Recent Progress in the Recovery and Recycling of Polymers from End-of-Life Silicon PV Modules
Abstract
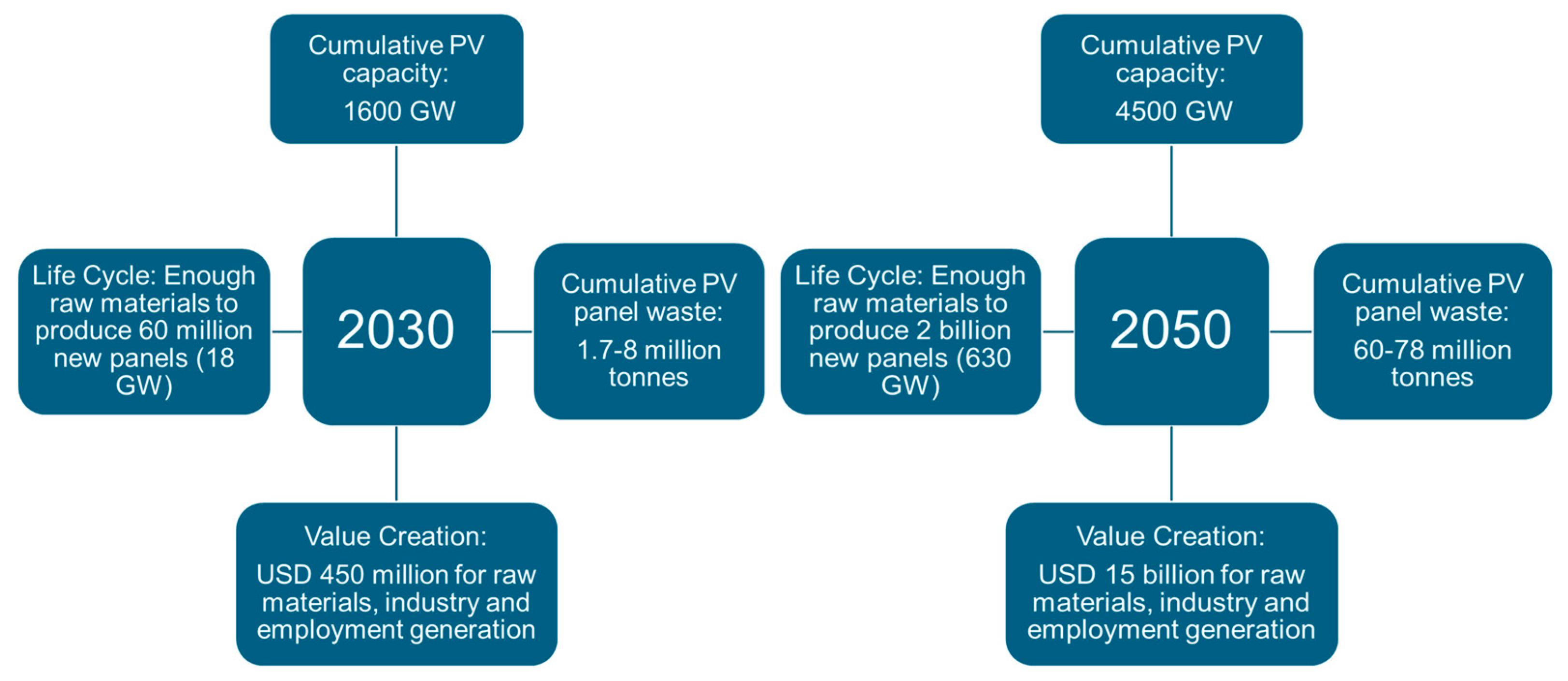
1. Introduction
2. Health, Safety, and Environmental Aspects of EOL PV Modules
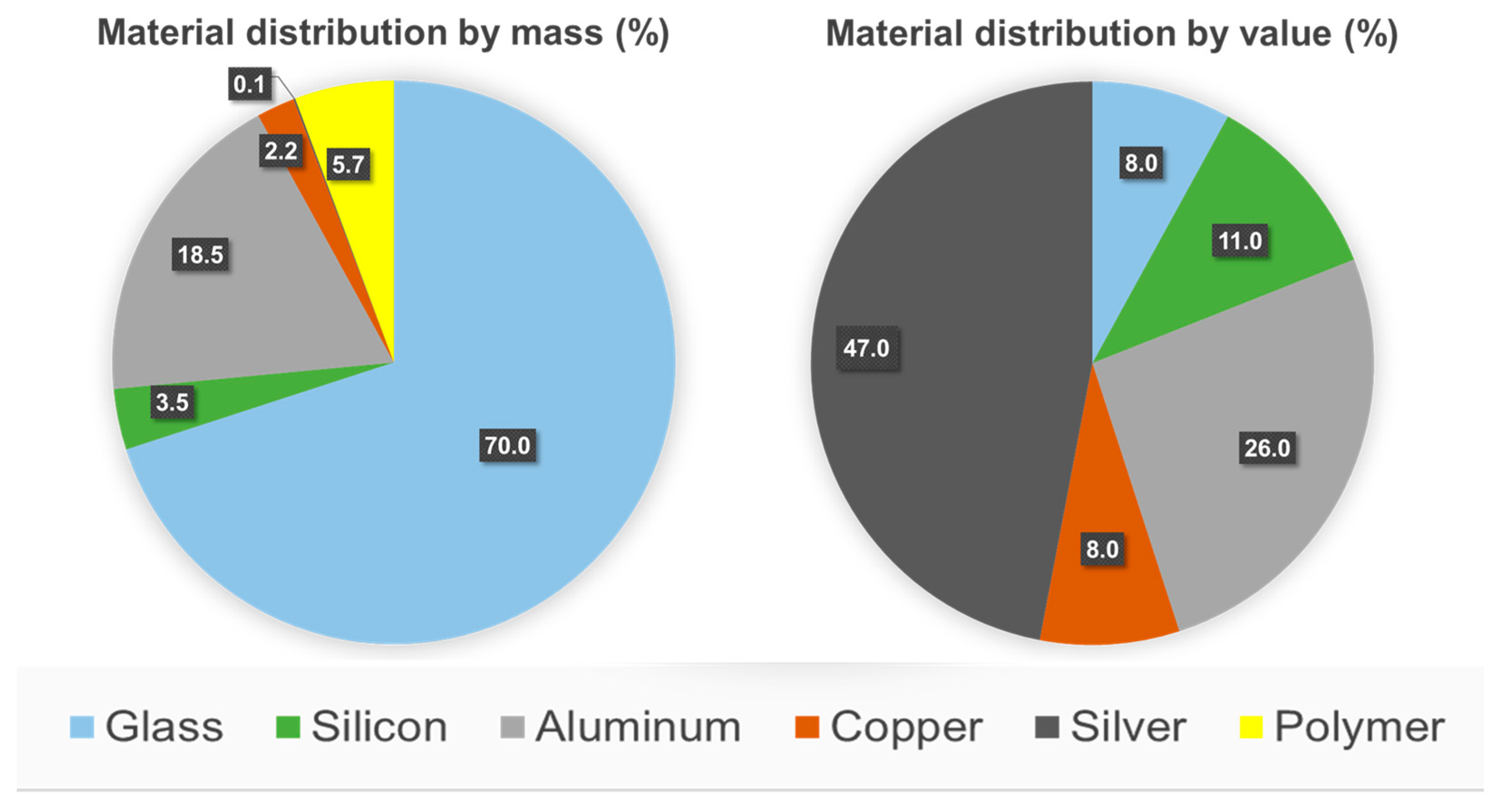
3. Polymer Materials Used in c-Si PV Panels
3.1. Encapsulants
3.2. Backsheets
4. Separation and Recovery of the Polymers
4.1. Chemical Delamination
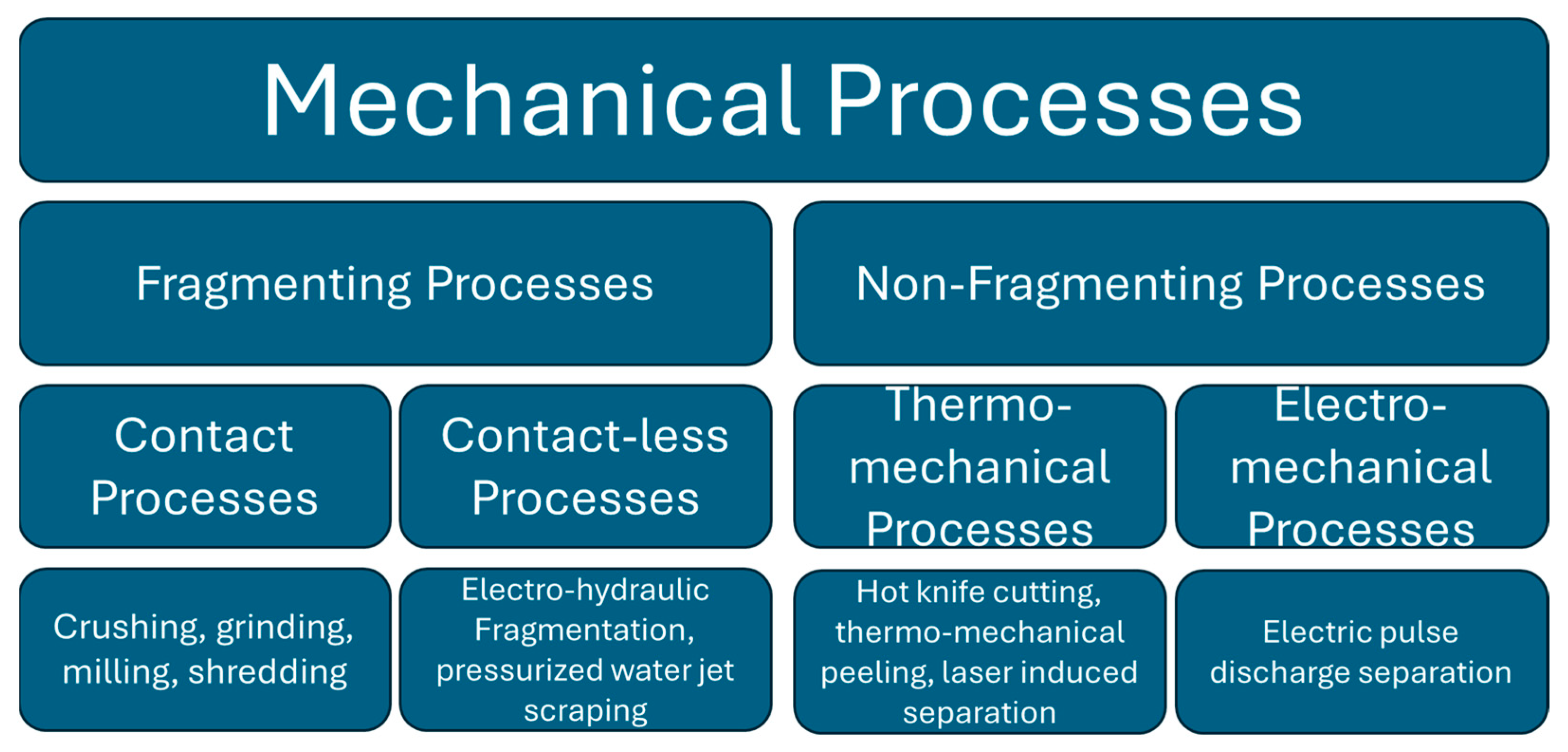
4.2. Mechanical, Thermo-Mechanical, and Electro-Mechanical Processes
4.3. Comparison of the Different Delamination Processes
5. Recycling of the Polymers
5.1. Classification of the Recycling Process
5.2. Recycling of the Encapsulant (EVA)
5.3. Recycling of the FP-Based Backsheet
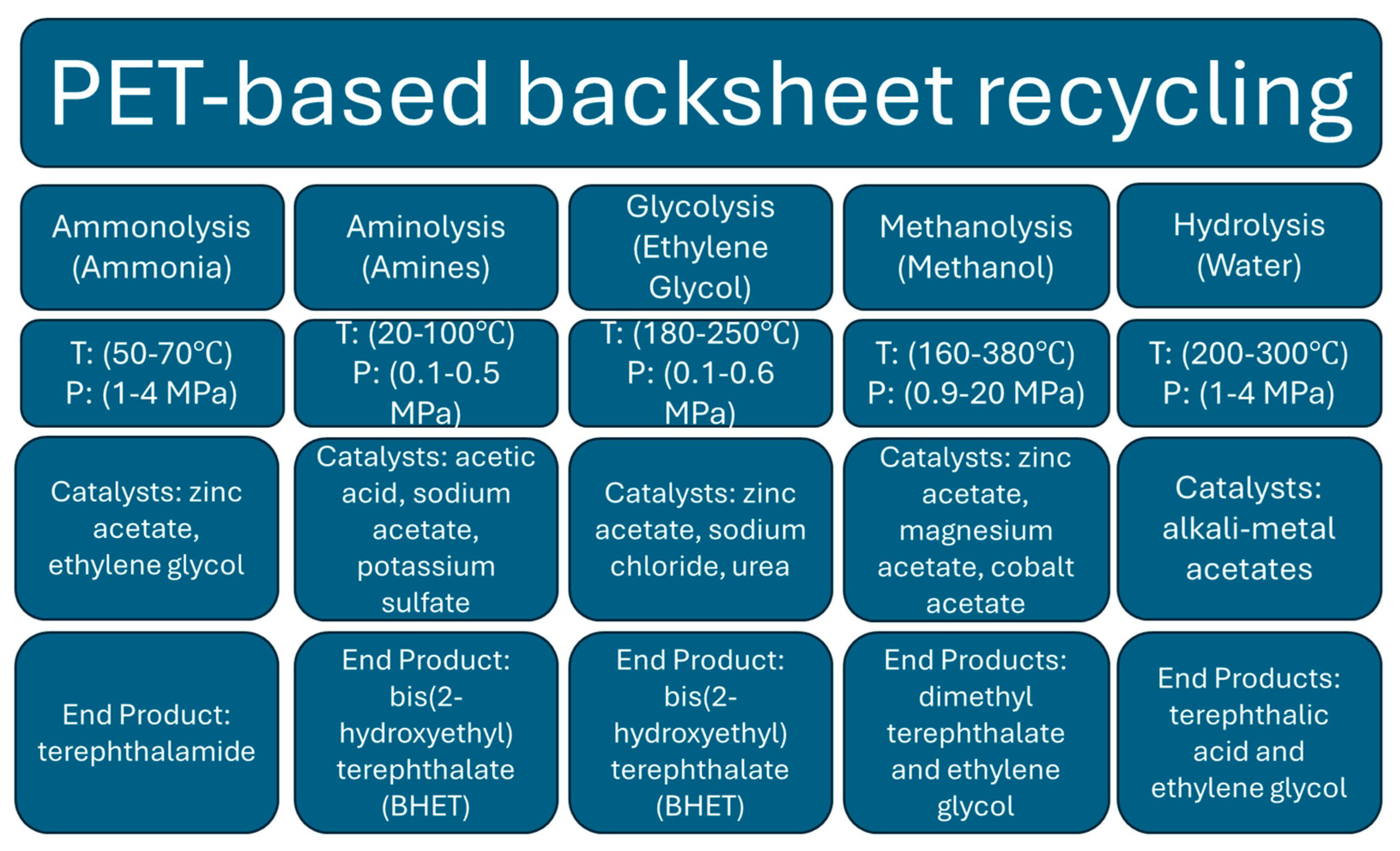
5.4. Recycling of the PET-Based Backsheet
6. Policies and Commercial Efforts Related to the Recycling of PV Panels
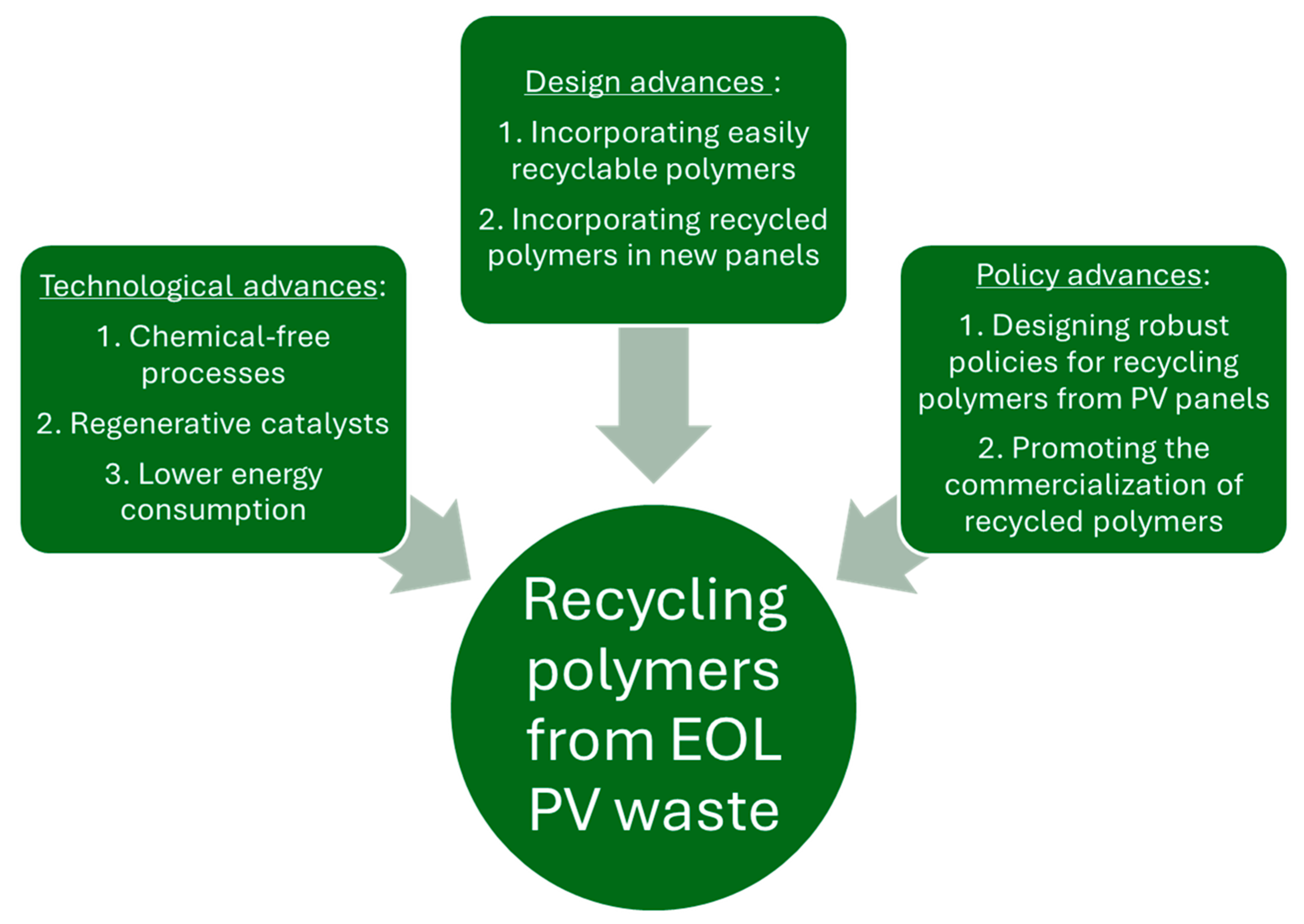
7. Challenges and Future Outlook
- Optimization of the neutral hydrolysis process to minimize the energy requirement and the process duration, while maximizing the process yield.
- Combining neutral hydrolysis with a complimentary process such as glycolysis to minimize effluent generation and maximize solvent recovery.
- The development of new environmentally benign catalysts that can minimize the energy consumption, maximize the process yield, and can be regenerated towards the end of the process without a complicated process.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Victoria, M.; Haegel, N.; Peters, I.M.; Sinton, R.; Jäger-Waldau, A.; Del Cañizo, C.; Breyer, C.; Stocks, M.; Blakers, A.; Kaizuka, I. Solar photovoltaics is ready to power a sustainable future. Joule 2021, 5, 1041–1056. [Google Scholar] [CrossRef]
- Jean, J.; Brown, P.R.; Jaffe, R.L.; Buonassisi, T.; Bulović, V. Pathways for solar photovoltaics. Energy Environ. Sci. 2015, 8, 1200–1219. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2023; IEA: Paris, France, 2023; Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 11 April 2024).
- Dale, M.; Benson, S.M. Energy balance of the global photovoltaic (PV) industry-is the PV industry a net electricity producer? Environ. Sci. Technol. 2013, 47, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Masson, G. IEA—2024 Snapshot Report of Global PV Markets; IEA: Paris, France, 2024. [Google Scholar] [CrossRef]
- Detollenaere, G.M.A.; Kaizuka, I.; Waldau, A.J.; Donoso, J. IEA-PVPS Snapshot of Global PV Markets 2021. 2021. Available online: https://iea-pvps.org/snapshot-reports/snapshot-2021/ (accessed on 8 September 2023).
- VDMA. International Technology Roadmap for Photovoltaic (ITRPV), 15th ed.; VDMA: Frankfurt am Main, Germany, 2024. [Google Scholar]
- Köntges, M.; Oreski, G.; Jahn, U.; Herz, M.; Hacke, P.; Weiß, K.-A. Assessment of Photovoltaic Module Failures in the Field: International Energy Agency Photovoltaic Power Systems Programme: IEA PVPS Task 13, Subtask 3; Report IEA-PVPS T13-09: 2017; International Energy Agency: Paris, France, 2017. [Google Scholar]
- IEA-PVPS Report Number: T12-06:2016, IEAPVPS Report on End-of-Life Solar PV Panels. 2016. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2016/IRENA_IEAPVPS_End-of-Life_Solar_PV_Panels_2016.pdf (accessed on 4 February 2025).
- Kontges, M.; Kurtz, S.; Packard, C.; Jahn, U.; Berger, K.; Kato, K.; Iseghem, M.V. Review of Failures of Photovoltaic Modules Report; IEA-PVPS T13-01: 2014; International Energy Agency-Photovoltaic Power Systems Programme: Paris, France, 2014. [Google Scholar]
- Kwak, J., II; Nam, S.-H.; Kim, L.; An, Y.-J. Potential environmental risk of solar cells: Current knowledge and future challenges. J. Hazard. Mater. 2020, 392, 122297. [Google Scholar] [CrossRef] [PubMed]
- Sanathi, R.; Banerjee, S.; Bhowmik, S. A technical review of crystalline silicon photovoltaic module recycling. Sol. Energy 2024, 281, 112869. [Google Scholar] [CrossRef]
- Komoto, K.; Held, M.; Agraffeil, C.; Alonso-Garcia, C.; Danelli, A.; Lee, J.S.; Lyu, F.; Bilbao, J.; Deng, R.; Heath, G. Status of PV Module Recycling in Selected IEA PVPS Task12 Countries; Report IEA-PVPS T12-24: 2022; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Wambach, K.; Libby, C.; Shaw, S. IEA PVPS Task 12 Report—Advances in Photovoltaic Module Recycling; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Task, I.P.V.S.; Heath, G.; Europe, A.W.S.; Komoto, B.A.K.; Lee, J.-S.; Zhang, C.J.; Sinha, P.; Heath, B.G. End-of-Life Management of Photovoltaic Panels: Trends in PV Module Recycling Technologies Operating Agent; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2018. [CrossRef]
- Stolz, P.; Frischknecht, R.; Wambach, K.; Sinha, P.; Heath, G. Life Cycle Assessment of Current Photovoltaic Module Recycling, IEA PVPS Task 12; Report IEA-PVPS T12 13 (2017); International Energy Agency Power Systems Programme: Paris, France, 2018. [Google Scholar]
- Padhamnath, P.; Ślęzak, M.; Karbowniczek, M. Disposing End of Life PV Modules—Reusing, Recycling and Upcycling. In EU PVSEC 2023; EUPVSEC: Lisbon, Portugal, 2023; pp. 1–8. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Santa, R.; Crnac, Z.; Bošnjaković, T. Environmental impact of PV power systems. Sustainability 2023, 15, 11888. [Google Scholar] [CrossRef]
