Influence of Different Binders on the Municipal Solid Waste Incineration Fly Ash Granulation-Based Stabilization Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods of Characterization
2.3.1. Compressive Strength Test
2.3.2. Methods for Leaching Test
2.3.3. Phase Composition and Microstructure
2.3.4. Resistance to Alkali, Acid Environment, and Freezing-Thawing Cycles
3. Results
3.1. Physical and Chemical Characteristics of MSWI FA Granules
3.1.1. Physical Properties of Manufactured MSWI FA Granules
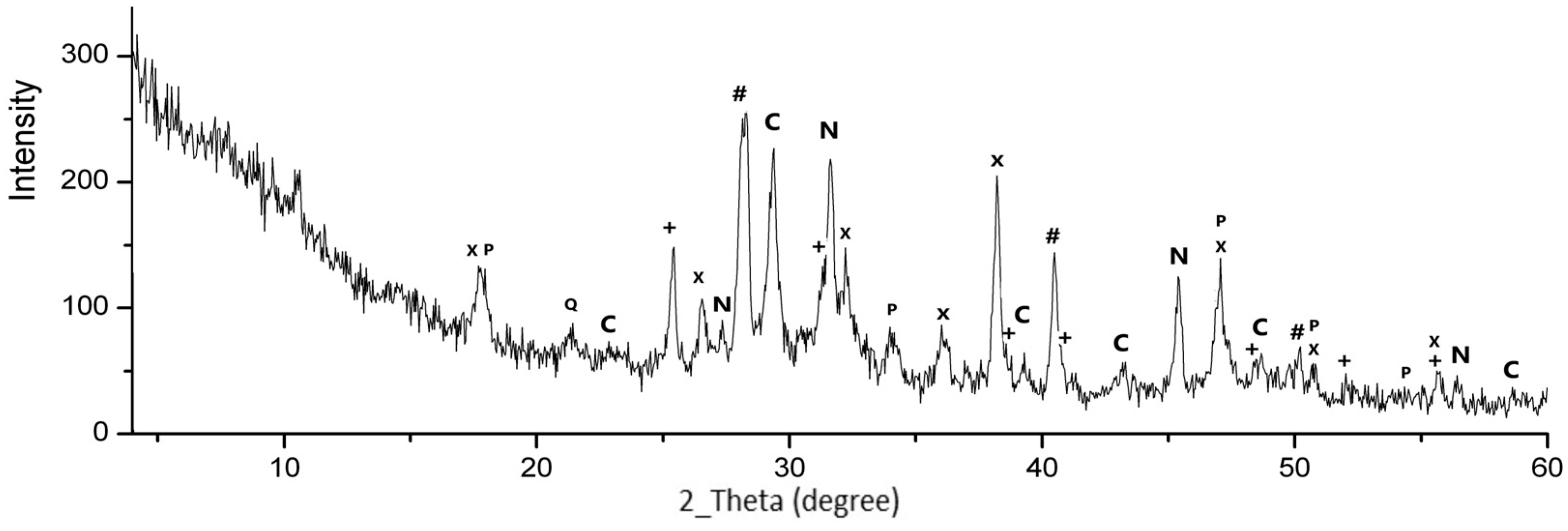
3.1.2. Elemental and Phase Compositions of MSWI FA Granules
3.2. Leaching Test
3.2.1. Leachability of Heavy Metals and Other Elements in MSWI FA Granules
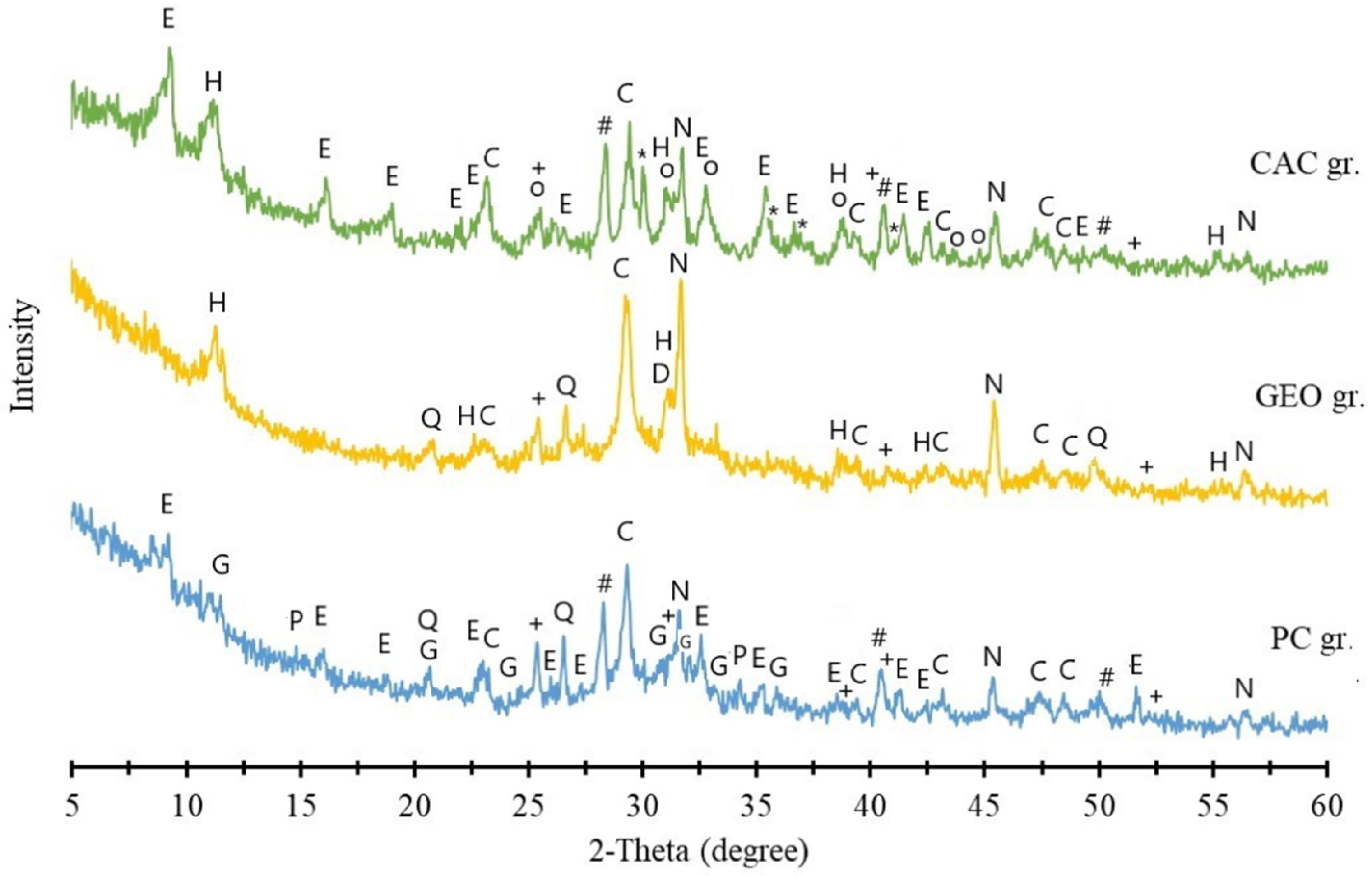
3.2.2. XRD Analysis of FA Granules After Leaching
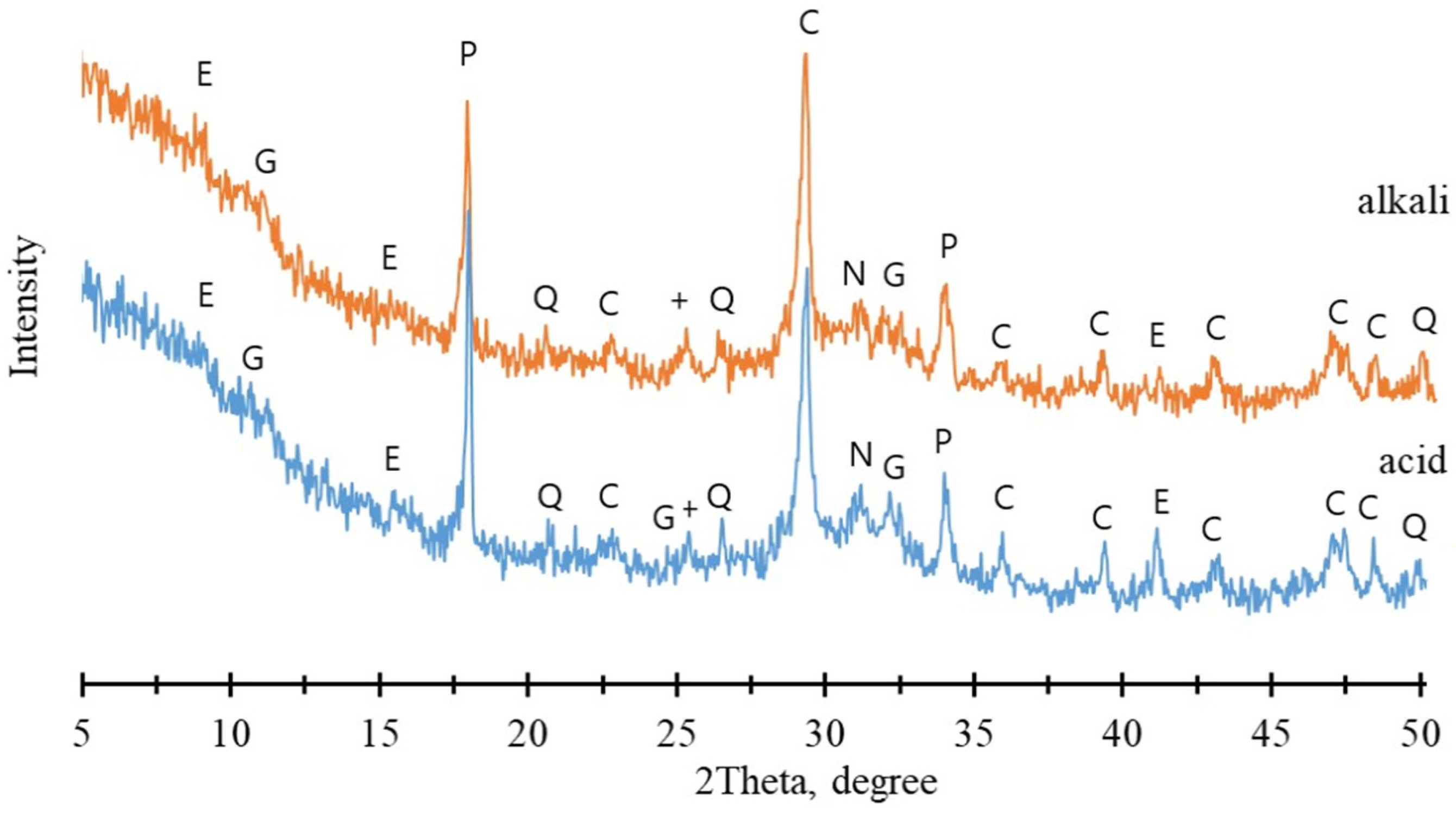
3.2.3. Chemical Characteristics of MSWI FA Granules After Keeping in Different Environments
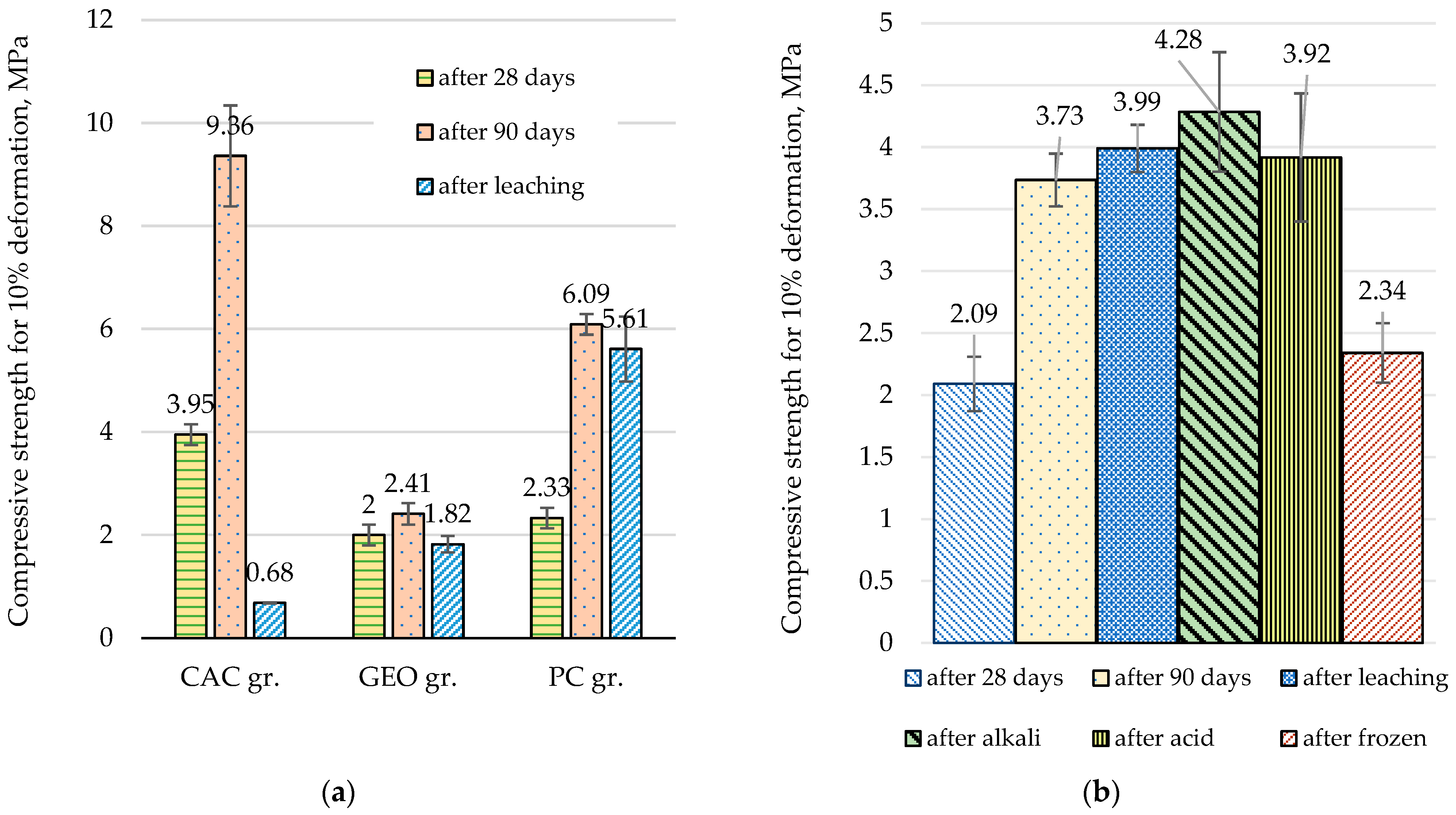
3.3. Compressive Strength of Granules After Influence of Different Environments
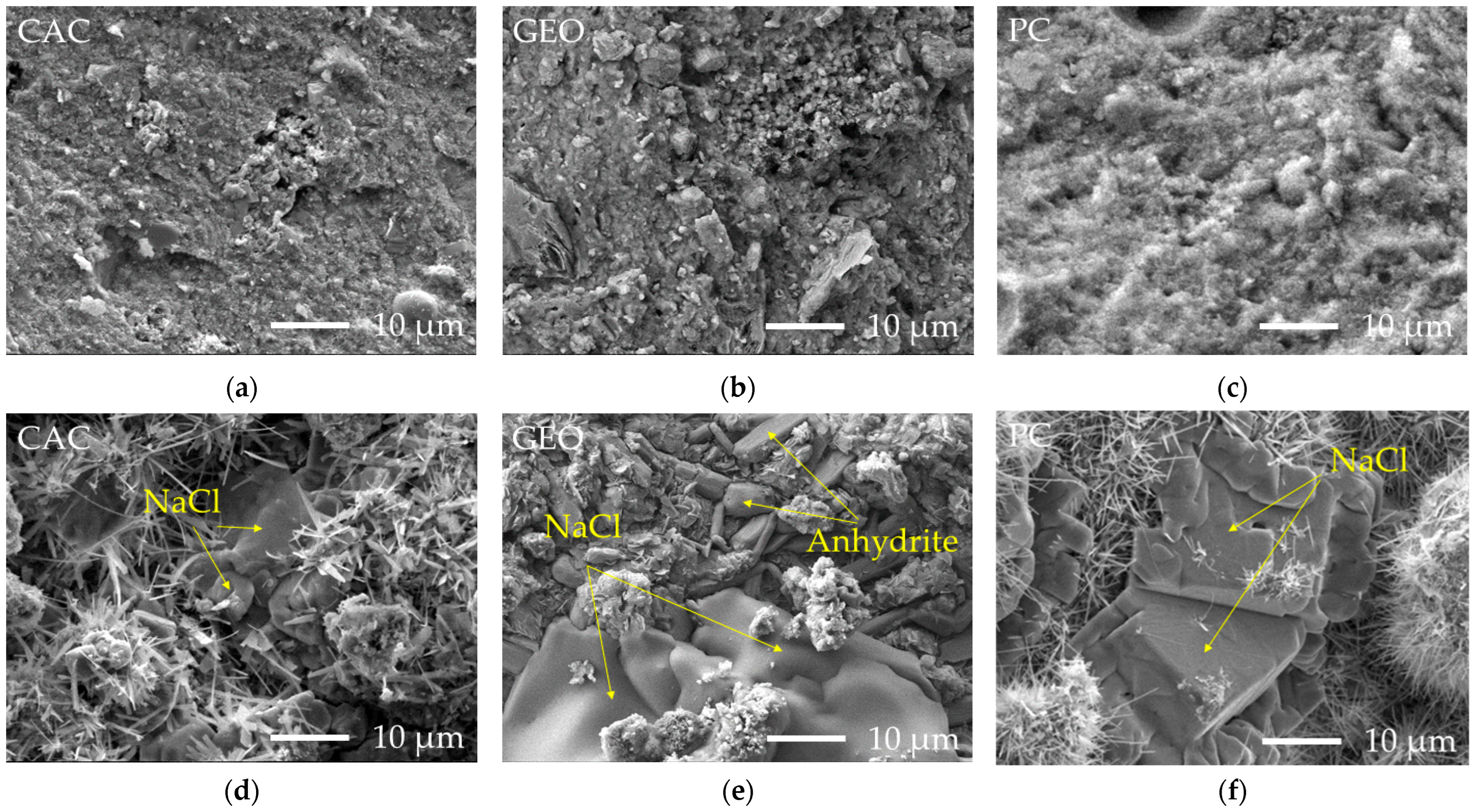
3.4. SEM Analysis of Granules Microstructure
4. Conclusions
- -
- CAC produced the densest granules (1165 kg/m3), capable of achieving high compressive strength (up to 9 MPa after 90 days). It showed a stronger ability to immobilize chlorides and sulfates. When 15% CAC and 35% water were used, typical CAC hydration products (CAH10, C2AH8, C3AH6) were not observed; instead, hydrocalumite and ettringite were formed, incorporating Cl and S. Upon water