A Novel Approach to Manufacturing an Antioxidant Material, GT-Ag@MSN, Using Recycled Silver and Silicon from Scrapped Photovoltaic Panels
Abstract
1. Introduction
2. Methods and Materials
2.1. Chemicals and Reagents
2.2. Solar Panel Separation and Preparation of MSN
2.3. Production of GT-Ag@MSN
2.4. Antibacterial Experiment
2.5. DPPH Free Radical Scavenging Experiment
2.6. GT-Ag@MSN Reduction Ability
2.7. Characterization
3. Results and Discussion
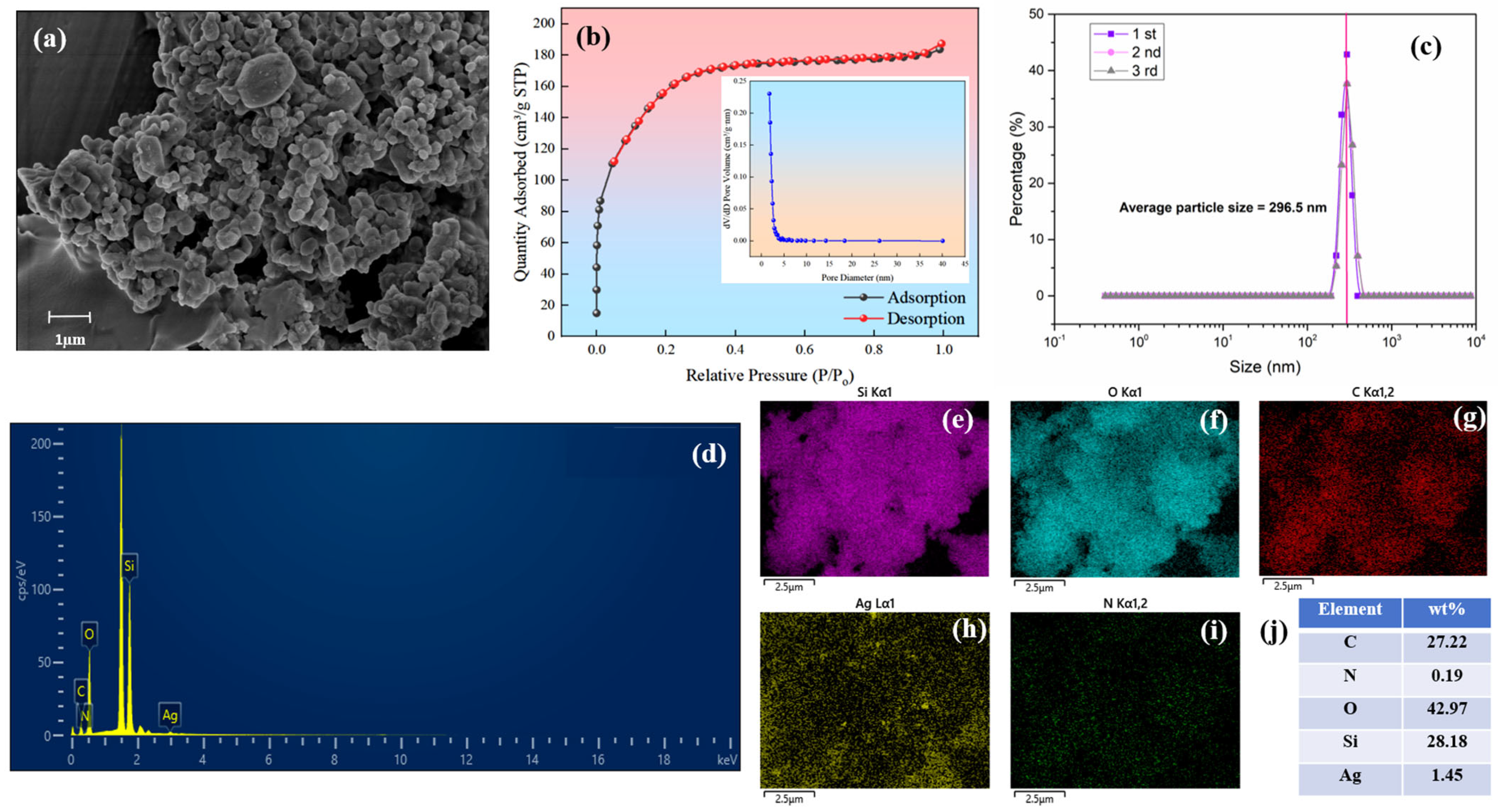
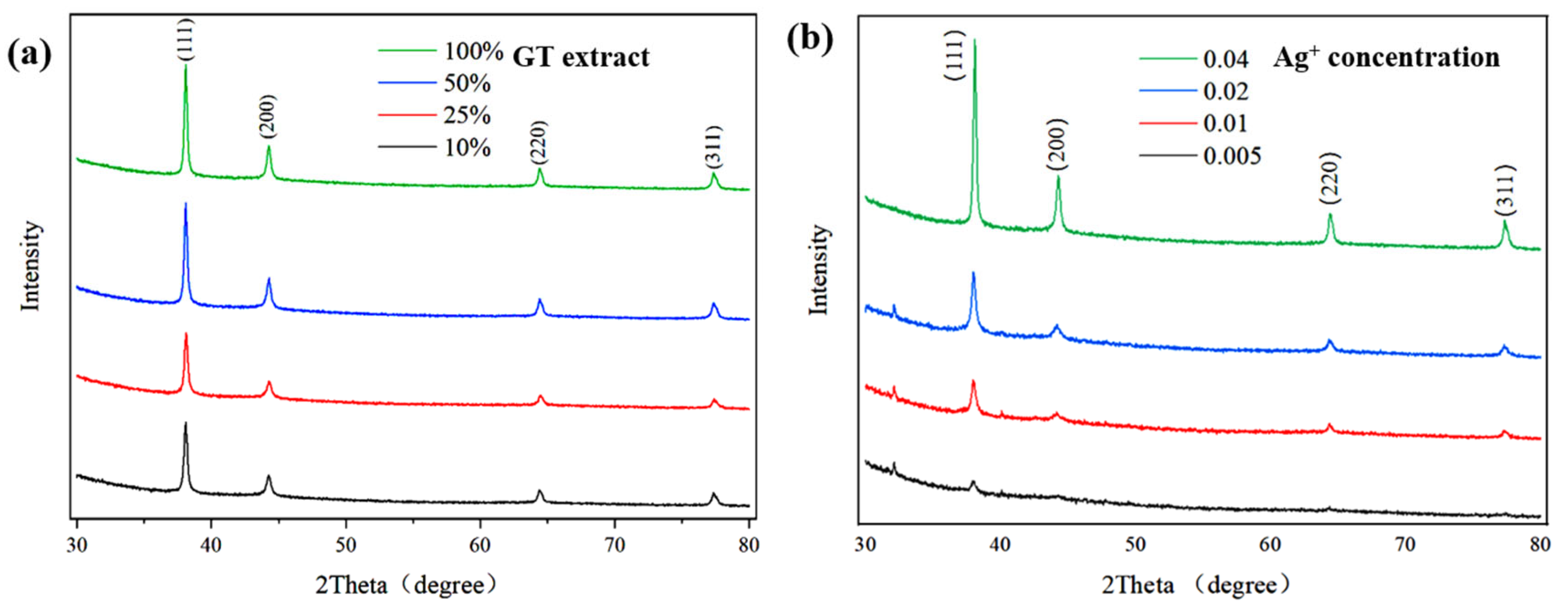
3.1. Characterization of GT-Ag@MSN
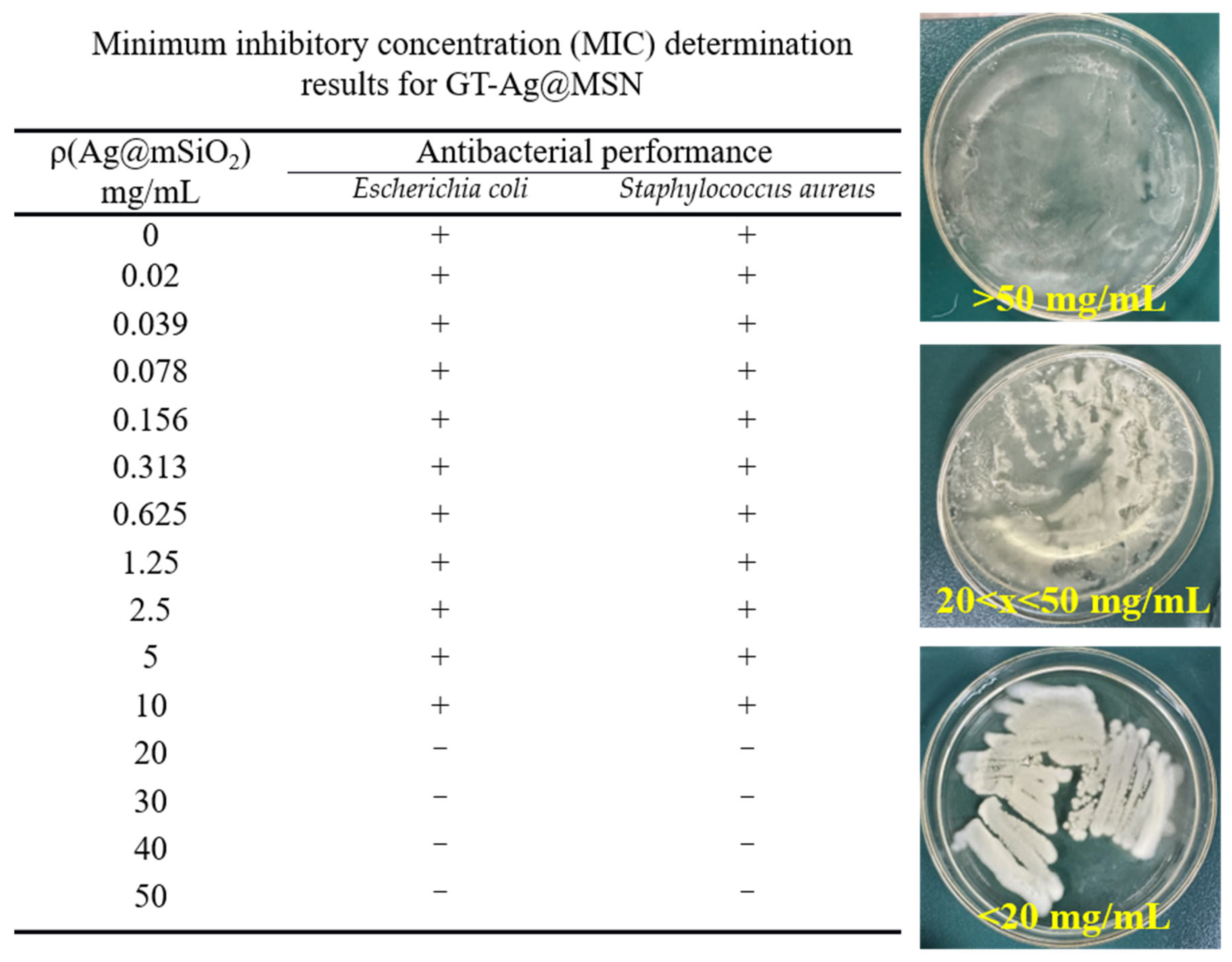
3.2. Antibacterial Performance of GT-Ag@MSN
3.3. DPPH Free Radical Scavenging Assay
3.4. GT-Ag@MSN Reducing Power
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tammaro, M.; Salluzzo, A.; Rimauro, J.; Schiavo, S.; Manzo, S. Experimental Investigation to Evaluate the Potential Environmental Hazards of Photovoltaic Panels. J. Hazard. Mater. 2016, 306, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, D.; Wang, G.; Wang, Y. Nondestructive Silicon Wafer Recovery by a Novel Method of Solvothermal Swelling Coupled with Thermal Decomposition. Chem. Eng. J. 2021, 418, 129457. [Google Scholar] [CrossRef]
- Zimmermann, Y.-S.; Schäffer, A.; Corvini, P.F.-X.; Lenz, M. Thin-Film Photovoltaic Cells: Long-Term Metal(Loid) Leaching at Their End-of-Life. Environ. Sci. Technol. 2013, 47, 13151–13159. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Yu, J.; Xu, C.; Wu, Y.; Pan, D.; Senthil, R.A. An Overview of the Comprehensive Utilization of Silicon-Based Solid Waste Related to PV Industry. Resour. Conserv. Recycl. 2021, 169, 105450. [Google Scholar] [CrossRef]
