The Mediterranean Dune–Beach–Banquette Ecosystem, Its Pivotal Role in Land–Sea Coupling and the Functioning of Coastal Systems, and Some Related Management Issues
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
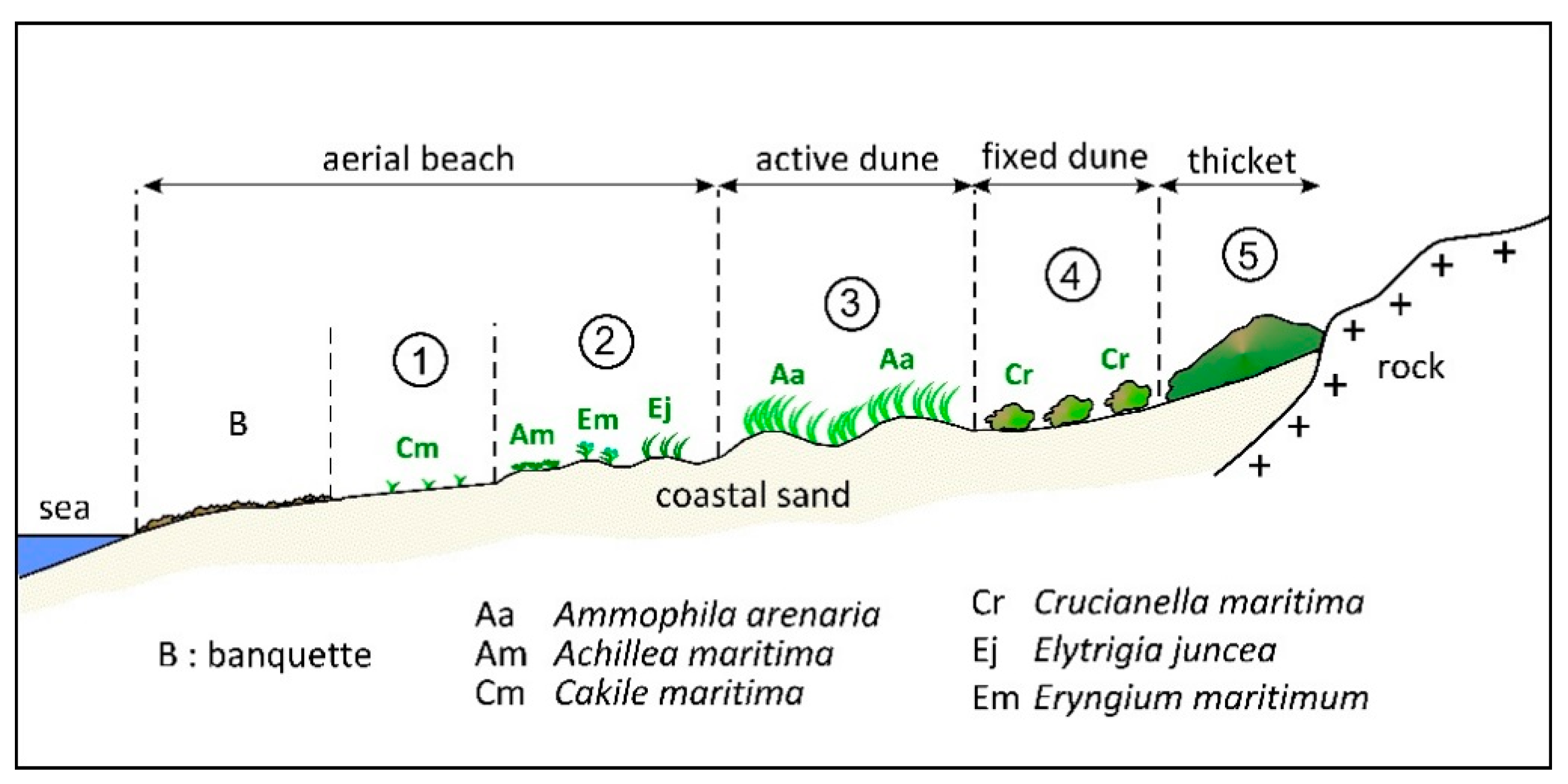
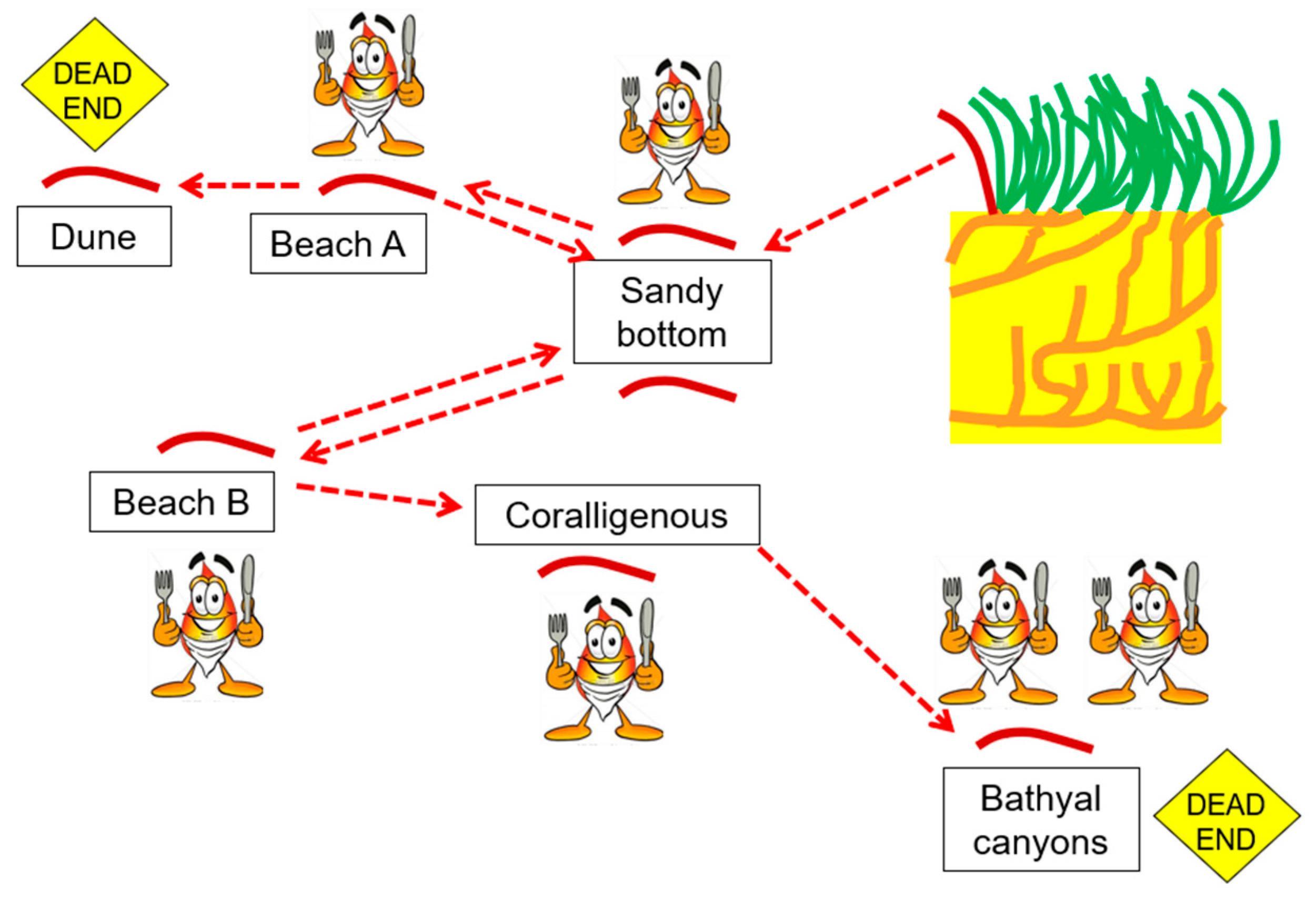
3.1. A Conceptual Model of the Dune–Beach–Banquette Ecosystem (DBB)
3.1.1. General Considerations
3.1.2. Primary Producers
3.1.3. Herbivorous Invertebrates
3.1.4. The Banquette
3.1.5. Drift Macroalgae
3.1.6. Carrion
3.1.7. Driftwood and Xylophagous Invertebrates
3.1.8. Bacteria
3.1.9. Detritus-Feeder Invertebrates
3.1.10. Sea Turtle Eggs and Juveniles
3.1.11. Predatory Invertebrates
3.1.12. Sea Birds and Terrestrial Birds
3.1.13. Terrestrial Mammals
3.2. Ecosystem Services
3.3. Threats to Ecosystem Services, Conservation and Management
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hedgpeth, J.W. Sandy beaches. Geol. Soc. Am. Mem. 1957, 67, 587–608. [Google Scholar]
- McLachlan, A.; Erasmus, T.; Dye, A.H.; Wooldridge, T.; Van der Horst, G.; Rossouw, G.; Lasiak, T.A.; McGwynne, L. Sand beach energetics: An ecosystem approach towards a high energy interface. Estuar. Coast. Shelf Sci. 1981, 13, 11–25. [Google Scholar] [CrossRef]
- Brown, A.C.; McLachlan, A. Sandy shore ecosystems and the threats facing them: Some predictions for the year 2025. Environ. Conserv. 2002, 29, 62–77. [Google Scholar] [CrossRef]
- Ley de la Vega, C.; Favennec, J.; Gallego-Fernández, J.; Pascual Vidal, C. (Eds.) Conservation des Dunes Côtières. Restauration et Gestion Durables en Méditerranée Occidentale; UICN: Gland, Switzerland; Malaga, Spain, 2012; pp. 1–124. [Google Scholar]
- Bisinicu, E.; Abaza, V.; Boicenco, L.; Adrian, F.; Harcota, G.E.; Marin, O.; Oros, A.; Pantea, E.; Spinu, A.; Timofte, F.; et al. Spatial cumulative assessment of impact risk-implementing ecosystem-based management for enhanced sustainability and biodiversity in the Black Sea. Sustainability 2024, 16, 4449. [Google Scholar] [CrossRef]
- Basterretxea, G.; Orfila, A.; Jordi, A.; Lynett, P.; Liu, P.L.F.; Duarte, C.M.; Tintoré, J. Seasonal dynamics of microtidal pocket beach with Posidonia oceanica seabeds (Mallorca, Spain). J. Coast. Res. 2004, 20, 1155–1164. [Google Scholar] [CrossRef]
- De Falco, G.; Molinaroli, E.; Conforti, A.; Simeone, S.; Tonielli, R. Biogenic sediments from coastal ecosystems to Beach-Dune Systems: Implications for the adaptation of mixed and carbonate beaches to future sea level rise. Biogeosci. Discuss 2017, 20, 1–27. [Google Scholar] [CrossRef]
- Simeone, S.; Molinaroli, E.; Conforti, A.; De Falco, G. Impact of ocean acidification on the carbonate sediment budget of a temperate mixed beach. Clim. Change 2018, 150, 227–242. [Google Scholar] [CrossRef]
- Monnier, B.; Lapaquellerie, J.; Boudouresque, C.F.; Cantaloube, F.; Mateo, M.Á.; Clabaut, P.; Pergent, G.; Pergent-Martini, C. The Posidonia oceanica matte: A reservoir of environmental information. In Proceedings of the Fourteenth International MEDCAST Congress on Coastal and Marine Sciences, Engineering, Management and Conservation, MECOAST 2019, Marmaris, Turkey, 22–26 October 2019; pp. 275–286. [Google Scholar]
- De Luca, M.; Chaiallah, A.; Andreucci, S.; Cossu, G.; Santonastaso, A.; Sechi, D.; Stelletti, M.; Pascucci, V. Seafloor map of the Alghero Bay (Sardinia, Italy). J. Maps 2020, 16, 669–679. [Google Scholar] [CrossRef]
