A Material Flow Analysis of Electric Vehicle Lithium-ion Batteries: Sustainable Supply Chain Management Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Flow Analysis (MFA) Method
2.1.1. Target Items and Scope
2.1.2. System Boundary
2.1.3. Quantification Method for Material Flow
2.2. Data Collection and Assumptions
2.2.1. Data Collection Method
2.2.2. Analysis Items and Methods
2.2.3. Assumptions
3. Results and Discussion
3.1. Mass-Based Material Flow Analysis for Electric Vehicle Lithium-ion Batteries
3.1.1. Manufacturing Stage
3.1.2. Consumption Stage
3.1.3. Discharge Collection Stage
3.1.4. Treatment Stage
Mass-Based MFA: Repurposing and Remanufacturing Process
Mass-Based MFA: Pretreatment Process
Mass-Based MFA: Hydrometallurgical Recycling Process
3.2. Substance Flow Analysis for Electric Vehicle Lithium-ion Batteries
3.2.1. Substance Flow Analysis of Recovery Rates During the Treatment Process
SFA: Repurposing and Remanufacturing Process
SFA: Pretreatment Process
SFA: Hydrometallurgical Recycling Process
3.2.2. Utilization of Recycled Products in Electric Vehicle Lithium-ion Battery Manufacturing
3.3. Policy and Technical Strategies for a Sustainable EV LIB Supply Chain
3.3.1. Policy Incentives and Regulatory Targets
3.3.2. Technical Standards for Recovery Rates
3.3.3. Process Optimization for Mn and Li Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, C.G.; Borghetti, F.; Longo, M.; Yaici, W.; Miraftabzadeh, S.M. Decarbonizing transportation: A data-driven examination of ICE vehicle to EV transition. Clean. Eng. Technol 2024, 21, 100782. [Google Scholar] [CrossRef]
- Littlejohn, C.; Proost, S. What role for electric vehicles in the decarbonization of the car transport sector in Europe? Econ. Transp. 2022, 32, 100283. [Google Scholar] [CrossRef]
- Lah, O. Decarbonizing the transportation sector: Policy options, synergies, and institutions to deliver on a low-carbon stabilization pathway. Wiley Interdiscip. Rev. Energy Environ. 2017, 6, e257. [Google Scholar] [CrossRef]
- Alšauskas, O.; Connelly, E.; Huismans, M.; Jenness, E.; Copier, J.J.; Le Marois, J.B.; Lombardo, T.; McDonagh, S.; O’Riordan, V.; Petropoulos, A. Global EV Outlook 2024: Moving Towards Increased Affordability; International Energy Agency: Paris, France, 2024. [Google Scholar] [CrossRef]
- IEA. Annual Battery Demand by Application and Scenario, 2023 and 2030; IEA: Paris, France, 2024; Available online: https://www.iea.org/data-and-statistics/charts/annual-battery-demand-by-application-and-scenario-2023-and-2030 (accessed on 29 August 2024).
- Habib, K.; Hamelin, L.; Wenzel, H. A dynamic perspective of the geopolitical supply risk of metals. J. Cleaner Prod. 2016, 133, 850–858. [Google Scholar] [CrossRef]
- Tan, J.; Keiding, J.K. Mapping the cobalt and lithium supply chains for e-mobility transition: Significance of overseas investments and vertical integration in evaluating mineral supply risks. Resour. Convserv. Recycl. 2024, 209, 107788. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, H.; Lin, F. Sustainable electric vehicle batteries for a sustainable world: Perspectives on battery cathodes, environment, supply chain, manufacturing, life cycle, and policy. Adv. Energy Mater. 2022, 12, 2200383. [Google Scholar] [CrossRef]
- Allesch, A.; Brunner, P.H. Material flow analysis as a decision support tool for waste management: A literature review. J. Ind. Ecol. 2015, 19, 753–764. [Google Scholar] [CrossRef]
- Choi, H.J.; Hwang, D.; Yoon, Y.S.; Jeon, T.W.; Rhee, S.W. Applying Material Flow Analysis for Sustainable Waste Management of Single-Use Plastics and Packaging Materials in the Republic of Korea. Sustainability 2024, 16, 6926. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Liu, C.; Wang, M.; Liu, L. Resilience assessment of the cobalt supply chain in China under the impact of electric vehicles and geopolitical supply risks. Resour. Policy 2023, 80, 103183. [Google Scholar] [CrossRef]
- Xiong, X.; Zeng, X.; Zhang, Z.; Pell, R.; Matsubae, K.; Hu, Z. China’s recycling potential of large-scale public transport vehicles and its implications. Commun. Eng. 2023, 2, 56. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-ion battery recycling—Overview of techniques and trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Buisson, E.; Dhir, S.; Hegarty, A.; Hwang, G.; Kim, Y.Y.; Michaels, K.C.; Bredariol, T.d.O.; Pospiech, R.; Raboca, J. Recycling of Critical Minerals: Strategies to Scale up Recycling and Urban Mining—World Energy Outlook Special Report; International Energy Agency: Paris, France, 2023; Available online: https://www.iea.org/reports/recycling-of-critical-minerals (accessed on 29 August 2024).
