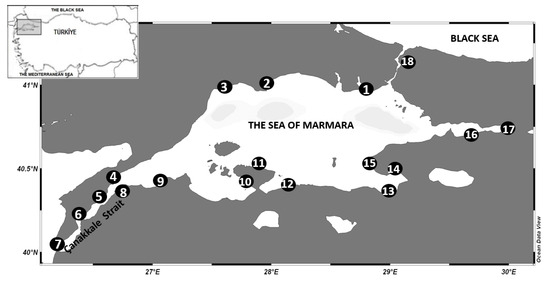
Figure 1.
Points for sampling of natural mussels along SoM.
The stations were numbered as follows: 1—Florya; 2—Marmara Ereğlisi; 3—Tekirdağ; 4—Gelibolu; 5—Burhanlı Village; 6—Bigalı; 7—Kumkale; 8—Çardak; 9—Kemer; 10—Erdek; 11—Turan Village; 12—Yenice Village; 13—Kurşunlu; 14—Büyükkumla; 15—Yalova; 16—Karamürsel; 17—Başiskele; 18—Beykoz.
Since mussels are organisms that survive by filtering water, water samples were taken at the points where the mussels were taken, and physicochemical parameters such as pH, dissolved oxygen (DO) (mg/L), temperature (°C) and salinity (PSU) values were measured on-site with an AQUAREAD (Aquaprobe—AP-700) multiparameter (UK Trade Mark) device (Aquaread Ltd., Broadstairs, UK). There is also a piece of research examining the relationship between these water data and various pollutant parameters [40]. DO values (mg/L) were also calibrated by Winkler’s titrimetric method [41]. In addition, the water samples were also analyzed for total suspended solids (TSS) using the method applied as specified in [42]. These measured parameters in the aquatic environment were also considered in this study for the statistical evaluation of MP concentrations in mussels.
2.2. Microplastic Extraction and Digestion
In order to reveal MP accumulation in mussels with different shell lengths, the mussels were grouped according to their availability at the stations. For this purpose, the groups were two different sizes, <5 cm (S) and >5 cm (L). After the mussel shells were thoroughly cleaned, they were rinsed in distilled water, the soft tissues were removed with the help of a stainless steel knife (Eisco Labs—Honeoye Falls, NY, USA) and the mussels in the same group were noted according to the number of individuals and their weights and placed in a clean glass beaker.
Potassium hydroxide (KOH, ≥85%, Merck, Darmstadt, Germany) has been widely recommended for the digestion of mussel tissues by numerous researchers [43,44,45,46], and it is frequently employed in tissue digestion protocols across various studies [31,32,47,48]. For the extraction of MPs, 180 mL (three times the average sample volume) of 10% KOH was admixed to the samples placed in beakers. The samples were kept in an ultrasonic water bath for 15–20 min and then placed in an incubator set at 50 °C for 48–72 h. During this period, the samples were manually mixed with a glass drumstick at maximum 6 h intervals. When the solution became completely clear, yellow in color and no organic residue remained, the digestion was considered complete, and the samples were taken out and left to cool at room temperature. The cooled samples were filtered with a vacuum pump using a 26 µm pore. The filter papers were placed separately in a clean Petri dish, sealed and dried in an oven set at 50 °C. The dried samples were taken outside, allowed to cool and kept until microscopic examination and FTIR (Fourier Transform Infrared Spectroscopy) analysis.
2.3. Visual Counts and FTIR Analysis of MPs
The filter papers were analyzed under a stereomicroscope (AccuScope—3075-LED-E Binocular Zoom Stereo Microscope, AccuScope Inc., Commack, NY, USA). In order to accurately identify the MPs, visual assessment was carried out according to the method of Hidalgo-Ruz et al. [49] in order to describe the morphological structure of the items. Following the assessment process, the particles visible to the naked eye were moved to a clean filter paper with a clean needle. The items were classified according to their shape as fiber, filament, piece, film, bead or foam.
The collected MP particles were analyzed by FTIR spectroscopy to determine their chemical characterization. Sample type and size are also important in FTIR analysis. The FTIR device in the laboratory could only efficiently determine samples with dimensions in the range of 0.5–5 mm. Smaller particles were controlled under a microscope using a hot needle test, and particles that raised suspicion of being plastic were disregarded, thereby minimizing the risk of the particles being plastic. Therefore, particles with dimensions in this size range were taken from all samples, and evaluations were made of these particles. Spectra were recorded with a wavelength range of 4000 to 400 cm−1 and a resolution of 4 cm−1, referencing similar studies [47,48,50,51]. Prior to each sample analysis, the sample holder and germanium crystal were carefully cleaned with ethanol, and a background measurement was taken again after each analysis. Particle spectra were analyzed, and the results were compared with library data.
2.4. Quality Assurance and Quality Control (QA/QC)
The risk of procedural contamination was reduced by actions such as avoiding plastic materials, using foil to minimize the contamination from air, and cleaning equipment before use, in accordance with Gwinett and Miller [52]. Analyses were performed under controlled and clean conditions wearing cotton lab coats and clean nitrile gloves (%98, Bluezen, Pottstown, PA, USA). The outer shells of the samples were cleansed and rinsed using filtrated MiliQ water (MP Mini Pure Super, Istanbul, Türkiye) to remove mud, sand and/or other substances. The steel equipment was meticulously decontaminated, filtered using GF/, 47 mm Whatman 1.2 µm paper before and after dissection and rinsed with ultrapure water. The stainless steel tools were wrapped in aluminum foil until the analysis.
A clean Petri dish was used as a blank sample, uncovered, for monitoring moist media contamination. If any MPs were recorded above the levels set as detection limits in the blank samples, the background was adjusted by subtracting that amount from the total number of MPs in that batch.
2.5. Microplastics Contamination Factor and Microplastic Pollution Load Index
MPCf (microplastics contamination factor) the contamination of represents MPs in the analyzed research material (MPi) relative to the background (baseline) level (MPb) [53]. The optimal baseline value would be the MP level measured in a sample prior to the rapid expansion of the plastics industry. However, since there was no plastic study conducted before the industrialization of the SoM, the lowest MPs value recorded in the study (0.84 items/gr) was taken as the MPb for this study. This value was considered as the value determined in the samples taken from Station 11, which is located at the top point of the Kapıdağ peninsula, which showed the lowest amount of MP, where there is little settlement and no industrialization. Furthermore, the MPLI was calculated as the nth root of the product of each MPCf [53].
MPCf = MPi/MPb
MPLIarea = (MPCf1 × MPCf2 × …… × MPCfn)1/n
In this study, MPLI values were calculated for 5 different regions: the SoM North Shelf (NS), SoM South Shelf (SS), Çanakkale Strait (ÇS), Gemlik Bay (GB) and the Gulf of İzmit (GI). The distribution of the stations according to the regions was NS (St 1, 2, 3), ES (St 4,5,6,7,8), SS (St 9,10,11,12), GB (St 13,14,15) and GI (St 16,17).
2.6. Risk Estimation
2.6.1. Chronic Daily Intake
Human exposure to MP contamination in mussels is through oral ingestion. Therefore, the chronic daily intake (CDI) based on the overall exposure to MPs from consumption of mussels contaminated with MPs was determined using its formula.
Using MP concentrations in mussels, oral exposures were calculated based on four different age groups: children, adolescents and female and male adults. For the estimation of CDI and other health risk parameters, the mean body weights for the four different age categories (children, adolescents, female adults, male adults) were considered as 21 kg for children (6 years), 51 kg for adolescents (14 years) [54], 75 kg for female adults (79.1 years) and 84 kg for male adults (73 years) [55]. The estimated CDI for each station’s MPs per mussel meal consumed was estimated using the following equation for the requisite age categories. Estimated weekly intake (EWI) and annual intake values were also calculated using the CDI values.
where C is the amount of microplastics (items/g ww); EF is the exposure frequency (365 days/year); ED is the exposure duration (life expectancy for men aged 73 years, women aged 79 years, children aged 6 years and adolescents aged 14 years); IR is the ingestion rate (seafood consumption rate; mg/person/day); AT is the averaging time for non-carcinogens (365 × ED days); LT is lifetime (exposure duration; years); and BW is the body weight of Turkish men and women, children and adolescents.
CDI = (C × EF × ED × IR)/(AT × LT × BW)
2.6.2. Microplastic Carcinogenic Risk
Within the scope of risk calculation, lifetime cancer risk for the four different risk groups mentioned above was also calculated according to cancer slope factor (CSF) values. The CSF is useful for estimating the quantitative risks of chemicals and components of agents categorized as carcinogens. It serves as an indicator of the risk of cancer associated with lifetime exposure to a substance. The CSFs were 0.24 for propylene (PP) and 1.9 for vinyl chloride (PVC) [56], while they were not listed for polyethylene terephthalate (PET) and polyethylene (PE) [57]. As polystyrene (PS) is classified as a non-carcinogenic polymer, its CSF has not been determined. However, given its role in the processing of plastics containing polyethylene, such as PE and PET, its CSF was taken to be 1.02 [58]. The CDI values were multiplied by the CSFs to find the MPCR [59]. MPCR is considered to be the increase in the likelihood of developing cancer within an individual over their lifetime. The MPCR equation caused by lifetime exposure to potential carcinogens is given below.
MPCR = CDI × CSF
In this way, CR the findings were also evaluated using the CDI values found according to the stations.
2.7. Statistical Analysis
Statistical analyses of the data were performed with SPSS Statistics (IBM, v22 program—Armonk, NY, USA). Spearman correlation analyses were performed to determine the relationship between MP concentrations and both MP shapes and the physicochemical parameters of the water. Mann–Whitney U tests were used to compare MP values between two differently sized groups of mussels (S and L). Kruskal–Wallis tests were used to compare MPs between regions. The cut-off value was accepted as p < 0.05 for the analyses to be considered statistically significant.
3. Results
3.1. Physicochemical Parameters of Water
The values of the physicochemical parameters of the water within the scope of this study are given in Table A2. In this study, salinity values ranged between 18.08 PSU and 23.93 PSU, with an average of 20.75 ± 1.42 PSU, and temperature values ranged between 17.4 and 19.90 °C with an average of 18.56 ± 0.66 °C. DO values were measured in the range of 6.97–10.36 mg/L, with an average of 9.01 ± 0.80 mg/L. The pH values were found to be between 8.01 and 8.31, with an average of 8.20 ± 0.09. The lowest total dissolved solids (TDS) concentrations ranged from 10.16 to 23.6 mg/L, with an average of 18.48 ± 2.44 mg/L. TSS values ranged between 12.3 and 55.4 mg/L, with a mean of 34.5 ± 11.50 mg/L [40].
3.2. Abundance and Distribution of MPs in Mussels
A total of 322 mussels were collected from eighteen different points along the SoM coastline, including the Çanakkale Strait, and analyzed. Mussels with shell lengths of 6–7 cm are considered commercial size. However, smaller mussels can also be collected and consumed individually by consumers. Additionally, mussels exhibit varying water filtration capacities depending on their shell size. To determine whether there were differences in their accumulation tendencies for MPs, samples were analyzed in two different size groups. Furthermore, biometric measurements such as height, length and width were also conducted for the collected mussels. Due to the varying sizes of mussels at the stations, samples were divided into either one size group (<5 cm or >5 cm) or two size groups (<5 cm and >5 cm) at each station. The biometric measurements of the mussels examined at each station are presented in Table 1.

Table 1.
Biometric measurements and MP occurrence in mussels in SoM.
Height, length and width values of samples were 4.4 ± 0.2 cm, 2.5 ± 0.1 cm and 1.8 ± 0.8 cm for the S group mussels and 5.9 ± 0.5 cm, 3.1 ± 0.3 cm and 2.2 ± 0.3 cm for the L group mussels, respectively. As seen in Table 1, a total of 1052 items were detected in the mussel samples. According to the mathematical ratio, the number of MPs is three times higher than the number of mussels. The distribution of MPs in the mussel samples ranged from 0.88 to 6.82 items/ind and 0.84 to 6.7 items/g ww across all regions. Values such as the average MP particle number, MP number per individual, MP number per gram and annual intake for children, adolescents, female adults and male adults differed based on the stations and regions where the mussels were obtained. While the southern shelf exhibited relatively higher MP amounts than the northern shelf, very high values per individual and per gram were also recorded, especially in the Çanakkale Strait. On the other hand, the lowest MP values per individual and per gram were 0.88 MP and 0.84 MP, respectively, in Turan Village (St 11), located at the top point of the Kapıdağ peninsula on the southern shelf of the SoM.