- Bialik, O.M.; Coletti, G.; Berndt, C.; Schmidt, M.; Micallef, A. Controls on mesophotic carbonate facies and sediment distribution across the Maltese shelf, central Mediterranean Sea. Facies 2024, 70, 16. [Google Scholar] [CrossRef]
- Inglis, G. The colonisation and degradation of stranded Macrocyctis pyrifera (L.) C. Ag. by the macrofauna of a New Zealand sandy beach. J. Exp. Mar. Biol. Ecol. 1989, 125, 203–217. [Google Scholar] [CrossRef]
- Colombini, I.; Chelazzi, L. Influence of marine allochtonous input on sandy beach communities. Oceanogr. Mar. Biol. Annu. Rev. 2003, 41, 113–159. [Google Scholar]
- Olabarria, C.; Lastra, M.; Garrido, J. Succession of macrofauna on macroalgal wrack of an exposed sandy beach: Effects of patch size and site. Mar. Environ. Res. 2007, 63, 19–40. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Ponel, P.; Astruch, A.; Barcelo, A.; Blanfuné, A.; Geoffroy, D.; Thibaut, T. The high heritage value of the Mediterranean sandy beaches, with a particular focus on the Posidonia oceanica ‘banquettes’: A review. Sci. Rep. Port-Cros Natl. Park 2017, 31, 23–70. [Google Scholar]
- Ragonese, S.; Norrito, G. Florilegio sulle possibili cause dell’erosione della spiaggia di Tonnarella (Mazara del Vallo; Sicilia sud occidentale). NTR—ITPP 2019, 98, 1–36. [Google Scholar]
- Chessa, L.A.; Fustier, V.; Fernandez, C.; Mura, F.; Pais, A.; Pergent, G.; Serra, S.; Vitale, L. Contribution to the knowledge of ‘banquettes’ of Posidonia oceanica (L.) Delile in Sardinia Island. Biol. Mar. Mediterr. 2000, 7, 35–38. [Google Scholar]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Protection and Conservation of Posidonia oceanica Meadows; RAMOGE and RAC/SPA: Tunis, Tunisia, 2012; pp. 1–202. [Google Scholar]
- Pergent, G.; Bazairi, H.; Bianchi, C.N.; Boudouresque, C.F.; Buia, M.C.; Clabaut, P.; Harmelin-Vivien, M.; Mateo, M.A.; Montefalcone, M.; Morri, C.; et al. Mediterranean Seagrass Meadows: Resilience and Contribution to Climate Change Mitigation. A Short Summary; IUCN: Gland, Switzerland; Málaga, Spain, 2012; pp. 1–40. [Google Scholar]
- Kirkman, H.; Kuo, J. Pattern and process in southern Western Australian seagrasses. Aquat. Bot. 1990, 37, 367–382. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Nieuwenhuize, J. Seagrass wrack-induced dune formation on a tropical coast (Banc d’Arguin, Mauritania). Estuar. Coast. Shelf Sci. 1990, 31, 499–502. [Google Scholar] [CrossRef]
- Bussotti, S.; Guidetti, P.; Rossi, F. Posidonia oceanica wrack beds as a fish habitat in the surf zone. Estuar. Coast. Shelf Sci. 2022, 272, 107882. [Google Scholar] [CrossRef]
- Piazza, C.; Paradis, G. Description phytosociologique et cartographique de la végétation du site protégé de Roccapina (Corse, France): Dune et zone humide. Doc. Phytosociol. 1995, 15, 211–233. [Google Scholar]
- Otero, M.M.; Simeone, S.; Aljinovic, B.; Salomidi, M.; Mossone, P.; Gerakaris, V.; Giunta Fornasin, M.E.; Milano, P.; Heurtefeux, H.; Issaris, Y.; et al. POSBEMED: Gouvernance et Gestion des Systèmes Plages/Dunes à Posidonie. Rapport Final; IUCN: Malaga, Spain, 2018; pp. 1–66 + Annexes. [Google Scholar]
- Feola, S.; Carranza, M.L.; Schamine, J.; Janssen, J.A.M.; Acosta, A.T.R. EU habitats of interest: An insight into Atlantic and Mediterranean beach and foredunes. Biodivers. Conserv. 2011, 20, 1457–1468. [Google Scholar] [CrossRef]
