Co-Production of Polysaccharides and Platform Sugars from Wheat Straw Fermented with Irpex lacteus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Pretreatment Methods
2.2. Experimental Design
2.3. Chemical Analysis and Enzymatic Saccharification
2.4. Analysis of Structural Changes
2.5. Statistical Analysis
3. Results and Discussion
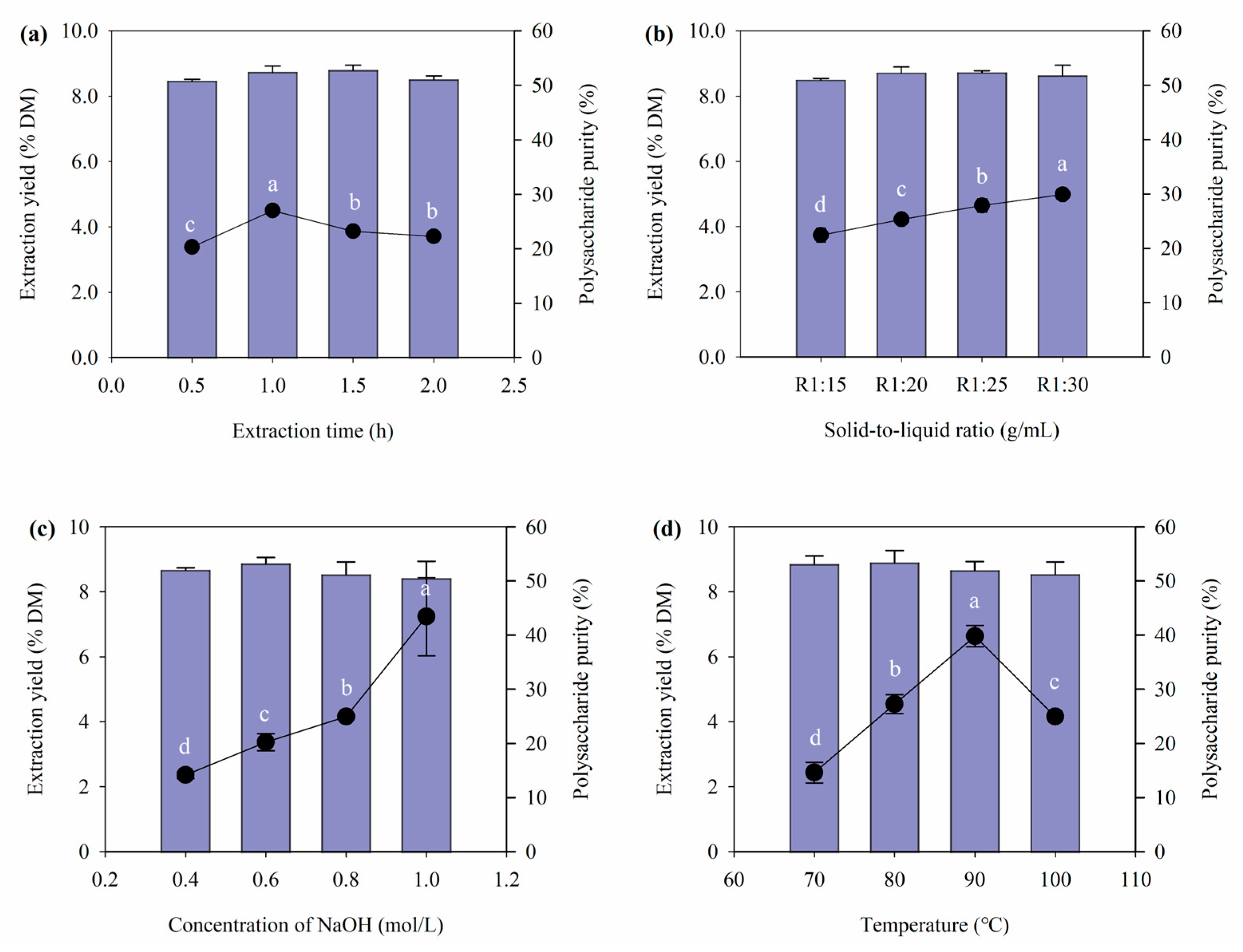
3.1. Extraction Yield and Purity of Polysaccharide
3.2. Chemical Composition and Sugar Yield Before and After Extraction
3.3. Changes in Morphological, Structural, and Thermal Characteristics
3.3.1. Morphology of Different Substrates
3.3.2. Chemical Bonds of Different Substrates
3.3.3. Crystallinity Analysis of the Wheat Straws
3.3.4. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes versicolor in lignocellulose-based bioeconomy: State of the art, challenges and opportunities. Bioresour. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Schier, F. Modelling Bioeconomy scenario pathways for the forest products markets with emerging lignocellulosic products. Sustainability 2020, 12, 10540. [Google Scholar] [CrossRef]
- Kovačević, Z.; Bischof, S.; Bilandžija, N.; Krička, T. Conversion of waste agricultural biomass from straw into useful bioproducts—Wheat fibers and biofuels. Sustainability 2024, 16, 4739. [Google Scholar] [CrossRef]
- Abbasi-Riyakhuni, M.; Hashemi, S.S.; Denayer, J.F.; Aghbashlo, M.; Tabatabaei, M.; Karimi, K. Integrated biorefining of rapeseed straw for ethanol, biogas, and mycoprotein production. Fuel 2025, 382, 133751. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Niu, D.; Yu, C.; Zheng, M.; Ren, J.; Li, C.; Xu, C. Effects of ensiling on Irpex lacteus fermentation in wheat straw: Chemical composition, in vitro rumen digestibility, and fungal community. Anim. Feed Sci. Technol. 2022, 292, 115433. [Google Scholar] [CrossRef]
- Jančíková, V.; Jablonský, M.; Voleková, K. Delignification of wheat straw using DES-like mixtures. Sustainability 2023, 15, 15343. [Google Scholar] [CrossRef]
- Wang, C.; He, G.; Meng, J.; Wang, S.; Kong, Y.; Jiang, J.; Hu, R.; Zhou, G. Improved lignocellulose saccharification of a Miscanthus reddish stem mutant induced by heavy-ion irradiation. GCB Bioenergy 2020, 12, 1066–1077. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, B.; Niu, Q.; Liu, Y.; Yang, Q. Enhancement of Liquid Hot Water Pretreatment on Corn Stover with Ball Milling to Improve Total Sugar Yields. Sustainability 2023, 15, 16426. [Google Scholar] [CrossRef]
- Niu, D.; Zuo, S.; Ren, J.; Huhetaoli; Zheng, M.; Jiang, D.; Xu, C. Novel strategy to improve the colonizing ability of Irpex lacteus in non-sterile wheat straw for enhanced rumen and enzymatic digestibility. Appl. Microbiol. Biotechnol. 2020, 104, 1347–1355. [Google Scholar] [CrossRef]