This study demonstrates that the extraction method applied for MPs achieves an efficiency of over 95% in mussel soft tissues. The rates of recovery of the three MP polymer types commonly found in biota (polyethylene—PE; polyethylene terephthalate—PET; and polypropylene—PP) were between 89% and 97%, with the highest rate for PE and the lowest rate for PET.
3.3. Morphology and Polymer Type of MPs in Mussels
The predominant size category for MPs was less than 0.5 mm. MPs were divided into six classes according to their shape: fibers, films, pellets, filaments, fragments and foams.
In the study, seven different MP colors were detected in all mussels, and the most common color was blue (mean 57.9%). Red (20.3%), followed by black (mean 16.3%). The colors with relatively lower rates were transparent (7.5%), white (4.9%) and purple (37%). The yellow color was detected at only one station, with a rate of 4%.
When MPs were evaluated according to their shape, the shape with the highest percentage was found to be fiber (61.1%). The Other MP shapes following the fiber type were fragment (18%), filament (12.7%) and film (10%), while the shapes with the lowest percentages were foam (4.9%) and pellet (1.6%). The station with the highest number of fiber MPs among the stations is Kurşunlu station (St 13) in Gemlik Bay, with 81.25%. Kurşunlu station was followed by Florya (St 1) with 79%. The lowest proportions of foam-type MPs were found at Yenice (St 12) (4%) on the southern shelf and Karamürsel (St 16) (5.7%) in the Gulf of İzmit, while pellet-type MPs were found only at Yenice station (1.6%).
Thirty-five sub-samples randomly selected from the MPs taken from mussel samples and ranging in size from 0.5 mm to 5 mm were analyzed using FTIR spectroscopy. The most common polymer type was polyethylene (PE), at 44%, followed by polyethylene terephthalate (PTE), polypropylene (PP), acrylonitrile butadiene styrene (ABS), polyvinyl chloride (PVC) and polystyrene (PS) with 28%, 11%, 8%, 6% and 3%, respectively (PE > PET > PP > ABS > PVC > PS).
3.4. Pollution Load Index and Microplastic Contamination Factor
MPCf values were calculated in mussels collected from different stations in order to reveal the pollution status. The lowest MPCf value was found at Beykoz (St 18) station, at 1.25, and the highest value was found at Yenice (St 12) station, with 7.98 across all length groups of mussels. It can be seen that the highest MPCf is 6 times higher than the lowest value. MPLI values, calculated regionally using MPCf values, were found to be the lowest in Gulf of İzmit, with 1.32, and the highest in the Çanakkale Strait.
3.5. Health Risk Assessment
M. galloprovincialis is a well known and frequently consumed species among crustaceans since it is a commercial mussel species. The consumption of Mediterranean mussels collected from coastal areas poses a risk of MP ingestion. Our risk assessments were made on mussels in two different size groups, S (<0.5 cm) and L (>5 cm). Biometric measurements according to the stations and the mussel groups in the stations are given in Table 1.
The estimated CDI risk of MP ingestion for was calculated on the basis of the consumption of a single mussel meal portion, equivalent to 100 g, 225 g and 1.19 kg per year, as reported by the WHO and FAO [60], EFSA [61] and EUMOFA [62], respectively. Since data on the number of consumed mussels used in the calculations (1.19 kg per year) include both soft tissue and shell, the total weight of the shelled mussels were calculated to accurately reflect these data.
4. Discussion
It is well known that plastic pollution in the marine environment today is considered a real threat due to its distribution on a global scale and its adverse effects on the molecular level, involving the physiological performance and health of organisms. Plastics in marine ecosystems have a long lifespan. Therefore, even if their production and disposal were to cease, their negative impacts on marine life would continue for decades [51].
Recently, substantial progress has been achieved in understanding MP accumulation in bivalves from various production sources, including aquaculture and fisheries [63]. On the other hand, research on that specific topic is limited compared to studies that assess all aspects of MPs (characterization, distribution, risk assessment). This lack of information on MPs in mussels and the associated detailed risk assessments weakens the sensitivity of risk estimations for the control of food safety and the management of the environment [64].
The pollution source of the SoM, especially the South Shelf, is river discharge. For example, the Simav River, the largest river flowing into the SoM, is one of these sources. The river flows through several settlements before reaching the SoM after passing Karacabey. During its course, the river is nourished by overflow from the Uluabat and Kuşgöl lakes, along with numerous large and small streams, including the Orhaneli Stream, Mustafakemalpaşa Stream and the Kocaçay and Nilüfer Streams. After passing through Balıkesir province, the Simav Stream is referred to as the Susurluk River. In the Susurluk Basin, where Kütahya, Balıkesir and Bursa provinces and districts are situated, there are many industrial organizations/facilities such as oil, cement, sugar, marble and meat–dairy integrated facilities, as well as agricultural areas [65,66]. The Simav Stream is known to be under intense pollution pressure [67]. The station with the highest MP per g among the stations is Yenice (St 12), located in the Susurluk Basin where the Simav River flows into the Susurluk Basin.
At Kumkale (St 7), another station with high values, concentrations were 5.17 items/g and 4.31 items/ind. Kumkale station is under the influence of multiple pollution sources. One of these influencing factors is that it is close to the point where the Hamamlik Stream and Küçük Menderes Stream flow. The station is also situated at the exit of the Çanakkale Strait. In addition to the pollution from the Black Sea, the upper current system of the SoM carries a heavy pollution load from the Çanakkale Strait to the Aegean Sea, also taking pollution through the SoM. Therefore, Kumkale station is thought to be affected by this pollution load as well as the nearby rivers.
TSS levels affect the presence of microplastic abundance and are associated with microplastic and suspended particle aggregation [68]. Microplastic particles have a lower density than water, allowing the particles to float in the water column. These properties cause microplastics to become part of the mass of suspended particles [69]. Several studies have examined the correlations between TSS and MP abundance in different environments. Peller et al. [70] showed that, although microplastics are part of the TSS, there is no clear correlation between microplastic and TSS concentrations. Similarly, Junior et al. [71], investigating the Surabaya River (Indonesia), Kwon et al. [72], investigating a wastewater treatment plant, and Ross et al. [73] investigating MP discharge through urban stormwater, did not find any correlation between these two parameters. On the other hand, Dhea et al. [74] tried to understand the correlations between MP and water quality parameters in the Brantas River (Indonesia) and underlined that there was a strong correlation between MPs and TSS. Wolff et al. [75] found a significant relationship between these two parameters in a wastewater treatment plant. Similarly, a significant positive correlation was found between MPs and PTS in this study (r = 0.509, p = 0.031). While Erkan et al. [27] indicated a positive correlation between TDS and MP abundance in water concentrations, in this study no correlation was found between MPs and other physicochemical parameters, including TDS. In sum, the relationships between TSS and MPs differ. This can be explained by the fact that the amount of MP and the parameters of water quality are complex parameters that can be influenced by several factors. For instance, the abundance of MPs can be affected by water currents, plastic-containing wastewater and anthropogenic activities [76,77]. Therefore, the relationship between TSS and MPs in different regions and environments should be further investigated, and additional data from different conditions should be collected and analyzed to confirm the significant relationship between water quality and microplastics. Moreover, it should be noted that the SoM is an oceanographic environment with a unique hydrodynamic structure, and it is exposed to different pollution sources incomparable to other natural environments.
CF values were calculated for each station. According to these results, the CF values varied between 1.25 and 6.15. The lowest value was found at Beykoz station, and the highest value was found at Kumkale station (Figure 2, Table 2). When the CF values are grouped according to regions—İzmit Gulf (GI), Gemlik Gulf (GB), Marmara North Shelf (NS), Marmara South Shelf (SS) and Çanakkale Strait (ÇS)—the average CF values are 1.36 (Moderate), 2.03 (Moderate), 2.28 (Moderate), 2.87 (Moderate) and 3.03 (High), respectively. In addition, the CFs calculated at Kumkale station, situated at the exit of the Çanakkale Strait, and Yenice station, located on the southern shelf, were found to be in the “Very High” class, with values of 6.15 and 7.78, respectively. When the PLI values were analyzed regionally, the highest values were found in the Çanakkale Strait with 3.28 and the South Shelf with 3.09, and were they classified as “High”. The other regions were evaluated as “Moderate” as they ranged between 1 and 3 (Table 2). It was calculated that MP values changed significantly depending on region. MP values were found to follow the order of CS, NS, SS, GB and GI, from high to low values (p < 0.05). Even the Beykoz station, which has the lowest CF value, is categorized as “Moderate”, since its PLI values are in the 1–3 range, and the PLI values of the North Shelf, Gemlik Bay and Gulf of İzmit regions are also in the 1–3 range, suggesting that the SoM coasts are under serious pollution threat.
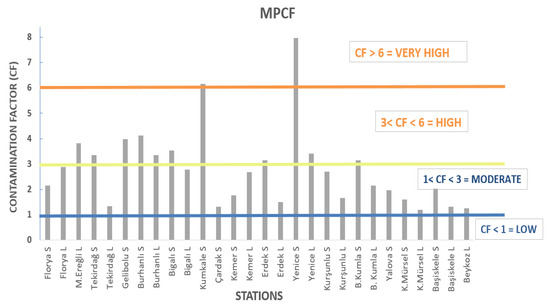
Figure 2.
MPCF values.

Table 2.
CF and PLI values of MPs in mussels according to stations and regions.
The classification of MP size is considered a key factor affecting their distribution, movement, uptake by mussels, bioavailability in the marine ecosystem, elimination and translocation. The plastic debris observed in this study were grouped into four different types according to their size: 3–5 mm, 1–3 mm, 0.5–1 mm and <0.5 mm (Figure 3a). In MP studies on mussels from various regions, the most predominant size ranges have been recorded as 0.5–1 mm [24,31], >1 mm [33] and 0.2–1 mm [34]. The most common size range in the present study was <0.5 mm, similar to the results for the coastal mussels of the SoM [22].
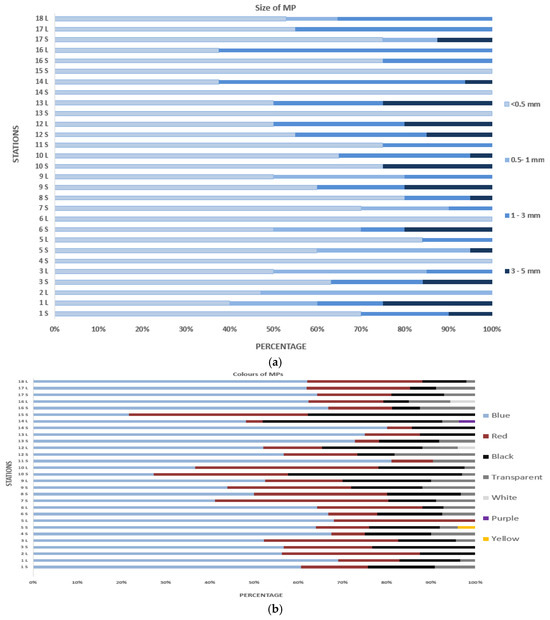
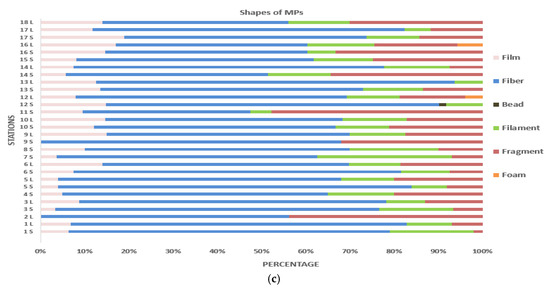
Figure 3.
Morphological properties of MPs in mussels according to station: (a) size of MPs; (b) colors of MPs; (c) shapes of MPs.
Microplastics were classified into seven color groups: blue (57.87%), red (20.27%), black (16.30%), transparent (7.48%), white (4.85%), purple (3.7%) and yellow (4%, found at one station). Blue was the most common color, consistent with a study in Bandırma Bay [30]. While Gedik et al. [22] did not specify colors in their study on SoM mussels, Tunçelli and Erkan [31] identified 16 colors, with black predominating. Other studies [33,78,79] also reported various colors, including blue, red, black, purple, transparent and yellow. The color of microplastics can indicate their degradation level and photodegradation in aquatic environments [80].