- Pinna, M.S.; Cogoni, D.; Fenu, G.; Bacchetta, G. The conservation status and anthropogenic impacts assessments of Mediterranean coastal dunes. Estuar. Coast. Shelf Sci. 2015, 167, 25–31. [Google Scholar] [CrossRef]
- Pinto, J.; Martí, C.; Fraguell, R.M. Assessing current conditions of coastal dune systems of Mediterranean developed shores. J. Coast. Res. 2014, 30, 832–842. [Google Scholar]
- Fenu, G.; Cogoni, D.; Ulian, T.; Bacchetta, G. The impact of human trampling on a threatened coastal Mediterranean plant: The case of Anchusa littorea Moris (Boraginaceae). Flora 2013, 208, 104–110. [Google Scholar] [CrossRef]
- Paradis, G.; Piazza, C.; Fenu, G.; Cogoni, D.; Bacchetta, G. Anchusa crispa. In The Top 50 Mediterranean Island Plants, Update 2017; Pasta, S., Perez-Graber, A., Fazan, L., Montmollin, B.D., Eds.; IUCN/SSC/Mediterranean Plant Specialist Group: Neuchâtel, Switzerland, 2017; E-Book and on Line; pp. 1–141. Available online: https://top50.iucn-mpsg.org/ (accessed on 10 May 2024).
- López-Pujol, J.; Orellana, M.R.; Bosch, M.; Simon, J.; Blanché, C. Effects of habitat fragmentation on allozyme diversity and conservation status of the coastal sand dune plant Stachys maritima (Lamiaceae) in the Iberian Peninsula. Plant Biol. 2003, 5, 504–512. [Google Scholar] [CrossRef]
- Ugo, J.; Burkhart, J.-A.; Dixon, L.; Diadema, K. Plan Régional D’action en Faveur de L’épiaire Maritime (Stachys maritima) 2024–2034; Conservatoire Botanique National Méditerranéen de Porquerolles: Hyères, France, 2023; pp. 1–79 + Annexes. [Google Scholar]
- Piazza, C.; Paradis, G.; Orsucci, D. Stachys maritima en Corse: État des connaissances en 2024. Carnets Bot. 2024, 207, 1–44 + errata (4p.). [Google Scholar]
- Ramage, T. Les îles, derniers bastions de la grande nébrie sur la côte atlantique française. Penn Ar Bed 2019, 233, 20–24. [Google Scholar]
- Lumaret, J.P. Atlas des Coléoptères Scarabéides Laparosticti de France; Série Inventaires de Faune et de Flore, fasc. 1; Secrétariat Faune Flore/MNHN: Paris, France, 1990. [Google Scholar]
- Verdugo Páez, A.; Drumont, A. Révision du genre Calicnemis Laporte, 1832: Approches morphologique et génétique (Coleoptera, Scarabaeidae, Dynastinae). Rev. Assoc. Roussillonnaise Entomol. 2015, 24, 1–60. [Google Scholar]
- Iorio, É.; Geoffroy, D.; Pétillon, J. Distribution and indicator value of intertidal centipedes from Mediterranean beaches within and around Port-Cros National Park (Southern France), with proposal of a simplified monitoring (Chilopoda). Bull. Soc. Entomol. Fr. 2020, 125, 41–62. [Google Scholar] [CrossRef]
- Bosmans, R.; Oger, P.; Ponel, P. Altella emilieae Lissner, 2016 (Aranae: Dictynidae) is a junior synonym of Charaea maritimus Simon, 1884 and a widespread Mediterranean species. Arachnology 2016, 17, 159–160. [Google Scholar] [CrossRef]
- UICN Comité Français; OFB; MNHN; AsFrA. La Liste rouge des espèces menacées en France. Chapitre Araignées de France Métropolitaine; MNHN: Paris, France, 2023; pp. 1–20. [Google Scholar]
- Dalkey, N.; Helmer, O. An experimental application of the Delphi method to the use of experts. Manag. Sci. 1963, 9, 458–467. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, University of Galway: Galway, Ireland. Available online: https://www.algaebase.org (accessed on 10 January 2025).