- Chen, H.; Yu, J.; Liu, X. Development strategies and policy trends of the next-generation vehicles battery: Focusing on the international comparison of China, Japan and South Korea. Sustainability 2022, 14, 12087. [Google Scholar] [CrossRef]
- Shafique, M.; Rafiq, M.; Azam, A.; Luo, X. Material flow analysis for end-of-life lithium-ion batteries from battery electric vehicles in the USA and China. Resour. Convserv. Recycl 2022, 178, 106061. [Google Scholar] [CrossRef]
- Bruno, M.; Fiore, S. Material flow analysis of lithium-ion battery recycling in Europe: Environmental and economic implications. Batteries 2023, 9, 231. [Google Scholar] [CrossRef]
- Rosenberg, S.; Kurz, L.; Huster, S.; Wehrstein, S.; Kiemel, S.; Schultmann, F.; Reichert, F.; Wörner, R.; Glöser-Chahoud, S. Combining dynamic material flow analysis and life cycle assessment to evaluate environmental benefits of recycling—A case study for direct and hydrometallurgical closed-loop recycling of electric vehicle battery systems. Resour. Convserv. Recycl. 2023, 198, 107145. [Google Scholar] [CrossRef]
- Cencic, O.; Rechberger, H. Material flow analysis with software STAN. J. Environ. Eng. Manag. 2008, 18, 3–7. [Google Scholar]
- Korea Environment Corporation. Waste Discharge and Treatment Performance Report (2022); Allbaro Operation Division, Korea Environment Corporation: Incheon, Republic of Korea, 2023; Available online: https://www.allbaro.or.kr (accessed on 7 May 2025).
- Financial Supervisory Service. Annual Report. 2023. Available online: https://dart.fss.or.kr/main.do (accessed on 29 August 2024).
- National Institute of Environmental Research. National Pollution Source Survey (2022). 2023. Available online: https://wems.nier.go.kr/ (accessed on 21 March 2024).
- Korea Ministry of Land, Infrastructure and Transport. Total Registered Motor Vehicles. 2024. Available online: https://stat.molit.go.kr/portal/cate/statMetaView.do?hRsId=58 (accessed on 29 August 2024).
- Korea International Trade Association. Import and Export by Item. 2024. Available online: http://stat.kita.net/stat/cstat/peri/itme/TiemList.screen (accessed on 21 March 2024).
- Danawa. Autombile Encyclopedia. 2024. Available online: https://auto.danawa.com/auto/ (accessed on 31 May 2024).
- Statista. Market Share of Different Types of Electric Vehicles Cathode Chemistries in 2020 with a Forecast for 2025 Through 2050. 2021. Available online: https://www.statista.com/statistics/1248519/distribution-of-different-electric-vehicle-batteries-on-theglobal-market/ (accessed on 21 March 2024).
- Korea Environmental Corporation. Circular Resources Information Center: Electric Waste Battery from Vehicle; Korea Environment Corporation: Incheon, Republic of Korea, 2024. [Google Scholar]
- Korea Environmental Corporation. Collection Performance for Waste Battery from Electric Vehicle (2022); Korea Environment Corporation: Incheon, Republic of Korea, 2024. [Google Scholar]
- Korea Environmental Corporation. Performance for Eco-Assurance System of Electrical and Electronic Equipment and Vehicles (2018–2022); Korea Environment Corporation: Incheon, Republic of Korea, 2023. [Google Scholar]
- Korea Insurance Development Institute. Repair Rate for EV LIB; Korea Insurance Development Institute: Seoul, Republic of Korea, 2024. [Google Scholar]
- Korea Environmental Corporation. Allbaro System Performance (2022); Korea Environmental Corporation: Incheon, Republic of Korea, 2024. [Google Scholar]
- Korea Ministry of Environment. Waste Generation and Treatment Status 2022. 2023. Available online: https://library.me.go.kr/#/search/detail/5912624 (accessed on 11 August 2023).