- Domínguez, A.; Geyer, R. Photovoltaic Waste Assessment of Major Photovoltaic Installations in the United States of America. Renew. Energy 2019, 133, 1188–1200. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, J.; Zheng, Y.; Yang, W.; Zhang, Y.; Cheng, W. Status Quo on Recycling of Waste Crystalline Silicon for Photovoltaic Modules and Its Implications for China’s Photovoltaic Industry. Front. Energy 2024, 18, 685–698. [Google Scholar] [CrossRef]
- Alshameri, A.W.; Owais, M. Antibacterial and Cytotoxic Potency of the Plant-Mediated Synthesis of Metallic Nanoparticles Ag NPs and ZnO NPs: A Review. OpenNano 2022, 8, 100077. [Google Scholar] [CrossRef]
- Munir, T.; Mahmood, A.; Peter, N.; Rafaqat, N.; Imran, M.; Ali, H.E. Structural, Morphological and Optical Properties at Various Concentration of Ag Doped SiO2-NPs via Sol Gel Method for Antibacterial and Anticancer Activities. Surf. Interfaces 2023, 102759. [Google Scholar] [CrossRef]
- Chang, Z.; Karmakar, B.; Lu, H.; Lou, X.; Salem Alkhayyat, S.; Mostafa-Hedeab, G.; El-Saber Batiha, G.; El-kott, A.F.; Elsaid, F.G.; Al-Kahtani, M.A.; et al. Preparation of Gelatin/Ag NPs under Ultrasound Condition: A Potent and Green Bio-Nanocomposite for the Treatment of Pleomorphic Hepatocellular Carcinoma, Morris Hepatoma, and Novikoff Hepatoma. Arab. J. Chem. 2022, 15, 103858. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Akpeji, B.H.; Azeez, S.O.; Ayipo, Y.O.; Abdulsalam, Z.A.; Adebayo, Z.F.; Ajao, A.T.; Zakariyah, A.T.; Elemike, E.E. Biosynthesis of Ag and TiO2 Nanoparticles and the Evaluation of Their Antibacterial Activities. Inorg. Chem. Commun. 2022, 141, 109503. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, L.; Li, Q.; Ai, J.; Liu, H.; Chen, Q. Rapid Hemostasis Accompanied by Antibacterial Action of Calcium Crosslinking Tannic Acid-Coated Mesoporous Silica/Silver Janus Nanoparticles. Mater. Sci. Eng. C 2021, 123, 111958. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.B.; Nayak, Y.; Garg, S.; Nayak, U.Y. Multifunctional Engineered Mesoporous Silica/Inorganic Material Hybrid Nanoparticles: Theranostic Perspectives. Coord. Chem. Rev. 2023, 478, 214977. [Google Scholar] [CrossRef]