- Seo, B.; Kim, J.Y.; Chung, J. Overview of global status and challenges for end-of-life crystalline silicon photovoltaic panels: A focus on environmental impacts. Waste Manag. 2021, 128, 45–54. [Google Scholar] [CrossRef]
- Komoto, K.; Oyama, S.; Sato, T.; Uchida, H. Recycling of PV modules and its environmental impacts. In Proceedings of the 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa, HI, USA, 10–15 June 2018; pp. 2590–2593. [Google Scholar]
- Padhamnath, P.; Nalluri, S.; Kuśmierczyk, F.; Kopyściański, M.; Karbowniczek, J.; Kozieł, T.; Leow, S.W.; Reindl, T. Development of PV panel recycling process enabling complete recyclability of end-of-life silicon photovoltaic panels. Sol. Energy Mater. Sol. Cells 2025, 286, 113571. [Google Scholar] [CrossRef]
- Klejnowska, K.; Mijal, W.; Golebiewska-Kurzawska, J.; Strzelczuk, J. Recycling of end-of-life PV panels-a review of technologies. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2024. [Google Scholar] [CrossRef]
- Preet, S.; Smith, S.T. A comprehensive review on the recycling technology of silicon based photovoltaic solar panels: Challenges and future outlook. J. Clean. Prod. 2024, 448, 141661. [Google Scholar] [CrossRef]
- Einhaus, R.; Madon, F.; Degoulange, J.; Wambach, K.; Denafas, J.; Lorenzo, F.R.; Abalde, S.C.; García, T.D.; Bollar, A. Recycling and Reuse potential of NICE PV-Modules. In Proceedings of the 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa, HI, USA, 10–15 June 2018; pp. 561–564. [Google Scholar] [CrossRef]
- Deng, R.; Zhuo, Y.; Shen, Y. Recent progress in silicon photovoltaic module recycling processes. Resour. Conserv. Recycl. 2022, 187, 106612. [Google Scholar] [CrossRef]
- Padhamnath, P.; Buatis, J.K.; Khanna, A.; Nampalli, N.; Nandakumar, N.; Shanmugam, V.; Aberle, A.G.; Duttagupta, S. Characterization of screen printed and fire-through contacts on LPCVD based passivating contacts in monoPolyTM solar cells. Sol. Energy 2020, 202, 73–79. [Google Scholar] [CrossRef]
- Mondon, A.; Jawaid, M.N.; Bartsch, J.; Glatthaar, M.; Glunz, S.W. Microstructure analysis of the interface situation and adhesion of thermally formed nickel silicide for plated nickel–copper contacts on silicon solar cells. Sol. Energy Mater. Sol. Cells 2013, 117, 209–213. [Google Scholar] [CrossRef]
- Lee, J.-S.; Ahn, Y.-S.; Kang, G.-H.; Wang, J.-P. Recovery of Pb-Sn Alloy and Copper from Photovoltaic Ribbon in Spent Solar Module. Appl. Surf. Sci. 2017, 415, 137–142. [Google Scholar] [CrossRef]
- Kraft, A.; Wolf, C.; Bartsch, J.; Glatthaar, M.; Glunz, S. Long term stability of copper front side contacts for crystalline silicon solar cells. Sol. Energy Mater. Sol. Cells 2015, 136, 25–31. [Google Scholar] [CrossRef]
- Murarka, S.P. Materials aspects of copper interconnection technology for semiconductor applications. Mater. Sci. Technol. 2001, 17, 749–758. [Google Scholar] [CrossRef]
- Lennon, A.; Colwell, J.; Rodbell, K.P. Challenges facing copper-plated metallisation for silicon photovoltaics: Insights from integrated circuit technology development. Prog. Photovolt. Res. Appl. 2019, 27, 67–97. [Google Scholar] [CrossRef]
- Perelman, A.; Barth, V.; Mandorlo, F.; Voroshazi, E. Critical materials and PV cells interconnection. EPJ Photovolt. 2024, 15, 4. [Google Scholar] [CrossRef]
- Zarmai, M.T.; Ekere, N.N.; Oduoza, C.F.; Amalu, E.H. A review of interconnection technologies for improved crystalline silicon solar cell photovoltaic module assembly. Appl. Energy 2015, 154, 173–182. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Denafas, J.; Makarevicius, V.; Lukošiūtė, S.-I.; Kruopienė, J. Sustainable industrial technology for recovery of Al nanocrystals, Si micro-particles and Ag from solar cell wafer production waste. Sol. Energy Mater. Sol. Cells 2019, 191, 493–501. [Google Scholar] [CrossRef]
- Yang, E.-H.; Lee, J.-K.; Lee, J.-S.; Ahn, Y.-S.; Kang, G.-H.; Cho, C.-H. Environmentally friendly recovery of Ag from end-of-life c-Si solar cell using organic acid and its electrochemical purification. Hydrometallurgy 2017, 167, 129–133. [Google Scholar] [CrossRef]
- de Oliveira, L.S.S.; Lima, M.; Yamane, L.H.; Siman, R.R. Silver recovery from end-of-life photovoltaic panels. Detritus 2020, 10, 62–74. [Google Scholar] [CrossRef]
- Han, Q.; Gao, Y.; Su, T.; Qin, J.; Wang, C.; Qu, Z.; Wang, X. Hydrometallurgy recovery of copper, aluminum and silver from spent solar panels. J. Environ. Chem. Eng. 2023, 11, 109236. [Google Scholar] [CrossRef]
- Moudir, N.; Boukennous, Y.; Moulaï-Mostefa, N.; Bozetine, I.; Maoudj, M.; Kamel, N.; Kamel, Z.; Moudir, D. Preparation of silver powder used for solar cell paste by reduction process. Energy Procedia 2013, 36, 1184–1191. [Google Scholar] [CrossRef]
- Rout, S.; Jana, P.; Borra, C.R.; Önal, M.A.R. Unlocking silver from end-of-life photovoltaic panels: A concise review. Renew. Sustain. Energy Rev. 2025, 210, 115205. [Google Scholar] [CrossRef]