- Trade Statistics Service. Statistics by Transaction Name. 2024. Available online: https://www.bandtrass.or.kr/theme/name.do?command=THE006View&viewCode=THE00601 (accessed on 2 October 2024).
- Korea Environmental Corporation. Performance Report on Export and Import Waste (2022). 2024. (Not publicly available).
- Korea Environmental Corporation. LIB Capacity and Weight by EV Type. 2024. (Not publicly available).
- Electric Vehicle Database. All Electric Vehicles. 2024. Available online: https://ev-database.org/ (accessed on 8 October 2024).
- Winjobi, O.; Dai, Q.; Kelly, J.C. Update of Bill-of-Materials and Cathode Chemistry addition for Lithium-ion Batteries in GREET 2020; Argonne National Laboratory: Lemont, IL, USA, 2020. [Google Scholar]
- Korea Ministry of Economy and Finance. Exchange Rate. 2023. Available online: https://www.index.go.kr/unity/potal/main/EachDtlPageDetail.do?idx_cd=1068 (accessed on 3 October 2024).
- YS/T 1342.1; Method for Chemical Analysis of Waste Batteries—Part 1: Determination of Nickel Content. Ministry of Industry and Information Technology of the People’s Republic of China: Beijing, China, 2019.
- YS/T 1342.2; Method for Chemical Analysis of Waste Secondary Battery—Part 2: Determination of Cobalt Content. Ministry of Industry and Information Technology of the People’s Republic of China: Beijing, China, 2019.
- YS/T 1229.3; Methods for Chemical Analysis of Crude Nickel Hydroxide—Part 3: Determination of Copper, Cobalt, Manganese Calcium, Zinc, Iron, Aluminum, Lead, Arsenic and Cadmium Contents. Ministry of Industry and Information Technology of the People’s Republic of China: Beijing, China, 2018.
- ES 04400.1e; Test Methods for Water Quality—Metals: Flame Atomic Absorption Spectrometry. National Institute of Environmental Research: Incheon, Republic of Korea, 2024.
- ISO 11885:2007; Water quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). International Organization for Standardization: Geneva, Switzerland, 2007.
- Shafique, M.; Akbar, A.; Rafiq, M.; Azam, A.; Luo, X. Global material flow analysis of end-of-life of lithium nickel manganese cobalt oxide batteries from battery electric vehicles. Waste Manag. Res. 2023, 41, 376–388. [Google Scholar] [CrossRef]
- Fallah, N.; Fitzpatrick, C. How will retired electric vehicle batteries perform in grid-based second-life applications? A comparative techno-economic evaluation of used batteries in different scenarios. J. Cleaner Prod. 2022, 361, 132281. [Google Scholar] [CrossRef]
- Kamran, M.; Raugei, M.; Hutchinson, A. A dynamic material flow analysis of lithium-ion battery metals for electric vehicles and grid storage in the UK: Assessing the impact of shared mobility and end-of-life strategies. Resour. Conserv. Recycl. 2021, 167, 105412. [Google Scholar] [CrossRef]
- Ma, X.; Azhari, L.; Wang, Y. Li-ion battery recycling challenges. Chem 2021, 7, 2843–2847. [Google Scholar] [CrossRef]
- Hammami, A.; Raymond, N.; Armand, M. Lithium-ion batteries: Runaway risk of forming toxic compounds. Nature 2003, 424, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Kirchhofer, M.; von Zamory, J.; Paillard, E.; Passerini, S. Separators for Li-ion and Li-metal battery including ionic liquid based electrolytes based on the TFSI- and FSI- anions. Int. J. Mol. Sci. 2014, 15, 14868–14890. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.M.; Mütze, T.; Peuker, U.A. Influence of cell opening methods on electrolyte removal during processing in lithium-ion battery recycling. Metals 2022, 12, 663. [Google Scholar] [CrossRef]
- Korea Mineral Resource Information Service. Mineral Prices. 2025. Available online: https://www.komis.or.kr/ (accessed on 20 January 2025).