exposure, ettringite and residual chlorides partially dissolved, leading to Cl and S leaching and a weakened granule structure, reducing strength to 0.66 MPa. Nonetheless, additional hydrocalumite and calcite formed, contributing to partial immobilization of chlorine and sulfur, without a decrease in heavy metal content.
- -
- Geopolymer contains Na⁺ ions in the alkaline activator, which suppresses NaCl dissolution from FA and promotes the more intensive dissolution of KCl instead. Due to the lower pH, ettringite formation was inhibited during granulation, while formed hydrocalumite dissolved during later water exposure, reducing the immobilization effect. Moreover, achieving effective reaction between solid and liquid geopolymer components during granulation is challenging, which likely contributed to the relatively low compressive strength (2.0–2.41 MPa) and diminished capacity for retaining hazardous substances.
- -
- PC demonstrated the most balanced performance, producing granules with good strength and long-term stability under various environmental conditions, including pH fluctuations and freeze-thaw cycles. Granulation resulted in the formation of ettringite and C-S-H gel, producing a dense structure and strength of ~2.3 MPa after 28 days, which increases to ~6.1 MPa after 90 days. Water storage led to ettringite dissolution and the formation of portlandite and calcite, while C-S-H gel remained, maintaining structural integrity and a strength of ~5.6 MPa. Chlorine leaching slightly increased under acidic or alkaline conditions, but granule integrity remained intact, with strength remaining around 4 MPa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSWI | Municipal solid waste incineration |