- Lim, S.; Imaizumi, Y.; Mochidzuki, K.; Koita, T.; Namihira, T.; Tokoro, C. Recovery of Silver From Waste Crystalline Silicon Photovoltaic Cells by Wire Explosion. IEEE Trans. Plasma Sci. 2021, 49, 2857–2865. [Google Scholar] [CrossRef]
- Yiwei, A.; Yunxia, Y.; Shuanglong, Y.; Lihua, D.; Guorong, C. Preparation of spherical silver particles for solar cell electronic paste with gelatin protection. Mater. Chem. Phys. 2007, 104, 158–161. [Google Scholar] [CrossRef]
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H.; Bernardes, A.M. Recycling WEEE: Extraction and concentration of silver from waste crystalline silicon photovoltaic modules. Waste Manag. 2016, 57, 220–225. [Google Scholar] [CrossRef]
- Gajare, O.; Jadhav, N.B.; Zele, S.; Lucas, N.; Gogate, N. Sustainable silver recovery by chemical treatment of metal rich fines from solar panel waste. Sol. Energy Mater. Sol. Cells 2025, 279, 113259. [Google Scholar] [CrossRef]
- Lee, S.; Frimpong, B.; Abbey, S.; Moon, Y.S.; Yoo, K.; Oh, Y.-M.; Kim, S.-K.; Kim, S.-J.; Oh, M.-W. Fabrication of conductive silver paste recovered from leaching of waste catalyst using hydrochloric acid. RSC Adv. 2022, 12, 9698–9703. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, S.; Gao, D.; Bao, G.; Wu, Z. A novel ion exchange method for recover silver and aluminum from waste crystalline silicon photovoltaic modules. Sol. Energy 2025, 286, 113142. [Google Scholar] [CrossRef]
- Zheng, R.; Luo, M.; Li, B.; Jia, M.; Zhang, H.; Liu, S.; Lu, Y.; Jiang, L.; Zhang, Z.; Liu, F. Eco-friendly recovery and preparation of high purity nano silver powders from retired photovoltaic solar cells. Sep. Purif. Technol. 2025, 359, 130343. [Google Scholar] [CrossRef]
- Heath, G.A.; Silverman, T.J.; Kempe, M.; Deceglie, M.; Ravikumar, D.; Remo, T.; Cui, H.; Sinha, P.; Libby, C.; Shaw, S. Research and development priorities for silicon photovoltaic module recycling to support a circular economy. Nat. Energy 2020, 5, 502–510. [Google Scholar] [CrossRef]
- Cui, H.; Heath, G.; Remo, T.; Ravikumar, D.; Silverman, T.; Deceglie, M.; Kempe, M.; Engel-Cox, J. Technoeconomic analysis of high-value, crystalline silicon photovoltaic module recycling processes. Sol. Energy Mater. Sol. Cells 2022, 238, 111592. [Google Scholar] [CrossRef]
- Li, W.; Adachi, T. Evaluation of long-term silver supply shortage for c-Si PV under different technological scenarios. Nat. Resour. Model. 2019, 32, e12176. [Google Scholar] [CrossRef]
- Veolia. Veolia Opens the First European Plant Entirely Dedicated to Recycling Photovoltaic Panels; Veolia: Aubervilliers, France, 2018. [Google Scholar]
- Dias, P.; Schmidt, L.; Lunardi, M.M.; Chang, N.L.; Spier, G.; Corkish, R.; Veit, H. Comprehensive recycling of silicon photovoltaic modules incorporating organic solvent delamination—Technical, environmental and economic analyses. Resour. Conserv. Recycl. 2021, 165, 105241. [Google Scholar] [CrossRef]
- Divya, A.; Adish, T.; Kaustubh, P.; Zade, P.S. Review on recycling of solar modules/panels. Sol. Energy Mater. Sol. Cells 2023, 253, 112151. [Google Scholar] [CrossRef]
- Lunardi, M.M.; Alvarez-Gaitan, J.P.; Bilbao, J.I.; Corkish, R. A Review of Recycling Processes for Photovoltaic Modules. In Solar Panels and Photovoltaic Materials; Books on Demand GMBH: Hamburg, Germany, 2018; pp. 9–29. [Google Scholar] [CrossRef]
- Martínez, M.; Barrueto, Y.; Jimenez, Y.P.; Vega-Garcia, D.; Jamett, I. Technological Advancement in Solar Photovoltaic Recycling: A Review. Minerals 2024, 14, 638. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Tan, Q.; Peters, A.L.; Yang, C. Global status of recycling waste solar panels: A review. Waste Manag. 2018, 75, 450–458. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, M.; Sun, Z.; Zhao, P.; Zhang, B. Recycling technology of end-of-life photovoltaic panels: A review, Energy Sources, Part A: Recovery. Util. Environ. Eff. 2023, 45, 10890–10908. [Google Scholar]
- Nain, P.; Kumar, A. A state-of-art review on end-of-life solar photovoltaics. J. Clean. Prod. 2022, 343, 130978. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Chen, X.; Ren, L.; Geng, C. A review of end-of-life crystalline silicon solar photovoltaic panel recycling technology. Sol. Energy Mater. Sol. Cells 2022, 248, 111976. [Google Scholar] [CrossRef]
- Mao, D.; Yang, S.; Ma, L.; Ma, W.; Yu, Z.; Xi, F.; Yu, J. Overview of life cycle assessment of recycling end-of-life photovoltaic panels: A case study of crystalline silicon photovoltaic panels. J. Clean. Prod. 2024, 434, 140320. [Google Scholar] [CrossRef]
- Singh, J.K.D.; Molinari, G.; Bui, J.; Soltani, B.; Rajarathnam, G.P.; Abbas, A. Life cycle assessment of disposed and recycled end-of-life photovoltaic panels in Australia. Sustainability 2021, 13, 11025. [Google Scholar] [CrossRef]
- Aryan, V.; Font-Brucart, M.; Maga, D. A comparative life cycle assessment of end-of-life treatment pathways for photovoltaic backsheets. Prog. Photovolt. Res. Appl. 2018, 26, 443–459. [Google Scholar] [CrossRef]
- Weckend, S.; Wade, A.; Heath, G. End-of-Life Management: Solar Photovoltaic Panels. 2016. Available online: https://www.irena.org/publications/2016/Jun/End-of-life-management-Solar-Photovoltaic-Panels (accessed on 8 September 2023).