- Rinne, M.; Aromaa-Stubb, R.; Elomaa, H.; Porvali, A.; Lundström, M. Evaluation of hydrometallurgical black mass recycling with simulation-based life cycle assessment. Int. J. Life Cycle Assest. 2024, 29, 1582–1597. [Google Scholar] [CrossRef]
- Asadi, A.; Kang, D.; Harandi, H.B.; Jung, J.C.Y.; Sui, P.C. Utilization of lithium sulphate electrodialysis for closed-loop LIB recycling: Experimental study and process simulation. Sep. Purif. Technol. 2024, 343, 126989. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z.H.I. Lithium carbonate recovery from cathode scrap of spent lithium-ion battery: A closed-loop process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef]
- Perdana, I.; Aprilianto, D.R.; Fadillah, F.A.; Fadli, R.; Petrus, H.T.B.M.; Astuti, W.; Muflikhum, M.A.; Oktaviano, H.S.; Fathoni, F.; Raihan, E.; et al. Lithium recovery from mixed spent LFP-NMC batteries through atmospheric water leaching. Sci. Rep. 2025, 15, 2591. [Google Scholar] [CrossRef]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective recovery of valuable metals from spent lithium-ion batteries—Process development and kinetics evaluation. J. Clean Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Cleaner Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Ku, H.; Jo, M.; Kim, S.; Song, J.; Yu, J.; Kwon, K. The effect of Fe as an impurity element for sustainable resynthesis of Li [Ni1/3Co1/3Mn1/3] O2 cathode material from spent lithium-ion batteries. Electrochim. Acta 2019, 296, 814–822. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, L.; Shen, L.; Ran, J.J.; Zhong, H.; Zhu, Y.R.; Chen, H. Recent advancements in hydrometallurgical recycling technologies of spent lithium-ion battery cathode materials. Rare Met. 2024, 43, 879–899. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial recycling of lithium-ion batteries—A critical review of metallurgical process routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Niese, N.; Pieper, C.; Arora, A.; Xie, A. The Case for a Circular Economy in Electric Vehicle Batteries; Boston Consulting Group: Boston, MA, USA, 2020; Available online: https://www.bcg.com/publications/2020/case-for-circular-economy-in-electric-vehicle-batteries (accessed on 27 March 2025).
- Regulation (EU) 1542/2023. Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC; European Parliament, Council of the European Union: Bruxelles, Belgium, 2023; Available online: https://data.europa.eu/eli/reg/2023/1542/oj (accessed on 29 August 2024).
- Wang, J.H.; Wang, Y.; Guo, Y.Z.; Liu, C.W.; Dan, L.L. Electrochemical characterization of AlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application. Rare Met. 2021, 40, 78–83. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, Z.; Ma, X.; Chen, M.; Chen, B.; Zheng, Y.; Yao, Z.; Vanaphuti, P.; Bong, S.; Yang, Z.; et al. Understanding fundamental effects of Cu impurity in different forms for recovered LiNi0.6Co0.2Mn0.2O2 cathode materials. Nano Energy 2020, 78, 105214. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, Y.; Vanaphuti, P.; Liu, Y.; Fu, J.; Yao, Z.; Ma, X.; Chen, M.; Yang, Z.; Lin, Y.; et al. Valence effects of Fe impurity for recovered LiNi0.6Co0.2Mn0.2O2 cathode materials. ACS Appl. Energy Mater. 2021, 4, 10356–10367. [Google Scholar] [CrossRef]
- GB/T 45203-2024; Recycled Black Powder for Lithium-ion Batteries. The Standardization Administration of the People’s Republic of China: Beijing, China, 2024.