| FA | Fly ash |
| PC | Portland cement |
| CAC | Calcium aluminate cement |
| GEO | Geopolymer |
| EDS | Energy dispersive spectroscopy |
References
- Order of the Minister of Environment of the Republic of Lithuania on the Approval of Minimum Requirements for Reducing Dustiness During the Storage, Loading, and Transportation of Loose Solid Materials. 11 November 2020. No. D1-682, Vilnius. Available online: https://www.e-tar.lt/portal/lt/legalAct/4b6141b0240a11eb932eb1ed7f923910 (accessed on 25 February 2025).
- European Commission. Integrated Pollution Prevention and Control. Reference Document on Best Available Techniques on Emissions from Storage. July 2006. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/esb_bref_0706.pdf (accessed on 25 February 2025).
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- El-Eswed, B.I. Chemical evaluation of immobilization of wastes containing Pb, Cd, Cu and Zn in alkali-activated materials: A critical review. J. Environ. Chem. Eng. 2020, 8, 104194. [Google Scholar] [CrossRef]
- Kozáková, L.; Bakalár, T.; Zelenák, M.; Prašcáková, M. Solidification of MSWI fly-ash with regard to hazardous metals leaching. Acta Montan Slovaca 2013, 18, 129–139. Available online: https://www.researchgate.net/publication/286314185_Solidification_of_MSWI_fly-ash_with_regard_to_hazardous_metals_leaching (accessed on 28 February 2025).