- Daniela-Abigail, H.-L.; Vega-De-Lille, M.I.; Sacramento-Rivero, J.C.; Ponce-Caballero, C.; El-Mekaoui, A.; Navarro-Pineda, F. Life cycle assessment of photovoltaic panels including transportation and two end-of-life scenarios: Shaping a sustainable future for renewable energy. Renew. Energy Focus. 2024, 51, 100649. [Google Scholar] [CrossRef]
- Fiandra, V.; Sannino, L.; Andreozzi, C.; Graditi, G. End-of-life of silicon PV panels: A sustainable materials recovery process. Waste Manag. 2019, 84, 91–101. [Google Scholar] [CrossRef]
- Latunussa, C.E.L.; Ardente, F.; Blengini, G.A.; Mancini, L. Life Cycle Assessment of an innovative recycling process for crystalline silicon photovoltaic panels. Sol. Energy Mater. Sol. Cells 2016, 156, 101–111. Available online: https://www.vdma.org/international-technology-roadmap-photovoltaic (accessed on 8 September 2023). [CrossRef]
- Duan, Y.; Guo, F.; Gardy, J.; Xu, G.; Li, X.; Jiang, X. Life cycle assessment of polysilicon photovoltaic modules with green recycling based on the ReCiPe method. Renew. Energy 2024, 236, 121407. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, Z. Life cycle assessment of decommissioned silicon photovoltaic module recycling using different technological configurations in China. J. Environ. Manag. 2024, 370, 122476. [Google Scholar] [CrossRef] [PubMed]
- Królikowski, M.; Fotek, M.; Żach, P.; Michałowski, M. Development of a Recycling Process and Characterization of EVA, PVDF, and PET Polymers from End-of-Life PV Modules. Materials 2024, 17, 821. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.; Malandrino, O.; Supino, S.; Testa, M.; Lucchetti, M.C. Management of end-of-life photovoltaic panels as a step towards a circular economy. Renew. Sustain. Energy Rev. 2018, 82, 2934–2945. [Google Scholar] [CrossRef]
- Hu, X.; An, A.K.J.; Chopra, S.S. Life cycle assessment of the polyvinylidene fluoride polymer with applications in various emerging technologies. ACS Sustain. Chem. Eng. 2022, 10, 5708–5718. [Google Scholar] [CrossRef]
- Padoan, F.C.S.M.; Altimari, P.; Pagnanelli, F. Recycling of end of life photovoltaic panels: A chemical prospective on process development. Sol. Energy 2019, 177, 746–761. [Google Scholar] [CrossRef]
- de Wild, P.; de Wild-Scholten, M.; Goudswaard, I. Life cycle assessment of photovoltaic module backsheets. Prog. Photovolt. Res. Appl. 2025, 33, 27–39. [Google Scholar] [CrossRef]
- Lunardi, M.M.; Alvarez-Gaitan, J.P.; Bilbao, J.I.; Corkish, R. Comparative life cycle assessment of end-of-life silicon solar photovoltaic modules. Appl. Sci. 2018, 8, 1396. [Google Scholar] [CrossRef]
- Oteng, D.; Zuo, J.; Sharifi, E. An evaluation of the impact framework for product stewardship on end-of-life solar photovoltaic modules: An environmental lifecycle assessment. J. Clean. Prod. 2023, 411, 137357. [Google Scholar] [CrossRef]
- Nain, P.; Kumar, A. Ecological and human health risk assessment of metals leached from end-of-life solar photovoltaics. Environ. Pollut. 2020, 267, 115393. [Google Scholar] [CrossRef]
- Espinosa, N.; Zimmermann, Y.-S.; Benatto, G.A.D.R.; Lenz, M.; Krebs, F.C. Outdoor fate and environmental impact of polymer solar cells through leaching and emission to rainwater and soil. Energy Environ. Sci. 2016, 9, 1674–1680. [Google Scholar] [CrossRef]
- Nain, P.; Kumar, A. Metal dissolution from end-of-life solar photovoltaics in real landfill leachate versus synthetic solutions: One-year study. Waste Manag. 2020, 114, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Nain, P.; Kumar, A. Understanding the possibility of material release from end-of-life solar modules: A study based on literature review and survey analysis. Renew. Energy 2020, 160, 903–918. [Google Scholar] [CrossRef]
- Liao, B.; Yang, L.; Ju, X.; Peng, Y.; Gao, Y. Experimental study on burning and toxicity hazards of a PET laminated photovoltaic panel. Sol. Energy Mater. Sol. Cells 2020, 206, 110295. [Google Scholar] [CrossRef]
- Chow, C.L.; Han, S.S.; Ni, X.M. A study on fire behaviour of combustible components of two commonly used photovoltaic panels. Fire Mater. 2017, 41, 65–83. [Google Scholar] [CrossRef]
- Liao, B.; Jiang, S.; Lai, D.; Yang, L. Investigation of combustion hazards of glass photovoltaic panels with multilayer material structures in fire scenarios. Sol. Energy 2025, 292, 113447. [Google Scholar] [CrossRef]
- Moskowitz, P.D.; Fthenakis, V.M. Toxic materials released from photovoltaic modules during fires: Health risks. Solar Cells 1990, 29, 63–71. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, F.; Li, Y.; Tan, W.; Yuan, Y.; Jiang, Y. Influence of landfill leachate microenvironment on the occurrence of microplastics: TOC changes are the main driving factor. J. Hazard. Mater. 2025, 492, 138080. [Google Scholar] [CrossRef]
- Kabir, M.S.; Wang, H.; Luster-Teasley, S.; Zhang, L.; Zhao, R. Microplastics in landfill leachate: Sources, detection, occurrence, and removal. Environ. Sci. Ecotechnol. 2023, 16, 100256. [Google Scholar] [CrossRef]
- Ma, J.; Ma, M.; Li, J.; Yang, Q.; Wan, Y.; Zhao, K.; Zhang, Y.; Liu, L.; Fei, X. Distribution and characteristics of Microplastics in leachate and underneath soil of two informal landfills. Waste Manag. 2025, 195, 155–166. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics?—Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Améduri, B.; Hori, H. Recycling and the end of life assessment of fluoropolymers: Recent developments, challenges and future trends. Chem. Soc. Rev. 2023, 52, 4208–4247. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, C. Components and Layers of a Typical Crystalline Si Module. 2023. Available online: https://commons.wikimedia.org/wiki/File:Components_and_layers_of_a_typical_crystalline_Si_module.jpg (accessed on 29 March 2025).
- Schnatmann, A.K.; Schoden, F.; Schwenzfeier-Hellkamp, E. Sustainable PV module design—Review of state-of-the-art encapsulation methods. Sustainability 2022, 14, 9971. [Google Scholar] [CrossRef]
- Kempe, M. Encapsulant Materials for PV Modules. In Photovoltaic Sol. Energy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 478–490. [Google Scholar] [CrossRef]
- Adothu, B.; Bhatt, P.; Chattopadhyay, S.; Zele, S.; Oderkerk, J.; Sagar, H.P.; Costa, F.R.; Mallick, S. Newly developed thermoplastic polyolefin encapsulant—A potential candidate for crystalline silicon photovoltaic modules encapsulation. Sol. Energy 2019, 194, 581–588. [Google Scholar] [CrossRef]
- Beaucarne, G.; Dupont, A.; Puthenmadom, D.; Shephard, N.; Sample, T. Material study of photovoltaic modules with silicone encapsulation after long-term outdoor exposure. Sol. Energy Mater. Sol. Cells 2021, 230, 111298. [Google Scholar] [CrossRef]
- Hara, K.; Ohwada, H.; Furihata, T.; Masuda, A. Durable crystalline Si photovoltaic modules based on silicone-sheet encapsulants. Jpn. J. Appl. Phys. 2018, 57, 027101. [Google Scholar] [CrossRef]
- Lewis, K.J. Encapsulant material requirements for photovoltaic modules. In Polymers in Solar Energy Utilization; ACS Publications: Washington, DC, USA, 1983. [Google Scholar] [CrossRef]
- Cuddihy, E.F.; Baum, B.; Willis, P. Low-cost encapsulation materials for terrestrial solar cell modules. Sol. Energy 1979, 22, 389–396. [Google Scholar] [CrossRef]
- Ketola, B.; McIntosh, K.R.; Norris, A.; Tomalia, M.K. Silicones for photovoltaic encapsulation. In Proceedings of the 23rd European Photovoltaic Solar Energy Conference, Valencia, Spain, 1–5 September 2008. [Google Scholar]
- Ketola, B.; Shirk, C.; Griffith, P.; Bunea, G. Demonstration of the benefits of silicone encapsulation of PV modules in a large scale outdoor array. In Proceedings of the 25th European Photovoltaic Solar Energy Conference and Exhibition, Valencia, Spain, 6–10 September 2010; pp. 346–353. [Google Scholar]
- Thompson, J.; Putzer, M.; Gonsior, N.; Miller, C. Silicone encapsulation enhances durability, efficiency, and enables new PV cell and modules technologies. In Proceedings of the Photovoltaic Module Reliability Workshop, Golden, CO, USA, 25–26 February 2014. [Google Scholar]
- Peike, C.; Hädrich, I.; Weiß, K.-A.; Dürr, I.; Ise, F. Overview of PV module encapsulation materials. Photovolt. Int. 2013, 19, 85–92. [Google Scholar]
- Knott, W.; Dudzik, H.; Schaefer, D. Process for Recycling Silicones. U.S. Patent 11286366B2, 29 March 2022. [Google Scholar]
- Kawamoto, T. Process for Recycling Silicone Compounds. U.S. Patent 6172253B1, 4 April 2001. [Google Scholar]
- Rupasinghe, B. Recycling Silicone-Based Materials: An Overview of Methods; IntechOpen: London, UK, 2022. [Google Scholar]
- Wolf, A.T.; Stammer, A. Chemical Recycling of Silicones—Current State of Play (Building and Construction Focus). Polymers 2024, 16, 2220. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.D.; Boulègue-Mondière, A.; Durand, N.; Raynaud, J.; Monteil, V. Back-to-cyclic monomers: Chemical recycling of silicone waste using a [polydentate ligand–potassium silanolate] complex. Green Chem. 2023, 25, 3869–3877. [Google Scholar] [CrossRef]
- Technology, P. Dow, Circusil Collaborating on Silicone Recycling Facility. 2023. Available online: https://www.ptonline.com/news/dow-circusil-collaborating-on-silicone-recycling-facility (accessed on 31 March 2025).
- Czanderna, A.W.; Pern, F.J. Encapsulation of PV modules using ethylene vinyl acetate copolymer as a pottant: A critical review. Sol. Energy Mater. Sol. Cells 1996, 43, 101–181. [Google Scholar] [CrossRef]
- Farias, G.M.G.; Agrawal, P.; Hanken, R.B.L.; de Araújo, J.P.; de Oliveira, A.D.B.; de Mélo, T.J.A. Effect of EVA copolymer containing different VA content on the thermal and rheological properties of bio-based high-density polyethylene/ethylene vinyl acetate blends. J. Therm. Anal. Calorim. 2021, 146, 2127–2139. [Google Scholar] [CrossRef]
- Ali, Z.I. Effect of electron beam irradiation and vinyl acetate content on the physicochemical properties of LDPE/EVA blends. J. Appl. Polym. Sci. 2007, 104, 2886–2895. [Google Scholar] [CrossRef]
- McLoughlin, K.M.; Oskouei, A.J.; Sing, M.K.; Bandegi, A.; Mitchell, S.; Kennedy, J.; Gray, T.G.; Manas-Zloczower, I. Thermomechanical properties of cross-linked EVA: A holistic approach. ACS Appl. Polym. Mater. 2023, 5, 1430–1439. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.; Kim, B.C.; Shin, B.-S.; Jeon, J.-Y.; Chae, D.W. Effect of VA and MWNT contents on the rheological and physical properties of EVA. Korea-Aust. Rheol. J. 2016, 28, 41–49. [Google Scholar] [CrossRef]
- Alothman, O.Y. Processing and characterization of high density polyethylene/ethylene vinyl acetate blends with different VA contents. Adv. Mater. Sci. Eng. 2012, 2012, 635693. [Google Scholar] [CrossRef]
- Agroui, K.; Maallemi, A.; Boumaour, M.; Collins, G.; Salama, M. Thermal stability of slow and fast cure EVA encapsulant material for photovoltaic module manufacturing process. Sol. Energy Mater. Sol. Cells 2006, 90, 2509–2514. [Google Scholar] [CrossRef]
- de Oliveira, M.C.C.; Cardoso, A.S.A.D.; Viana, M.M.; Lins, d.F.V. The causes and effects of degradation of encapsulant ethylene vinyl acetate copolymer (EVA) in crystalline silicon photovoltaic modules: A review. Renew. Sustain. Energy Rev. 2018, 81, 2299–2317. [Google Scholar] [CrossRef]
- Desai, U.; Sharma, B.K.; Singh, A.; Singh, A. Improvement in the reliability of photovoltaic mini-modules through modifying the structural composition of EVA encapsulant. Sol. Energy 2022, 242, 246–255. [Google Scholar] [CrossRef]
- Lange, R.F.M.; Luo, Y.; Polo, R.; Zahnd, J. The lamination of (multi) crystalline and thin film based photovoltaic modules. Progress Photovolt. Res. Appl. 2011, 19, 127–133. [Google Scholar] [CrossRef]
- Kim, N.; Lee, S.; Zhao, X.G.; Kim, D.; Oh, C.; Kang, H. Reflection and durability study of different types of backsheets and their impact on c-Si PV module performance. Sol. Energy Mater. Sol. Cells 2016, 146, 91–98. [Google Scholar] [CrossRef]
- Geretschläger, K.J.; Wallner, G.M.; Fischer, J. Structure and basic properties of photovoltaic module backsheet films. Sol. Energy Mater. Sol. Cells 2016, 144, 451–456. [Google Scholar] [CrossRef]
- DuPont, DuPontTM Tedlar® surface protection film provides superior surface protection for a variety of materials and industries. Tedlar® by DuPontTM (2025). Available online: https://www.dupont.com/brands/tedlar.html (accessed on 31 March 2025).