- Xiong, S.; Martín, C.; Eilertsen, L.; Wei, M.; Myronycheva, O.; Larsson, S.H.; Lestander, T.A.; Atterhem, L.; Jönsson, L.J. Energy-efficient substrate pasteurisation for combined production of shiitake mushroom (Lentinula edodes) and bioethanol. Bioresour. Technol. 2019, 274, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Niu, D.; Zhang, S.; Li, C.; Yin, D.; Zhi, J.; Zhang, L.; Jiang, X.; Ren, J. Enhanced delignification and production of bioactive compounds in wheat straw by optimizing sterilization methods for Irpex lacteus fermentation. Food Chem. 2024, 435, 137570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Zhao, Z.; Wang, M.; Liu, P. Effect of alkali-treated birch sawdust on the lignocellulase secretion and exo-polysaccharide production by Inonotus obliquus under submerged fermentation and its lignocellulose degradation patterns. J. Biosci. Bioeng. 2022, 133, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Montoya, S. Assessment of Polysaccharide and Biomass Production from Three White-Rot Fungi by Solid-State Fermentation Using Wood and Agro-Industrial Residues: A Kinetic Approach. Forests 2020, 11, 1055. [Google Scholar] [CrossRef]
- Brethauer, S.; Robert Lawrence, S.; Michael Hans-Peter, S. Enhanced simultaneous saccharification and fermentation of pretreated beech wood by in situ treatment with the white rot fungus Irpex lacteus in a membrane aerated biofilm reactor. Bioresour. Technol. 2017, 237, 135–138. [Google Scholar] [CrossRef]
- Shankar, A.; Saini, S.; Sharma, K.K. Fungal-integrated second-generation lignocellulosic biorefinery: Utilization of agricultural biomass for co-production of lignocellulolytic enzymes, mushroom, fungal polysaccharides, and bioethanol. Biomass Convers. Biorefinery 2022, 14, 1117–1131. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Huang, Y.; Yuan, Y.; Cai, Y. Extraction of Polysaccharide from Spirulina and Evaluation of Its Activities. Evid. Based Complement. Altern. Med. 2018, 2018, 3425615. [Google Scholar] [CrossRef]
- Huang, S.Q.; Li, J.W.; Wang, Z.; Pan, H.-X.; Chen, J.-X.; Ning, Z.-X. Optimization of Alkaline Extraction of Polysaccharides from Ganoderma lucidum and Their Effect on Immune Function in Mice. Molecules 2010, 15, 3694–3708. [Google Scholar] [CrossRef]
- Liang, Z.; Xiong, L.; Zang, Y.; Tang, Z.; Shang, Z.; Zhang, J.; Jia, Z.; Huang, Y.; Ye, X.; Liu, H.; et al. Extraction Optimization and Anti-Tumor Activity of Polysaccharides from Chlamydomonas reinhardtii. Mar. Drugs 2024, 22, 356. [Google Scholar] [CrossRef]
- Shah, T.A.; Khalid, S.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M. Sodium hydroxide hydrothermal extraction of lignin from rice straw residue and fermentation to biomethane. Sustainability 2023, 15, 8755. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Zhang, K.; Guo, Z.; Xu, G.; Huang, L.; Wang, L.; Li, J. Ultrasound-Assisted Enzyme Extraction, Physicochemical Properties and Antioxidant Activity of Polysaccharides from Cordyceps militaris Solid Medium. Molecules 2024, 29, 4560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr. Polym. 2020, 227, 115332. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Resch, M.G.; Baker, J.O.; Decker, S.R. Low Solids Enzymatic Saccharification of Lignocellulosic Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2015. [Google Scholar]