- Bie, R.; Chen, P.; Song, X.; Ji, X. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J. Energy Inst. 2016, 89, 704–712. [Google Scholar] [CrossRef]
- Yang, D.; Kow, K.; Wang, W.; Meredith, W.; Zhang, G.; Mao, Y.; Xu, M. Co-treatment of municipal solid waste incineration fly ash and alumina-/silica-containing waste: A critical review. J. Hazard. Mater. 2024, 479, 135677. [Google Scholar] [CrossRef]
- Luna Galiano, Y.; Fernández Pereira, C.; Vale, J. Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J. Hazard. Mater. 2011, 185, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Czop, M. Evaluation of the Leachability of Contaminations of Fly Ash and Bottom Ash from the Combustion of Solid Municipal Waste before and after Stabilization Process. Sustainability 2018, 11, 5384. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, C.; Rao, Y.; Yu, C.; Luo, Z.; Zhao, H.; Wang, X.; Wu, C.; Wang, Q. Solidification/stabilization and risk assessment of heavy metals in municipal solid waste incineration fly ash: A review. Sci. Total Environ. 2023, 892, 164451. [Google Scholar] [CrossRef] [PubMed]
- Berber, H.; Tamm, K.; Leinus, L.; Kuusik, R.; Tõnsuaadu, K.; Paaver, P.; Uibu, M. Accelerated carbonation technology granulation of industrial waste: Effects of mixture composition on product properties. Waste Manag. Res. 2020, 38, 142–155. [Google Scholar] [CrossRef]
- Sivakumar, A.; Gomathi, P. Pelletized fly ash lightweight aggregate concrete: A promising material. J. Civil Eng. Constr. Technol. 2012, 3, 42–48. Available online: https://academicjournals.org/journal/JCECT/article-full-text-pdf/A6B644E3378 (accessed on 3 March 2025). [CrossRef]
- Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Manufacture of artificial aggregate using MSWI bottom ash. Waste Manag. 2011, 31, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ke, Y.; Min, X.; Liang, Y.; Wang, Z.; Li, Y.; Fei, J.; Yao, L.; Xu, H.; Jiang, G. Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System. Int. J. Environ. Res. Public Health 2018, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Chen, J.N.; Ginder-Vogol, M.; Edil, T.B. Effect of pH and Grain Size on the Leaching Mechanism of Elements from Recycled Concrete Aggregate. In Proceedings of GeoShanghai 2018 International Conference: Geoenvironment and Geohazard; GSIC: Madrid, Spain, 2018. [Google Scholar] [CrossRef]
- Bui Viet, D.; Chan, W.; Phua, Z.; Ebrahimi, A.; Abbas, A.; Lisak, G. The use of fly ashes from waste-to-energy processes as mineral CO2 sequesters and supplementary cementitious materials. J. Hazard. Mater. 2020, 398, 122906. [Google Scholar] [CrossRef] [PubMed]
- Wi, K.; Sahin, O.; Wang, K.; Lee, Y. Characterization and evaluation of cement-based systems containing solution-treated municipal solid-waste incineration fly ash. Constr. Build. Mater. 2024, 416, 135230. [Google Scholar] [CrossRef]
- Li, H.; Peng, Y.; Xu, M.; Wang, Y.; Ding, J.; Ma, B.; Jin, L.; Lu, S.; Yan, J. Use of municipal solid waste incineration fly ash as a supplementary cementitious material: CO2 mineralization coupled with mechanochemical pretreatment. Environ. Res. 2024, 242, 117799. [Google Scholar] [CrossRef]
- Wu, K.; Shi, H.; Schutter, G.D.; Guo, X.; Ye, G. Preparation of alinite cement from municipal solid waste incineration fly ash. Cem. Concr. Compos. 2012, 34, 322–327. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, S.; Singh, N. Characterization and Sustainable Utilization of Municipal Solid Waste Incineration Ash: A Review. Recent Dev. Energy Environ. Eng. Lect. Notes Civil Eng. 2023, 333, 383–395. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, A.; Zhang, J.; Tang, Y.; Shi, P.; Tang, Q.; Huang, Y. Physical and Chemical Properties, Pretreatment, and Recycling of Municipal Solid Waste Incineration Fly Ash and Bottom Ash for Highway Engineering: A Literature Review. Adv. Civil Eng. 2020, 2020, 8886134. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef]
- Colangelo, F.; Messina, F.; Cioffi, R. Recycling of MSWI fly ash by means of cementitious double step cold bonding pelletization: Technological assessment for the production of lightweight artificial aggregates. J. Hazard. Mater. 2015, 299, 181–191. [Google Scholar] [CrossRef]
- Brännvall, E.; Kumpiene, J. Fly ash in landfill top covers—A review. Environ. Sci. Process. Impacts 2016, 18, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, B.; Ai, H.; Qi, Y.; Liu, Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. J. Clean. Prod. 2021, 319, 128790. [Google Scholar] [CrossRef]
- Zheng, L.; Xia, Z.; Zhang, X.Y. Comparison between geopolymer reaction and cement hydration in solidification of fly ash generated in municipal solid waste incineration. Rev. Compos. Matér. Av. 2018, 28, 395–403. [Google Scholar] [CrossRef]
- Mikoč, M.; Bjelobrk, I.; Korajac, J. Alkali-activated fly ash concrete (Concrete without cement). Teh. Vjesn. 2011, 18, 99–102. Available online: https://hrcak.srce.hr/65933 (accessed on 3 March 2025).
- Guo, X.; Shi, H. Calcium sulfoaluminate (CSA) blended cements. Mag. Concr. Res. 2016, 68, 208–215. [Google Scholar] [CrossRef]
- Xiong, Y.; Xia, Y.; Meng, Y.; Huang, G.; Ma, Z.; Wang, L.; Yan, J. Recycling of municipal solid waste incineration fly ash into cement clinker. In Treatment and Utilization of Combustion and Incineration Residues; Elsevier: Amsterdam, The Netherlands, 2024; pp. 191–204. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, Y.; Baxtiyarovich, A.F.; Zhang, Y.; Wang, L.; Yan, J. Transforming MSWI fly ash into low-carbon and high-compatibility composites via designed calcium sulfoaluminate-based binders. J. Environ. Manag. 2024, 371, 123023. [Google Scholar] [CrossRef]
- Lan, T.; Meng, Y.; Ju, T.; Song, M.; Chen, Z.; Shen, P.; Du, Y.; Deng, Y.; Han, S.; Jiang, J. Manufacture of alkali-activated and geopolymer hybrid binder (AGHB) by municipal waste incineration fly ash incorporating aluminosilicate supplementary cementitious materials (ASCM). Chemosphere 2022, 303, 134978. [Google Scholar] [CrossRef]
- Su, L.; Wu, S.; Zhu, W.; Liang, B.; Zhang, X.; Yang, J. Enhanced geopolymerization of MSWI fly ash through combined activator pretreatment: A sustainable approach to heavy metal encapsulation and resource recovery. J. Environ. Manag. 2024, 370, 122870. [Google Scholar] [CrossRef]
- Tamilarasan, A.; Om, S. Effect of varying molarity and curing conditions on the mechanical and microstructural characteristics of alkali activated GGBS binder. Mater. Res. Express 2023, 10, 095305. [Google Scholar] [CrossRef]
- Sitarz, M.; Hager, I.; Choińska, M. Evolution of Mechanical Properties with Time of Fly-Ash-Based Geopolymer Mortars under the Effect of Granulated Ground Blast Furnace Slag Addition. Energies 2019, 13, 1135. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W. Influence of different curing temperatures and alkali activators on properties of GBFS geopolymer mortars containing fly ash and palm-oil fuel ash. Constr. Build. Mater. 2016, 125, 1229–1240. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Mechanical and durability properties of fly ash geopolymer concrete containing recycled coarse aggregates. Int. J. Sustain. Built Environ. 2016, 5, 277–287. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, W.; Zhao, Q.; Zhang, N.; Xue, C.; Wang, S.; Song, Y. Performance deterioration of municipal solid waste incineration fly ash-based geopolymer under sulfuric acid attack. Constr. Build. Mater. 2023, 391, 131847. [Google Scholar] [CrossRef]
- Calgaro, L.; Contessi, S.; Bonetto, A.; Badetti, E.; Ferrari, G.; Artioli, G.; Marcomini, A. Calcium aluminate cement as an alternative to ordinary Portland cement for the remediation of heavy metals contaminated soil: Mechanisms and performance. J. Soils Sediments 2021, 21, 1755–1768. [Google Scholar] [CrossRef]
- Wei, G.X.; Liu, H.Q.; Zhang, S.G. Using of Different Type Cement in Solidification/Stabilization of MSWI Fly Ash. AMR 2011, 291, 1870–1874. [Google Scholar] [CrossRef]
- Ponduru, S.A.; Han, T.; Huang, J.; Neithalath, N.; Sant, G.; Kumar, A. Understanding roles and evaluating reactivity of fly ashes in calcium aluminate binders. Constr. Build. Mater. 2024, 414, 135062. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.; Jia, Z. Solidification/Stabilization of Waste Incineration Fly Ash by Modified Calcium Aluminate Cement. Water Air Soil Pollut. 2024, 235, 163. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Memon, S.A.; Dong, Z.; Li, D.; Cui, H. Effect of calcium sulfate type and dosage on properties of calcium aluminate cement-based self-leveling mortar. Constr. Build. Mater. 2018, 167, 253–262. [Google Scholar] [CrossRef]
- Xu, L.; Wang, P.; Zhang, G. Calorimetric study on the influence of calcium sulfate on the hydration of Portland cement–calcium aluminate cement mixtures. J. Therm. Anal. Calorim. 2012, 110, 725–731. [Google Scholar] [CrossRef]
- EN 1097-11; Tests for Mechanical and Physical Properties of Aggregates—Part 11: Determination of Compressibility and Confined Compressive Strength of Lightweight Aggregates. European Standards s.r.o.: Brussel, Belgium, 2013.