- Vaishak, S.; Bhale, P.V. Investigation the effect of different backsheet materials on performance characteristics of a photovoltaic/thermal (PV/T) system. Renew. Energy 2021, 168, 160–169. [Google Scholar] [CrossRef]
- Xu, X.; Lai, D.; Wang, G.; Wang, Y. Nondestructive silicon wafer recovery by a novel method of solvothermal swelling coupled with thermal decomposition. Chem. Eng. J. 2021, 418, 129457. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Atia, T.A.; Toro, L. Photovoltaic panel recycling: From type-selective processes to flexible apparatus for simultaneous treatment of different types. Miner. Process. Extr. Metall. 2016, 125, 221–227. [Google Scholar] [CrossRef]
- Doi, T.; Tsuda, I.; Unagida, H.; Murata, A.; Sakuta, K.; Kurokawa, K. Experimental study on PV module recycling with organic solvent method. Sol. Energy Mater. Sol. Cells 2001, 67, 397–403. [Google Scholar] [CrossRef]
- Chen, W.S.; Chen, Y.J.; Chen, Y.A. The application of organic solvents and thermal process for eliminating EVA resin layer from waste photovoltaic modules. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012012. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Wang, D.; Cao, J.; Ma, W.; Wei, K.; Yun, L. Recovery of Silicon via Using KOH-Ethanol Solution by Separating Different Layers of End-of-Life PV Modules. JOM 2020, 72, 2624–2632. [Google Scholar] [CrossRef]
- Rubino, A.; Schiavi, P.G.; Altimari, P.; Pagnanelli, F. Valorization of polymeric fractions and metals from end of life photovoltaic panels. Waste Manag. 2021, 122, 89–99. [Google Scholar] [CrossRef]
- Budakoğlu, R.; Akın, D.A.; İrey, O.İ.; Gökçen, E.; Kasapoğlu, G.; Yılmaz, D. Environmentally sustainable methodology for the extraction of ethyene vinyl acetate (EVA) residue from EoL PV panel dismantled by hot knife. Renew. Energy 2025, 246, 122872. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J. Dissolution of ethylene vinyl acetate in crystalline silicon PV modules using ultrasonic irradiation and organic solvent. Sol. Energy Mater. Sol. Cells 2012, 98, 317–322. [Google Scholar] [CrossRef]
- Pang, S.; Yan, Y.; Wang, Z.; Wang, D.; Li, S.; Ma, W.; Wei, K. Enhanced separation of different layers in photovoltaic panel by microwave field. Sol. Energy Mater. Sol. Cells 2021, 230, 111213. [Google Scholar] [CrossRef]
- Lovato, E.S.; Donato, L.M.; Lopes, P.P.; Tanabe, E.H.; Bertuol, D.A. Application of supercritical CO2 for delaminating photovoltaic panels to recover valuable materials. J. CO2 Util. 2021, 46, 101477. [Google Scholar] [CrossRef]
- Aravelli, S.L.K.G.; Ramavathu, S.N. Smart and sustainable technologies for recycling photovoltaic panels. Environ. Chall. 2021, 2, 100020. [Google Scholar] [CrossRef]
- Granata, G.; Pagnanelli, F.; Moscardini, E.; Havlik, T.; Toro, L. Recycling of photovoltaic panels by physical operations. Sol. Energy Mater. Sol. Cells 2014, 123, 239–248. [Google Scholar] [CrossRef]
- Azeumo, M.F.; Germana, C.; Ippolito, N.M.; Franco, M.; Luigi, P.; Settimio, S. Photovoltaic module recycling, a physical and a chemical recovery process. Sol. Energy Mater. Sol. Cells 2019, 193, 314–319. [Google Scholar] [CrossRef]
- Del Pero, F.; Delogu, M.; Berzi, L.; Escamilla, M. Innovative device for mechanical treatment of End of Life photovoltaic panels: Technical and environmental analysis. Waste Manag. 2019, 95, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Pagnanelli, F.; Moscardini, E.; Granata, G.; Atia, T.A.; Altimari, P.; Havlik, T.; Toro, L. Physical and chemical treatment of end of life panels: An integrated automatic approach viable for different photovoltaic technologies. Waste Manag. 2017, 59, 422–431. [Google Scholar] [CrossRef]
- dos Santos Martins Padoan, F.C.; Schiavi, P.G.; Belardi, G.; Altimari, P.; Rubino, A.; Pagnanelli, F. Material flux through an innovative recycling process treating different types of end-of-life photovoltaic panels: Demonstration at pilot scale. Energies 2021, 14, 5534. [Google Scholar] [CrossRef]
- Dias, P.; Dias, P.; Veit, H. Recycling crystalline silicon photovoltaic modules. In Emerging Photovoltaic Materials: Silicon & Beyond; Kurinec, S.K., Ed.; Scrivener Publishing LLC: Berverly, MA, USA, 2018; Print ISBN: 9781119407546; Online ISBN: 9781119407690. [Google Scholar]
- Dias, P.; Schmidt, L.; Gomes, L.B.; Bettanin, A.; Veit, H.; Bernardes, A.M. Recycling waste crystalline silicon photovoltaic modules by electrostatic separation. J. Sustain. Metall. 2018, 4, 176–186. [Google Scholar] [CrossRef]
- Dias, P.R.; Schmidt, L.; Chang, N.L.; Lunardi, M.M.; Deng, R.; Trigger, B.; Gomes, L.B.; Egan, R.; Veit, H. High yield; low cost, environmentally friendly process to recycle silicon solar panels: Technical, economic and environmental feasibility assessment. Renew. Sustain. Energy Rev. 2022, 169, 112900. [Google Scholar] [CrossRef]
- Granata, G.; Paltrinieri, N.; Mingotti, N. Dust Hazards And Safety Measures Related To Photovoltaic Panel Recycling. In 2016 SUSTAINABLE INDUSTRIAL PROCESSING SUMMIT AND EXHIBITION Volume 10: Battery, Recycling, Environmental, Mining; Flogen Star OUTREACH: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Akimoto, Y.; Iizuka, A.; Shibata, E. High-voltage pulse crushing and physical separation of polycrystalline silicon photovoltaic panels. Miner. Eng. 2018, 125, 1–9. [Google Scholar] [CrossRef]
- Weh, A. High Voltage Pulse Fragmentation Technology to Recycle Fibre-Reinforced Composites; European Commission: Geneva, Switzerland, 2015; p. 12. [Google Scholar]
- Song, B.-P.; Zhang, M.-Y.; Fan, Y.; Jiang, L.; Kang, J.; Gou, T.-T.; Zhang, C.-L.; Yang, N.; Zhang, G.-J.; Zhou, X. Recycling experimental investigation end of life photovoltaic panels by application of high voltage fragmentation. Waste Manag. 2020, 101, 180–187. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, J.; Yan, G.; Zhu, G.; Zhu, X.; Zhang, Z.; Zhang, B. A novel and efficient method for resources recycling in waste photovoltaic panels: High voltage pulse crushing. J. Clean. Prod. 2020, 257, 120442. [Google Scholar] [CrossRef]
- Pestalozzi, F.; Eisert, S.; Woidasky, J. Benchmark comparison of high voltage discharge separation of photovoltaic modules by electrohydraulic and electrodynamic fragmentation. Recycling 2018, 3, 13. [Google Scholar] [CrossRef]
- Padhamnath, P.; Nalluri, S.; Kuśmierczyk, F.; Kopyściański, M.; Karbowniczek, J.; Leow, S.W.; Reindl, T. Electrohydraulic fragmentation processing enabling separation and recovery of all components in end-of-life silicon photovoltaic panels. Sol. Energy 2025, 289, 113329. [Google Scholar] [CrossRef]
- Nevala, S.-M.; Hamuyuni, J.; Junnila, T.; Sirviö, T.; Eisert, S.; Wilson, B.P.; Serna-Guerrero, R.; Lundström, M. Electro-hydraulic fragmentation vs conventional crushing of photovoltaic panels–Impact on recycling. Waste Manag. 2019, 87, 43–50. [Google Scholar] [CrossRef]
- Wahman, M.; Surowiak, A.; Ebin, B.; Berent, K. PV back sheet recovery from c-Si modules using hot knife technique. Sol. Energy Mater. Sol. Cells 2024, 276, 113067. [Google Scholar] [CrossRef]
- Frischknecht, R.; Komoto, K.; Doi, T. Life cycle assessment of crystalline silicon photovoltaic module delamination with hot knife technology, IEA PVPS Task 12; Report IEA-PVPS T12; International Energy Agency Power Systems Programme: Paris, France, 2023. [Google Scholar]
- Tokoro, C.; Lim, S.; Sawamura, Y.; Kondo, M.; Mochidzuki, K.; Koita, T.; Namihira, T.; Kikuchi, Y. Copper/silver recovery from photovoltaic panel sheet by electrical dismantling method. Int. J. Autom. Technol. 2020, 14, 966–974. [Google Scholar] [CrossRef]
- Fiandra, V.; Sannino, L.; Andreozzi, C.; Corcelli, F.; Graditi, G. Silicon photovoltaic modules at end-of-life: Removal of polymeric layers and separation of materials. Waste Manag. 2019, 87, 97–107. [Google Scholar] [CrossRef]
- Wang, R.; Song, E.; Zhang, C.; Zhuang, X.; Ma, E.; Bai, J.; Yuan, W.; Wang, J. Pyrolysis-based separation mechanism for waste crystalline silicon photovoltaic modules by a two-stage heating treatment. RSC Adv. 2019, 9, 18115–18123. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-K.; Ahn, Y.-S.; Yeo, J.-G.; Lee, J.-S.; Kang, G.-H.; Cho, C.-H. Peeling behavior of backsheet according to surface temperature of photovoltaic module. Korean J. Mater. Res. 2019, 29, 703–708. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; You, J.; Diao, H.; Zhao, L.; Wang, W. Back EVA recycling from c-Si photovoltaic module without damaging solar cell via laser irradiation followed by mechanical peeling. Waste Manag. 2022, 137, 312–318. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Altimari, P.; Padoan, F.C.S.M.; Atia, T.A.; Beolchini, F.; Amato, A.; Toro, L. Solvent versus thermal treatment for glass recovery from end of life photovoltaic panels: Environmental and economic assessment. J. Environ. Manag. 2019, 248, 109313. [Google Scholar] [CrossRef]
- Emersleben, A.; Meyer, N. The use of recycled glass for the construction of pavements. In GeoCongress 2012: State of the Art and Practice in Geotechnical Engineering; American Society of Civil Engineers: Reston, VA, USA, 2012; pp. 1642–1649. [Google Scholar]
- Robert, D.; Baez, E.; Setunge, S. A new technology of transforming recycled glass waste to construction components. Constr. Build. Mater. 2021, 313, 125539. [Google Scholar] [CrossRef]
- Scelsi, L.; Hodzic, A.; Soutis, C.; Hayes, S.A.; Rajendran, S.; AlMa’adeed, M.A.; Kahraman, R. A review on composite materials based on recycled thermoplastics and glass fibres, Plastics. Rubber Compos. 2011, 40, 1–10. [Google Scholar] [CrossRef]
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Blaesing, L.; Walnsch, A.; Hippmann, S.; Modrzynski, C.; Weidlich, C.; Pavón, S.; Bertau, M. Ferrosilicon Production from Silicon Wafer Breakage and Red Mud. ACS Sustain. Resour. Manag. 2024, 1, 404–416. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Sahajwalla, V. Characteristics of waste automotive glasses as silica resource in ferrosilicon synthesis. Waste Manag. Res. 2016, 34, 113–121. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Sahajwalla, V. Synthesis of ferrosilicon alloy using waste glass and plastic. Mater. Lett. 2014, 116, 101–103. [Google Scholar] [CrossRef]
- Lu, M.; Jia, J.; Gao, Q.; Xia, M.; Li, J.; Wang, J.; Park, C.B.; Zhang, R. Sustainable utilization of disposed photovoltaic backsheet to microcellular thermal-insulation foam through two-steps supercritical CO2 foaming. J. Supercrit. Fluids 2025, 222, 106616. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Mezgebe, B.; Dietrich, J.; Shan, Y.; Harmon, S.; Lee, C.C. Material recovery from electronic waste using pyrolysis: Emissions measurements and risk assessment. J. Environ. Chem. Eng. 2021, 9, 104943. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez, A.; Menargues, S. TG/FTIR study of the thermal pyrolysis of EVA copolymers. J. Anal. Appl. Pyrolysis 2005, 74, 224–230. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Huang, Q.; He, Y.; Shi, M. Unraveling the pyrolysis behavior and co-pyrolysis characteristics of EVA and backsheet in spent crystalline silicon photovoltaic modules. Chem. Eng. J. 2025, 506, 160164. [Google Scholar] [CrossRef]
- Zeng, D.; Born, M.; Wambach, K. Pyrolysis of EVA and its application in recycling of photovoltaic modules. J. Environ. Sci. 2004, 16, 889–893. [Google Scholar]