- Tison, J.M.; Foucault, B.D. (Eds.) Flora Gallica. Flore de France; Biotope: Mèze, France, 2014. [Google Scholar]
- Colombini, I.; Brilli, M.; Fallaci, M.; Gagnarli, E.; Chelazzi, L. Food webs of a sandy beach macroinvertebrate community using stable isotopes analysis. Acta Oecol. 2011, 37, 422–432. [Google Scholar] [CrossRef]
- Cardona, L.; García, M. Beach-cast seagrass material fertilizes the foredune vegetation of Mediterranean coastal dunes. Acta Oecol. 2008, 34, 97–103. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Marbà, N.; Acosta, A.; Vignolo, C.; Traveset, A. Effects of Posidonia oceanica beach-cast on germination, growth and nutrient uptake of coastal dune plants. PLoS ONE 2013, 8, e70607. [Google Scholar] [CrossRef] [PubMed]
- Colombini, I.; Brilli, M.; Fallaci, M.; Gagnarli, E.; Chelazzi, L. Habitat partitioning and trophic levels of terrestrial macroinvertebrates of a Tyrrhenian coastal ecosystem (Grosseto, Italy). Trav. Inst. Sci. Rabat Sér. Gén. 2011, 6, 25–35. [Google Scholar]
- Tassin, C. Paysages Végétaux du Domaine Méditerranéen; IRD Éditions: Marseille, France, 2012; pp. 1–420. [Google Scholar]
- Paradis, G.; Piazza, C. Étude phytosociologique et cartographique en 2017 et 2018 du site dunaire de Tenutella (commune d’Olmeto, Corse-du-Sud), inscrit dans le réseau Natura 2000. Evaxiana 2019, 5, 196–240. [Google Scholar]
- Paradis, G.; Piazza, C. Flore et végétation d’une portion de côte en accrétion: Sud du port de Taverna (côte orientale de Corse). Bull. Soc Bot. Centre-Ouest. N.S. 2020, 51, 248–307. [Google Scholar]
- Díez-Garretas, B.; Asensi, A. The coastal plant communities of Juniperus macrocarpa in the Mediterranean region. Plant Biosyst. 2014, 148, 429–438. [Google Scholar] [CrossRef]
- Hugot, L.; Juillet, N.; Bacchetta, G. Anchusa crispa. In The IUCN Red List of Threatened Species 2011: E.T61654A12533631. Available online: https://www.iucnredlist.org/species/61654/12533631 (accessed on 3 May 2021).
- Paradis, G.; Piazza, C. État des lieux en 2018 du site littoral très dégradé de Capu Laurosu (Propriano, Corse), avant sa réhabilitation par le Conservatoire du Littoral. Evaxiana 2020, 7, 249–301. [Google Scholar]
- Kutiel, P. Conservation and management of the Mediterranean coastal sand dunes in Israel. J. Coast. Conserv. 2001, 7, 183–192. [Google Scholar] [CrossRef]
- Vanden Berghen, C. Observations sur la végétation de l’île de Djerba (Tunisie méridionale). Note 1: Introduction et végétation des dunes mobiles. Bull. Soc. Roy. Belg. 1977, 110, 217–227. [Google Scholar]
- Vanden Berghen, C. Observations sur la végétation de l’île de Djerba (Tunisie méridionale). Note 2: Les dunes fixées. L’association à Imperata cylindrica et Ononis angustissima. Bull. Soc. Roy. Belg. 1978, 111, 227–236. [Google Scholar]
- Vázquez, X.A. European Fauna of Oedemeridae; Argania Edition: Barcelona, Spain, 2002; pp. 117 + 179. [Google Scholar]
- Jaulin, S.; Soldati, F. Les Dunes Littorales du Languedoc-Roussillon: Guide Méthodologique D’évaluation de Leur état de Conservation à travers l’étude Des Cortèges Spécialisés de Coleopteres; Office Pour Les Insectes et Leur Environnement du Languedoc-Roussillon (OPIE-LR); DIREN-LR: Millas, France, 2005; pp. 1–58. [Google Scholar]
- Pérès, J.M.; Picard, J. Nouveau manuel de bionomie benthique de la Mer Méditerranée. Rec. Trav. Stat. Mar. Endoume 1964, 31, 3–137. [Google Scholar]
- Ott, J.A. Growth and production in Posidonia oceanica (L.) Delile. Mar. Ecol. PSZN 1980, 1, 47–64. [Google Scholar] [CrossRef]
- Vela, A.; Leoni, V.; Pergent, G.; Pergent-Martini, C. Relevance of leaf matter loss in the functioning of Posidonia oceanica system. Biol. Mar. Mediterr. 2006, 13, 102–106. [Google Scholar]
- Pergent, G.; Romero, J.; Pergent-Martini, C.; Mateo, M.A.; Boudouresque, C.F. Primary production, stocks and fluxes in the Mediterranean seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 1994, 106, 139–146. [Google Scholar] [CrossRef]
- Cebrián, J.; Duarte, C.M.; Marbà, N.; Enríquez, S. Magnitude and fate of the production of four co-occurring Western Mediterranean seagrass species. Mar. Ecol. Prog. Ser. 1997, 155, 29–44. [Google Scholar] [CrossRef]
- Personnic, S.; Boudouresque, C.F.; Astruch, P.; Ballesteros, E.; Blouet, S.; Bellan-Santini, D.; Bonhomme, P.; Thibault-Botha, D.; Feunteun, E.; Harmelin-Vivien, M.; et al. An ecosystem-based approach to assess the status of a Mediterranean ecosystem, the Posidonia oceanica seagrass meadow. PLoS ONE 2014, 9, e98994. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Thibaut, T.; Verlaque, M. The necromass of the Posidonia oceanica seagrass meadow: Fate, role, ecosystem services and vulnerability. Hydrobiologia 2016, 781, 25–42. [Google Scholar] [CrossRef]
- Prado, P.; Tomas, F.; Alcoverro, T.; Romero, J. Extensive direct measurements of Posidonia oceanica defoliation confirm the importance of herbivory in temperate seagrass meadows. Mar. Ecol. Prog. Ser. 2007, 340, 63–71. [Google Scholar] [CrossRef]
- Pinna, S.; Pais, S.; Chessa, L.; Sechi, N.; Ceccherelli, G. Leaf partitioning of the seagrass Posidonia oceanica between two herbivores: Is Sarpa salpa herbivory underestimated because of Paracentrotus lividus grazing? Estuar. Coast. Shelf Sci. 2009, 84, 21–27. [Google Scholar] [CrossRef]
- Shakman, E.; Boedeker, C.; Bariche, M.; Kinzelbach, R. Food and feeding habits of the Lessepsian migrants Siganus luridus Rüppell, 1928 and Siganus rivulatus Forsskål 1775 (Teleostei: Siganidae) in the Southern Mediterranean (Libyan coast). J. Biol. Res. Thessaloniki 2009, 12, 115–124. [Google Scholar]