- EN 12457-1; Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 1: One Stage Batch Test at a Liquid to Solid Ratio of 2 L/kg for Materials with High Solid Content and with Particle Size Below 4 mm (Without or with Size Reduction). European Standards s.r.o.: Brussel, Belgium, 2003.
- Agrela, F.; Cabrera, M.; Galvín, A.; Barbudo, A.; Ramirez, A. Influence of the sulphate content of recycled aggregates on the properties of cement-treated granular materials using Sulphate-Resistant Portland Cement. Constr. Build. Mater. 2014, 68, 127–134. [Google Scholar] [CrossRef]
- Jewell, R.B.; Rathbone, R.F.; Duvallet, T.Y.; Robl, T.L.; Mahboub, K.C. Fabrication and Testing of Low-Energy Calcium Sulfoaluminate-Belite Cements that Utilize Circulating Fluidized Bed Combustion By-Products. Coal Combust. Gasif. Prod. 2015, 7, 9–18. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Xu, Y. Solidification/stabilization and immobilization mechanism of Pb(II) and Zn(II) in ettringite. Cem. Concr. Res. 2023, 174, 107350. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: Literature review and experimental study. J. Hazard. Mater. 2006, 136, 20–33. [Google Scholar] [CrossRef]
- Gevers, B.R.; Labuschagné, F.J. Green Synthesis of Hydrocalumite (CaAl-OH-LDH) from Ca(OH)2 and Al(OH)3 and the Parameters That Influence Its Formation and Speciation. Crystals 2020, 10, 672. [Google Scholar] [CrossRef]
- Jiménez, A.; Trujillano, R.; Gil, A.; Rives, V.; Vicente, M.Á. Hydrocalumite and hydrocalumite-type compounds: A special type of layered double hydroxides. Appl. Clay Sci. 2025, 267, 107707. [Google Scholar] [CrossRef]
- Qian, G.; Feng, L.; Zhou, J.Z.; Xu, Y.; Liu, J.; Zhang, J.; Xu, Z.P. Solubility product (Ksp)-controlled removal of chromate and phosphate by hydrocalumite. Chem. Eng. J. 2012, 181, 251–258. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, F.; He, W.; Zheng, D.; Cui, H.; Lv, L.; Tang, W.; Han, N. Experimental Investigation of Chloride Uptake Performances of Hydrocalumite-Like Ca-Al LDHs with Different Microstructures. Appl. Sci. 2019, 10, 3760. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- ED (2003) 2003/33/EC: Council Decision of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Available online: https://assets.publishing.service.gov.uk/media/65fd73e8a6c0f70011ef9278/CD1VCO_1.PDF (accessed on 10 April 2025).
- Ding, X.; Luo, B.; Zhou, H.; Chen, Y. Generalized solutions for advection–dispersion transport equations subject to time- and space-dependent internal and boundary sources. Comput. Geotech. 2025, 178, 106944. [Google Scholar] [CrossRef]
- Stawski, T.; Karafiludis, S.; Pimentel, C.; Montes-Hernandez, G.; Kochovski, Z.; Bienert, R.; Weimann, K.; Emmerling, F.; Scoppola, E.; Van Driessche, A.E.S. Solution-driven processing of calcium sulfate: The mechanism of the reversible transformation of gypsum to bassanite in brines. J. Clean. Prod. 2024, 440, 141012. [Google Scholar] [CrossRef]