- Boonsombuti, A.; Luengnaruemitchai, A.; Wongkasemjit, S. Enhancement of enzymatic hydrolysis of corncob by microwave-assisted alkali pretreatment and its effect in morphology. Cellulose 2013, 20, 1957–1966. [Google Scholar] [CrossRef]
- Qi, J.; Li, F.; Jia, L.; Zhang, X.; Deng, S.; Luo, B.; Zhou, Y.; Fan, M.; Xia, Y. Fungal Selectivity and Biodegradation Effects by White and Brown Rot Fungi for Wood Biomass Pretreatment. Polymers 2023, 15, 1957. [Google Scholar] [CrossRef]
- Timung, R.; Mohan, M.; Chilukoti, B.; Sasmal, S.; Banerjee, T.; Goud, V.V. Optimization of dilute acid and hot water pretreatment of different lignocellulosic biomass: A comparative study. Biomass Bioenergy 2015, 81, 9–18. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Wang, J.; Yang, C.; Wang, Y.; Hu, Z.; Cai, W.; Liu, L. Optimization of Polysaccharide Extraction from Polygonatum cyrtonema Hua by Freeze–Thaw Method Using Response Surface Methodology. Molecules 2024, 29, 4879. [Google Scholar] [CrossRef]
- Liu, F.; Chen, H.; Qin, L.; Al-Haimi, A.A.N.M.; Xu, J.; Zhou, W.; Zhu, S.; Wang, Z. Effect and characterization of polysaccharides extracted from Chlorella sp. by hot-water and alkali extraction methods. Algal Res. 2023, 70, 102970. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, J. Fermenting and lignin degradability of a white-rot fungus Coriolopsis trogii using industrial lignin as substrate. Appl. Biochem. Biotechnol. 2022, 194, 5220–5235. [Google Scholar] [CrossRef]
- Niu, D.; An, W.; Yu, C.; Zhu, P.; Li, C.; Yin, D.; Zhi, J.; Jiang, X.; Ren, J. Pre-pasteurization enhances the fermentation of wheat straw by Irpex lacteus: Chemical composition, enzymatic hydrolysis, and microbial community. Ind. Crops Prod. 2023, 202, 116962. [Google Scholar] [CrossRef]
- García-Torreiro, M.; Pallín, M.Á.; López-Abelairas, M.; Lu-Chau, T.A.; Lema, J.M. Alkali treatment of fungal pretreated wheat straw for bioethanol production. Bioethanol 2016, 2, 32–43. [Google Scholar] [CrossRef]
- Li, X.; Park, A.; Estrela, R.; Kim, S.R.; Jin, Y.S.; Cate, J.H. Comparison of xylose fermentation by two high-performance engineered strains of Saccharomyces cerevisiae—ScienceDirect. Biotechnol. Rep. 2016, 9, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Xu, J.-L.; Zhang, Y.; Liang, C.-Y.; He, M.-C.; Yuan, Z.-H.; Xie, J. Reinforced alkali-pretreatment for enhancing enzymatic hydrolysis of sugarcane bagasse. Fuel Process. Technol. 2016, 143, 1–6. [Google Scholar] [CrossRef]
- Xie, C.; Gong, W.; Yang, Q.; Zhu, Z.; Yan, L.; Hu, Z.; Peng, Y. White-rot fungi pretreatment combined with alkaline/oxidative pretreatment to improve enzymatic saccharification of industrial hemp. Bioresour. Technol. 2017, 243, 188–195. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- Zhang, L.; You, T.; Zhou, T.; Zhang, L.; Xu, F. Synergistic effect of white-rot fungi and alkaline pretreatments for improving enzymatic hydrolysis of poplar wood. Ind. Crops Prod. 2016, 86, 155–162. [Google Scholar] [CrossRef]