- Shi, P.; Yang, S.; Wu, G.; Chen, H.; Chang, D.; Jie, Y.; Fang, G.; Mo, C.; Chen, Y. Efficient separation and recovery of lithium and manganese from spent lithium-ion batteries powder leaching solution. Sep. Purif. Technol. 2023, 309, 123063. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Giannika, V. Decarbonized and circular brine management/valorization for water & valuable resource recovery via minimal/zero liquid discharge (MLD/ZLD) strategies. J. Environ. Manag. 2022, 324, 116239. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z. Recovery of lithium from spent lithium-ion batteries using precipitation and electrodialysis techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Keller, A.; Hlawitschka, M.W. Recovery of excess sulfuric acid in the lithium-ion batteries recycling process. Sep. Purif. Technol. 2024, 341, 126965. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and avenues for acid mine drainage treatment, beneficiation, and valorisation in circular economy: A review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
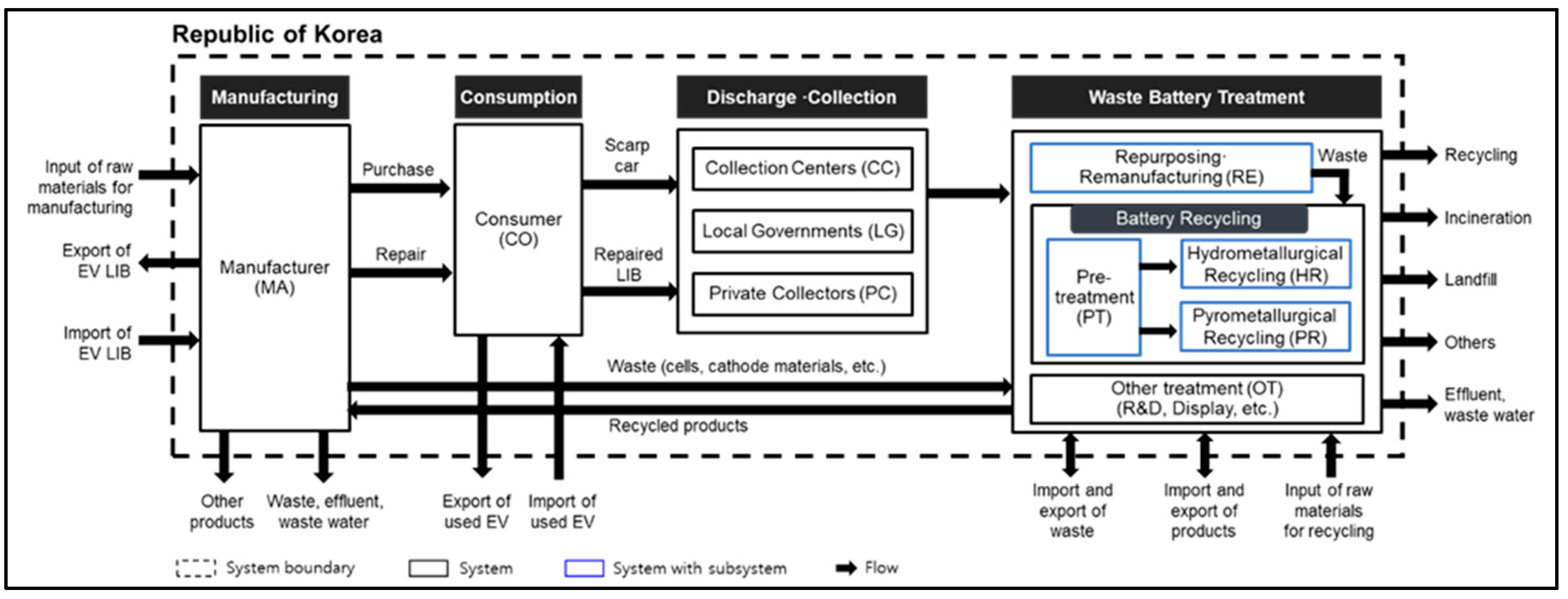
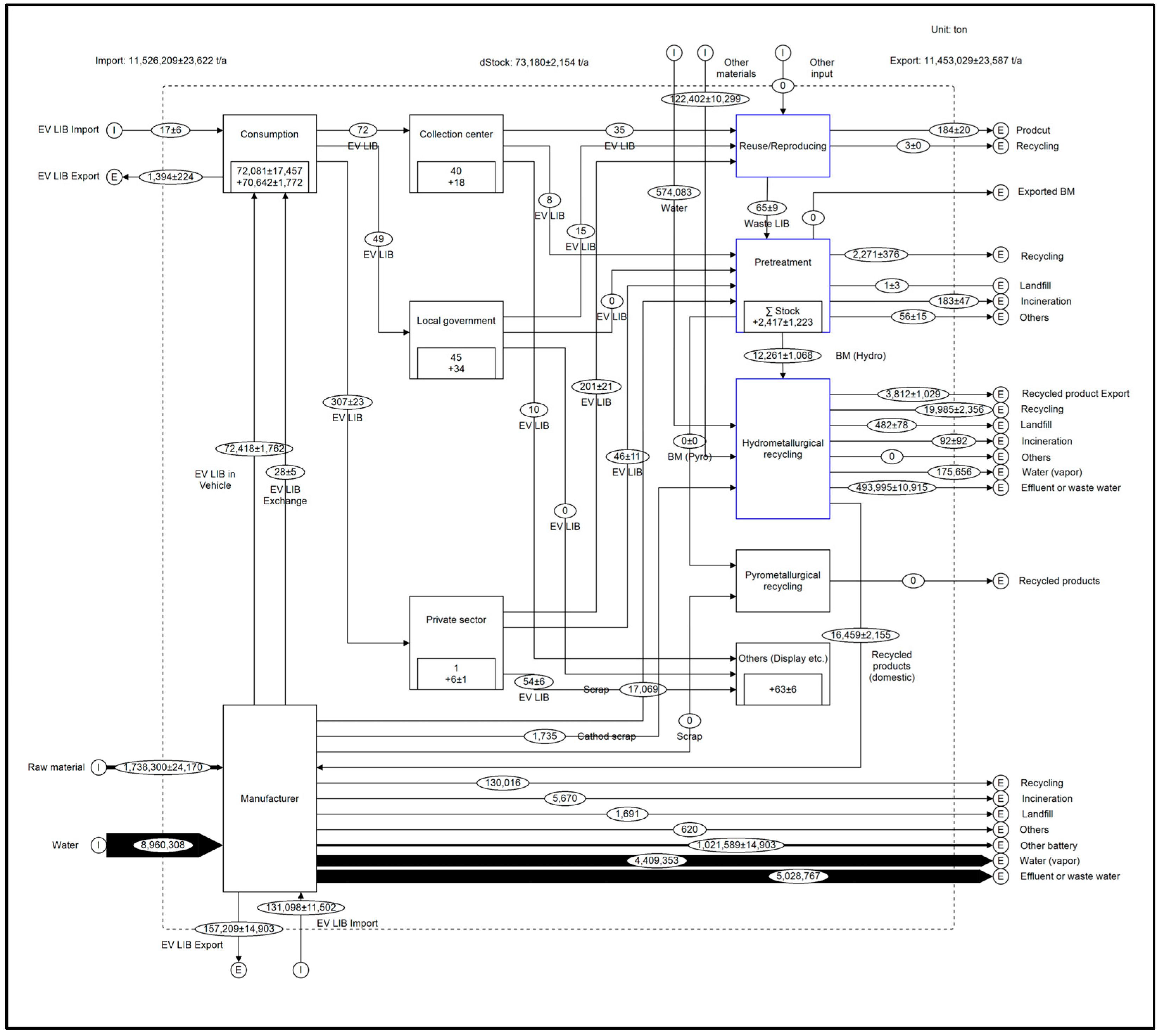

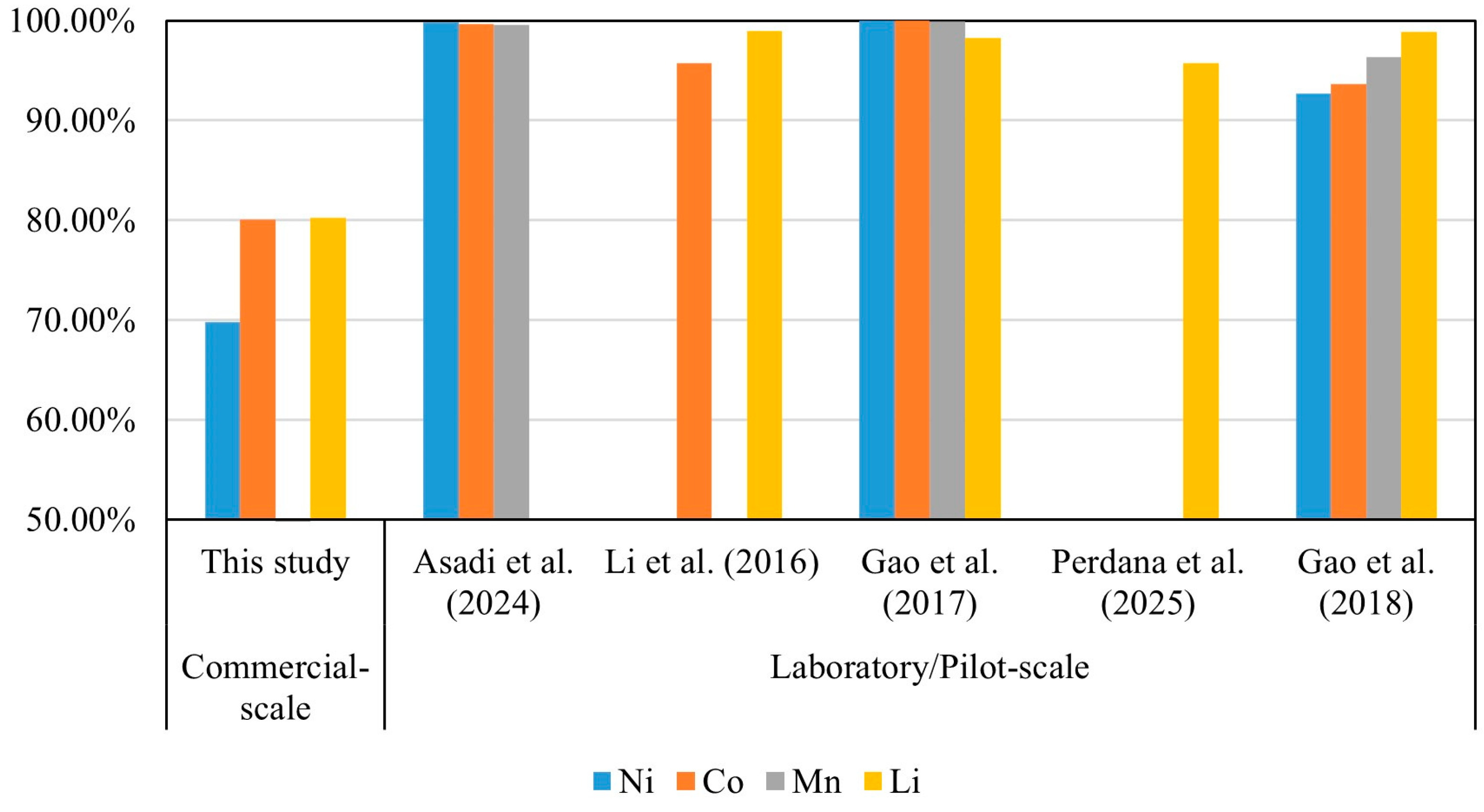
| Life Cycle | No. | Type of Data | Data Quality | References |
|---|---|---|---|---|
| Manufacturing | 1-1 | Waste generation and treatment | Secondary data (National statistics) | [20] |
| 1-2 | Product sales | Secondary data (Industrial report) | [21] | |
| 1-3 | Evaporation, wastewater generation | Secondary data (National statistics) | [22] | |
| Consumption | 2-1 | EV registration and scrapping | Secondary data (National statistics) | [23] |