Another morphological characteristic of the MPs found in the mussels collected for this study was their shape. The most dominant shape identified in this study is fiber, with an average of 61.08%. The next most prevalent shapes after fiber are fragment (17.93%), filament (12.66%), film (10.02%), foam (4.85%) and pellet (1.6%) (Figure 3c). Similarly, while fiber has been reported to be the most predominant shape among the microplastics examined in mussels [22,31,33,81,82,83,84,85,86,87,88], film [32] and fragment [22,89,90] have been recorded as the most dominant shapes in other studies. The discharge of tiny fibers originating from textiles into marine environment close to highly populated regions is notably affected by wastewater from washing machines [91].
The stations with the highest presence of fibers were Yenice (St 12) 75.4%, Kurşunlu (St 13) 81.25% and Bigalı (St 6). Kurşunlu station is located in Gemlik Bay, and high MP contamination has been reported in fish and sediment samples from this bay [92]. It is worth stating that Gemlik Bay is a center of industry and trade, with the presence of commercial ports [92], and major ports such as Gemport, Borusan and Roda are located in the south of the bay [93]. Moreover, it was reported that the sediments of this region were exposed to significant MP pollution due to the effect of the Karsak Stream flowing into Gemlik Bay [94]. The other two stations with high fiber contents (St 6 and 12) have the fact that they are located close to rivers in common. In particular, Yenice station is close to the Simav River, while Bigalı station is close to the Kayaaltı Stream that flows into the Çanakkale Strait. The fact that all three stations mentioned in this study have high fiber-type MP ratios indicates that fiber-type MPs, which are predominant in rivers, are directly transported to the sea, and that the seas are also seriously contaminated and exposed to MPs through rivers.
The study also evaluated any correlation between MPs with shapes such as film, fiber, fragment and filament and total MP values. Accordingly, when the correlation between MPs and shapes was examined, a very strong (r = 0.957, p < 0.001) positive correlation was found between total MPs and fiber, while a strong (r = 0.746, p < 0.001) positive significant correlation was found for filament. Yıbar et al. [89] also conducted a similar statistical evaluation. These researchers found a high positive correlation between fragment and line MP values (r = 0.868; p = 0.001), they also found that total MP values were influenced by fragment and line MP values and that there was a high positive correlation between fragment MP values (r = 0.976; p < 0.001) and line MP values (r = 0.888; p = 0.001).
It was noted that the high proportion of fibers found in these studies could originate from rivers/streams flowing into the sea near the stations [93,95]. Likewise, while emphasizing that rivers flowing into the sea are one of the major contributors of MPs into the marine environment [96], it has also been reported that plastic waste produced on land flows into the sea through rivers and contributes to MP pollution in the marine environment, in addition to the impact of the maritime industry [97]. Another study conducted in the Gulf of Thailand similarly revealed an increase in river-derived MP values [98]. Considering that they act as a sink for plastic debris, the oceans receive a significant amount of plastic waste through rivers each year, with the amount estimated to range from 4.8 to 12.7 million tons [99].
Microfibers, primarily from personal care and textiles, are major pollutants. The textile industry accounts for 14% of global plastic production, with synthetic fibers making up 63% of fiber production [100]. These fibers accumulate in the environment due to wear and tear, and household washing sheds large quantities, entering marine environments through waste, sludge and wastewater. A single garment can shed over 1900 fibers per wash, commonly found in wastewater treatment plants. Over 14 million tons of microfibers have accumulated on the ocean floor, with 200,000–500,000 tons entering the oceans annually [101]. They are present in all ecosystems, including oceans [102] and freshwater [103], and in organisms like fish, birds and humans [104]. An estimated 1.4 trillion microfibers in the ocean threaten biodiversity [105], and their widespread presence explains their frequent detection in bivalves globally.
The process by which mussels absorb synthetic fibers is still unclear, and it is generally assumed that mussels do not exhibit color preferences when selecting food. Plastics also have a wide range of uses, including their use in ropes, mussel farming, suspension buoys and net bags [45]. Studies have found significantly higher concentrations of blue and colorless MPs in bivalves in comparison to other colors, with transparent MPs being the most dominant [64]. Variations in sampling methods may result in differences in the concentrations and colors of MPs found in bivalves, as levels of MP contamination and mussels’ habitats can vary greatly. Additionally, research across various regions has shown that mussels ingest MPs present in coastal waters [106], and MPs smaller than <1000 µm have been found to have considerably toxigenic features for invertebrates [107]. Moreover, ingestion of filamentous particles has been shown to cause more various toxic impacts compared to pellets and fragments [108].
In this study, MPs were determined separately in the S and L size groups of mussels, and there was no significant difference between the accumulation of total MP values in the S and L size groups of mussels (p > 0.05). Similarly, Gedik et al. [22] and Gedik and Eryaşar [24] reported that they had not found any significant relationship between MP concentrations and shell length. When it was examined whether there was a significant difference between MP shapes and mussel sizes, it was calculated that filament values were significantly higher in the S size group of mussels (p < 0.05).
Regions characterized by high human activity, such as Beykoz, Erdek and Kurşunlu, exhibited significantly higher levels of MP abundance compared to regions with less human influence, such as Turan Village. In other studies, MP contamination in Adriatic Sea [80] and SoM [31] mussels has been linked to anthropogenic activities. A higher risk of MP ingestion was found in bivalve samples from areas with high anthropogenic activity [33,109].
Coastal areas face significant pollution from dense settlement, urbanization and industrialization, leading to widespread littoral zone pollution [110]. Besides human influences, environmental factors like substrate type and transport, coastal morphology, river proximity, water currents, prevailing waves and wind can affect MP concentrations and contribute to variations in pollution sources [111]. This may explain the station and regional differences in this study.
Table 1 shows that MPs were found in 100% of the mussels from the all sampling points. Reported incidence rates in the literature vary widely, in the range of 0–100%, and rates vary based on factors like location, time of sampling, sample size and species. The absence of a standard method for the analysis and extraction of MPs contributes to inconsistencies in stated data, especially regarding shape, polymer composition and particle size [112]. Tunçelli and Erkan [31] emphasize that their findings strongly indicate the pervasive presence of MPs. Similarly, this study aligns with previous research, as MPs were found at every sampled station, raising concerns about their impact on the SoM’s ecosystem. To address data gaps across various seafood classes, standardized methodologies are essential for ensuring consistent and reliable exposure assessments.
According to Andrady [113], plastic debris carried from terrestrial areas to the marine environment account for 80% of the total marine garbage, while particles smaller than 5 mm, called MPs, represent 93% of plastic marine litter [114]. Since MPs are a significant concern because of their global distribution and the potential threats they pose to the health of humans, animals and the environment [13,115]. Information on the presence of MPs in biota and aquatic/marine environments is being continually renewed [116,117,118]. This is particularly important for MP contamination in seafood [119,120,121]. The Ellen MacArthur Foundation predicts that, if waste management strategies are not addressed, by 2050, the amount of plastic in the oceans will exceed the number of fish and more than one million marine animals will die annually from causes related to plastics and MPs [122,123].
PP, a common material in food packaging, is known for its fragile features. Both PP and PE experience wear and tear from fishing equipment due to intense fishing facilities [124,125]. Additionally, PE is widely utilized in the manufacturing of plastic bottles and bags, food packaging and agriculture. With densities lower than water, both materials can be transported to the ocean via ocean currents [126]. PP, in particular, has a low resistance to UV radiation and oxidation, causing it to degrade more rapidly in marine environments. As a result, smaller particles are more easily released [127,128].
Acrylonitrile butadiene styrene (ABS)-type plastics were found at two stations (Erdek and Kemer) in this study. ABS is a material used in injection molding. As the name suggests, it is composed of three different materials: acrylonitrile, butadiene and styrene. This combination makes the plastic very strong, with excellent impact resistance and other desirable properties. Acrylonitrile provides high chemical and heat resistance. Butadiene is hard and strong, while styrene adds rigidity and workability to the material. It can be assumed that the increase in pollution in Erdek Bay in recent years has led to this result. Moreover, the presence of this polymer species in both Erdek and Kemer stations suggests that this contamination originated from Gönen Stream, which flows into the SoM between these two regions.
The ability of mussels to efficiently filter high volumes of water while capturing a wide and diverse range of pollutants and particles from the aquatic environment makes them important for MP-oriented research [25,129,130]. Furthermore, because they are sessile and large, they can be easily collected both in the environment and in the coastal area. Due to these benefits, mussels have been utilized for biomonitoring contaminants in oceans [13]. These bivalves have also recently been analyzed to investigate the abundance of MPs in marine ecosystems [22,24,25,31,33,39,131].
Microplastics in the water column exhibit complex movement patterns influenced by waves, wind and currents [132]. Microplastics such as polyethylene (PE), polypropylene (PP) and polystyrene (PS), due to their low density, tend to accumulate and float on the seawater surface with the help of the high surface tension of seawater [133]. Farmed shellfish, which are suspended below the surface in the water column, may ingest small amounts of these microplastics, posing a risk to aquatic organisms [134]. Thus, the findings of this study in wild mussels collected from near the surface are important in terms of revealing the pollution in the environment.
Mussels are an important and highly popular seafood in most of countries around the world and are considered a valuable source of nutrients due to their high content of omega-3 fatty acids, protein and vitamins [135]. Consequently, MP contamination in mussels is a significant concern [13,136,137]. MPs have also been reported in abundance in M. galloprovincialis, a commercially important mussel species in several regions [22,24,31,33,119,138,139,140,141,142].
Mussels are eaten in various culinary preparations worldwide. In Türkiye, M. galloprovincialis mussels are generally consumed as stuffed mussels [143] or/and fried in a batter prepared with flour and egg to make a dish called “fried mussels”. In Spain, mussels are often used in dishes like paella [144], whereas in Italy, they is usually prepared by boiling or steaming [145]. Various studies have assessed annual exposures to MPs from mussel consumption. In our study, the highest exposure from wild mussel consumption was estimated to be 4366.96 items/year. Tunçelli and Erkan [31] reported this amount as 8456 items/year in their study, while Cox et al. [146] reported values as high as 52,000 MPs per year. Furthermore, due to the lack of arranged TDI (tolerable daily intake) limits for MP intake in seafood, it is currently impractical to make a direct comparison using a threshold value.
The CDI values calculated for four different risk groups (children, adolescents, female adults and male adults), two different height groups (S and L) and the rates of 100 g/day, 225 g/day and 1.19 kg/year according to different sources are given in Table A3. When both height groups were evaluated together, the CDI findings calculated for 100 g daily servings ranged from 5.82 × 10−1 to 5.32 for children, 1.04 × 10−1–9.38 × 10−1 for adolescents, 1.25 × 10−2–1.09 × 10−1 for female adults and 1.21 × 10−2–1.09 × 10−1 for male adults.
The CDI values calculated for 225 g per day were found in the same order of 1.31–1.20 × 101, 2.33 × 10−1–2.11, 2.81 × 10−2–2.54 × 10−1, 2.72 × 10−2–2.46 × 10−1 items/day/person. Based on an annual consumption rate of 1.19 kg, all the CDI findings were less than 1 for all the risk groups. According to a daily consumption rate of 100 g, the results were below 1 in all risk groups except children. This suggests that the daily intake of MPs is low and therefore may not pose any risk from daily consumption. However, when calculated based on daily consumptions of both 100 g and 225 g, all the CDI values for children and adolescents varying by station were higher than 1, indicating that the daily intake is high and may pose a risk to the consumer. Studies generally show that MPs are taken up by children at higher rates than adults [147]. The findings in this study also confirm this conclusion. Based on these results, it can be concluded that children are more vulnerable to the negative consequences of exposure to pollution. Therefore, adolescents, particularly children, should exercise caution regarding the consumption of mussels, and measures such as controlling consumption frequency and avoiding products obtained from unconfirmed clean or contaminated regions may contribute to minimizing these risks.