- Choi, S.-S.; Kim, E. Analysis of pyrolysis products of ethylene-vinyl acetate coploymer (EVA) using pre-deacetylation. J. Anal. Appl. Pyrolysis 2017, 127, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, R.; Luo, G.; Liu, Q.; Pan, S.; Zhao, T.; Li, X.; Yao, H. Isothermal pyrolysis and gasification characteristics and gas products of ethylene vinyl acetate and back sheets in end-of-life photovoltaic module thermal treatment recovery. Fuel 2025, 386, 134233. [Google Scholar] [CrossRef]
- Patel, K.K.; Mallick, S. Analysis of the Degradation Products of the Organic Materials Present in the c-Si PV Modules, Emitted during a Pyrolysis Treatment and Recycling Strategy for Circular and Sustainable Development. Circ. Econ. Sustain. 2025. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Shi, M.; He, Y. A comparative study of mechanical crushing and pyrolysis techniques for separation and recovery of discarded polycrystalline silicon photovoltaic modules. Sol. Energy Mater. Sol. Cells 2024, 275, 113020. [Google Scholar] [CrossRef]
- Yao, Z.; Tong, J.; Gonçalves, R.F.B.; Kumar, A.; Manić, N.; Vegliò, F.; Romano, P.; Jiang, J.; Cui, J.; Liu, J.; et al. Recycling of end-of-life solar panels: Focusing on the pyrolysis conversion of back sheet from a micro perspective. J. Clean. Prod. 2025, 490, 144796. [Google Scholar] [CrossRef]
- Duan, C.; Wang, Z.; Zhou, B.; Yao, X. Global polyethylene terephthalate (PET) plastic supply chain resource metabolism efficiency and carbon emissions co-reduction strategies. Sustainability 2024, 16, 3926. [Google Scholar] [CrossRef]
- Tamoor, M.; Samak, N.A.; Yang, M.; Xing, J. The cradle-to-cradle life cycle assessment of polyethylene terephthalate: Environmental perspective. Molecules 2022, 27, 1599. [Google Scholar] [CrossRef]
- Danz, P.; Aryan, V.; Möhle, E.; Nowara, N. Experimental study on fluorine release from photovoltaic backsheet materials containing PVF and PVDF during pyrolysis and incineration in a technical lab-scale reactor at various temperatures. Toxics 2019, 7, 47. [Google Scholar] [CrossRef]
- Huber, S.; Moe, M.K.; Schmidbauer, N.; Hansen, G.H.; Herzke, D. Emissions from Incineration of Fluoropolymer Materials, a Literature Survey; Norwegian Institute for Air Research: Kjeller, Norway, 2009; 58p. [Google Scholar]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Krasucka, P.; Charmas, B.; Stefaniak, W.; Goworek, J. Swelling effects in cross-linked polymers by thermogravimetry. J. Therm. Anal. Calorim. 2017, 130, 85–93. [Google Scholar] [CrossRef]
- Gugliuzza, A. Solvent swollen polymer. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1801–1802. [Google Scholar]
- Trivedi, H.K.; Yadav, R.K.; Meshram, A.; Gupta, R. Removal of encapsulant ethylene-vinyl acetate for recycling of photovoltaic modules: Hansen solubility parameters analysis. Process Saf. Environ. Prot. 2024, 186, 1397–1409. [Google Scholar] [CrossRef]
- Farrell, C.; Osman, A.I.; Zhang, X.; Murphy, A.; Doherty, R.; Morgan, K.; Rooney, D.W.; Harrison, J.; Coulter, R.; Shen, D. Assessment of the energy recovery potential of waste Photovoltaic (PV) modules. Sci. Rep. 2019, 9, 5267. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, S.; Yu, W. Recyclable ethylene-vinyl acetate copolymer vitrimer foams. Polymer 2021, 222, 123662. [Google Scholar] [CrossRef]
- Sulkan, C.; Thakur, P.K.; Sharma, S.; Das, N. Optimized EVA Decomposition in Bifacial Solar Panels: Sustainable Recovery of Polymerized Oil and Materials. Adv. Sustain. Syst. 2025, 9, 2500004. [Google Scholar] [CrossRef]
- Uličná, S.; Owen-Bellini, M.; Moffitt, S.L.; Sinha, A.; Tracy, J.; Roy-Choudhury, K.; Miller, D.C.; Hacke, P.; Schelhas, L.T. A study of degradation mechanisms in PVDF-based photovoltaic backsheets. Sci. Rep. 2022, 12, 14399. [Google Scholar] [CrossRef]
- Moffitt, S.L.; Pan, P.-C.; Perry, L.; Tracy, J.; Choudhury, K.R.; Kempe, M.D.; Gu, X. Microstructure changes during failure of PVDF-based photovoltaic backsheets. Prog. Photovolt. Res. Appl. 2023, 31, 26–35. [Google Scholar] [CrossRef]
- Fairbrother, A.; Phillips, N.; Gu, X. 7—Degradation Processes and Mechanisms of Backsheets. In Durability and Reliability of Polymers and Other Materials in Photovoltaic Modules; Yang, H.E., French, R.H., Bruckman, L.S., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 153–174. [Google Scholar] [CrossRef]
- Kempe, M.D.; Miller, D.C.; Zielnik, A.; Montiel-Chicharro, D.; Zhu, J.; Gottschalg, R. Survey of mechanical durability of PV backsheets. In Proceedings of the 2017 IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017; pp. 3208–3213. [Google Scholar]
- Markert, J.; Kotterer, S.; Mansour, D.E.; Philipp, D.; Gebhardt, P. Advanced analysis of backsheet failures from 26 power plants. EPJ Photovolt. 2021, 12, 7. [Google Scholar] [CrossRef]
- Pascual, J.; García, M.; Marcos, J.; Marroyo, L. Analysis of polyamide and fluoropolymer backsheets: Degradation and insulation failure in field-aged photovoltaic modules. Prog. Photovolt. Res. Appl. 2023, 31, 494–505. [Google Scholar] [CrossRef]
- Morita, Y.; Saito, Y.; Kumagai, S.; Kameda, T.; Shiratori, T.; Yoshioka, T. Alkaline hydrolysis of photovoltaic backsheet containing PET and PVDF for the recycling of PVDF. J. Mater. Cycles Waste Manag. 2023, 25, 674–683. [Google Scholar] [CrossRef]
- Tian, C.; Chen, J.; Li, X.; Dai, R.; Wang, Z. Chemical cleaning−solvent treatment−hydrophilic modification strategy for regenerating end-of-life PVDF membrane. J. Memb. Sci. 2023, 669, 121325. [Google Scholar] [CrossRef]
- Yao, Z.; Cui, J.; Jiang, J.; Tong, J.; Kumar, A.; Romano, P.; Vegliò, F.; Kumar, S.; Liu, J.; Qi, W. End-of-life solar panels recycling: Focusing on the kinetic and thermodynamic compensation effects during back sheets pyrolysis. Process Saf. Environ. Prot. 2025, 195, 106838. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Miller, C. Polyethylene Terephthalate. Waste Age 2002, 33, 104. [Google Scholar]
- Vane, L.M.; Rodriguez, F. Selected Aspects of Poly (ethylene terephthalate) Solution Behavior: Application to a Selective Dissolution Process for the Separation of Mixed Plastics. In Emerging Technologies in Plastics Recycling; ACS Publications: Washington, DC, USA, 1992. [Google Scholar]
- Poulakis, J.G.; Papaspyrides, C.D. Dissolution/reprecipitation: A model process for PET bottle recycling. J. Appl. Polym. Sci. 2001, 81, 91–95. [Google Scholar] [CrossRef]
- Fried, J.R. Polymer Science and Technology; Pearson Education: London, UK, 2014. [Google Scholar]
- Paszun, D.; Spychaj, T. Chemical Recycling of Poly(ethylene terephthalate). Ind. Eng. Chem. Res. 1997, 36, 1373–1383. [Google Scholar] [CrossRef]
- Farahat, M.S.; Abdel-Azim, A.-A.A.; Abdel-Raowf, M.E. Modified unsaturated polyester resins synthesized from poly(ethylene terephthalate) waste, 1. Synthesis and curing characteristics. Macromol. Mater. Eng. 2000, 283, 1–6. [Google Scholar] [CrossRef]
- Geyer, B.; Lorenz, G.; Kandelbauer, A. Recycling of poly (ethylene terephthalate)—A review focusing on chemical methods. Express Polym. Lett. 2016, 10, 559–586. [Google Scholar] [CrossRef]
- Lotz, R.; Wick, G.; Neuhaus, C. Process for the Recovery of Dimethyl Terephthalate from Polyethylene Terephthalate. U.S. Patent 3,321,510, 23 May 1967. [Google Scholar]
- Marathe, M.N.; Dabholkar, D.A.; Jain, M.K. Process for the Recovery of Dimethyl Terephthalate From Polyethylene Terephthalate Polymer Waste. GB Patent 2 041 916, 16 February 1980. [Google Scholar]
- Michel, R.E. Recovery of Methyl Esters of Aromatic Acids and Glycols from Thermoplastic Polyester Scrap Using Methanol Vapor. Eur. Patent 484 963, 10 May 1992. [Google Scholar]
- Socrate, C.; Vosa, R. Continuous Process for the Recovery of Terephthalic Acid from Waste or Used Polyalkylene Terephthalate Polymers. Eur. Patent 662 466, 12 July 1995. [Google Scholar]
- Laldinpuii, Z.T.; Khiangte, V.; Lalhmangaihzuala, S.; Lalmuanpuia, C.; Pachuau, Z.; Lalhriatpuia, C.; Vanlaldinpuia, K. Methanolysis of PET Waste Using Heterogeneous Catalyst of Bio-waste Origin. J. Polym. Environ. 2022, 30, 1600–1614. [Google Scholar] [CrossRef]
- Goto, M.; Koyamoto, H.; Kodama, A.; Hirose, T.; Nagaoka, S. Depolymerization of polyethylene terephthalate in supercritical methanol. J. Phys. Condens. Matter 2002, 14, 11427. [Google Scholar] [CrossRef]
- Umdagas, L.; Orozco, R.; Heeley, K.; Thom, W.; Al-Duri, B. Advances in chemical recycling of polyethylene terephthalate (PET) via hydrolysis: A comprehensive review. Polym. Degrad. Stab. 2025, 234, 111246. [Google Scholar] [CrossRef]
- Cao, F.; Wang, L.; Zheng, R.; Guo, L.; Chen, Y.; Qian, X. Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products. RSC Adv. 2022, 12, 31564–31576. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Sato, M.; Murakami, M. Depolymerization of Polyester Scraps. Japan Patent 06 248 646, 20 May 1985. [Google Scholar]
- Fujita, A.; Sato, M.; Murakami, M. Process for the Depolymerization of Polyester Scrap. U.S. Patent 4,609,680, 2 September 1986. [Google Scholar]
- Baliga, S.; Wong, W.T. Depolymerization of Poly(Ethylene Terephthalate) Recycled from Post-Consumer Soft-drink Bottles. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 2071–2082. [Google Scholar] [CrossRef]
- Vaidya, U.R.; Nadkarni, V.M. Unsaturated Polyester Resins from PET Waste. 1. Synthesis and Characterization. Ind. Eng. Chem. Res. 1987, 26, 194. [Google Scholar] [CrossRef]
- Nevrekar, N.A.; Sheth, N.S. Depolymerization of Poly(ethylene terephthalate)a Study. Man-Made Text. India 1990, 33, 7. [Google Scholar]
- Johnson, P.L.; Teeters, D. A Kinetic Study of the Depolymerization of Poly(ethylene terephthalate) Recycled from Soft-drink Bottles. Polym. Prepr. Am. Chem. Soc. Div. Polym. Chem. 1991, 32, 144. [Google Scholar]
- Tersac, G.; Laurencon, G.; Roussel, H. Synthesis of Insulating Foams from Poly(ethylene terephthalate) Bottles. Caoutch. Plast. 1991, 68, 81. [Google Scholar]
- Ostrysz, R. Unsaturated Polyester Resins with Thixotropic Properties Prepared by a Block Condensation Method. Polimery 1970, 15, 406. [Google Scholar]
- Vaidya, U.R.; Nadkarni, V.M. Unsaturated Polyesters from PET Waste: Kinetics of Polycondensation. J. Appl. Polym. Sci. 1987, 34, 235. [Google Scholar] [CrossRef]
- Vaidya, U.R.; Nadkarni, V.M. Polyester Polyols from Glycolyzed PET Waste: Effect of Glycol Type on Kinetics of Polyesterification. J. Appl. Polym. Sci. 1989, 38, 1179. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.S.; Park, T.S.; Kim, J.; Kim, K.U. Preparation and Viscosity of Unsaturated Polyester Resins Based on Recycled Poly(ethylene terephthalate). Pollimo 1995, 19, 353. [Google Scholar]
- Guo, Z.; Adolfsson, E.; Tam, P.L. Nanostructured micro particles as a low-cost and sustainable catalyst in the recycling of PET fiber waste by the glycolysis method. Waste Manag. 2021, 126, 559–566. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Hamid, M.K.A.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green. Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Khoonkari, M.; Haghighi, A.H.; Sefidbakht, Y.; Shekoohi, K.; Ghaderian, A. Chemical Recycling of PET Wastes with Different Catalysts. Int. J. Polym. Sci. 2015, 2015, 124524. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the catalytic glycolysis of polyethylene terephthalate. J. Environ. Manag. 2021, 296, 113267. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lindqvist, K.; de la Motte, H. An efficient recycling process of glycolysis of PET in the presence of a sustainable nanocatalyst. J. Appl. Polym. Sci. 2018, 135, 46285. [Google Scholar] [CrossRef]