| 2-2 | Import and export of used EVs | Secondary data (Industrial report) | [24] | |
| 2-4 | EV sales | Secondary data (Industrial statistics) | [25] | |
| 2-5 | EV market share | Secondary data (Industrial statistics) | [26] | |
| Discharge and Collection | 3-1 | EV LIB collection and sales at central collection centers and local governments | Secondary data (National statistics) | [27] |
| 3-2 | Information on EV LIB bidding companies | Secondary data (National statistics) | [28] | |
| 3-3 | Scrap performance of vehicles containing LFP batteries | Secondary data (National statistics) | [29] | |
| 3-4 | Repair rate for EV LIBs | Secondary data (National statistics) | [30] | |
| Waste battery treatment | 4-1 | Waste generation and treatment performance | Secondary data (National statistics) | [31,32] |
| 4-2 | Input materials for recycling, waste generation, recycled products | Primary data (Survey) | - | |
| 4-3 | Valuable metal content (concentration) in waste (wastewater) | Primary data (Sampling and analysis) | - | |
| 4-4 | Evaporation, wastewater generation | Secondary data (National statistics) | [22] | |
| 4-5 | Import and export of black mass | Secondary data (National statistics) | [33] | |
| 4-6 | Performance report on export and import waste | Secondary data (National statistics) | [34] | |
| Common (unit conversion factors, etc.) | 6-1 | Tolerance weight and LIB weight by EV | Secondary data (National report, industrial data) | [25,35,36] |
| 6-2 | Component materials by LIB type | Secondary data (Previous study) | [37] | |
| 6-3 | Exchange rate | Secondary data (National statistics) | [38] |