The study also calculated microplastic carcinogenic risk (MPCR). Representative values were calculated for 100 g. A detailed breakdown of the values is shown in Table A4. MPCR values were calculated by averaging the CDI values for two different mussel sizes. Accordingly, MPCR values were recorded in the range of 2.49 × 10−2–7.19 with an average of 1.41 ± 1.04 for PVC, in the range of 1.04 × 10−2–9.85 × 10−1 with an average of 2.43 × 10−1 ± 8.59 × 10−2 for PP and in the range of 1.23 × 10−2–7.95 × 10−2 with an average of 3.70 × 10−2 ± 3.86 × 10−1 for PE and PET. When MPCR was evaluated for all age groups and polymer types, the highest risk was found in children, similar to the other risk calculations. According to US Environmental Protection Agency regulations, the acceptable cancer risk range is 1 × 10−6 to 1 × 10−4 [148]. The MPCR is above this range for all polymer types and all risk groups. In this way, populations in all risk groups appear to be at risk of carcinogenic effects from the oral ingestion of different MP polymers. The findings from this study point to the potential cancer risk from MPs in the digestive exposure pathway through the consumption of mussels.
According to the WHO and FAO [60], with a the daily consumption of 100 g, the annual digestion of MPs was found to be 212.60–1940.87 MPs for children, 37.83–342.51 MPs for adolescents, 4.56–41.27 MPs for female adults and 4.40–39.88 MPs for male adults. According to the EFSA values [61], annual intakes for the same risk groups were 478.36–4366.96 MPs, 85.12–770.64 MPs, 10.26–92.87 MPs and 9.91–89.73 MPs, respectively, based on the 225 g serving size. Based on the EUMOFA values [62], an estimated annual mussel consumption of 1.19 kg, the children, adolescent, female adult and male adult risk groups may be exposed to amounts of 6.93–63.27 MPs, 1.23–11.17 MPs, 0.15–1.35 MPs and 0.14–1.30 MPs, respectively. According to our regional assessment, the highest MP consumption risks are in Yenice on the southern shelf and the Kumkale and Burhanlı stations in the Çanakkale Strait.
It has been reported that consuming a 250 g portion of mussels can result in the ingestion of 90 plastic particles [149]. Risk assessment also suggests that acceptable limits should not exceed 1000 MPs per 250 g of mussel tissue (one serving), taking into account the highest concentration of MPs (4 items/g) and the highest rate of consumption for mussels [150]. Accordingly, our calculation was based on the amount of MP per g of mussel soft tissue found. Although the average amount of MP per g in this study was below 4 items/g for both the S and L group mussels, when calculated for 250 g mussel portions, it was found to be 1292.5 items/portion, only at Kumkale station in the Çanakkale Strait.
Various studies [151,152] have reported considerable variation in estimated human dietary exposure to microplastics, likely due to ambiguities in estimation methods and data origins. The EFSA [61] estimated that consuming a medium-sized portion of mussels (225 g of soft tissue) results in the ingestion of approximately 900 MP particles, equivalent to 7 µg of plastic. Senathirajah et al. [152] assessed global MP intake rates, with estimates ranging from 2602 to 16,288 particles per person per year. Another study found that high shellfish consumers in Europe ingest around 11,000 MP particles annually, while low consumers are exposed to approximately 1800 particles per year [153]. Human exposure through the consumption of mussels is reported to average 751 microplastics/person/year globally, with the maximum intake reported in China [154].
Table 3 presents a comparison of MP content in M. galloprovincialis mussels and annual intake across different global regions and risk groups. The MP levels in this study were higher than those in Italy and northern Portugal, but lower than on Spain’s Catalan coast. Within the study area, mussels from the Çanakkale Strait and southern shelf contained slightly more MPs than those from the northern shelf and bays. The annual MP intake was highest in children, followed by adolescents and adults, consistent with previous studies. However, adult intake in this study was lower, likely due to differences in calculation methods and seafood consumption patterns, which vary by country.

Table 3.
A comparison of risk assessment for the consumption of mussels—M. gallorprovincialis—in different regions of the world. (Since other studies did not distinguish between men and women in their adult risk groups, averages of our adult women and men values were calculated for this table.)
The risk assessment approach for mussels and bivalves containing MPs is different from that for fish and other seafood. This distinction arises because mussels are consumed whole, including all tissues and organs that may be contaminated with MPs. In contrast, in seafood such as fish, tissues and organs that can accumulate MPs, such as digestive and excretory organs and even skin, are typically separated from muscle tissues [156]. Although studies assessing MP-related risks in M. galloprovincialis remain limited, research in this area is steadily increasing. A research on the risk of consumption-associated MP pollution in M. galloprovincialis in Italy found an average of 6.7–7.2 items/g in cooked mussels, compared with 2.67 items/g in raw tissue. According to the highest MP concentration found in mussels, consuming a 225 g portion was estimated to result in an intake of 1395 (cooked) to 1620 (raw) MP particles [144].
Information on the risks of MPs on the health of children and adults is unclear [61]. Previous studies have largely overlooked the fate of ingested microplastics in the human body, including their intestinal absorption and biliary excretion. The accumulation of MPs in body tissues can lead to stress, irritation, oxidative damage and subtle physiological responses [157]. Current studies show limited evidence that microplastics (MPs) significantly affect human cell viability, and there is debate over whether tested exposure levels reflect real tissue accumulation. Further research is needed to assess their health impacts, including potential links to reduced IQ, dwarfism and the slowing of organ growth in children. Given these uncertainties, more detailed studies on the risks of consuming seafood, particularly mussels, are warranted.
Variations in MP analysis methods across studies lead to inconsistent results, even within the same region, underscoring the need for an internationally standardized protocol. Additionally, the long-term monitoring of MP pollution in the heavily contaminated Sea of Marmara is essential in order to more accurately assess the risks associated with consuming its mussels and fish.
5. Conclusions
A detailed potential risk assessment was conducted for consumers of different age groups in the event of mussel consumption, and it was confirmed once again that children are the most vulnerable risk group and are at serious risk. In areas with high MP concentrations, not only anthropogenic sources but also rivers flowing into the sea are present, once again highlighting that MPs can also reach the marine environment via rivers.
In waste management practices, the contamination of soil and other environmental media with microplastics (MPs) is inevitable due to composting and regular disposal. Therefore, it is crucial to implement long-term, sustainable measures such as banning single-use plastic products and ensuring that plastic waste is collected and recycled before it becomes microplastics. It is important to emphasize that all risk groups, especially children and adolescents, should be cautious about consuming mussels. In addition, not consuming products sourced from areas that are not clean or of uncertain cleanliness could minimize these risks.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the author upon request.
Acknowledgments
As the author, I am grateful to Fatih İLHAN (Yıldız Technical University, İstanbul, Türkiye) and Gökhan BALCIOĞLU (Gelişim University, Istanbul, Türkiye) who worked devotedly with me during the field sampling as if it was their own work; Hande ÇAVUŞ ARSLAN (Haliç University, Istanbul, Türkiye) for statistical analysis; Nuray ÇAĞLAR (Istanbul University, Istanbul, Türkiye) for scientific contributions; and Servet BAYRAM (Medipol University, Istanbul, Türkiye) for proofreading and English editing. I am grateful to Fügen BALCIOĞLU (RIP), who I believe is always watching over me from heaven.
Conflicts of Interest
The author declares no conflicts of interest.
Appendix A

Table A1.
Coordinates of sampling points.
Table A1.
Coordinates of sampling points.
| Station No | Station Name | Coordinates |
|---|---|---|
| 1 | Florya | 40°58′39″ N 28°46′22″ E |
| 2 | M. Ereğlisi | 40°58′09″ N 27°57′37″ E |
| 3 | Tekirdağ | 40°00′04″ N 26°14′00″ E |
| 4 | Gelibolu | 40°59′04″ N 27°34′43″ E |
| 5 | Burhanlı | 40°18′23″ N 26°33′45″ E |
| 6 | Bigalı | 40°12′42″ N 26°23′19″ E |
| 7 | Kumkale | 40°00′04″ N 26°14′00″ E |
| 8 | Çardak | 40°22′34″ N 26°42′43″ E |
| 9 | Kemer | 40°24′50″ N 27°03′45″ E |
| 10 | Erdek | 40°22′47″ N 27°47′58″ E |
| 11 | Turan Village | 40°30′35″ N 27°47′03″ E |
| 12 | Yenice | 40°23′22″ N 28°06′49″ E |
| 13 | Kurşunlu | 40°21′51″ N 29°01′51″ E |
| 14 | B. Kumla | 40°28′36″ N 29°05′01″ E |
| 15 | Yalova | 40°30′32″ N 28°50′18″ E |
| 16 | K. Mürsel | 40°41′34″ N 29°36′24″ E |
| 17 | Başiskele | 40°42′59″ N 26°55′10″ E |
| 18 | Beykoz | 41°10′02″ N 29°04′54″ E |

Table A2.
Physicochemical parameters of stations used by Balcıoğlu İlhan et al. [40].
Table A2.
Physicochemical parameters of stations used by Balcıoğlu İlhan et al. [40].
| Station No | DO (mg/L) | pH | Temperature (°C) | Salinity (PSU) | TSS (mg/L) | TDS (mg/L) |
|---|---|---|---|---|---|---|
| 1 | 8.9 | 8.24 | 19.10 | 19.87 | 55.4 | 17.95 |
| 2 | 8.26 | 8.06 | 18.5 | 19.35 | 37 | 17.34 |
| 3 | 8.21 | 8.18 | 18.8 | 20.98 | 29.4 | 18.14 |
| 4 | 8.51 | 8.21 | 17.9 | 21.74 | 32 | 18.89 |
| 5 | 9.69 | 8.3 | 18 | 21.96 | 54.2 | 19.05 |
| 6 | 9.08 | 8.31 | 17.4 | 22.76 | 35.2 | 19.68 |
| 7 | 8.99 | 8.22 | 19.4 | 23.93 | 48 | 23.60 |
| 8 | 8.44 | 8.21 | 18.50 | 21.29 | 28.2 | 19.25 |
| 9 | 9.1 | 8.29 | 19.9 | 21.67 | 27.2 | 19.56 |
| 10 | 9.77 | 8.25 | 19.2 | 21 | 22.6 | 19.90 |
| 11 | 9.05 | 8.2 | 18.7 | 21.3 | 29 | 19.21 |
| 12 | 8.79 | 8.3 | 19.40 | 21.73 | 39.4 | 19.67 |
| 13 | 9.99 | 8.27 | 17.80 | 19.05 | 22.3 | 17.26 |
| 14 | 8.39 | 8.12 | 18.30 | 20.11 | 30.1 | 18.20 |
| 15 | 10.36 | 8.18 | 17.70 | 19.74 | 25.3 | 17.67 |
| 16 | 9.8 | 8.03 | 18.20 | 19.32 | 48 | 18.63 |
| 17 | 9.89 | 8.16 | 18.40 | 19.66 | 45.4 | 18.56 |
| 18 | 6.97 | 8.01 | 18.9 | 18.08 | 12.3 | 10.16 |
| Min | 6.97 | 8.01 | 17.40 | 18.08 | 12.30 | 10.16 |
| Max | 10.36 | 8.31 | 19.90 | 23.93 | 55.40 | 23.60 |
| Mean | 9.01 | 8.20 | 18.56 | 20.75 | 34.50 | 18.48 |
| Sd | 0.80 | 0.09 | 0.66 | 1.42 | 11.50 | 2.44 |

Table A3.
Chronic daily intake (CDI) values of microplastics in mussels (items/day/person).
Table A3.