- Shirazimoghaddam, S.; Amin, I.; Albanese, J.A.F.; Shiju, N.R. Chemical Recycling of Used PET by Glycolysis Using Niobia-Based Catalysts. ACS Eng. Au 2023, 3, 37–44. [Google Scholar] [CrossRef]
- Raheem, A.B.; Uyigue, L. The conversion of post-consumer polyethylene terephthalate (PET) into a thermosetting polyester resins. Arch. Appl. Sci. Res. 2010, 2, 240. [Google Scholar]
- Kijo-Kleczkowska, A.; Gnatowski, A. Recycling of plastic waste, with particular emphasis on thermal methods. Energies 2022, 15, 2114. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Pusztaszeri, S.F. Method for Recovery of Terephthalic Acid from Polyester Scrap. U.S. Patent 4 355 175, 19 October 1982. [Google Scholar]
- Sharma, N.D.; Vaidya, A.A.; Sharma, P. Recovery of Pure Terephthalic Acid from Polyester Materials. Ind. Patent 163 385, 18 May 1985. [Google Scholar]
- Brown, G.E., Jr. Method for Recovering Terephthalic Acid and Ethylene Glycol from Polyester Materials. U.S. Patent 3,952,053, 20 April 1976. [Google Scholar]
- Yoshioka, T.; Sato, T.; Okuwaki, A. Hydrolysis of Waste PET by Sulfuric Acid at 150 °C for a Chemical Recycling. J. Appl. Polym. Sci. 1994, 52, 1353. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.-S. Strategic possibility routes of recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Lazarus, S.D.; Twilley, J.C.; Snider, O.E. Simultaneous Depolymerization of Polycaproamide and Polyester with Recovery of Caprolactam. U.S. Patent 3 317 519, 1967. [Google Scholar]
- Namboori, C.G.G.; Haith, M.S. Steric Effects in the Basic Hydrolysis of Poly(ethylene terephthalate). J. Appl. Polym. Sci. 1968, 12, 1999. [Google Scholar] [CrossRef]
- Alter, H. Disposal and Reuse of Plastics. In Encyclopedia of Polymer Science and Engineering; Wiley Interscience: New York, NY, USA, 1986; Volume 5, pp. 103–128. [Google Scholar]
- Kosmidis, V.A.; Achilias, D.S.; Karayannidis, G.P. Poly(ethylene terephthalate) Recycling and Recovery of Pure Terephthalic Acid. Kinetics of a Phase Transfer Catalyzed Alkaline Hydrolysis. Macromol. Mater. Eng. 2001, 286, 640–647. [Google Scholar] [CrossRef]
- Yanik, J.; Karayildirim, T. Liquefaction of Municipal Waste Plastics over Acidic and Nonacidic Catalysts. In Feedstock Recycling and Pyrolysis of Waste Plastics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 209–224. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Umar, A.; Mehta, S.K.; Bhatti, M.S.; Kansal, S.K. Recycling of waste poly (ethylene terephthalate) bottles by alkaline hydrolysis and recovery of pure nanospindle-shaped terephthalic acid. J. Nanosci. Nanotechnol. 2018, 18, 5804–5809. [Google Scholar] [CrossRef]
- Barredo, A.; Asueta, A.; Amundarain, I.; Leivar, J.; Miguel-Fernández, R.; Arnaiz, S.; Epelde, E.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I. Chemical recycling of monolayer PET tray waste by alkaline hydrolysis. J. Environ. Chem. Eng. 2023, 11, 109823. [Google Scholar] [CrossRef]
- Mandoki, J.W. Depolymerization of Condensation Polymers. U.S. Patent 4,605,762, 12 August 1986. [Google Scholar]
- Rosen, B.J. Preparation of Purified Terephthalic Acid from Waste PET. U.S. Patent 5,095,145, 10 March 1992. [Google Scholar]
- Royall, D.J.; Harvie, J.L. Process for the Production of Terephthalic Acid. Eur. Patent 550 979, 17 September 1993. [Google Scholar]
- Campanelli, J.R.; Cooper, D.G.; Kamal, M.R. The Kinetics of High-Temperature Hydrolytic Depolymerization of Poly(ethylene terephthalate). ANTEC 1992, 50, 270. [Google Scholar]
- Campanelli, J.R.; Cooper, D.G.; Kamal, M.R. Catalyzed Hydrolysis of Poly(ethylene terephthalate) Melts. J. Appl. Polym. Sci. 1994, 53, 985. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of Water for Chemical Reactions in High-Temperature Water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant: Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Queiroz, A.; Pedroso, G.B.; Kuriyama, S.N.; Fidalgo-Neto, A.A. Subcritical and supercritical water for chemical recycling of plastic waste. Curr. Opin. Green Sustain. Chem. 2020, 25, 100364. [Google Scholar] [CrossRef]
- Kozlov, N.S.; Korotysko, G.P.; Kashinskii, A.V.; Gavrilenko, N.D. Preparation of Terephthalic and Benzoic Acid by Simultaneous Alkaline Hydrolysis of Wastes from PET and Methyl Benzoate. Vesti Akad. Nauk BSSR Ser. Khim. Nauk. 1984, 5, 91. [Google Scholar]
- Michalski, A. Hydrolysis of Poly(ethylene terephthalate) Waste to Obtain Terephthalic Acid. Wl. Chem. 1987, 49, 144. [Google Scholar]
- Darzi, R.; Dubowski, Y.; Posmanik, R. Hydrothermal processing of polyethylene-terephthalate and nylon-6 mixture as a plastic waste upcycling treatment: A comprehensive multi-phase analysis. Waste Manag. 2022, 143, 223–231. [Google Scholar] [CrossRef] [PubMed]
- GOTO, M. Subcritical and Supercritical Fluid Technology for Recycling Waste Plastics. J. Jpn. Pet. Inst. 2016, 59, 254–258. [Google Scholar] [CrossRef]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green. Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Awodi, Y.W.; Johnson, A.; Peters, R.H.; Popoola, A.V. The Aminolysis of Poly(ethylene terephthalate). J. Appl. Polym. Sci. 1987, 33, 2503. [Google Scholar] [CrossRef]
- Popoola, V.A.; Formation, P. Mechanisms of the Reaction. J. Appl. Polym. Sci. 1988, 36, 1677. [Google Scholar] [CrossRef]
- Collins, M.J.; Zeronian, S.H.; Marshall, M.L. Analysis of the Molecular Weight Distributions of Aminolyzed Poly(ethylene terephthalate) by Using Gel Permeation Chromatography. J. Macromol. Sci. Chem. A 1991, 28, 775. [Google Scholar] [CrossRef]
- Ellison, M.S.; Fisher, L.D.; Alger, K.W.; Zeronian, S.H. Physical Properties of Polyester Fibers Degraded by Aminolysis and by Alkaline Hydrolysis. J. Appl. Polym. Sci. 1982, 27, 247. [Google Scholar] [CrossRef]
- Liang, J.; Fu, J.; Lin, H.; Chen, J.; Peng, S.; Sun, Y.; Xu, Y.; Kang, S. Valorization of polyethylene terephthalate wastes to terephthalamide via catalyst-free ammonolysis. J. Ind. Eng. Chem. 2024, 132, 578–587. [Google Scholar] [CrossRef]
- Gupta, P.; Bhandari, S. 6—Chemical Depolymerization of PET Bottles via Ammonolysis and Aminolysis. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Thomas, A.V.K.M.G., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 109–134. [Google Scholar] [CrossRef]
- Peterson, R.-J.L.; Neppel, E.P.; Peereboom, L.; Trinh, P.A.; Ofoli, R.Y.; Dorgan, J.R. Upcycling Waste PET: I. Ammonolysis Kinetics of Model Dimethyl Terephthalate and the Catalytic Effects of Ethylene Glycol. ACS Sustain. Chem. Eng. 2025, 13, 4120–4131. [Google Scholar] [CrossRef]
- Department of Economic and Social Affairs, United Nations, Sustainable Development Goals. 2015. Available online: https://sdgs.un.org/goals (accessed on 3 February 2025).
- Cucchiella, F.; Rosa, P. End-of-Life of used photovoltaic modules: A financial analysis. Renew. Sustain. Energy Rev. 2015, 47, 552–561. [Google Scholar] [CrossRef]
- Deng, R.; Chang, N.; Lunardi, M.M.; Dias, P.; Bilbao, J.; Ji, J.; Chong, C.M. Remanufacturing end-of-life silicon photovoltaics: Feasibility and viability analysis. Prog. Photovolt. Res. Appl. 2021, 29, 760–774. [Google Scholar] [CrossRef]
- Nithya, R.; Sivasankari, C.; Thirunavukkarasu, A. Electronic waste generation, regulation and metal recovery: A review. Environ. Chem. Lett. 2021, 19, 1347–1368. [Google Scholar] [CrossRef]
- Serpe, A.; Purchase, D.; Bisschop, L.; Chatterjee, D.; De Gioannis, G.; Garelick, H.; Kumar, A.; Peijnenburg, W.; Piro, V.M.I.; Cera, M. 2002–2022: 20 years of e-waste regulation in the European Union and the worldwide trends in legislation and innovation technologies for a circular economy. RSC Sustain. 2025, 3, 1039–1083. [Google Scholar] [CrossRef]
- Kastanaki, E. Dynamic assessment of photovoltaic waste streams in the EU-27 countries under the circular economy principles of ‘Reduce, Reuse and Recycle. Resour. Conserv. Recycl. 2025, 214, 108033. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, T.; Gupta, A.K. End-of-life management of solar PV waste in India: Situation analysis and proposed policy framework. Renew. Sustain. Energy Rev. 2022, 153, 111774. [Google Scholar] [CrossRef]
- Curtis, T.L.; Buchanan, H.; Heath, G.; Smith, L.; Shaw, S. Solar Photovoltaic Module Recycling: A Survey of US Policies and Initiatives; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2021.
- Ali, A.; Malik, S.A.; Shafiullah, M.; Malik, M.Z.; Zahir, M.H. Policies and regulations for solar photovoltaic end-of-life waste management: Insights from China and the USA. Chemosphere 2023, 340, 139840. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Islam, M.T.; Rehman, S.; Qadir, S.A.; Shahid, M.; Khan, M.W.; Zahir, M.H.; Islam, A.; Khalid, M. Solar Photovoltaic Module End-of-Life Waste Management Regulations: International Practices and Implications for the Kingdom of Saudi Arabia. Sustainability 2024, 16, 7215. [Google Scholar] [CrossRef]
- Majewski, P.; Al-shammari, W.; Dudley, M.; Jit, J.; Lee, S.-H.; Myoung-Kug, K.; Sung-Jim, K. Recycling of solar PV panels-product stewardship and regulatory approaches. Energy Policy 2021, 149, 112062. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Huda, N.; Behnia, M. Multi-levels of photovoltaic waste management: A holistic framework. J. Clean. Prod. 2021, 294, 126252. [Google Scholar] [CrossRef]
- Sharma, A.; Pandey, S.; Kolhe, M. Global review of policies & guidelines for recycling of solar PV modules. Int. J. Smart Grid Clean Energy 2019, 8, 597–610. [Google Scholar]
- Held, M.; Wessendorf, C. Status of PV Module Take-Back and Recycling in Germany; International Energy Agency (IEA): Paris, France, 2024. [Google Scholar]
- First Solar. First Solar Recycling Platform, (n.d.). Available online: https://www.firstsolar.com/en/Solutions/Recycling (accessed on 5 May 2025).
- Ercole, P. FRELP 2 project–Full recovery end-of-life photovoltaic. In Proceedings of the 32nd European Photovoltaic Solar Energy Conference and Exhibition, Munich, Germany, 20–24 June 2016; pp. 1775–1783. [Google Scholar]
- Rosi Solar. Rosi Solar PV Recycling, (n.d.). Available online: https://www.rosi-solar.com/?r=0 (accessed on 5 May 2025).
- Werecyclesolar. Werecyclesolar PV Recycling, (n.d.). Available online: https://werecyclesolar.com/recycle/ (accessed on 5 May 2025).
- ReSolar. ReSolar PV Recycling, (n.d.). Available online: https://www.resolartech.com/en/ (accessed on 5 May 2025).
- Suny Group. Suny Group PV Recycling, (n.d.). Available online: https://www.sunyrecycle.com/Scrap-PV-Solar-Panel-Recycling-Plant/?campaignid=18479209652&adgroupid=144441507480&feeditemid=&targetid=kwd-1875817506443&device=c&creative=625302061324&keyword=solar%20panels%20recycling%20line&gad_source=1&gad_campaignid=18479209652&gbraid=0AAAAAC8CqAJ1uiIk3GUbtzVIh3MvrWwY0&gclid=EAIaIQobChMIg_K5hcaMjQMV6SqiAx3RNBg0EAAYAyAAEgL3D_D_BwE (accessed on 5 May 2025).
- Peacock, B. Australia’s Most Recognisable Solar Recycler Handed Windup Notice by Victorian Court, PV Magazine. 2023. Available online: https://www.pv-magazine-australia.com/2023/09/15/australias-most-recognisable-solar-recycler-handed-windup-notice-by-victorian-court/ (accessed on 5 May 2025).