- Zhuang, J.; Yu, Y.; Lu, R. Mesoporous Silica Nanoparticles as Carrier to Overcome Bacterial Drug Resistant Barriers. Int. J. Pharm. 2023, 631, 122529. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Yu, X.; Liu, H.; Li, K.; He, Y.; Tian, H.; Kumar, R. Material, Antibacterial and Anticancer Properties of Natural Polyphenols Incorporated Soy Protein Isolate: A Review. Eur. Polym. J. 2021, 152, 110494. [Google Scholar] [CrossRef]
- Riccucci, G.; Cazzola, M.; Ferraris, S.; Gobbo, V.A.; Guaita, M.; Spriano, S. Surface Functionalization of Ti6Al4V with an Extract of Polyphenols from Red Grape Pomace. Mater. Des. 2021, 206, 109776. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly)Phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Cheng, W.; Wen, J. Now and Future: Development and Perspectives of Using Polyphenol Nanomaterials in Environmental Pollution Control. Coord. Chem. Rev. 2022, 473, 214825. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Li, P.; Wang, G.; Wei, J. Controllable Deposition of Ag Nanoparticles on Various Substrates via Interfacial Polyphenol Reduction Strategy for Antibacterial Application. Colloids Surf. Physicochem. Eng. Asp. 2022, 655, 130287. [Google Scholar] [CrossRef]
- Ebrahim Mohammadzadeh, S.; Faghiri, F.; Ghorbani, F. Green Synthesis of Phenolic Capping Ag NPs by Green Walnut Husk Extract and Its Application for Colorimetric Detection of Cd2+ and Ni2+ Ions in Environmental Samples. Microchem. J. 2022, 179, 107475. [Google Scholar] [CrossRef]
- Hao, R.; Li, D.; Zhang, J.; Jiao, T. Green Synthesis of Iron Nanoparticles Using Green Tea and Its Removal of Hexavalent Chromium. Nanomaterials 2021, 11, 650. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, J.; Hu, X.; Xing, L.; Yan, C. Immobilization of Cr(VI) Contaminated Soil Using Green-Tea Impregnated Attapulgite. J. Clean. Prod. 2021, 278, 123967. [Google Scholar] [CrossRef]