| Valuable Metal | Treatment Type | Input (ton) | Output (ton) | Recovery Rate (B/A) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Waste (A) | Recycled Products (B) | Secondary Waste | Wastewater | ||||||
| Recycling | Incineration | Landfill | Others | ||||||
| Ni | RE | 32 | 22 | 10 | 0 | 0 | 0 | 0 | 69.2% |
| PT | 2284 | 2256 | 13 | 11 | 0 | 4 | 0 | 98.8% | |
| HR | 2772 | 1936 | 815 | 6 | 15 | 0 | 1 | 69.8% | |
| Total | 2822 | 1958 | 828 | 17 | 15 | 4 | 1 | 69.4% | |
| Co | RE | 12 | 8 | 4 | 0 | 0 | 0 | 0 | 69.0% |
| PT | 348 | 344 | 2 | 1 | 0 | 0 | 0 | 98.9% | |
| HR | 519 | 417 | 98 | 1 | 2 | 0 | 1 | 80.4% | |
| Total | 531 | 425 | 101 | 2 | 2 | 0 | 1 | 80.1% | |
| Mn | RE | 10 | 7 | 3 | 0 | 0 | 0 | 0 | 69.5% |
| PT | 396 | 393 | 2 | 1 | 0 | 0 | 0 | 99.2% | |
| HR | 571 | 0 | 548 | 1 | 22 | 0 | 0 | 0.0% | |
| Total | 581 | 7 | 550 | 2 | 22 | 0 | 0 | 1.1% | |
| Li | RE | 7 | 5 | 2 | 0 | 0 | 0 | 0 | 69.6% |
| PT | 569 | 562 | 3 | 3 | 0 | 1 | 0 | 98.9% | |
| HR | 695 | 557 | 7 | 1 | 0 | 0 | 129 | 80.2% | |
| Total | 706 | 562 | 10 | 4 | 0 | 1 | 129 | 79.6% | |
| Valuable Metal | Amount of Valuable Metals Used in Domestic EV LIB Manufacturing [A (ton)] | Production and Utilization of Recycled Products | |||
|---|---|---|---|---|---|
| Recycled Product Production [B (ton)] | Domestic Consumption of Recycled Products [C (ton)] | Potential Substitution Rate of Recycled Products [B/A (%)] | Domestic Utilization Rate of Recycled Products [C/A (%)] | ||
| Ni | 12,325 ± 4372 | 1936 ± 293 | 1101 ± 162 | 15.7 ± 6.1 | 8.9 ± 3.4 |
| Co | 4565 ± 2259 | 417 ± 111 | 416 ± 110 | 9.1 ± 1.5 | 9.1 ± 1.5 |
| Mn | 3684 ± 2832 | 0 ± 0 | 0 ± 0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Li | 2697 ± 562 | 557 ± 240 | 119 ± 51 | 20.6 ± 2.5 | 4.4 ± 0.5 |