- Reigl, S.; Van Driessche, A.E.S.; Ullrich, T.; Koltzenburg, S.; Kunz, W.; Kellermeier, M. Organic solvent-free synthesis of calcium sulfate hemihydrate at room temperature. Chemical 2024, 57, 58. [Google Scholar] [CrossRef] [PubMed]
- Oproiu, C.; Parvan, M.; Voigu, G.; Badanoiu, A.; Trusca, R. Influence of a Chromium-rich Industrial Waste on the Hydration and Hardening Processes of Portland Cements with Slag and Limestone Additions. Rev. Chim. 2020, 71, 252–261. [Google Scholar] [CrossRef]
- Guo, X.; Ma, H.; Zhou, J.; Cheng, W.; Ba, M. Stabilization mechanism of hexavalent chromium ions in portland cement-based materials. Case Stud. Constr. Mater. 2024, 20, e03110. [Google Scholar] [CrossRef]
- Ferreira, A.V.; Righi, A.; Araújo, F.G.S.; Espinosa, D.C.R.; Tenório, J.A.S. Applications of the Rietveld method to quantify the crystalline phases of Portland cement clinker doped with nickel and chromium. Powder Diffr. 2008, 23, S42–S45. [Google Scholar] [CrossRef]
- Vollpracht, A.; Brameshuber, W. Binding and leaching of trace elements in Portland cement pastes. Cem. Concr. Res. 2015, 79, 76–92. [Google Scholar] [CrossRef]
- Matejka, L.; Siler, P.; Novotny, R.; Svec, J.; Masilko, J.; Soukal, F. Negative effect of zinc compounds on hydration kinetics of ordinary Portland cement. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1039, 012004. [Google Scholar] [CrossRef]
- Xu, L.; Sun, Z.; Chen, Y.; Yang, K.; Yang, X.; Wu, K.; Lothenbach, B. Retardation mechanism of zinc on Portland cement and alite hydration. Cem. Concr. Res. 2024, 184, 107571. [Google Scholar] [CrossRef]
- Dyer, T.; Jones, R.; Garvin, S. Exposure of Portland cement to multiple trace metal loadings. Mag. Concr. Res. 2009, 61, 57–65. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, C.; Zhao, M.; Liu, Z.; Yang, Y.; Liu, G.; Ma, X. Solidification mechanisms of copper in ferrite-rich Portland cement and its action mechanism in mineral phase. J. Build. Eng. 2022, 58, 104962. [Google Scholar] [CrossRef]
- Zezulová, A.; Staněk, T.; Opravil, T. The Influence of Barium Sulphate and Barium Carbonate on the Portland Cement. Procedia Eng. 2015, 151, 42–49. [Google Scholar] [CrossRef]
- Prlić-Kardum, J.; Sander, A.; Glasnović, A. Batch Crystallization of KCl: The Influence of the Cooling and Mixing Rate on the Granulometric Properties of Obtained Crystals. Chem. Biochem. Eng. Q. 2005, 19, 39–47. [Google Scholar]
- Wang, P.; Lee, J.; Park, S.; Yoon, H.; Shin, S.; Kim, E.; Koh, H.; Park, H. Calcium Sulfate Hemihydrate Powders with a Controlled Morphology for Use as Bone Cement. J. Am. Ceram. Soc. 2008, 91, 2039–2042. [Google Scholar] [CrossRef]