- Hu, X.; Wen, J.; Zhang, H.; Wang, Q.; Yan, C.; Xing, L. Can Epicatechin Gallate Increase Cr(VI) Adsorption and Reduction on ZIF-8? Chem. Eng. J. 2020, 391, 123501. [Google Scholar] [CrossRef]
- Hou, D.-Z.; Ling, P.; Zhu, Y.; Ouyang, Y.-M.; Karmakar, B. White Tea Extract Modified Green Synthesis of Magnetite Supported Ag Nanoparticles: Evaluation of Its Catalytic Activity, Antioxidant and Anti-Colon Cancer Effects. Arab. J. Chem. 2022, 15, 104219. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Reda, E.M.; Ghazal, H.; Othman, H.A. Synthesis of AgNPs and ZnONPs Using Tea Leaves Extract and Their Utilization to Improve Dyeability, Printability and Functionality of Cotton and Wool Fabrics. Inorg. Chem. Commun. 2023, 150, 110525. [Google Scholar] [CrossRef]
- Hossain, S.S.; Bae, C.-J.; Roy, P.K. Recent Progress of Wastes Derived Nano-Silica: Synthesis, Properties, and Applications. J. Clean. Prod. 2022, 377, 134418. [Google Scholar] [CrossRef]
- Fu, P.; Yang, T.; Feng, J.; Yang, H. Synthesis of Mesoporous Silica MCM-41 Using Sodium Silicate Derived from Copper Ore Tailings with an Alkaline Molted-Salt Method. J. Ind. Eng. Chem. 2015, 29, 338–343. [Google Scholar] [CrossRef]
- Yue, Q.; Wen, J.; Zhou, Y.; Zheng, Y. Resource Utilization of Waste Solar Photovoltaic Panels for Preparation of Microporous Silicon Nanoparticles. Waste Manag. 2025, 193, 495–505. [Google Scholar] [CrossRef]
- Wang, O.; Ma, X. Innovating the Recycling of Silicon-Based Solar Panels with an Eco-Friendly Alkaline Leaching Process. Resour. Conserv. Recycl. 2024, 211, 107887. [Google Scholar] [CrossRef]
- Negi, A.; Rana, P.; Kumar vishwakarma, R.; Singh Negi, D. Multi Dye Degradation, Antibacterial, Antidiabetic and Antioxidant Assessment of Silver Nanoparticles (Ag-NPs) Derived via Leaves of Smilax Aspera. Inorg. Chem. Commun. 2022, 143, 109703. [Google Scholar] [CrossRef]