- Vizzini, S. Analysis of the trophic role of Mediterranean seagrasses in marine coastal ecosystems: A review. Bot. Mar. 2009, 52, 383–393. [Google Scholar] [CrossRef]
- Bourcart, J. Compte rendu des missions de géologie sous-marine accomplies en 1949. Com. Centr. Océanogr. Étude Côtes, Bull. Inform. 1949, 8, 6–9. [Google Scholar]
- Bourcart, J. Mission océanographique sur le plateau continental de la côte de Provence, en avril 1952, à bord de l’aviso ‘Ingénieur Elie Monnier’. Bull. Inform. (Service Hydrographique de la Marine) 1952, 4, 279–282 + 1 map. [Google Scholar]
- Fourt, M.; Goujard, A. Rapport Scientifique de la Campagne MESDEACAN (Têtes des Canyons Méditerranéens Continentaux) Novembre 2008-avril 2010; GIS Posidonie: Marseille, France, 2012; pp. 1–75. [Google Scholar]
- Ott, J.A.; Maurer, L. Strategies of energy transfer from marine macrophytes to consumer levels: The Posidonia oceanica example. In Biology of Benthic Organisms; Keegan, B.F., Ceidighn, P.O., Boaden, P.J.S., Eds.; Pergamon Press: Oxford, UK, 1977; pp. 493–502. [Google Scholar]
- Cardona, L.; Revelles, M.; Sales, M.; Aguilar, A.; Borrell, A. Meadows of the seagrass Posidonia oceanica are a significant source of organic matter for adjoining ecosystems. Mar. Ecol. Prog. Ser. 2007, 335, 123–131. [Google Scholar] [CrossRef]
- Boncagni, P.; Rakaj, A.; Fianchini, A.; Vizzini, S. Preferential assimilation of seagrass detritus by two coexisting Mediterranean sea cucumbers: Holothuria polii and Holothuria tubulosa. Estuar. Coast. Shelf Sci. 2019, 231, 106464. [Google Scholar] [CrossRef]
- Dimech, M.; Borg, J.A.; Schembri, P.J. Motile macroinvertebrate assemblages associated with submerged Posidonia oceanica litter accumulations. Biol. Mar. Mediterr. 2006, 13, 130–133. [Google Scholar]
- Mascart, T.; Agusto, L.; Lepoint, G.; Remy, F.; De Troch, M. How do harpacticoid copepods colonize detrital seagrass leaves? Mar. Biol. 2015, 162, 929–943. [Google Scholar] [CrossRef]
- Mascart, T.; Lepoint, G.; Deschoemaeker, S.; Binard, M.; Remy, F.; De Troch, M. Seasonal variability of meiofauna, especially harpacticoid copepods, in Posidonia oceanica macrophytodetritus accumulations. J. Sea Res. 2015, 95, 149–160. [Google Scholar] [CrossRef]
- Remy, F.; Mascart, T.; De Troch, M.; Michel, L.N.; Lepoint, G. Seagrass organic matter transfer in Posidonia oceanica macrophytodetritus accumulations. Estuar. Coast. Shelf Sci. 2018, 212, 73–79. [Google Scholar]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [PubMed]
- Garcias-Bonet, N.; Sherman, T.D.; Duarte, C.M.; Marbá, N. Distribution and pathogenicity of the protist Labyrinthula sp. in western Mediterranean seagrass meadows. Estuar. Coasts 2011, 34, 1161–1168. [Google Scholar] [CrossRef]
- Andromede Oceanologie. La Méditerranée Dévoile ses Dessous—Cartographie Continue des Habitats Marins; Agence de l’Eau RMC: Marseille, France, 2014. [Google Scholar]
- Pergent-Martini, C.; Valette, A.; Damier, É.; Pergent, G. L’évaluation surfacique des habitats est-elle un indicateur fiable de la dynamique spatio-temporelle en milieu marin? In Colloque National de Cartographie des Habitats Marins, 3rd ed.; CARAMB’AR: Brest, France, 2017; pp. 98–101. [Google Scholar]
- Pergent, G.; Barralon, E.; Monnier, B.; Pergent-Martini, C.; Valette-Sansevin, A. Strategy to study blue carbon ecosystems in Corsica. In Proceedings of the 6th Mediterranean Symposium on marine Vegetation, Antalya, Turkey, 14–15 January 2019; Langar, H., Ouerghi, A., Eds.; RAC/SPA: Tunis, Tunisia, 2019; pp. 15–20. [Google Scholar]
- Calvo, S.; Tomasello, A.; Di Maida, G.; Pirrotta, M.; Buia, M.C.; Cinelli, F.; Cormaci, M.; Furnari, G.; Giaccone, G.; Luzzu, F.; et al. Seagrasses along the Sicilian coasts. Chem. Ecol. 2010, 26, 249–266. [Google Scholar] [CrossRef]
- Hattour, A.; Ben Mustapha, K. Le Couvert Végétal Marin du Golfe de Gabès: Cartographie et Réseau de Surveillance de l’herbier de Posidonie; Institut National des Sciences et Technologies de la Mer: Le Kram, Tunisia, 2013; pp. 1–164. [Google Scholar]
- Traganos, D.; Aggarwal, B.; Poursanidis, D.; Topouzelis, K.; Chrysoulakis, N.; Reinartz, P. Towards global-scale mapping and monitoring using Sentinel-2 on Google Earth engine: The case study of the Aegean and Ionian seas. Rem. Sens. 2018, 10, 1227. [Google Scholar] [CrossRef]
- Kletou, D.; Kleitou, P.; Savva, I.; Attrill, M.J.; Charalambous, S.; Loucaides, A.; Hall-Spencer, J.M. Seagrasses of Vasiliko Bay, eastern Mediterranean: Lost cause or priority conservation habitat? J. Mar. Sci. Engin. 2020, 8, 717. [Google Scholar] [CrossRef]
- Guillén Nieto, J.E.; Martínez Vidal, J.; Triviño Pérez, A.; Soler Capdepón, G. Preliminary study of the management of Posidonia oceanica banquettes in Spanish coastal beaches. Rapp. Comm. Intl. Mer Méditerr. 2013, 40, 807. [Google Scholar]
- Cancemi, G.; Buron, K. Érosion du Littoral et Suivi des Banquettes de Posidonie Sur Les Plages de Corse; DIREN Corse and EVEMar: Porto-Vecchio, France, 2008; pp. 1–45. [Google Scholar]
- Gómez-Pujol, L.; Orfila, A.; Álvarez-Ellacuria, A.; Terrados, J.; Tintoré, J. Posidonia oceanica beach-cast litter in Mediterranean beaches: A coastal videomonitoring study. J. Coast. Res. 2013, 65, 1768–1773. [Google Scholar] [CrossRef]
- Simeone, S.; De Muro, S.; De Falco, G. Seagrass berm deposition on a Mediterranean embayed beach. Estuar. Coast. Shelf Sci. 2013, 135, 171–181. [Google Scholar] [CrossRef]
- Roig-Munar, F.X.; Rodríguez-Perea, A.; Martín Prieto, J.Á.; Galaber Ferrer, B. Cuantificación de la pérdida de sedimento por la retirada mecánica de bermas (banquettes) de Posidonia oceanica en las playas de las Islas Baleares: Consecuencias geomorfológicas. Rev. Soc. Geol. Esp. 2019, 32, 73–86. [Google Scholar]