| Valuable Metal | Baseline (2022) | Short-Term | Long-Term | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2025 | 2027 | 2029 | 2031 | 2032 | 2033 | 2034 | 2035 | 2036 | ||
| Ni | 5.5% | - | - | 5.5% | 6.0% | 7.8% | 9.6% | 11.4% | 13.2% | 15.0% |
| Co | 7.6% | 8.5% | 11.0% | 13.5% | 16.0% | 18.0% | 20.0% | 22.0% | 24.0% | 26.0% |
| Li | 3.9% | 4.2% | 4.8% | 5.4% | 6.0% | 7.2% | 8.4% | 9.6% | 10.8% | 12.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-J.; Kim, M.; Roh, H.J.; Hwang, D.; Yoon, Y.-S.; Kang, Y.-Y.; Jeon, T.-W. A Material Flow Analysis of Electric Vehicle Lithium-ion Batteries: Sustainable Supply Chain Management Strategies. Sustainability 2025, 17, 4560. https://doi.org/10.3390/su17104560
Choi H-J, Kim M, Roh HJ, Hwang D, Yoon Y-S, Kang Y-Y, Jeon T-W. A Material Flow Analysis of Electric Vehicle Lithium-ion Batteries: Sustainable Supply Chain Management Strategies. Sustainability. 2025; 17(10):4560. https://doi.org/10.3390/su17104560
Chicago/Turabian StyleChoi, Hyeong-Jin, Minjung Kim, Hyung Joo Roh, Donggun Hwang, Young-Sam Yoon, Young-Yeul Kang, and Tae-Wan Jeon. 2025. "A Material Flow Analysis of Electric Vehicle Lithium-ion Batteries: Sustainable Supply Chain Management Strategies" Sustainability 17, no. 10: 4560. https://doi.org/10.3390/su17104560
APA StyleChoi, H.-J., Kim, M., Roh, H. J., Hwang, D., Yoon, Y.-S., Kang, Y.-Y., & Jeon, T.-W. (2025). A Material Flow Analysis of Electric Vehicle Lithium-ion Batteries: Sustainable Supply Chain Management Strategies. Sustainability, 17(10), 4560. https://doi.org/10.3390/su17104560