- Ariff, I.N.M.; Bahrin, E.K.; Ramli, N.; Abd-Aziz, S. Direct Use of Spent Mushroom Substrate from Pleurotus pulmonarius as a Readily Delignified Feedstock for Cellulase Production. Waste Biomass Valorization 2017, 10, 839–850. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, J.; Xuan, X.; Liu, L.; Xie, M.; Liu, H.; Yu, S.; Zheng, G. Study on untreated and alkali treated rice straw reinforced geopolymer composites. Mater. Chem. Phys. 2021, 262, 124304. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Shi, S.; Lin, M. Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresour. Technol. 2017, 241, 415–423. [Google Scholar] [CrossRef]
- Castoldi, R.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; Correa, R.C.G.; Peralta, R.A.; Moreira, R.d.F.P.M.; Polizeli, M.d.L.T.d.M.; de Souza, C.G.M.; Peralta, R.M. Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: Study of degradation patterns and saccharification kinetics. Chem. Eng. J. 2014, 258, 240–246. [Google Scholar] [CrossRef]
- Thota, S.P.; Badiya, P.K.; Yerram, S.; Vadlani, P.V.; Pandey, M.; Golakoti, N.R.; Belliraj, S.K.; Dandamudi, R.B.; Ramamurthy, S.S. Macro-micro fungal cultures synergy for innovative cellulase enzymes production and biomass structural analyses. Renew. Energ. 2017, 103, 766–773. [Google Scholar] [CrossRef]
- Lu, X.; Li, F.; Zhou, X.; Hu, J.; Liu, P. Biomass, lignocellulolytic enzyme production and lignocellulose degradation patterns by Auricularia auricula during solid state fermentation of corn stalk residues under different pretreatments. Food Chem. 2022, 384, 132622. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yue, H.; Li, H.; Zhang, J.; Zhang, Y.; Wang, X.; Gong, S.; Liu, G.-Q. Selective delignification of poplar wood with a newly isolated white-rot basidiomycete Peniophora incarnata T-7 by submerged fermentation to enhance saccharification. Biotechnol. Biofuels 2021, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Chutani, P.; Kumar, P.; Sharma, K.K. Development of an eco-friendly deinking process for the production of bioethanol using diverse hazardous paper wastes. Renew. Energ. 2020, 146, 2362–2373. [Google Scholar] [CrossRef]
- Devi, R.; Kapoor, S.; Thakur, R.; Sharma, E.; Tiwari, R.K.; Joshi, S.J. Lignocellulolytic enzymes and bioethanol production from spent biomass of edible mushrooms using Saccharomyces cerevisiae and Pachysolen tannophilus. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Barman, D.N.; Haque, M.A.; Hossain, M.M.; Paul, S.K.; Yun, H.D. Deconstruction of Pine Wood (Pinus sylvestris) Recalcitrant Structure Using Alkali Treatment for Enhancing Enzymatic Saccharification Evaluated by Congo Red. Waste Biomass Valorization 2018, 11, 1755–1764. [Google Scholar] [CrossRef]