Chronic daily intake (CDI) values of microplastics in mussels (items/day/person).
| St No Mussel Size | CDI Values (MP/Day/Person) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 g/Day | 225 g/Day | 1.19 kg/Year | ||||||||||
| C | A | WA | MA | C | A | WA | MA | C | A | WA | MA | |
| 1S | 1.43 | 2.52 × 10−1 | 3.04 × 10−2 | 2.94 × 10−2 | 3.21 | 5.67 × 10−1 | 6.84 × 10−2 | 6.60 × 10−2 | 4.66 × 10−2 | 8.22 × 10−3 | 9.90 × 10−4 | 9.57 × 10−4 |
| 1L | 1.91 | 3.40 × 10−1 | 4.10 × 10−2 | 3.96 × 10−2 | 4.30 | 7.66 × 10−1 | 9.23 × 10−2 | 8.92 × 10−2 | 6.24 × 10−2 | 1.11 × 10−2 | 1.34 × 10−3 | 1.29 × 10−3 |
| 2L | 2.53 | 4.50 × 10−1 | 5.42 × 10−2 | 5.23 × 10−2 | 5.69 | 1.01 | 1.22 × 10−1 | 1.18 × 10−1 | 8.24 × 10−2 | 1.47 × 10−2 | 1.77 × 10−3 | 1.71 × 10−3 |
| 3S | 2.23 | 3.94 × 10−1 | 4.74 × 10−2 | 4.58 × 10−2 | 5.02 | 8.86 × 10−1 | 1.07 × 10−1 | 1.03 × 10−1 | 7.27 × 10−2 | 1.28 × 10−2 | 1.55 × 10−3 | 1.49 × 10−3 |
| 3L | 8.89 × 10−1 | 1.58 × 10−1 | 1.91 × 10−2 | 1.84 × 10−2 | 2.00 | 3.56 × 10−1 | 4.29 × 10−2 | 4.15 × 10−2 | 2.90 × 10−2 | 5.16 × 10−3 | 6.22 × 10−4 | 6.01 × 10−4 |
| 4S | 2.65 | 4.68 × 10−1 | 5.64 × 10−2 | 5.45 × 10−2 | 5.96 | 1.05 | 1.27 × 10−1 | 1.23 × 10−1 | 8.64 × 10−2 | 1.52 × 10−2 | 1.84 × 10−3 | 1.78 × 10−3 |
| 5S | 2.75 | 4.86 × 10−1 | 5.86 × 10−2 | 5.66 × 10−2 | 6.20 | 1.09 | 1.32 × 10−1 | 1.27 × 10−1 | 8.98 × 10−2 | 1.58 × 10−2 | 1.91 × 10−3 | 1.84 × 10−3 |
| 5L | 2.22 | 3.95 × 10−1 | 4.76 × 10−2 | 4.60 × 10−2 | 4.99 | 8.89 × 10−1 | 1.07 × 10−1 | 1.03 × 10−1 | 7.24 × 10−2 | 1.29 × 10−2 | 1.55 × 10−3 | 1.50 × 10−3 |
| 6S | 2.36 | 4.16 × 10−1 | 5.01 × 10−2 | 4.84 × 10−2 | 5.30 | 9.36 × 10−1 | 1.13 × 10−1 | 1.09 × 10−1 | 7.68 × 10−2 | 1.36 × 10−2 | 1.63 × 10−3 | 1.58 × 10−3 |
| 6L | 1.83 | 3.26 × 10−1 | 3.93 × 10−2 | 3.80 × 10−2 | 4.13 | 7.34 × 10−1 | 8.85 × 10−2 | 8.55 × 10−2 | 5.98 × 10−2 | 1.06 × 10−2 | 1.28 × 10−3 | 1.24 × 10−3 |
| 7S | 4.10 | 7.24 × 10−1 | 8.73 × 10−2 | 8.43 × 10−2 | 9.23 | 1.63 | 1.96 × 10−1 | 1.90 × 10−1 | 1.34 × 10−1 | 2.36 × 10−2 | 2.84 × 10−3 | 2.75 × 10−3 |
| 8S | 9.29 × 10−1 | 1.64 × 10−1 | 1.97 × 10−2 | 1.91 × 10−2 | 2.09 | 3.69 × 10−1 | 4.44 × 10−2 | 4.29 × 10−2 | 3.03 × 10−2 | 5.34 × 10−3 | 6.44 × 10−4 | 6.22 × 10−4 |
| 9S | 1.18 | 2.09 × 10−1 | 2.51 × 10−2 | 2.43 × 10−2 | 2.66 | 4.70 × 10−1 | 5.66 × 10−2 | 5.47 × 10−2 | 3.86 × 10−2 | 6.80 × 10−3 | 8.20 × 10−4 | 7.92 × 10−4 |
| 9L | 1.77 | 3.15 × 10−1 | 3.80 × 10−2 | 3.67 × 10−2 | 3.98 | 7.09 × 10−1 | 8.54 × 10−2 | 8.26 × 10−2 | 5.77 × 10−2 | 1.03 × 10−2 | 1.24 × 10−3 | 1.20 × 10−3 |
| 10S | 2.10 | 3.71 × 10−1 | 4.47 × 10−2 | 4.32 × 10−2 | 4.73 | 8.35 × 10−1 | 1.01 × 10−1 | 9.72 × 10−2 | 6.86 × 10−2 | 1.21 × 10−2 | 1.46 × 10−3 | 1.41 × 10−3 |
| 10L | 7.64 × 10−1 | 1.36 × 10−1 | 1.64 × 10−2 | 1.58 × 10−2 | 1.72 | 3.06 × 10−1 | 3.68 × 10−2 | 3.56 × 10−2 | 2.49 × 10−2 | 4.43 × 10−3 | 5.34 × 10−4 | 5.16 × 10−4 |
| 11S | 1.18 | 2.09 × 10−1 | 2.51 × 10−2 | 2.43 × 10−2 | 2.66 | 4.70 × 10−1 | 5.66 × 10−2 | 5.47 × 10−2 | 3.86 × 10−2 | 6.80 × 10−3 | 8.20 × 10−4 | 7.92 × 10−4 |
| 12S | 5.32 | 9.38 × 10−1 | 1.13 × 10−1 | 1.09 × 10−1 | 1.20 × 101 | 2.11 | 2.54 × 10−1 | 2.46 × 10−1 | 1.73 × 10−1 | 3.06 × 10−2 | 3.69 × 10−3 | 3.56 × 10−3 |
| 12L | 2.25 | 4.01 × 10−1 | 4.83 × 10−2 | 4.66 × 10−2 | 5.07 | 9.01 × 10−1 | 1.09 × 10−1 | 1.05 × 10−1 | 7.34 × 10−2 | 1.31 × 10−2 | 1.57 × 10−3 | 1.52 × 10−3 |
| 13S | 1.80 | 3.18 × 10−1 | 3.83 × 10−2 | 3.70 × 10−2 | 4.05 | 7.15 × 10−1 | 8.62 × 10−2 | 8.33 × 10−2 | 5.87 × 10−2 | 1.04 × 10−2 | 1.25 × 10−3 | 1.21 × 10−3 |
| 13L | 1.07 | 1.90 × 10−1 | 2.30 × 10−2 | 2.22 × 10−2 | 2.41 | 4.29 × 10−1 | 5.16 × 10−2 | 4.99 × 10−2 | 3.49 × 10−2 | 6.21 × 10−3 | 7.48 × 10−4 | 7.23 × 10−4 |
| 14S | 2.10 | 3.70 × 10−1 | 4.46 × 10−2 | 4.31 × 10−2 | 4.71 | 8.32 × 10−1 | 1.00 × 10−1 | 9.69 × 10−2 | 6.83 × 10−2 | 1.21 × 10−2 | 1.45 × 10−3 | 1.40 × 10−3 |
| 14L | 1.42 | 2.52 × 10−1 | 3.04 × 10−2 | 2.94 × 10−2 | 3.19 | 5.67 × 10−1 | 6.84 × 10−2 | 6.60 × 10−2 | 4.62 × 10−2 | 8.22 × 10−3 | 9.90 × 10−4 | 9.57 × 10−4 |
| 15S | 1.31 | 2.31 × 10−1 | 2.78 × 10−2 | 2.69 × 10−2 | 2.95 | 5.20 × 10−1 | 6.27 × 10−2 | 6.05 × 10−2 | 4.27 × 10−2 | 7.53 × 10−3 | 9.08 × 10−4 | 8.77 × 10−4 |
| 16S | 1.06 | 1.88 × 10−1 | 2.26 × 10−2 | 2.19 × 10−2 | 2.39 | 4.22 × 10−1 | 5.09 × 10−2 | 4.92 × 10−2 | 3.47 × 10−2 | 6.12 × 10−3 | 7.37 × 10−4 | 7.12 × 10−4 |
| 16L | 7.87 × 10−1 | 1.40 × 10−1 | 1.69 × 10−2 | 1.63 × 10−2 | 1.77 | 3.15 × 10−1 | 3.80 × 10−2 | 3.67 × 10−2 | 2.57 × 10−2 | 4.57 × 10−3 | 5.50 × 10−4 | 5.32 × 10−4 |
| 17S | 1.35 | 2.38 × 10−1 | 2.87 × 10−2 | 2.77 × 10−2 | 3.04 | 5.36 × 10−1 | 6.46 × 10−2 | 6.24 × 10−2 | 4.40 × 10−2 | 7.76 × 10−3 | 9.35 × 10−4 | 9.04 × 10−4 |
| 17L | 8.66 × 10−1 | 1.54 × 10−1 | 1.86 × 10−2 | 1.79 × 10−2 | 1.95 | 3.47 × 10−1 | 4.18 × 10−2 | 4.04 × 10−2 | 2.82 × 10−2 | 5.02 × 10−3 | 6.05 × 10−4 | 5.85 × 10−4 |
| 18L | 5.82 × 10−1 | 1.04 × 10−1 | 1.25 × 10−2 | 1.21 × 10−2 | 1.31 | 2.33 × 10−1 | 2.81 × 10−2 | 2.72 × 10−2 | 1.90 × 10−2 | 3.38 × 10−3 | 4.07 × 10−4 | 3.93 × 10−4 |
| Min | 5.82 × 10−1 | 1.04 × 10−1 | 1.25 × 10−2 | 1.21 × 10−2 | 1.31 | 2.33 × 10−1 | 2.81 × 10−2 | 2.72 × 10−2 | 1.90 × 10−2 | 3.38 × 10−3 | 4.07 × 10−4 | 3.93 × 10−4 |
| Max | 5.32 | 9.38 × 10−1 | 1.13 × 10−1 | 1.09 × 10−1 | 1.20 × 101 | 2.11 | 2.54 × 10−1 | 2.46 × 10−1 | 1.73 × 10−1 | 3.06 × 10−2 | 3.69 × 10−3 | 3.56 × 10−3 |
| Mean | 1.82 | 3.22 × 10−1 | 3.88 × 10−2 | 3.75 × 10−2 | 4.09 | 7.24 × 10−1 | 8.73 × 10−2 | 8.44 × 10−2 | 5.93 × 10−2 | 1.05 × 10−2 | 1.26 × 10−3 | 1.22 × 10−3 |

Table A4.
Microplastic carcinogenic risk (MPCR) values for 100 g intake.
Table A4.
Microplastic carcinogenic risk (MPCR) values for 100 g intake.
| Station No | MPCR | |||||
| Children | Adolescents | |||||
| PVC | PP | PE-PET | PVC | PP | PE-PET | |
| 1 | - | - | 1.70 | - | - | 3.02 × 10−1 |
| 3 | - | - | 1.59 | - | - | 2.81 × 10−1 |
| 5 | - | - | 2.54 | - | - | 4.49 × 10−1 |
| 6 | - | 5.03 × 10−1 | 2.14 | - | 8.91 × 10−2 | 3.79 × 10−1 |
| 7 | - | 9.85 × 10−1 | 4.19 | - | 1.74 × 10−1 | 7.39 × 10−1 |
| 9 | - | - | 1.51 | - | - | 2.67 × 10−1 |
| 10 | - | - | 1.46 | - | - | 2.59 × 10−1 |
| 12 | 7.19 | 9.08 × 10−1 | 3.86 | 1.27 | 1.61 × 10−1 | 6.83 × 10−1 |
| 13 | - | - | 1.46 | - | - | 2.59 × 10−1 |
| 14 | - | - | 1.79 | - | - | 3.17 × 10−1 |
| 17 | 2.10 | - | 1.13 | 3.73 × 10−1 | - | 2.00 × 10−1 |
| 18 | - | - | 5.94 × 10−1 | - | - | 1.06 × 10−1 |
| Min | 2.10 | 5.03 × 10−1 | 5.94 × 10−1 | 3.73 × 10−1 | 8.91 × 10−2 | 1.06 × 10−1 |
| Max | 7.19 | 9.85 × 10−1 | 4.19 | 1.27 | 1.74 × 10−1 | 7.39 × 10−1 |
| Mean | 4.65 | 7.99 × 10−1 | 1.87 | 8.22 × 10−1 | 1.41 × 10−1 | 3.31 × 10−1 |
| Sd | 2.55 | 2.11 × 10−1 | 9.49 × 10−1 | 4.49 × 10−1 | 3.73 × 10−2 | 1.68 × 10−1 |
| Station No | MPCR | |||||
| Female Adults | Male Adults | |||||
| PVC | PP | PE-PET | PVC | PP | PE-PET | |
| 1 | - | - | 3.64 × 10−2 | - | - | 3.52 × 10−2 |
| 3 | - | - | 3.39 × 10−2 | - | - | 3.28 × 10−2 |
| 5 | - | - | 5.41 × 10−2 | - | - | 5.23 × 10−2 |
| 6 | - | 1.07 × 10−2 | 4.56 × 10−2 | - | 1.04 × 10−2 | 4.41 × 10−2 |
| 7 | - | 2.09 × 10−2 | 8.90 × 10−2 | - | 2.02 × 10−2 | 8.60 × 10−2 |
| 9 | - | - | 3.22 × 10−2 | - | - | 3.11 × 10−2 |
| 10 | - | - | 3.12 × 10−2 | - | - | 3.01 × 10−2 |
| 12 | 1.53 × 10−1 | 1.94 × 10−2 | 8.23 × 10−2 | 8.50 × 10−2 | 1.87 × 10−2 | 7.95 × 10−2 |
| 13 | - | - | 3.12 × 10−2 | - | - | 3.02 × 10−2 |
| 14 | - | - | 3.82 × 10−2 | - | - | 3.69 × 10−2 |
| 17 | 4.49 × 10−2 | - | 2.41 × 10−2 | 2.49 × 10−2 | - | 2.33 × 10−2 |
| 18 | - | - | 1.27 × 10−2 | - | - | 1.23 × 10−2 |
| Min | 4.49 × 10−2 | 1.07 × 10−2 | 1.27 × 10−2 | 2.49 × 10−2 | 1.04 × 10−2 | 1.23 × 10−2 |
| Max | 1.53 × 10−1 | 2.09 × 10−2 | 8.90 × 10−2 | 8.50 × 10−2 | 2.02 × 10−2 | 7.95 × 10−2 |
| Mean | 9.90 × 10−2 | 1.70 × 10−2 | 3.99 × 10−2 | 5.50 × 10−2 | 1.64 × 10−2 | 3.70 × 10−2 |
| Sd | 5.41 × 10−2 | 4.50 × 10−3 | 2.02 × 10−2 | 3.01 × 10−2 | 4.31 × 10−3 | 1.67 × 10−2 |
References
- IUCN (International Union for Conservation of Nature and Natural Resources). Plastic Pollution. Issues Brief. 2024. Available online: https://www.iucn.org (accessed on 23 March 2025).