- Pergent-Martini, C.; Leoni, V.; Pergent, G.; Vela, A. Étude finale de faisabilité pour la réutilisation des banquettes de feuilles de Posidonia oceanica: Du ramassage au recyclage—PORIME; Rapport d’activité 2005–2006—Région Corse. Programme INTERREG IIIA Sardaigne/Corse/Toscane; GIS Posidonie: Marseille, France, 2006; pp. 1–54. [Google Scholar]
- Simeone, S.; De Falco, G. Morphology and composition of beach-cast Posidonia oceanica litter on beaches with different exposures. Geomorphology 2012, 151–152, 224–233. [Google Scholar] [CrossRef]
- Mateo, M.A.; Sánchez-Lizaso, J.L.; Romero, J. Posidonia oceanica ‘banquettes’: A preliminary assessment of the relevance for meadow carbon and nutrient budget. Estuar. Coast. Shelf Sci. 2003, 56, 85–90. [Google Scholar] [CrossRef]
- Fontaine, Q.; Paradis, G.; Fullgrabe, L.; Blayac, H.; Marengo, M.; Piazza, C.; Cancemi, G.; Lejeune, P. Caractérisation des Dépôts de Banquettes de Posidonie et Etude Des Communautés Végétales Présentes sur Trois Plages du Parc Naturel Marin du Cap Corse et de l’Agriate; Contrat Stareso-OEC E09-20; Stareso: Calvi, France, 2020; pp. 1–159. [Google Scholar]
- Vitale, L.; Chessa, L.A. Indagini sulle banquettes di Posidonia oceanica (L.) Delile del litorale di Stintino (Sardegna NW). Biol. Mar. Mediterr. 1998, 5, 657–660. [Google Scholar]
- Cantasano, N. Management plan for the beach-cast seagrass in Calabria. In Marine Research at CNR, Chapter: DTA/06-2011, Department of Earth and Environment; National Research Council of Italy Ed.: Roma, Italy, 2011; pp. 1173–1182. [Google Scholar]
- De Falco, G.; Simeone, S.; Baroli, M. Management of beach-cast Posidonia oceanica seagrass on the Island of Sardinia (Italy, western Mediterranean). J. Coast. Res. 2008, 26, 69–75. [Google Scholar] [CrossRef]
- Deidun, A.; Saliba, S.; Schembri, P.J. Banquette faunal assemblages from groomed and ungroomed beaches on the Maltese Islands. Rapp. Comm. Intl. Mer Méditerr. 2007, 38, 456. [Google Scholar]
- Boudouresque, C.F. Des plages aux grands fonds: L’importance des herbiers de posidonie de Marseille et du Parc national des Calanques. In Plongées et Science en Provence. Une Histoire de L’exploration Sous-Marine sur les Côtes des Bouches-du-Rhône et du Var; L’Ancre de Marine: Marseille, France, 2024; pp. 96–107 + 230–231. [Google Scholar]
- Bellan-Santini, D. Conclusions d’une étude quantitative dans la biocoenose des algues photophiles en Méditerranée sur les côtes de Provence (France). Mar. Biol. 1968, 1, 250–256. [Google Scholar] [CrossRef]
- Sala, E.; Ballesteros, E.; Dendrinos, P.; Di Franco, A.; Ferretti, F.; Foley, D.; Fraschetti, S.; Friedlander, A.; Garrabou, J.; Güçlüsoy, H.; et al. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 2012, 7, e32742. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Personnic, S.; Ruitton, R.; Ballesteros, E.; Bellan-Santini, D.; Bianchi, C.N.; Bussotti, S.; Cebrian, E.; et al. An ecosystem-based approach to assess the status of Mediterranean algae-dominated shallow rocky reefs. Mar. Pollut. Bull. 2017, 117, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, F.; Gómez, M.; De la Huz, R.; Lastra, M.; López, J. Wrack algae of exposed sandy beach: Effect on the nutrient supply to the coastal environment. Trav. Inst. Sci. Rabat Sér. Gén. 2011, 6, 103–104. [Google Scholar]
- Ribera, M.A.; Gomez Garreta, A.; Gallardo, T.; Cormaci, M.; Furnari, G.; Giaccone, G. Checklist of Mediterranean seaweeds. I. Fucophyceae (Warming, 1884). Bot. Mar. 1992, 35, 109–130. [Google Scholar] [CrossRef]
- Ni-Ni-Win; Hanyuda, T.; Draisma, S.G.A.; Meinesz, A.; Kawai, H. Padina ditristromatica sp. nov. and Padina pavonicoides sp. nov. (Dictyotales, Phaeophyceae), two new species from the Mediterranean Sea based on morphological and molecular markers. Eur. J. Phycol. 2011, 46, 327–341. [Google Scholar] [CrossRef]
- Orellana, S.; Hernández, M.; Sansón, M. Diversity of Cystoseira sensu lato (Fucales, Phaeophyceae) in the eastern Atlantic and Mediterranean based on morphological and DNA evidence, including Carpodesmia gen. emend. and Treptacantha gen. emend. Eur. J. Phycol. 2019, 54, 447–465. [Google Scholar] [CrossRef]
- Molinari Novoa, E.A.; Guiry, M.D. Reinstatement of the genera Gongolaria Boehmer and Ericaria Stackhouse (Sargassaceae, Phaeophyceae). Not. Alg. 2020, 172, 1–10. [Google Scholar]
- Redondo-Gómez, D.; Quaggiotto, M.M.; Bailey, D.M.; Eguía, S.; Morales-Reyes, Z.; López-Pastor, B.D.L.N.; Martín-Vega, D.; Martínez-Carrasco, C.; Sebastián-González, E.; Sánchez-Zapata, J.; et al. Comparing scavenging in marine and terrestrial ecosystems: A case study with fish and gull carcasses in a small Mediterranean island. Basic Appl. Ecol. 2022, 59, 92–104. [Google Scholar] [CrossRef]
- Ricci, S.; Colombini, I.; Fallaci, M.; Scoccianti, C.; Chelazzi, L. Arthropods as bioindicators of the red fox foraging activity in a Mediterranean beach-dune system. J. Arid Environ. 1998, 38, 335–348. [Google Scholar] [CrossRef]
- Conti, E.; Costa, G.; Petralia, A.; Petralia, E.; Russo, C. Eco-ethology of Parallelomorphus laevigatus (Coleoptera, Carabidae): A species to protect. Atti Mem. Ente Fauna Sicil. 2014, 11, 41–49. [Google Scholar]
- Carpaneto, G.M.; Baviera, C.; Biscaccianti, A.B.; Brandmayr, P.; Mazzei, A.; Mason, F.; Battistoni, A.; Teofili, C.; Rondinini, C.; Fattorini, S.; et al. A red list of Italian saproxylic beetles: Taxonomic overview, ecological features and conservation issues (Coleoptera). Fragm. Entomol. 2015, 47, 53–126. [Google Scholar] [CrossRef]