- Gao, Y.; Dou, H.; Ma, Y.; Tian, G.; Weragoda, D.M.; Li, S.; Yang, X.; Zhang, Z.; Fan, G.; Chen, B. Antibacterial Performance Effects of Ag NPs in Situ Loaded in MOFs Nano-Supports Prepared by Post-Synthesis Exchange Method. J. Environ. Chem. Eng. 2024, 12, 112133. [Google Scholar] [CrossRef]
- Manickam, V.; Mani, G.; Muthuvel, R.; Pushparaj, H.; Jayabalan, J.; Pandit, S.S.; Elumalai, S.; Kaliappan, K.; Tae, J.H. Green Fabrication of Silver Nanoparticles and It’s in Vitro Anti-Bacterial, Anti-Biofilm, Free Radical Scavenging and Mushroom Tyrosinase Efficacy Evaluation. Inorg. Chem. Commun. 2024, 162, 112199. [Google Scholar] [CrossRef]
- Ye, L.; Li, H.; Xiang, J.; Chen, J.; Zhang, X.; Zheng, S.; Liao, W. Synthesis of Ag@SiO2 Core-Shell Nanoparticles for Antibacterial Application in Water-Based Acrylic Polymer Coatings. Surf. Interfaces 2024, 51, 104490. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Li, D.; Le, Y.; Hou, X.-Y.; Chen, J.-F.; Shen, Z.-G. Colored Nanoparticles Dispersions as Electronic Inks for Electrophoretic Display. Synth. Met. 2011, 161, 1270–1275. [Google Scholar] [CrossRef]
- Bukhary, H.A.; Zaman, U.; ur Rehman, K.; Alissa, M.; Rizg, W.Y.; Khan, D.; Almehizia, A.A.; Naglah, A.M.; Al-Wasidi, A.S.; Alharbi, A.S.; et al. Acid Protease Functionalized Novel Silver Nanoparticles (APTs-AgNPs): A New Approach towards Photocatalytic and Biological Applications. Int. J. Biol. Macromol. 2023, 242, 124809. [Google Scholar] [CrossRef]
- Chinnasamy, R.; Chinnaperumal, K.; Venkatesan, M.; Jogikalmat, K.; Cherian, T.; Willie, P.; Malafaia, G. Eco-Friendly Synthesis of Ag-NPs Using Endostemon Viscosus (Lamiaceae): Antibacterial, Antioxidant, Larvicidal, Photocatalytic Dye Degradation Activity and Toxicity in Zebrafish Embryos. Environ. Res. 2023, 218, 114946. [Google Scholar] [CrossRef]