- WWF. Bending the Curve of Biodiversity Loss. In Living Planet Report 2020; Almond, R.E.A., Grooten, M., Petersen, T., Eds.; WWF: Gland, Switzerland, 2020. [Google Scholar]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants, 1st ed.; Elsevier Limited: Oxford, UK, 2016; p. 330. [Google Scholar]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Cowger, W.; Erdle, L.M.; Coffin, S.; Villarrubia-Gomez, P.; Moore, C.J.; Wilcox, C. A Growing Plastic Smog, Now Estimated to Be Over 170 Trillion Plastic Particles Afloat in the World’s Oceans—Urgent Solutions Required. PLoS ONE 2023, 18, e0281596. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Frias, J.P.G.L.; Booth, A.M.; Vieira, L.R.; Masura, J.; Baker, J.; Foster, G.; Guilhermino, L. Microplastics Pollution in the Marine Environment. In World Seas: An Environmental Evaluation. Volume III: Ecological Issues and Environmental Impacts, 2nd ed.; Sheppard, C., Ed.; Academic Press (Elsevier): London, UK, 2019; Volume 3, pp. 329–351. [Google Scholar]
- Kane, I.A.; Clare, M.A. Dispersion, Accumulation, and the Ultimate Fate of Microplastics in Deep-Marine Environments: A Review and Future Directions. Front. Earth Sci. 2019, 7, 80. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic Polymers in Mediterranean Surface Waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef] [PubMed]
- Strungaru, S.A.; Jijie, R.; Nicoara, M.; Plavan, G.; Faggio, C. Micro (Nano) Plastics in Freshwater Ecosystems: Abundance, Toxicological Impact and Quantification Methodology. Trends Anal. Chem. 2018, 110, 116–128. [Google Scholar] [CrossRef]
- Djekoun, M.; Gaaied, S.; Romdhani, I.; Rida, A.M.; Missaoui, Y.; Boubekeur, M.S.; Trea, F.; Lakbar, C.; Ouali, K.; Banni, M. Abundance and Distribution of Environmental Microplastic in Edible Fish and Mussels from the South Mediterranean Coasts. Mar. Pollut. Bull. 2024, 206, 116705. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine Litter Plastics and Microplastics and Their Toxic Chemicals Components: The Need for Urgent Preventive Measures. Environ. Sci. Eur. 2018, 30, 1314. [Google Scholar] [CrossRef]
- Zitouni, N.; Cappello, T.; Missawi, O.; Boughattas, I.; De Marco, G.; Belbekhouche, S.; Mokni, M.; Alphonse, V.; Guerbej, H.; Bousserrhine, N.; et al. Metabolomic Disorders Unveil Hepatotoxicity of Environmental Microplastics in Wild Fish Serranus Scriba (Linnaeus 1758). Sci. Total Environ. 2022, 838 Pt 1, 155872. [Google Scholar] [CrossRef]
- Missawi, O.; Bousserrhine, N.; Belbekhouche, S.; Zitouni, N.; Alphonse, V.; Boughattas, I.; Banni, M. Abundance and Distribution of Small Microplastics (≤3 μm) in Sediments and Seaworms from the Southern Mediterranean Coasts and Characterisation of Their Potential Harmful Effects. Environ. Pollut. 2020, 263 Pt A, 114634. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, J. Adverse Outcome Pathways Potentially Related to Hazard Identification of Microplastics Based on Toxicity Mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Sureda, A.; Capo, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic Ingestion by Mullus surmuletus Linnaeus, 1758 Fish and Its Potential for Causing Oxidative Stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bricker, S.; Lauenstein, G.; Maruya, K. NOAA’s Mussel Watch Program: Incorporating Contaminants of Emerging Concern (CECs) into a Long-Term Monitoring Program. Mar. Pollut. Bull. 2014, 81, 289–290. [Google Scholar] [CrossRef]
- Rubini, S.; Munari, M.; Baldini, E.; Barsi, F.; Meloni, D.; Pussini, N.; Barchiesi, F.; Di Francesco, G.; Losasso, C.; Cocumelli, C.; et al. Microplastics in Mussels (Mytilus galloprovincialis): Understanding Pollution in Italian Seas. Toxics 2025, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, I.; Štefanko, K.; Špada, V.; Pustijanac, E.; Buršić, M.; Burić, P. Microplastics in Mediterranean Mussel Mytilus galloprovincialis: Comparison between Cultured and Wild-Type Mussels from the Northern Adriatic. Appl. Sci. 2024, 14, 2056. [Google Scholar] [CrossRef]
- Gedik, K.; Eryaşar, A.R.; Gözler, A.M. The Microplastic Pattern of Wild-Caught Mediterranean Mussels from the Marmara Sea. Mar. Pollut. Bull. 2022, 175, 113331. [Google Scholar] [CrossRef]
- De Simone, S.; Perošević-Bajčeta, A.; Joksimović, D.; Beccherelli, R.; Zografopoulos, D.C.; Mussi, V. Study of Microplastics and Inorganic Contaminants in Mussels from the Montenegrin Coast, Adriatic Sea. J. Mar. Sci. Eng. 2021, 9, 544. [Google Scholar] [CrossRef]
- Gedik, K.; Eryaşar, A.R. Microplastic Pollution Profile of Mediterranean Mussels (Mytilus galloprovincialis) Collected along the Turkish Coasts. Chemosphere 2020, 260, 127570. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using Mussel as a Global Bioindicator of Coastal Microplastic Pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef] [PubMed]
- TurkStat (Turkish Statistics). Population and Housing Census. 2021. Available online: https://data.tuik.gov.tr/Bulten/Index?p=Nufus-ve-Konut-Sayimi-2021-45866 (accessed on 28 June 2023).
- Erkan, H.S.; Turan, N.B.; Albay, M.; Engin, G.O. A Preliminary Study on the Distribution and Morphology of Microplastics in the Coastal Areas of Istanbul, the Metropolitan City of Turkey: The Effect of Location Differences. J. Clean. Prod. 2021, 307, 127320. [Google Scholar] [CrossRef]
- Öztürk, I.; Seker, M. Ecology of the Marmara Sea: Formation and Interactions of Marine Mucilage, and Recommendations for Solutions. In Turkish Academy of Sciences; TÜBA: Ankara, Türkiye, 2021. [Google Scholar]
- Li, Q.; Ma, C.; Zhang, Q.; Shi, H. Microplastics in Shellfish and Implications for Food Safety. Curr. Opin. Food Sci. 2021, 40, 192–197. [Google Scholar] [CrossRef]
- Mutlu, T.; Eryaşar, A.R.; Karaoğlu, K.; Veske, E.; Gedik, K. Microplastics Pollution in Gulf of Bandırma, Sea of Marmara: Biota and Sediment. Mar. Pollut. Bull. 2025, 213, 117667. [Google Scholar] [CrossRef]
- Tunçelli, İ.C.; Erkan, N. Microplastic Pollution in Wild and Aquacultured Mediterranean Mussels from the Sea of Marmara: Abundance, Characteristics, and Health Risk Estimations. Environ. Res. 2024, 242, 117787. [Google Scholar] [CrossRef] [PubMed]
- Galyon, F.; Ünver Alçay, A. Microplastic Contamination in Raw Mussels Collected in Istanbul. Reg. Stud. Mar. Sci. 2023, 68, 103280. [Google Scholar] [CrossRef]
- Yozukmaz, A. Investigation of Microplastics in Edible Wild Mussels from İzmir Bay (Aegean Sea, Western Turkey): A Risk Assessment for the Consumers. Mar. Pollut. Bull. 2021, 171, 112733. [Google Scholar] [CrossRef]
- Provincial Directorate of Environment, Urbanization and Climate Change, Çanakkale Governorship. Çanakkale Province Environmental Status Report for 2023; Provincial Directorate of Environment, Urbanization and Climate Change, Çanakkale Governorship: Çanakkale, Türkiye, 2023; SS.183. [Google Scholar]
- Beşiktepe, Ş.T.; Sur, H.I.; Özsoy, E.; Latif, M.A.; Oǧuz, T.; Ünlüata, Ü. The Circulation and Hydrography of the Marmara Sea. Prog. Oceanogr. 1994, 34, 285–334. [Google Scholar] [CrossRef]
- Beşiktepe, Ş.T. Density Currents in the Two-Layer Flow: An Example of Dardanelles Outflow. Oceanol. Acta 2003, 26, 243–253. [Google Scholar] [CrossRef]
- Özsoy, E.; Tuğrul, S.; Delfanti, R.; Sannino, G. Dynamics of High Energy Environment: Processes at the Turkish Straits System (DEEP), TUBITAK1001. Available online: https://open.metu.edu.tr/bitstream/handle/11511/49585/TVRVeU16UTA.pdf (accessed on 6 March 2025).