- Delnatte, J. À propos d’Isidus moreli Mulsant & Rey, 1874, en France (Coleoptera, Elateridae, Elaterinae, Pomachiliini). Bull. Soc. Entomol. Fr. 2010, 115, 325–338. [Google Scholar]
- Harrison, P.G.; Mann, K.H. Detritus formation from eelgrass (Zostera marina L.): The relative effects of fragmentation, leaching and decay. Limnol. Oceanogr. 1975, 20, 924–934. [Google Scholar] [CrossRef]
- Walker, D.I.; Pergent, G.; Fazi, S. Seagrass decomposition. In Global Seagrass Research Methods; Short, F.T., Coles, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 313–324. [Google Scholar]
- Odum, W.E.; Heald, E.J. The detritus-based food web of an estuarine mangrove community. In Estuarine Research. Volume I, Chemistry, Biology, and the Estuarine System; Cronin, L.E., Ed.; Elsevier: Amsterdam, The Netherlands, 1975; pp. 265–286. [Google Scholar]
- Romero-Martinengo, J. Vegetació submarina de les illes Medes. II. Espermatófits: Posidonia oceanica. In Els sistemes Naturals de les Illes Medes; Ros, J., Olivella, I., Gili, J.M., Eds.; Institut d’Estudis Catalans: Barcelona, Spain, 1984; pp. 373–382. [Google Scholar]
- Hall Jr, R.O.; Meyer, J.L. The trophic significance of bacteria in a detritus-based stream food web. Ecology 1998, 79, 1995–2012. [Google Scholar] [CrossRef]
- Veyret, P. Contribution à l’étude de la faune entomologique de Port-Cros, îles d’Hyères (Var). Première partie: Coléoptères. Ann Soc. Sci. Nat. Toulon Var. 1951, 3, 18–38. [Google Scholar]
- Ponel, P. Recherches sur la communauté des arthropodes terrestres des sables littoraux de la plage de La Palud (île de Port-Cros, Var). Trav. Sci. Parc Natl. Port-Cros 1984, 10, 109–117. [Google Scholar]
- Colombini, I.; Mateo, M.Á.; Serrano, O.; Fallaci, M.; Gagnarli, E.; Serrano, L.; Chelazzi, L. On the role of Posidonia oceanica beach wrack for macroinvertebrates of a Tyrrhenian sandy shore. Acta Oecol. 2009, 35, 32–44. [Google Scholar] [CrossRef]
- Audisio, P.; Vigna Taglianti, A. Insecta Coleoptera. Biol. Mar. Mediterr. 2010, 17 (Suppl. S1), 547–571. [Google Scholar]
- Frank, J.H.; Ahn, K.J. Coastal Staphylinidae (Coleoptera): A worldwide checklist, biogeography and natural history. ZooKeys 2011, 107, 1–98. [Google Scholar]
- Deidun, A.; Saliba, S.; Schembri, P.J. Considerations on the ecological role of wrack accumulations on sandy beaches in the Maltese Islands and recommendations for their conservation management. J. Coast. Res. 2009, 56, 410–414. [Google Scholar]
- Lourenço, F.; Prado e Castro, C.; Ameixa, O.M.C.C. First record of Fucellia maritima (Haliday, 1838) (Diptera, Anthomyiidae) populations in Portugal. Norw. J. Entomol. 2020, 67, 246–248. [Google Scholar]
- Berville, L.; Bazin, N.; Ponel, P.; Pavon, D.; Vidal, P.; Durand, J.-P.; Cuchet, T.; Fiquet, P.; Imbert, M.; Lambret, P. Données nouvelles sur la répartition de Pseudomogoplistes squamiger (Fischer, 1853) en Provence et en Corse (Orthoptera Mogoplistidae). L’Entomologiste 2012, 68, 69–72. [Google Scholar]
- Dusoulier, F. Redécouverte du grillon maritime Pseudomogoplistes squamiger (Fisher, 1853) (Orthoptera: Mogoplistidae) sur le territoire du Parc national de Port-Cros (département du Var, France) et premiers éléments de recherche sur son écologie. Sci. Rep. Port-Cros Natl. Park 2017, 31, 81–103. [Google Scholar]
- Chapelin-Viscardi, J.D.; Braud, Y.; Ponel, P. Bilan des connaissances et éléments nouveaux concernant la répartition d’Anisolabis maritima (Bonelli, 1832) en France (Dermaptera Anisolabididae). L’Entomologiste 2012, 68, 115–119. [Google Scholar]
- Travé, J. Contribution à l’étude des oribates (acariens) de l’île de Port-Cros (Parc national). Trav. Sci. Parc Natl. Port-Cros 1984, 10, 119–150. [Google Scholar]
- Noël, P.Y. Les crustacés du Parc National de Port-Cros et de la région des îles d’Hyères (Méditerranée), France. État actuel des connaissances. Sci. Rep. Port-Cros Natl. Park 2003, 19, 135–306. [Google Scholar]
- Colombini, I.; Bouslama, M.F.; Elgtari, M.; Fallaci, M.; Scapini, F.; Chelazzi, L. Study of the community structure of terrestrial arthropods of a Mediterranean sandy beach ecosystem of Morocco. Trav. Inst. Sci. Rabat Sér. Gén. 2005, 4, 43–54. [Google Scholar]
- Sauve, A.; Ichter, J.; Argagnon, O.; Bellan-Santini, D.; Bioret, F.; Cavallin, P.; Cottaz, C.; Delaugerre, M.J.; Delbosc, P.; Dumoulin, J.; et al. La Liste rouge des Ecosystèmes en France—Les Littoraux Méditerranéens de France Métropolitaine, Vol. 2: Côtes Rocheuses, Rivages de Galets et Graviers, Rapport Technique; Comité Français de l’UICN, OFB & MNHN: Montreuil, France, 2022; pp. 1–151. [Google Scholar]
- Miller, J.D. Reproduction in sea turtles. In The Biology of Sea Turtles, Volume I; CRC Press: Boca Raton, FL, USA, 2017; pp. 51–81. [Google Scholar]
- Bourjea, J.; Sauvignet, H.; Ciccione, S. Les Tortues Marines. 80 clés Pour Comprendre; Éditions Quae: Versailles, France, 2023; pp. 1–120. [Google Scholar]
- Groombridge, B. Les Tortues Marines en Méditerranée: Distribution, Populations, Protection; Conseil de l’Europe: Strasbourg, France, 1990; pp. 1–116. [Google Scholar]
- Casale, P.; Margaritoulis, D. Sea Turtles in the Mediterranean: Distribution, Threats and Conservation Priorities; IUCN: Gland, Swtzerland, 2010; pp. 1–294. [Google Scholar]
- Simantiris, N.; Andreanidou, K.; Sampson, G. Over 30 years of monitoring and implementing the Bern Convention’s recommendations for the protection of Mediterranean sea turtles. Mar. Pol. 2024, 168, 106319. [Google Scholar] [CrossRef]