- Ganesan, T.; Muthukrishnan, S.; Albeshr, M.F.; Selvankumar, T.; Pradeepkumar, S.; Indumathi, K.P.; Anto, B. Aerva Lanata Flower Extract Mediated Green Synthesis of Silver Nanoparticles: Their Characterization, in Vitro Antioxidants and Antimicrobial Investigations. Polym. Adv. Technol. 2024, 35, 1–9. [Google Scholar] [CrossRef]
- Khan, A.H.; Hassan, S.; Aamir, M.; Khan, M.W.; Haq, F.; Hayat, J.; Rizwan, M.; Ullah, A.; Ullah, I.; Zengin, G.; et al. Exploring the Therapeutic Properties of Alga-Based Silver Nanoparticles: Anticancer, Antibacterial, and Free Radical Scavenging Capabilities. Chem. Biodivers. 2023, 20, e202301068. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Losetty, V. Synthesis of Sustainable Silver Nanoparticles Using Plant Extract and Their Antimicrobial, Anticancer, and Photocatalytic Dye Degradation Efficiency Analysis. Process Biochem. 2024, 144, 64–78. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Yue, Q.; Qi, Z.; Gong, Z.; Ba, Y. A Novel Approach to Manufacturing an Antioxidant Material, GT-Ag@MSN, Using Recycled Silver and Silicon from Scrapped Photovoltaic Panels. Sustainability 2025, 17, 4557. https://doi.org/10.3390/su17104557
Wen J, Yue Q, Qi Z, Gong Z, Ba Y. A Novel Approach to Manufacturing an Antioxidant Material, GT-Ag@MSN, Using Recycled Silver and Silicon from Scrapped Photovoltaic Panels. Sustainability. 2025; 17(10):4557. https://doi.org/10.3390/su17104557
Chicago/Turabian StyleWen, Jia, Qing Yue, Zhifei Qi, Zhixuan Gong, and Yujiao Ba. 2025. "A Novel Approach to Manufacturing an Antioxidant Material, GT-Ag@MSN, Using Recycled Silver and Silicon from Scrapped Photovoltaic Panels" Sustainability 17, no. 10: 4557. https://doi.org/10.3390/su17104557
APA StyleWen, J., Yue, Q., Qi, Z., Gong, Z., & Ba, Y. (2025). A Novel Approach to Manufacturing an Antioxidant Material, GT-Ag@MSN, Using Recycled Silver and Silicon from Scrapped Photovoltaic Panels. Sustainability, 17(10), 4557. https://doi.org/10.3390/su17104557