- Özgür, S. The Pollutants Accumulated in the Marmara Sea Basin. Master’s Thesis, Gebze Institute of Advanced Technology, Graduate School, Gebze, Türkiye, 2006. [Google Scholar]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Mussels along the Coastal Waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Balcıoğlu İlhan, E.B.; İlhan, F.; Balcıoğlu, G.; Taşkın Kaya, D.; Ismayilova, I.J.; Çağlar Balkıs, N. Correlation between Water Quality and Pollution Parameters in the Surface Waters of the Coastal Areas of the Sea of Marmara, Türkiye. Int. J. Environ. Geoinform. 2025, 12, 28–42. [Google Scholar] [CrossRef]
- Winkler, L.W. The Determination of Oxygen Dissolved in Water. Rep. Ger. Chem. Soc. 1888, 21, 2843–2855. [Google Scholar]
- APHA; AWWA; WPCP. Standard Methods for the Examination of Water and Wastewater, 15th ed.; APHA: Washington, DC, USA, 1980. [Google Scholar]
- Hermsen, E.; Mintenig, S.M.; Besseling, E.; Koelmans, A.A. Quality Criteria for the Analysis of Microplastic in Biota Samples: A Critical Review. Environ. Sci. Technol. 2018, 52, 10230–10240. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Van Werven, B.; Van Oyen, A.; Meijboom, A.; Rebolledo, E.L.B.; Van Franeker, J.A. The Use of Potassium Hydroxide (KOH) Solution as a Suitable Approach to Isolate Plastics Ingested by Marine Organisms. Mar. Pollut. Bull. 2017, 115, 86–90. [Google Scholar] [CrossRef]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, Isolating and Identifying Microplastics Ingested by Fish and Invertebrates. Anal. Methods 2017, 9, 1346–1360. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in Seafood: Benchmark Protocol for Their Extraction and Characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Sparks, C.; Awe, A.; Maneveld, J. Abundance and Characteristics of Microplastics in Retail Mussels from Cape Town, South Africa. Mar. Pollut. Bull. 2021, 166, 112186. [Google Scholar] [CrossRef]
- Wakkaf, T.; El Zrelli, R.; Kedzierski, M.; Balti, R.; Shaiek, M.; Mansour, L.; Tlig-Zouari, S.; Bruzaud, S.; Rabaoui, L. Microplastics in Edible Mussels from a Southern Mediterranean Lagoon: Preliminary Results on Seawater-Mussel Transfer and Implications for Environmental Protection and Seafood Safety. Mar. Pollut. Bull. 2020, 158, 111355. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Zhao, S.; Ward, J.E.; Danley, M.; Mincer, T.J. Field-Based Evidence for Microplastic in Marine Aggregates and Mussels: Implications for Trophic Transfer. Environ. Sci. Technol. 2018, 52, 11038–11048. [Google Scholar] [CrossRef]
- Avio, C.G.; Cardelli, L.R.; Gorbi, S.; Pellegrini, D.; Regoli, F. Microplastics Pollution after the Removal of the Costa Concordia Wreck: First Evidences from a Biomonitoring Case Study. Environ. Pollut. 2017, 227, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gwinnett, C.; Miller, R.Z. Are We Contaminating Our Samples? A Preliminary Study to Investigate Procedural Contamination During Field Sampling and Processing for Microplastic and Anthropogenic Microparticles. Mar. Pollut. Bull. 2021, 173 Pt B, 113095. [Google Scholar] [CrossRef]
- Kabir, A.H.M.E.; Sekine, M.; Imai, T.; Yamamoto, K.; Iwasaki, Y.; Kodera, K. Assessing Small-Scale Freshwater Microplastics Pollution, Land-Use, Source-to-Sink Conduits, and Pollution Risks: Perspectives from Japanese Rivers Polluted with Microplastics. Sci. Total Environ. 2021, 768, 144655. [Google Scholar] [CrossRef]
- Vieira, A.R.; Collignon, P.; Aarestrup, F.M.; McEwen, S.A.; Hendriksen, R.S.; Hald, T.; Wegener, H.C. Association Between Antimicrobial Resistance in Escherichia coli Isolates from Food Animals and Blood Stream Isolates from Humans in Europe: An Ecological Study. Foodborne Pathog. Dis. 2011, 8, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- World Data. Average Height and Weight by Country. Available online: https://www.worlddata.info/average-bodyheight.php#by-country (accessed on 21 July 2024).
- USEPA. Health Assessment Summary Tables. Annual FY-92; Prepared by the Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, Cincinnati, OH, for the Office of Emergency and Remedial Response, Washington, DC; USEPA: Washington, DC, USA, 1992. [Google Scholar]
- USEPA. Toxics Release Inventory Relative Risk-Based Environmental Indicators: Interim Toxicity Weighting Summary Document. pp. 1–252. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P1003WSB.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1995+Thru+1999&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C95thru99%5CTxt%5C00000023%5CP1003WSB.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 3 August 2024).
- Salamone, J.C. (Ed.) Polymeric Materials Encyclopedia, v.8 P; CRC Press: Boca Raton, FL, USA, 1996; pp. 6036–6037. [Google Scholar]
- Enyoh, C.E.; Verla, A.W.; Rakib, M.R.J. Application of Index Models for Assessing Freshwater Microplastics Pollution. World News Nat. Sci. 2021, 38, 37–48. [Google Scholar]
- WHO; FAO. Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption, 25–29 January 2010; No. FIPM/R978 (En); World Health Organization: Rome, Italy, 2011. [Google Scholar]
- EFSA. Statement on the Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 2016, 14, 4501. [Google Scholar] [CrossRef]
- EUMOFA. The EU Fish Market. EUMOFA (European Market Observatory for Fisheries and Aquaculture Products, Luxembourg); FAO, The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2022. [Google Scholar]
- Song, K.; Wang, R.; Yang, G.; Xie, S.; Chen, Y.; Yang, F.; Huang, W.; Zhang, T.; Feng, Z. Pollution Concerns in Mariculture Water and Cultured Economical Bivalves: Occurrence of Microplastics under Different Aquaculture Modes. J. Clean. Prod. 2023, 406, 136913. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in Four Bivalve Species and Basis for Using Bivalves as Bioindicators of Microplastic Pollution. Sci. Total Environ. 2021, 782, 146830. [Google Scholar] [CrossRef]
- Gürkan, Ü.; Tekin Özan, S. Susurluk Çayı Bursa-Balıkesir’deki Tatlı Su Kefali Squalias cephalus L’nin Helmint Faunası. SDU J. Sci. 2012, 7, 77–85. [Google Scholar]
- Kudun, K. Hydrogeochemistry of the Simav Brook. Master’s Thesis, Istanbul Technical University, Graduate School of Natural and Applied Sciences, Istanbul, Türkiye, 1994. [Google Scholar]
- Çiçek, N.L.; Güçlü, S.S.; Erdoğan, Ö.; Küçük, F. Water Quality Assessment of Simav River (Susurluk Basin/Turkey) According to Seasons and Stations. Int. J. Comput. Exp. Sci. Eng. 2023, 9, 68–80. [Google Scholar] [CrossRef]
- Besseling, E.; Quik, J.T.; Sun, M.; Koelmans, A.A. Fate of Nano- and Microplastic in Freshwater Systems: A Modeling Study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Kooi, M.; Besseling, E.; Kroeze, C.; Wezel, A.P.V.; Koelmans, A.A. Modeling the Fate and Transport of Plastic Debris in Freshwaters: Review and Guidance. Freshw. Microplast. 2018, 58, 125–152. [Google Scholar]
- Peller, J.R.; Eberhardt, L.; Clark, R.; Nelson, C.; Kostelnik, E.; Iceman, C. Tracking the Distribution of Microfiber Pollution in a Southern Lake Michigan Watershed through the Analysis of Water, Sediment and Air. Environ. Sci. Process. Impacts 2019, 21, 1549–1559. [Google Scholar] [CrossRef]
- Junior, T.; Nisaa, A.F.; Karnaningroem, N.; Mardyanto, M.A. The Presence of Microplastics in Surabaya Coastal Area and Its Correlation with Conventional Water Quality Parameters. IOP Conf. Ser. Earth Environ. Sci. 2024, 1414, 012038. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hidayaturrahman, H.; Peera, S.G.; Lee, T.G. Elimination of Microplastics at Different Stages in Wastewater Treatment Plants. Water 2022, 14, 2404. [Google Scholar] [CrossRef]
- Ross, M.S.; Loutan, A.; Groeneveld, T.; Molenaar, D.; Kroetch, K.; Bujaczek, T.; Kolter, S.; Moon, S.; Huynh, A.; Khayam, R.; et al. Estimated Discharge of Microplastics via Urban Stormwater During Individual Rain Events. Front. Environ. Sci. 2023, 11, 1090267. [Google Scholar] [CrossRef]
- Dhea, L.A.; Kurniawan, A.; Ulfa, S.M.; Karimah, K. Correlation of Microplastic Size Distribution and Water Quality Parameters in the Upstream Brantas River. J. Penelit. Pendidik. IPA 2023, 9, 520–526. [Google Scholar] [CrossRef]
- Wolff, S.; Weber, F.; Kerpen, J.; Winklhofer, M.; Engelhart, M.; Barkmann, L. Elimination of Microplastics by Downstream Sand Filters in Wastewater Treatment. Water 2021, 13, 33. [Google Scholar] [CrossRef]
- Cordova, M.R.; Hernawan, U.E. Microplastics in Sumba Waters, East Nusa Tenggara. IOP Conf. Ser. Earth Environ. Sci. 2018, 162, 012023. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-Term Aging and Degradation of Microplastic Particles: Comparing in Situ Oceanic and Experimental Weathering Patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality Assessment of the Blue Mussel (Mytilus edulis): Comparison Between Commercial and Wild Types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Otegui, M.B.P.; Castro, M.A.; Yuvero, M.C.; Giménez, J. Spatial and Temporal Variation of Microplastic in Mussels from Intertidal and Subtidal Banks in the Southwestern Atlantic Ocean. Sci. Total Environ. 2025, 958, 177957. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Nomura, M.; Horie, Y.; Okamura, H. Color Preferences and Gastrointestinal-Tract Retention Times of Microplastics by Freshwater and Marine Fishes. Environ. Pollut. 2022, 304, 119253. [Google Scholar] [CrossRef]
- Bošković, N.; Joksimović, D.; Bajt, O. Microplastics in Mussels from the Boka Kotorska Bay (Adriatic Sea) and Impact on Human Health. Food Chem. Toxicol. 2023, 173, 113641. [Google Scholar] [CrossRef]
- Hossain, M.B.; Banik, P.; Nur, A.A.; Choudhury, T.R.; Liba, S.I.; Albeshr, M.F.; Yu, J.; Arai, T. Microplastics in Fish Culture Ponds: Abundance, Characterization, and Contamination Risk Assessment. Front. Environ. Sci. 2023, 11, 1251158. [Google Scholar] [CrossRef]
- Lestari, P.; Trihadiningrum, Y.; Firdaus, M.; Warmadewanthi, I.D.A.A. Microplastic Pollution in Surabaya River Water and Aquatic Biota, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012054. [Google Scholar] [CrossRef]
- Alam, F.C.; Sembiring, E.; Muntalif, B.E.; Suendo, V. Microplastic Distribution in Surface Water and Sediment River Around Slum and Industrial Area (Case Study: Ciwalengke River, Majalaya District, Indonesia). Chemosphere 2019, 224, 637–645. [Google Scholar] [CrossRef]
- Fang, C.; Zheng, R.; Chen, H.; Hong, F.; Lin, L.; Lin, H.; Guo, H.; Bailey, C.; Segner, H.; Mu, J.; et al. Comparison of Microplastic Contamination in Fish and Bivalves from Two Major Cities in Fujian Province, China, and the Implications for Human Health. Aquaculture 2019, 512, 734322. [Google Scholar] [CrossRef]
- Patterson, J.; Jeyasanta, K.I.; Sathish, N.; Booth, A.M.; Edward, J.K.P. Profiling Microplastics in the Indian Edible Oyster, Magallana bilineata Collected from the Tuticorin Coast, Gulf of Mannar, Southeastern India. Sci. Total Environ. 2019, 691, 727–735. [Google Scholar] [CrossRef]
- Ding, J.; Li, J.; Sun, C.; He, C.; Jiang, F.; Gao, F.; Zheng, L. Separation and Identification of Microplastics in Digestive System of Bivalves. Chin. J. Anal. Chem. 2018, 46, 690–697. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Li, L.; Jabeen, K.; Shi, H. Microplastics in Commercial Bivalves from China. Environ. Pollut. 2015, 207, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Yıbar, A.; Nur Genc, M.; Ceylan, A.; Suzer, B.; Duman, M. Determination of Microplastic and Mold Species in Mussels from the Marmara Sea, Turkey. Acta Veter. Eurasia 2024, 50, 196–209. [Google Scholar]
- Bonello, G.; Varrella, P.; Pane, L. First Evaluation of Microplastic Content in Benthic Filter-Feeders of the Gulf of La Spezia (Ligurian Sea). J. Aquat. Food Prod. Technol. 2018, 27, 284–291. [Google Scholar] [CrossRef]
- Rochman, C.; Tahir, A.; Williams, S.; Baxa, D.V. Anthropogenic Debris in Seafood: Plastic Debris and Fibers from Textiles in Fish and Bivalves Sold for Human Consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- Ünlü, S.; Alpar, B. Distribution and Sources of Hydrocarbons in Surface Sediments of Gemlik Bay (Marmara Sea, Turkey). Chemosphere 2006, 64, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Urban-Malinga, B.; Zalewski, M.; Jakubowska, A.; Wodzinowski, T.; Malinga, M.; Pałys, B.; Dąbrowska, A. Microplastics on Sandy Beaches of the Southern Baltic Sea. Mar. Pollut. Bull. 2020, 155, 111170. [Google Scholar] [CrossRef]
- Yücedağ, E.; Mülayim, A.; Kecel Gündüz, S. Investigation of Microplastic Pollution in the Sediment and Commercial Fish Species of Gemlik Bay (Marmara Sea) by Microscopic and Spectroscopic Methods. Turk. J. Fish. Aquat. Sci. 2024, 23, 15032. [Google Scholar]
- Lots, F.A.E.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A Large-Scale Investigation of Microplastic Contamination: Abundance and Characteristics of Microplastics in European Beach Sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef]
- Terzi, Y.; Oztürk, R.Ç.; Eryaşar, A.R.; Yandı, İ.; Şahin, A.; Yılmaz, F.; Gündoğdu, S. Riverine Microplastic Discharge Along the Southern Black Sea Coast of Turkey. Environ. Res. Lett. 2025, 20, 024061. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in Water Systems: A Review of Their Impacts on the Environment and Their Potential Hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Phaksopa, J.; Sukhsangchan, R.; Keawsang, R.; Tanapivattanakul, K.; Asvakittimakul, B.; Thamrongnawasawat, T.; Worachananant, S. Assessment of Microplastics in Green Mussel (Perna viridis) and Surrounding Environments around Sri Racha Bay, Thailand. Sustainability 2023, 15, 9. [Google Scholar] [CrossRef]
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The Vertical Distribution and Biological Transport of Marine Microplastics Across the Epipelagic and Mesopelagic Water Column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Henninger, C.E.; Wood, J.; Garforth, A.; Asuquo, E. Designing Out Microplastic Pollution Released from Textiles and Apparel During Laundering. Camb. Prism. Plast. 2024, 2, e20. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). Microplastics from Textiles: Towards a Circular Economy for Textiles in Europe. Available online: https://www.eea.europa.eu/publications/microplastics-from-textiles-towards-a (accessed on 27 March 2025).