- Jo, H.; Jang, Y.; Young, J.H. Influence of NaCl on mineral carbonation of CO2 using cement material in aqueous solutions. Chem. Eng. Sci. 2012, 80, 232–241. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.; Glasser, F. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]

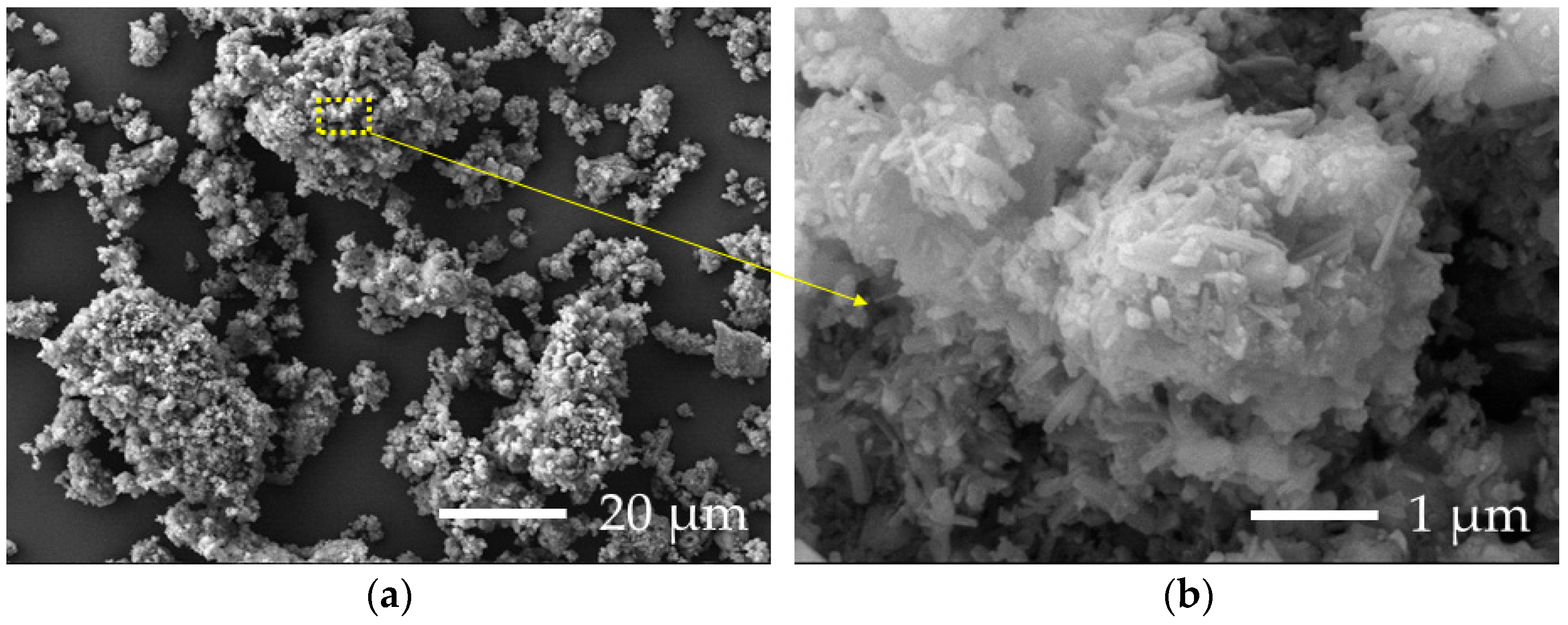

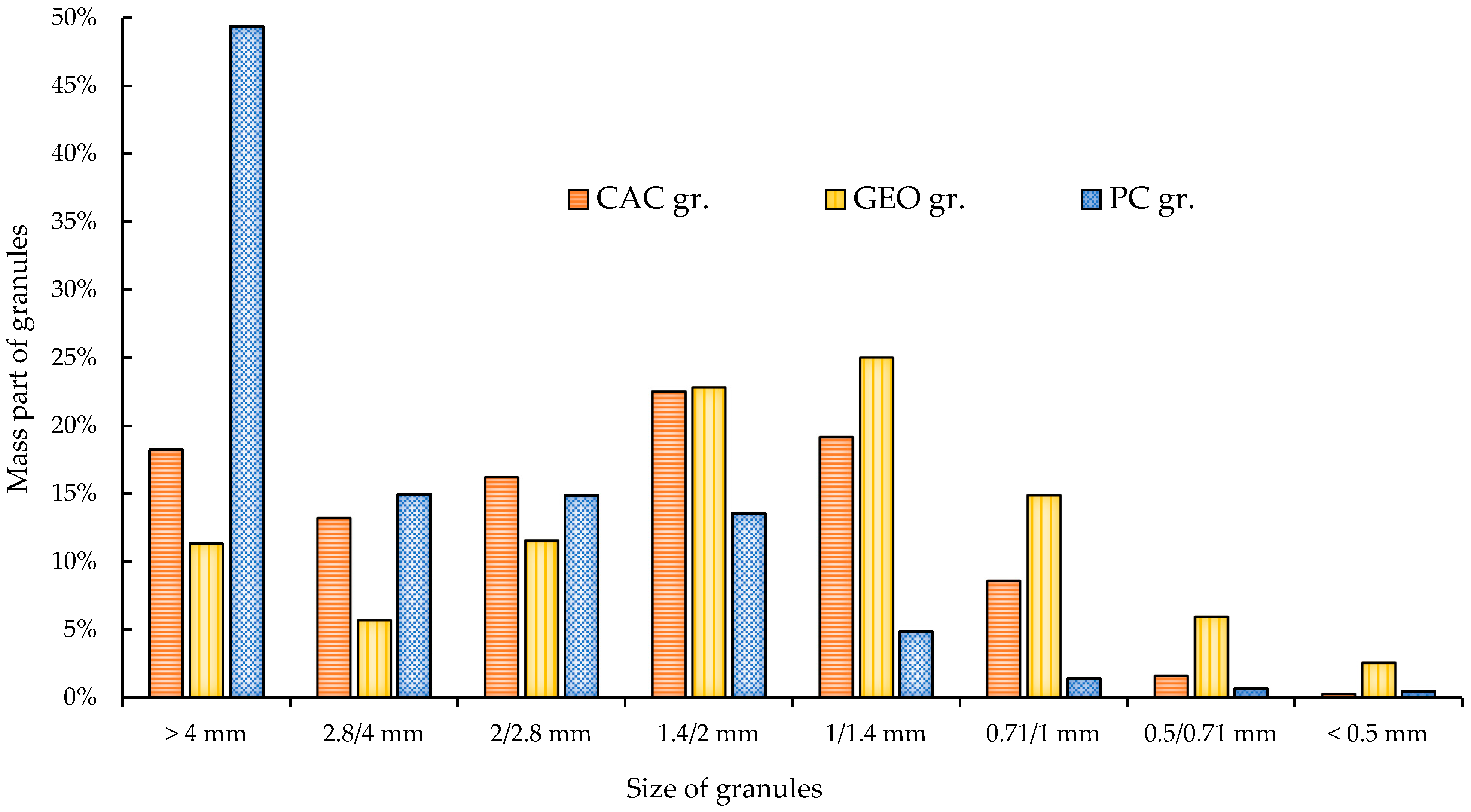
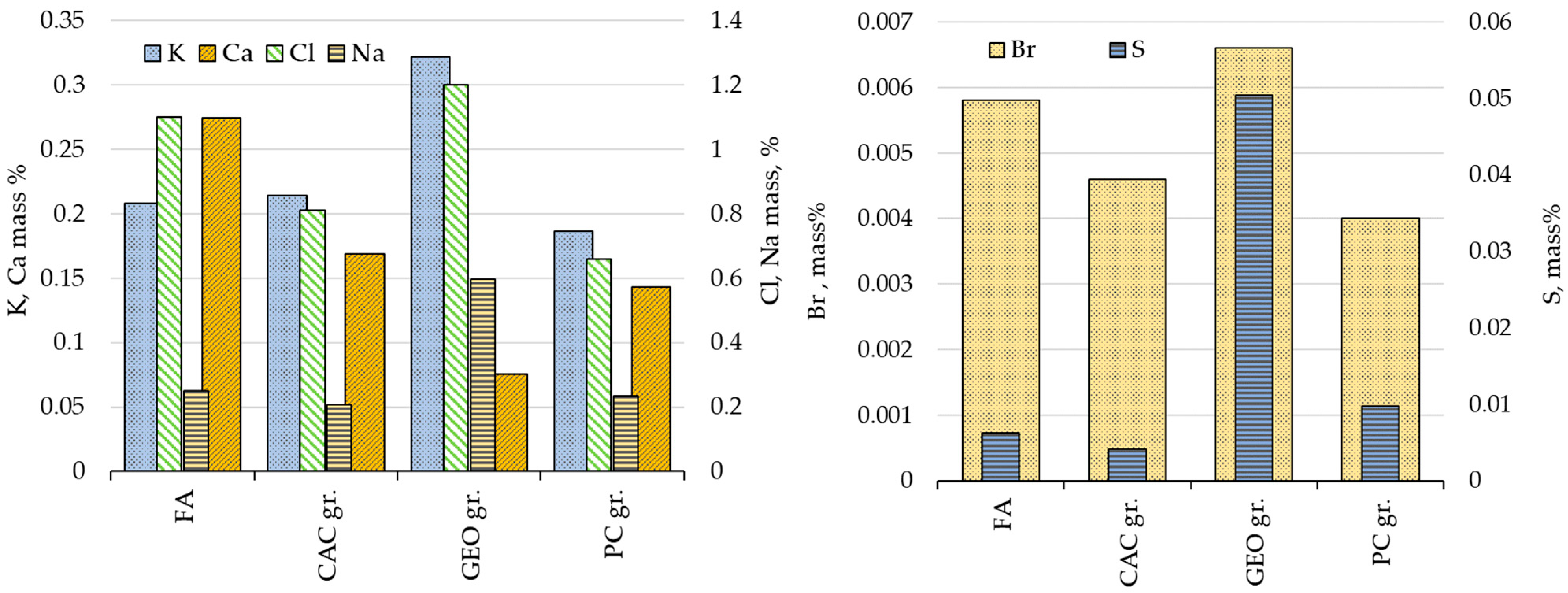
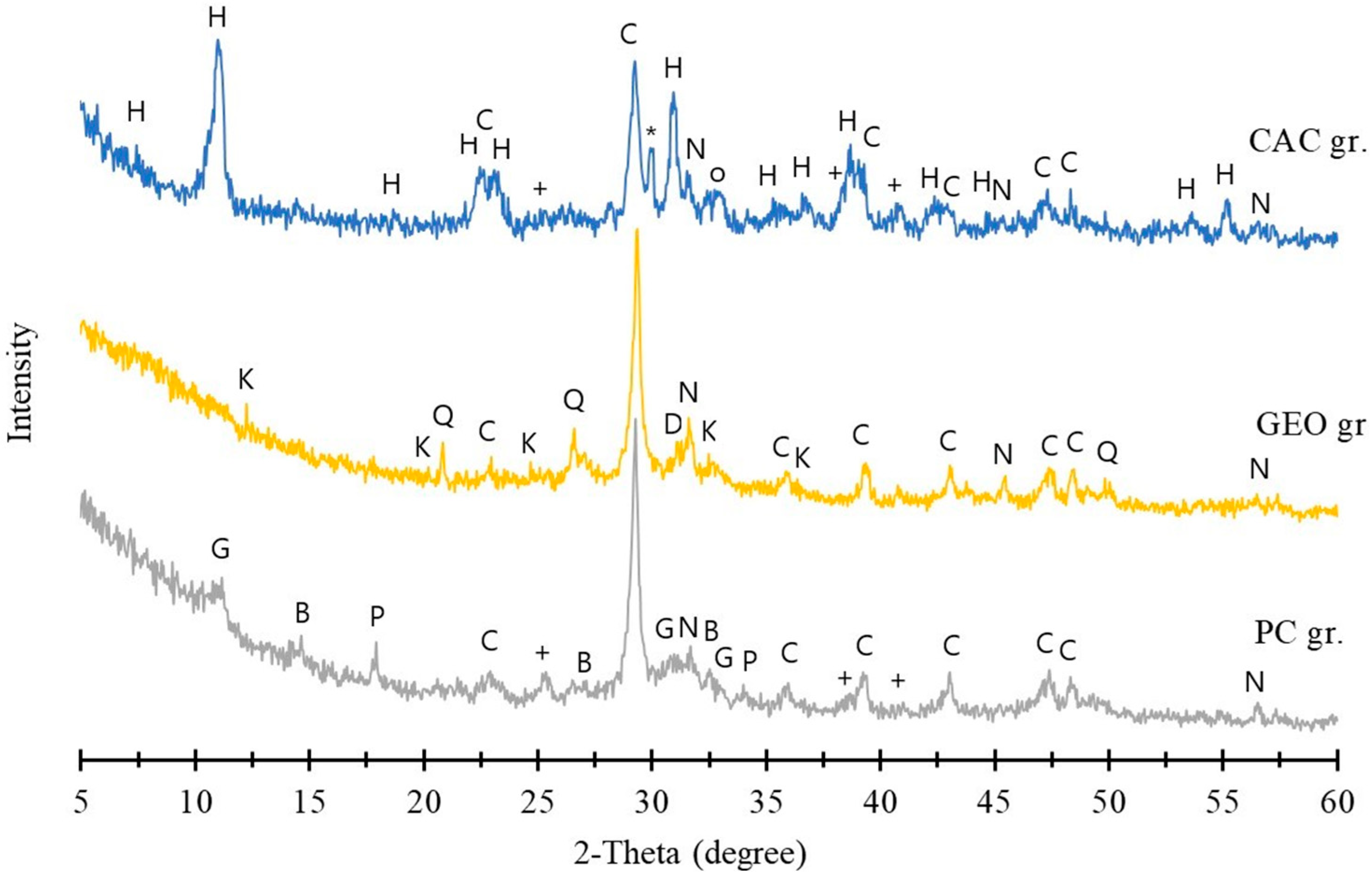

| Category | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | K2O | MgO | Cl | Na2O | TiO2 | ZnO | CO2 | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSWI FA | 49.9 | 2.99 | 0.98 | 0.95 | 3.66 | 4.14 | 0.54 | 12.0 | 4.62 | 0.47 | 1.36 | 17.3 | 1.09 |
| CAC | 27.2 | 0.30 | 67.8 | 0.08 | 0.02 | 0.01 | 0.13 | 0.01 | 0.17 | - | 0.01 | 4.18 | 0.09 |
| GEO 1 | 12.4 | 47.0 | 18.8 | 1.53 | 0.18 | 1.47 | 1.80 | 0.04 | 7.09 | 0.66 | 0.01 | 8.63 | 0.39 |
| PC | 57.3 | 16.2 | 4.53 | 2.54 | 2.30 | 0.97 | 2.42 | 0.06 | 0.26 | - | 0.04 | 12.8 | 0.58 |
| Sample | FA Amount | Binder | Binder Amount | Water Amount * |
|---|---|---|---|---|
| CAC gr. | 85 | CAC Gorkal 70 | 15 | 35 |
| GEO gr. | 75 | Geopolymer | 25 | 10 |
| PC gr. | 75 | Portland cement | 25 | 35 |
| Element 1 | FA | CAC gr. | CAC gr. (Leaching) | GEO gr. | GEO gr. (Leaching) | PC gr. | PC gr. (Leaching) | PC gr. (Acid) | PC gr. (Alkali) |
|---|---|---|---|---|---|---|---|---|---|
| C | 4.54 | 3.93 | 4.41 | 3.83 | 3.85 | 4.08 | 4.56 | 4.66 | 4.6 |
| O | 39.2 | 44.4 | 48.9 | 45.1 | 50.3 | 45.0 | 50.6 | 50.7 | 50.6 |
| Na | 3.30 | 2.50 | 0.282 | 4.69 | 1.66 | 2.01 | 0.365 | 0.211 | 0.176 |
| Mg | 0.31 | 0.45 | 0.39 | 0.59 | 0.767 | 0.76 | 0.832 | 0.827 | 0.81 |
| Al | 0.50 | 5.61 | 6.41 | 3.23 | 3.56 | 1.33 | 1.43 | 1.43 | 1.51 |
| Si | 1.33 | 1.73 | 1.72 | 8.86 | 9.62 | 3.10 | 3.62 | 3.61 | 3.7 |
| P | 0.14 | 0.19 | 0.188 | 0.14 | 0.149 | 0.17 | 0.184 | 0.189 | 0.189 |
| S | 1.38 | 1.26 | 1.63 | 1.07 | 1.11 | 1.29 | 1.38 | 1.59 | 1.59 |
| Cl | 11.2 | 7.92 | 3.55 | 6.03 | 2.36 | 6.66 | 2.53 | 1.87 | 1.67 |
| K | 3.17 | 2.35 | 0.529 | 2.56 | 1.15 | 2.24 | 0.551 | 0.329 | 0.278 |
| Ca | 32.5 | 27.7 | 29.9 | 21.46 | 23.4 | 31.0 | 31.6 | 32.2 | 32.4 |
| Cr | 0.025 | 0.020 | 0.0243 | 0.052 | 0.0169 | 0.018 | 0.0212 | 0.0213 | 0.0246 |
| Ti | 0.252 | 0.237 | 0.272 | 0.639 | 0.287 | 0.319 | 0.319 | 0.338 | 0.352 |
| Mn | 0.035 | 0.033 | 0.0407 | 0.052 | 0.0581 | 0.046 | 0.0488 | 0.0524 | 0.0512 |
| Fe | 0.590 | 0.510 | 0.582 | 0.639 | 0.688 | 0.883 | 0.939 | 0.938 | 0.952 |
| Ni | 0.005 | 0.004 | - | 0.004 | 0.0038 | 0.004 | 0.0049 | 0.0049 | 0.005 |
| Cu | 0.051 | 0.039 | 0.0436 | 0.029 | 0.0335 | 0.039 | 0.0403 | 0.0406 | 0.0403 |
| Zn | 0.968 | 0.731 | 0.824 | 0.578 | 0.614 | 0.659 | 0.688 | 0.691 | 0.68 |
| Br | 0.119 | 0.086 | 0.0482 | 0.066 | 0.0282 | 0.074 | 0.0368 | 0.0319 | 0.0307 |
| F | - | - | - | 0.138 | 0.141 | - | - | - | - |