- Granata, G.; Altimari, P.; Pagnanelli, F.; De Greef, J. Recycling of solar photovoltaic panels: Techno-economic assessment in waste management perspective. J. Clean. Prod. 2022, 363, 132384. [Google Scholar] [CrossRef]
- Deng, R.; Chang, N.L.; Ouyang, Z.; Chong, C.M. A techno-economic review of silicon photovoltaic module recycling. Renew. Sustain. Energy Rev. 2019, 109, 532–550. [Google Scholar] [CrossRef]
- Rubino, A.; Granata, G.; Moscardini, E.; Baldassari, L.; Altimari, P.; Toro, L.; Pagnanelli, F. Development and techno-economic analysis of an advanced recycling process for photovoltaic panels enabling polymer separation and recovery of Ag and Si. Energies 2020, 13, 6690. [Google Scholar] [CrossRef]
- Crespo, B.; Cavanaugh, C.; Potter, A.; Yaniger, S.; Gaustad, G.; Wilkinson, C. Technoeconomic feasibility of photovoltaic recycling. Int. J. Appl. Glass Sci. 2024, 15, 381–390. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Recent Developments in the Chemical Recycling of Postconsumer Poly(ethylene terephthalate) Waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Čorak, I.; Tarbuk, A.; Đorđević, D.; Višić, K.; Botteri, L. Sustainable alkaline hydrolysis of polyester fabric at low temperature. Materials 2022, 15, 1530. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Fthenakis, V.; Ebin, B.; Steenari, B.; Butler, E.; Sinha, P.; Corkish, R.; Wambach, K.; Simon, E.S. Major challenges and opportunities in silicon solar module recycling. Prog. Photovolt. Res. Appl. 2020, 28, 1077–1088. [Google Scholar] [CrossRef]
- Valdivia, C.E.; Li, C.T.; Russell, A.; Haysom, J.E.; Li, R.; Lekx, D.; Sepeher, M.M.; Henes, D.; Hinzer, K.; Schriemer, H.P. Bifacial photovoltaic module energy yield calculation and analysis. In Proceedings of the 2017 IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017; pp. 1094–1099. [Google Scholar]
- Mühleisen, W.; Loeschnig, J.; Feichtner, M.; Burgers, A.R.; Bende, E.E.; Zamini, S.; Yerasimou, Y.; Kosel, J.; Hirschl, C.; Georghiou, G.E. Energy yield measurement of an elevated PV system on a white flat roof and a performance comparison of monofacial and bifacial modules. Renew. Energy 2021, 170, 613–619. [Google Scholar] [CrossRef]
- Wang, S.; Wilkie, O.; Lam, J.; Steeman, R.; Zhang, W.; Khoo, K.S.; Siong, S.C.; Rostan, H. Bifacial photovoltaic systems energy yield modelling. Energy Procedia 2015, 77, 428–433. [Google Scholar] [CrossRef]
- Loughlin, D.H.; Barlaz, M.A. Policies for strengthening markets for recyclables: A worldwide perspective. Crit. Rev. Environ. Sci. Technol. 2006, 36, 287–326. [Google Scholar] [CrossRef]
- Schwanse, E. Recycling policies and programmes for PET drink bottles in Mexico. Waste Manag. Res. 2011, 29, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Y.; Wu, Y.; Zhou, G.; Wang, H.; Han, H.; Chang, T. Performance simulation and policy optimization of waste polyethylene terephthalate bottle recycling system in China. Resour. Conserv. Recycl. 2020, 162, 105014. [Google Scholar] [CrossRef]
- Smith, R.L.; Takkellapati, S.; Riegerix, R.C. Recycling of plastics in the United States: Plastic material flows and polyethylene terephthalate (PET) recycling processes. ACS Sustain. Chem. Eng. 2022, 10, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.M.; Castro, R.; Jr, J.A.G. PET containers in Brazil: Opportunities and challenges of a logistics model for post-consumer waste recycling. Resour. Conserv. Recycl. 2011, 55, 291–299. [Google Scholar] [CrossRef]
- Kuan, S.H.; Low, F.S.; Chieng, S. Towards regional cooperation sustainable plastic recycling: Comparative analysis of plastic waste recycling policies and legislations in Japan and Malaysia. Clean. Technol. Environ. Policy 2022, 24, 761–777. [Google Scholar] [CrossRef]
- Er-Raji, O.; Lange, S.; Hartwig, C.E.; Prasetio, A.; Bivour, M.; Hermle, M.; Turek, M.; De Wolf, S.; Glunz, S.W.; Borchert, J. Tuning Self-Assembly of Hole-Selective Monolayers for Reproducible Perovskite/Silicon Tandem Solar Cells. Small Methods 2025, 2401758. [Google Scholar] [CrossRef]
- Kore, B.P.; Er-Raji, O.; Fischer, O.; Callies, A.; Schultz-Wittmann, O.; Schulze, P.S.C.; Bivour, M.; De Wolf, S.; Glunz, S.W.; Borchert, J. Efficient fully textured perovskite silicon tandems with thermally evaporated hole transporting materials. Energy Environ. Sci. 2025, 18, 354–366. [Google Scholar] [CrossRef]
- Zanetta, A.; Larini, V.; Vikram; Toniolo, F.; Vishal, B.; Elmestekawy, K.A.; Du, J.; Scardina, A.; Faini, F.; Pica, G.; et al. Vertically oriented low-dimensional perovskites for high-efficiency wide band gap perovskite solar cells. Nat. Commun. 2024, 15, 9069. [Google Scholar] [CrossRef] [PubMed]
- Moody, N. Assessing the Regulatory Requirements of Lead-Based Perovskite Photovoltaics. Joule 2020, 4, 970. [Google Scholar] [CrossRef]
- Suo, J.; Pettersson, H.; Yang, B. Sustainable Approaches to Address Lead Toxicity in Halide Perovskite Solar Cells: A Review of Lead Encapsulation and Recycling Solutions. EcoMat 2025, 7, e12511. [Google Scholar] [CrossRef]
- Binek, A. Recycling Perovskite Solar Cells To Avoid Lead Waste. ACS Appl. Mater. Interfaces 2016, 8, 12881. [Google Scholar] [CrossRef]
- Dou, J.; Bai, Y.; Chen, Q. Challenges of lead leakage in perovskite solar cells. Mater. Chem. Front. 2022, 6, 2779. [Google Scholar] [CrossRef]
- Akram, W.; Li, X.; Ahmed, S.; Ouyang, Z.; Li, G. A review of life cycle assessment and sustainability analysis of perovskite/Si tandem solar cells. RSC Sustain. 2025, 3, 21–36. [Google Scholar] [CrossRef]
- Bao, Z.; Luo, Y.; Wang, L.; Dou, J.; Wang, L.; Ma, Y.; Du, Y.; Lan, Y.; Zhu, C.; Chen, H.; et al. A Shortcut for Commercialization of Perovskites Solar Cells by a Recycling and Remanufacturing Strategy. ACS Energy Lett. 2025, 10, 1474–1482. [Google Scholar] [CrossRef]
- Tian, X.; Stranks, S.D.; Huang, J.; Fthenakis, V.M.; Yang, Y.; You, F. Perspectives for sustainability analysis of scalable perovskite photovoltaics. Energy Environ. Sci. 2025, 18, 194–213. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, N.; Tian, X.; Zhang, T.; Wang, B.; Wang, X.; Xian, Y.; Lu, C.; Ou, X.; Yan, Y.; et al. Aqueous-based recycling of perovskite photovoltaics. Nature 2025, 638, 670–675. [Google Scholar] [CrossRef]
- Gallegos, M.V.; Gil-Escrig, L.; Zanoni, K.P.S.; Bolink, H.J.; Damonte, L.C. Recycling and reusing ITO substrates from perovskite solar cells: A sustainable perspective. Sol. Energy Mater. Sol. Cells 2024, 277, 113117. [Google Scholar] [CrossRef]
- Kim, S.-J.; Jeong, E.-J.; Seo, J.-Y. Sustainable Recycling of Perovskite Solar Cells: Green Solvent-Based Recovery of ITO Substrates. Int. J. Chem. Kinet. 2025, 57, 235–241. [Google Scholar] [CrossRef]
- Prince, K.J.; Mirletz, H.M.; Gaulding, E.A.; Wheeler, L.M.; Kerner, R.A.; Zheng, X.; Schelhas, L.T.; Tracy, P.; Wolden, C.A.; Berry, J.J.; et al. Sustainability pathways for perovskite photovoltaics. Nat. Mater. 2025, 24, 22–33. [Google Scholar] [CrossRef]
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; van Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From Defects to Degradation: A Mechanistic Understanding of Degradation in Perovskite Solar Cell Devices and Modules. Adv. Energy Mater. 2020, 10, 1904054. [Google Scholar] [CrossRef]
- Cheacharoen, R.; Boyd, C.C.; Burkhard, G.F.; Leijtens, T.; Raiford, J.A.; Bush, K.A.; Bent, S.F.; McGehee, M.D. Encapsulating perovskite solar cells to withstand damp heat and thermal cycling. Sustain. Energy Fuels 2018, 2, 2398–2406. [Google Scholar] [CrossRef]



| Year | Chemical Used | Process Used | Results | Comments | Reference |
|---|---|---|---|---|---|
| 2001 | Lacquer thinner | Immersion in the liquid at room temperature for 48 h, followed by heating the liquid and polymer to 80 °C for 10 min | Only swelling but no separation | Standalone test for dissolving EVA. Samples were not from actual modules. | [122] |
| Acetone | No effect | ||||
| Toluene | Only swelling but no separation | ||||
| Petroleum benzene | Only swelling but no separation | ||||
| Ethanol | No effect | ||||
| Isopropanol | No effect | ||||
| Methyl ethyl Ketone | Only swelling but no separation | ||||
| Methyl isobutyl Ketone | Only swelling but no separation | ||||
| Tetrahydrofuran | Only swelling but no separation | ||||
| Ethylene glycol | No effect | ||||
| Trichloroethylene | Separation achieved, complete dissolution after 10 days | ||||
| Glycerine | No effect | ||||
| 2016 | Cyclohexane | Immersion in 70 °C for two hours | Separation achieved in 120 min | EVA and polymers were burned to recover Si. | [121] |
| 2019 | D-Limonene | Immersion in stationary solvent at 90 °C | Separation achieved in 30–60 min | Aimed to recover glass, no attempts were made to recover the polymers. Polymer fractions were later burnt to recover the glass. | [123] |
| Tetrahydrofuran | Separation achieved in 60–120 min | ||||
| Toluene | Separation achieved in 30–60 min | ||||
| Chloroform | Only swelling but no separation | ||||
| Acetone | No effect | ||||
| Ethyl alcohol | No effect | ||||
| 2020 | NaOH + Ethanol | Immersing in a hydrothermal kettle inside a muffle furnace at 200 °C, dried at 130 °C for 8 h | Separation achieved after 4 h of heating in chemical bath | Aimed at recovering silicon. | [124] |
| KOH + Ethanol | Separation achieved after 4 h of heating in chemical bath | ||||
| 2021 | Toluene | Immersing at atmospheric pressure for 99 h | Complete separation | Recovery of EVA was not attempted. | [51] |
| 2021 | Cyclohexane | 10 g of crushed material for 120 min at 70 °C in a jacketed cell reactor (250 mL) equipped with a bubble condenser | The separation of Tedlar (backsheet) and EVA. | Tedlar recovered while EVA was combusted to recover metal. | [125] |
| 2025 | CHCl3 | Immersing in atmospheric pressure at 50 °C for 96 h | Swelling, but no separation between EVA and PET | Aimed at recovering the polymers for recycling. No attempts were made to recycle PET. EVA was partially recovered by spin coating. | [21] |
| Toluene | Immersing in atmospheric pressure at 90 °C for 96 h | Swelling and partial separation between EVA and PET | |||
| CHCl3, NaOH + (EG) | Immersing in CHCl3 at atmospheric pressure at 50 °C for 24 h, followed by immersing in NaOH + EG at 150 °C for two hours | 75% of the samples were delaminated | |||
| Toluene, NaOH + EG | Immersing in toluene at atmospheric pressure at 80 °C for 24 h, followed by immersing in NaOH + EG at 150 °C for two hours | 99% of the samples were delaminated | |||
| 2025 | KOH + Methyl alcohol | Glass + EVA layer dipped in the chemical at room temperature in the presence of a non-ionic surfactant (Triton X100) | Complete removal of EVA from the glass | No attempts were made to recover or recycle the EVA. | [126] |
| KOH + Ethyl alcohol | |||||
| KOH + Propanol |
| Delamination Process | Potential of Recovering Polymers | Advantages | Disadvantages | Possible Improvements |
|---|---|---|---|---|
| Thermo-mechanical |
|
|
|
|
| Chemical |
|
|
|
|
| Mechano-chemical |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padhamnath, P. Recent Progress in the Recovery and Recycling of Polymers from End-of-Life Silicon PV Modules. Sustainability 2025, 17, 4583. https://doi.org/10.3390/su17104583
Padhamnath P. Recent Progress in the Recovery and Recycling of Polymers from End-of-Life Silicon PV Modules. Sustainability. 2025; 17(10):4583. https://doi.org/10.3390/su17104583
Chicago/Turabian StylePadhamnath, Pradeep. 2025. "Recent Progress in the Recovery and Recycling of Polymers from End-of-Life Silicon PV Modules" Sustainability 17, no. 10: 4583. https://doi.org/10.3390/su17104583
APA StylePadhamnath, P. (2025). Recent Progress in the Recovery and Recycling of Polymers from End-of-Life Silicon PV Modules. Sustainability, 17(10), 4583. https://doi.org/10.3390/su17104583