- Li, X.; Tang, Z.; Sun, Z.; Simonsen, J.; Luo, Z.; Li, X.; Morrell, J.J. Chemical and Enzymatic Fiber Modification to Enhance the Mechanical Properties of CMC Composite Films. Polymers 2022, 14, 4127. [Google Scholar] [CrossRef]
- Irfan, M.; Asghar, U.; Nadeem, M.; Nelofer, R.; Syed, Q.; Shakir, H.A.; Qazi, J.I. Statistical Optimization of Saccharification of Alkali Pretreated Wheat Straw for Bioethanol Production. Waste Biomass Valorization 2016, 7, 1389–1396. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, Q.; He, Z.; Wang, Z.; Wang, X.; Sun, J. Effect of biodegradation on thermogravimetric and chemical characteristics of hardwood and softwood by brown-rot fungus. Bioresour. Technol. 2016, 211, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zeng, Y.; Wang, J.; Yang, Y.; Yang, X.; Zhang, X. Thermogravimetric study and kinetic analysis of fungal pretreated corn stover using the distributed activation energy model. Bioresour. Technol. 2013, 128, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, X.; Yu, H.; Zhang, X.; Ma, F. The delignification effects of white-rot fungal pretreatment on thermal characteristics of moso bamboo. Bioresour. Technol. 2012, 114, 437–442. [Google Scholar] [CrossRef]




| Items 1 | WS 2 | FWS | AE-WS | AE-FWS |
|---|---|---|---|---|
| Hemicellulose (% DM) | 17.49 ± 0.994 a | 15.24 ± 0.743 b | 7.99 ± 0.411 c | 5.20 ± 0.230 d |
| Cellulose (% DM) | 30.70 ± 1.789 c | 32.49 ± 1.167 d | 50.30 ± 0.993 b | 60.21 ± 1.913 a |
| ASL (% DM) | 3.79 ± 0.075 b | 5.21 ± 0.091 a | 2.40 ± 0.080 c | 2.54 ± 0.023 c |
| ADL (% DM) | 15.03 ± 0.365 a | 12.34 ± 0.090 b | 9.11 ± 0.866 c | 7.28 ± 0.487 d |
| Ash (% DM) | 9.87 ± 0.031 c | 12.20 ± 0.006 a | 8.76 ± 0.174 d | 10.92 ± 0.203 b |
| Glucose yield (mg/g DM) | 108.08 ± 2.08 d | 177.81 ± 1.94 c | 491.50 ± 4.709 b | 585.93 ± 12.477 a |
| Xylose yield (mg/g DM) | 30.61 ± 2.70 d | 51.24 ± 3.01 b | 53.33 ± 3.183 a | 42.31 ± 0.874 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, J.; Huhe, T.; Ding, X.; Yuan, R.; Zhang, S.; Ren, J.; Niu, D. Co-Production of Polysaccharides and Platform Sugars from Wheat Straw Fermented with Irpex lacteus. Sustainability 2025, 17, 4581. https://doi.org/10.3390/su17104581
Pu J, Huhe T, Ding X, Yuan R, Zhang S, Ren J, Niu D. Co-Production of Polysaccharides and Platform Sugars from Wheat Straw Fermented with Irpex lacteus. Sustainability. 2025; 17(10):4581. https://doi.org/10.3390/su17104581
Chicago/Turabian StylePu, Jun, Taoli Huhe, Xiao Ding, Ruling Yuan, Sainan Zhang, Jianjun Ren, and Dongze Niu. 2025. "Co-Production of Polysaccharides and Platform Sugars from Wheat Straw Fermented with Irpex lacteus" Sustainability 17, no. 10: 4581. https://doi.org/10.3390/su17104581
APA StylePu, J., Huhe, T., Ding, X., Yuan, R., Zhang, S., Ren, J., & Niu, D. (2025). Co-Production of Polysaccharides and Platform Sugars from Wheat Straw Fermented with Irpex lacteus. Sustainability, 17(10), 4581. https://doi.org/10.3390/su17104581