| Rb | 0.012 | - | - | - | 0.0042 | 0.008 | 0.0033 | - | - |
| Sr | 0.039 | 0.032 | 0.0249 | 0.031 | 0.0321 | 0.035 | 0.0258 | 0.0243 | 0.024 |
| Zr | 0.003 | 0.002 | 0.0034 | 0.004 | 0.0043 | 0.038 | 0.0047 | 0.0056 | 0.0053 |
| I | 0.020 | 0.017 | 0.0141 | 0.016 | 0.0096 | 0.018 | 0.0124 | 0.0136 | 0.0105 |
| Pb | 0.158 | 0.126 | 0.137 | 0.099 | 0.106 | 0.106 | 0.112 | 0.109 | 0.107 |
| Cd | 0.013 | 0.013 | 0.0103 | 0.008 | 0.0061 | 0.009 | 0.008 | 0.008 | 0.0126 |
| Sn | 0.043 | 0.028 | 0.0342 | 0.018 | 0.0262 | 0.023 | 0.0313 | 0.0312 | 0.0299 |
| Sb | 0.040 | 0.032 | 0.0387 | 0.023 | 0.0247 | 0.026 | 0.026 | 0.0272 | 0.0304 |
| Ba | 0.058 | 0.054 | 0.0269 | 0.044 | 0.0481 | 0.060 | 0.0561 | 0.0582 | 0.0605 |
| Element → ↓ Binder | C | O | Ca | Al | Si | S | Cl | K | Na |
|---|---|---|---|---|---|---|---|---|---|
| After 28 days on the surface of granule (inside the granule) | |||||||||
| CAC gr. | 18.7 (13.3) | 30.5 (44.4) | 13.3 (19.8) | 0.4 (2.8) | 0.2 (0.8) | 0.4 (4.1) | 21.1 (11.1) | 8.0 (1.8) | 6.5 (1.5) |
| GEO gr. | 24.5 (19.1) | 28.7 (29.8) | 11.7 (12.4) | 1.5 (1.8) | 0.31 (2.9) | 4.9 (3.8) | 17.6 (17.8) | 1.4 (2.1) | 8.8 (10.2) |
| PC gr. | 17.1 (14.7) | 43.1 (33.9) | 24.5 (20.3) | 0.0 (0.4) | 0.45 (1.7) | 2.3 (1.6) | 9.6 (16.8) | 1.2 (1.8) | 1.1 (8.2) |
| After leaching on the surface of granule (inside the granule) | |||||||||
| CAC gr. | 17.1 (16.7) | 46.2 (47.7) | 23.2 (23.3) | 4.0 (3.40) | 0.4 (0.7) | 2.5 (3.28) | 4.8 (3.52) | 0.4 (0.1) | 0.6 (0.5) |
| GEO gr. | 11.1 (19.8) | 59.1 (50.4) | 18.8 (14.4) | 2.9 (2.0) | 0.6 (7.16) | 7.1 (1.4) | 0.4 (2.0) | 0.0 (0.9) | 0.0 (1.6) |
| PC gr. | 19.4 (16.4) | 51.6 (50.5) | 24.0 (23.2) | 0.8 (0.7) | 0.4 (2.1) | 2.1 (3.3) | 0.2 (2.5) | 0.8 (0.3) | 0.7 (0.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevtsova, M.; Malaiškienė, J.; Škamat, J.; Antonovič, V. Influence of Different Binders on the Municipal Solid Waste Incineration Fly Ash Granulation-Based Stabilization Process. Sustainability 2025, 17, 4573. https://doi.org/10.3390/su17104573
Shevtsova M, Malaiškienė J, Škamat J, Antonovič V. Influence of Different Binders on the Municipal Solid Waste Incineration Fly Ash Granulation-Based Stabilization Process. Sustainability. 2025; 17(10):4573. https://doi.org/10.3390/su17104573
Chicago/Turabian StyleShevtsova, Maryna, Jurgita Malaiškienė, Jelena Škamat, and Valentin Antonovič. 2025. "Influence of Different Binders on the Municipal Solid Waste Incineration Fly Ash Granulation-Based Stabilization Process" Sustainability 17, no. 10: 4573. https://doi.org/10.3390/su17104573
APA StyleShevtsova, M., Malaiškienė, J., Škamat, J., & Antonovič, V. (2025). Influence of Different Binders on the Municipal Solid Waste Incineration Fly Ash Granulation-Based Stabilization Process. Sustainability, 17(10), 4573. https://doi.org/10.3390/su17104573