- Mishra, S.; Rath, C.; Das, A.P. Marine microfiber pollution: A review on present status and future challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Athey, S.N.; Erdle, L.M. Are we underestimating anthropogenic microfiber pollution? A critical review of occurrence, methods, and reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A real global threat for environment and food safety: A state of the art review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Halpern, S. Sebin Project Tackling Microfibers Head on. Available online: http://www.seabinproject.com/Seabin-Project-Tackling-Microfibers-Head-On (accessed on 27 March 2025).
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Manolaki, S.M.; Chatzivasileiou, D.; Lampa, M.; Dimitriou, P.D.; Philippidis, A.; Karakassis, I.; Papageorgiou, N. Microplastic contamination in cultured mussels and pearl oysters in Greece. Microplastics 2023, 2, 168–181. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Underwood, A.J.; Chapman, M.G.; Williams, R.; Thompson, R.C.; van Franeker, J.A. Linking effects of anthropogenic debris to ecological impacts. Proc. Biol. Sci. 2015, 282, 20142929. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dietary and Inhalation Exposure to Nano-And Microplastic Particles and Potential Implications for Human Health; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Trevisan, R.; Ranasinghe, P.; Merril, G.B.; Santos, J.; Hong, A.; Edward, W.C.; Jayasundara, N.; Somarelli, J.A. A growing crisis for One Health: Impacts of plastic pollution across layers of biological function. Front. Mar. Sci. 2022, 9, 980705. [Google Scholar] [CrossRef]
- Noman, A.; Adyel, T.M.; Trevathan-Tackett, S.; Macreadie, P.I. Plastic paradox in blue carbon ecosystems. Environ. Sci. Technol. 2024, 58, 4469–4475. [Google Scholar] [CrossRef]
- Li, H.-X.; Shi, M.; Tian, F.; Lin, L.; Liu, S.; Hou, R.; Peng, J.-P.; Xu, X.-P. Microplastics contamination in bivalves from the Daya Bay: Species variability and spatio-temporal distribution and human health risks. Sci. Total Environ. 2022, 841, 156749. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Gimenez, B.C.G. Microplastics in the marine environment: Current trends and future perspectives. Mar. Pollut. Bull. 2015, 97, 5–12. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lourenço, S.C.; Aleluia, A.; dos Santos, N.C.L.; Huang, M.; Wang, J.; Guilhermino, L. A global synthesis of microplastic contamination in wild fish species: Challenges for conservation, implications for sustainability of wild fish stocks and future directions. Adv. Mar. Biol. 2023, 94, 159–200. [Google Scholar]
- Catarino, A.I.; Asselman, J.; Khan, F.R.; Everaert, G. Editorial: Plastic Pollution in a Changing Marine Environment: Effects and Risk. Front. Mar. Sci. 2023, 10, 1213393. [Google Scholar] [CrossRef]
- Kibria, G. Global Review and Analysis of the Presence of Microplastics in Fish. Asian Fish. Sci. 2022, 35, 191–256. [Google Scholar] [CrossRef]
- Azoulay, D.; Villa, P.; Arellano, Y.; Gordon, M.; Moon, D.; Miller, K.; Thompson, K. Plastic and Health: The Hidden Costs of a Plastic Planet; Center for International Environmental Law (CIEL): Washington, DC, USA, 2019. [Google Scholar]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential Toxicity of Polystyrene Microplastic Particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wang, J.; Xu, K.; Huang, Y.; Yan, M. Microplastic Pollution in Water and Fish Samples Around Nanxun Reef in Nansha Islands, South China Sea. Sci. Total Environ. 2019, 696, 134022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Bai, H.; Chen, B.; Sun, X.; Qu, K.; Xia, B. Microplastic Pollution in North Yellow Sea, China: Observations on Occurrence, Distribution and Identification. Sci. Total Environ. 2018, 636, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The Plastic in Microplastics: A Review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Tripathi, D.; Ebrary, I. Practical Guide to Polypropylene; Rapra Technology Limited: Shrewsbury, UK, 2002. [Google Scholar]
- Multisanti, C.R.; Merola, C.; Perugini, M.; Aliko, V.; Faggio, C. Sentinel Species Selection for Monitoring Microplastic Pollution: A Review on One Health Approach. Ecol. Indic. 2022, 145, 109587. [Google Scholar] [CrossRef]
- Harris, N.L.; Gibbs, D.A.; Baccini, A.; Birdsey, R.A.; de Bruin, S.; Farina, M.; Fatoyinbo, L.; Hansen, M.C.; Herold, M.; Houghton, R.A.; et al. Global Maps of Twenty-First Century Forest Carbon Fluxes. Nat. Clim. Chang. 2021, 11, 234–240. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in Mussels and Fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Zhang, H. Transport of Microplastics in Coastal Seas. Estuar. Coast. Shelf Sci. 2017, 199, 74–86. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U. Endocrine Disruption in Invertebrates. Pure Appl. Chem. 2003, 75, 2207–2218. [Google Scholar] [CrossRef]
- Hongsawat, P.; Thinjong, W.; Chouychai, B.; Punyapalakul, P.; Prarat, P. Microplastics in Retail Shellfish from a Seafood Market in Eastern Thailand: Occurrence and Risks to Human Food Safety. Mar. Pollut. Bull. 2024, 201, 116228. [Google Scholar] [CrossRef]
- Aakre, I.; Næss, S.; Kjellevold, M.; Markhus, M.W.; Alvheim, A.R.; Dalane, J.Ø.; Kielland, E.; Dahl, L. New Data on Nutrient Composition in a Large Selection of Commercially Available Seafood Products and Its Impact on Micronutrient Intake. Food Nutr. Res. 2019, 63, 3573. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Y.; He, C.; Li, J.; Li, F. Towards Risk Assessments of Microplastics in Bivalve Mollusks Globally. J. Mar. Sci. Eng. 2022, 10, 288. [Google Scholar] [CrossRef]
- Garrido Gamarro, E.; Costanzo, V. Microplastics in Food Commodities—A Food Safety Review on Human Exposure through Dietary Sources. Food Safety and Quality Series No. 18; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Abelouah, M.R.; Romdhani, I.; Ben-Haddad, M.; Hajji, S.; De-la-Torre, G.E.; Gaaied, S.; Barra, I.; Banni, M.; Ait Alla, A. Binational Survey Using Mytilus galloprovincialis as a Bioindicator of Microplastic Pollution: Insights into Chemical Analysis and Potential Risk on Humans. Sci. Total Environ. 2023, 870, 161894. [Google Scholar] [CrossRef]
- Ferreira, O.; Barboza, L.G.A.; Rudnitskaya, A.; Moreirinha, C.; Vieira, L.R.; Botelho, M.J.; Vale, C.; Fernandes, J.O.; Cunha, S.; Guilhermino, L. Microplastics in Marine Mussels, Biological Effects and Human Risk of Intake: A Case Study in a Multi-Stressor Environment. Mar. Pollut. Bull. 2023, 197, 115704. [Google Scholar] [CrossRef] [PubMed]
- Trani, A.; Mezzapesa, G.; Piscitelli, L.; Mondelli, D.; Nardelli, L.; Belmonte, G.; Toso, A.; Piraino, S.; Panti, C.; Baini, M.; et al. Microplastics in Water Surface and in the Gastrointestinal Tract of Target Marine Organisms in Salento Coastal Seas (Italy, Southern Puglia). Environ. Pollut. 2023, 316 Pt 1, 120702. [Google Scholar] [CrossRef]
- Expόsito, N.; Rovira, J.; Sierra, J.; Gimenez, G.; Domingo, J.L.; Schuhmacher, M. Levels of Microplastics and Their Characteristics in Molluscs from North-West Mediterranean Sea: Human Intake. Mar. Pollut. Bull. 2022, 181, 113843. [Google Scholar] [CrossRef] [PubMed]
- Vital, S.A.; Cardoso, C.; Avio, C.; Pittura, L.; Regoli, F.; Bebianno, M.J. Do Microplastic Contaminated Seafood Consumption Pose a Potential Risk to Human Health? Mar. Pollut. Bull. 2021, 171, 112769. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Çevik, C.; Ataş, N.T. Stuffed with Microplastics: Microplastic Occurrence in Traditional Stuffed Mussels Sold in the Turkish Market. Food Biosci. 2020, 37, 100715. [Google Scholar] [CrossRef]
- Monfort, M.C. The European Market for Mussels. Globefish Res. Progr. 2014, 115, 2014. [Google Scholar]
- Renzi, M.; Guerranti, C.; Blašković, A. Microplastic Contents from Maricultured and Natural Mussels. Mar. Pollut. Bull. 2018, 131, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Nor, H.M.N.; Merel, K.; Noël, J.D.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar]
- USEPA. Recommended Use of BW3/4 as the Default Method in Derivation of the Oral Reference Dose. Available online: https://www.epa.gov/sites/default/files/2013-09/documents/recommended-use-of-bw34.pdf (accessed on 25 March 2025).
- Terrazas-López, R.; Guadarrama-Guzman, P.; Sujitha, S.B.; Arreola-Mendoza, L.; Ponniah, J.M. The Occurrence of Microplastics in the Marine Food Web in Latin America: Insights on the Current State of Knowledge and Future Perspectives. Sustainability 2024, 16, 5905. [Google Scholar] [CrossRef]
- FAO. FAO Aquaculture Newsletter. No. 57 (September); FAO: Rome, Italy, 2017. [Google Scholar]
- Pletz, M. Ingested Microplastics: Do Humans Eat One Credit Card per Week? J. Hazard. Mater. Lett. 2022, 3, 100071. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the Mass of Microplastics Ingested—A Pivotal First Step Towards Human Health Risk Assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; Li, J.; Shi, H.; Xu, X.; Ju, P.; Jiang, F.; Li, F. Microplastics in Global Bivalve Mollusks: A Call for Protocol Standardization. J. Hazard. Mater. 2022, 438, 129490. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Otero, X.L.; Guilhermino, L. Microplastic Contamination in Marine Mussels from the Atlantic Coast of North Portugal and Human Risk of Microplastic Intake through Mussel Consumption. Environ. Pollut. 2024, 352, 124133. [Google Scholar] [CrossRef]
- Rist, S.; Carney Almroth, B.; Hartmann, N.B.; Karlsson, T.M. A Critical Perspective on Early Communications Concerning Human Health Aspects of Microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Enyoh, C.E.; Chowdhury, T.; Chowdhury, M.A.H. Analytical Techniques, Occurrence and Health Effects of Micro and Nano Plastics Deposited in Street Dust. Int. J. Environ. Anal. Chem. 2022, 102, 6435–6453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).