- Kasparek, M.; Godley, B.J.; Broderick, A.C. Nesting of the green turtle, Chelonia mydas, in the Mediterranean: A review of status and conservation needs. Zool. Middle East 2001, 24, 45–74. [Google Scholar] [CrossRef]
- Girard, F.; Catteau, S.; Gambaiani, D.; Gérigny, O.; Sénégas, J.B.; Moisson, P.; Claro, F. Shift in demographic structure and increased reproductive activity of loggerhead turtles in the French Mediterranean Sea revealed by long-term monitoring. Sci. Rep. 2021, 11, 23164. [Google Scholar] [CrossRef]
- Denaro, M.; Malito, T.; Mancuso, C.; Parise, G.; Urso, S. Nesting activity of the loggerhead sea turtle, Caretta caretta, in Calabria during the 2016-2020. Mediterr. Mar. Sci. 2022, 23, 46–54. [Google Scholar]
- Mancino, C.; Hochscheid, S.; Maiorano, L. Increase of nesting habitat suitability for green turtles in a warming Mediterranean Sea. Sci. Rep. 2023, 13, 19906. [Google Scholar] [CrossRef]
- Luna-Ortiz, A.; Marin-Capuz, G.; Abella, E.; Crespo-Picazo, J.L.; Escribano, F.; Felix, G.; Giralt, S.; Tomas, J.; Pegueroles, C.; Pascual, M.; et al. New colonisers drive the increase of the emerging loggerhead turtle nesting in Western Mediterranean. Sci. Rep. 2024, 14, 1506. [Google Scholar] [CrossRef]
- Colombini, I.; Chelazzi, L. Environmental factors influencing the surface activity of Eurynebria complanata (Coleoptera, Carabidae). Rev. Chilena Hist. Nat. 1996, 69, 511–537. [Google Scholar]
- Ponel, P.; Braschi, J.; Reeb, C. Observation récente de Scarites buparius (Forster, 1771) sur la presqu’île de Giens (Var, France) [Coleoptera, Carabidae, Scaritinae]. Sci. Rep. Port-Cros Natl. Park 2017, 31, 331–336. [Google Scholar]
- Lissner, J.; Chatzaki, M. Description of a new Altella Simon, 1884 (Aranae: Dictynidae) from Greece. Arachnology 2016, 17, 39–42. [Google Scholar] [CrossRef]
- Trotta, A. Note on spiders collected in coastal habitats and first Italian record of Chaerea maritimus (Arachnida, Araneae). Bull. Environ. Life Sci. 2019, 1, 3336. [Google Scholar]
- Iorio, É.; Noël, F. Découverte de deux géophilomorphes halobies rares dans le Parc national de Port-Cros (Var) (Chilopoda, Geophilomorpha). Bull. Soc. Linn. Bordeaux 2017, 152, 183–194. [Google Scholar]
- Lourenço, P.M.; Catry, T.; Piersma, T.; Granadeiro, J.P. Comparative feeding ecology of shorebirds wintering at Banc d’Arguin, Mauritania. Estuar. Coasts 2016, 39, 855–865. [Google Scholar] [CrossRef]
- Lourenço, P.M.; Catry, T.; Granadeiro, J.P. Diet and feeding ecology of the wintering shorebird assemblage in the Bijagós archipelago, Guinea-Bissau. J. Sea Res. 2017, 128, 52–60. [Google Scholar] [CrossRef]
- Kersten, M.; Piersma, T. High levels of energy expenditure in shorebirds; metabolic adaptations to an energetically expensive way of life. Ardea 1987, 55, 175–187. [Google Scholar] [CrossRef]
- Bairlein, F. Nutritional strategies in migratory birds. In Avian Migration; Berthold, P., Gwinner, E., Sonnenschein, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Najdenski, H.; Dimova, T.; Zaharieva, M.M.; Nikolov, B.; Petrova-Dinkova, G.; Dalakchieva, S.; Popov, K.; Hristova-Nikolova, I.; Zehtindjiev, P.; Peev, S. Migratory birds along the Mediterranean–Black Sea Flyway as carriers of zoonotic pathogens. Can. J. Microbiol. 2018, 64, 915–924. [Google Scholar] [CrossRef]
- Viana, D.S.; Santamaría, L.; Figuerola, J. Migratory birds as global dispersal vectors. Trends Ecol. Evol. 2016, 31, 763–775. [Google Scholar] [CrossRef]
- Viana, D.S.; Gangoso, L.; Bouten, W.; Figuerola, J. Overseas seed dispersal by migratory birds. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152406. [Google Scholar] [CrossRef]
- Figuerola, J.; Green, A.J.; Michot, T.C. Invertebrate eggs can fly: Evidence of waterfowl-mediated gene flow in aquatic invertebrates. Am. Nat. 2005, 165, 274–280. [Google Scholar] [CrossRef]
- Nathan, R.; Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A. Mechanisms of long-distance seed dispersal. Trends Ecol. Evol. 2008, 23, 638–647. [Google Scholar] [CrossRef]
- Viana, D.S.; Santamaría, L.; Michot, T.C.; Figuerola, J. Migratory strategies of waterbirds shape the continental-scale dispersal of aquatic organisms. Ecography 2013, 36, 430–438. [Google Scholar] [CrossRef]
- Battisti, C. Sepia cuttlebones pecked by birds along a Mediterranean beach: Patterns, frequency and a possible conservation implication. Avocetta 2020, 44, 95–99. [Google Scholar] [CrossRef]
- Costanza, R.; D’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’neill, R.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Costanza, R.; De Groot, R.; Sutton, P.; Van Der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- De Groot, R.; Brander, L.; Van Der Ploeg, S.; Costanza, R.; Bernard, F.; Braat, L.; Christie, M.; Crossman, N.; Ghermandi, A.; Hein, L.; et al. Global estimates of the value of ecosystems and their services in monetary units. Ecosyst. Serv. 2012, 1, 50–61. [Google Scholar] [CrossRef]
- Scemama, P.; Kermagoret, C.; Astruch, P.; Boudouresque, C.F.; Changeux, T.; Harmelin-Vivien, M.; Ourgaud, M.; Ruitton, S.; Verlaque, M.; Charbonnel, E.; et al. Impact assessment of multiple pressures on ecosystem services with a state and transition model: Application to Posidonia oceanica seagrass meadows. J. Environ. Manag. 2024, 367, 121888. [Google Scholar] [CrossRef]
- Carranza, M.L.; Drius, M.; Malavasi, M.; Frate, L.; Stanisci, A.; Acosta, A.T.R. Assessing land take and its effects on dune carbon pools. An insight into the Mediterranean coastline. Ecol. Indic. 2018, 85, 951–955. [Google Scholar] [CrossRef]
- Drius, M.; Jones, L.; Marzialetti, F.; De Francesco, M.C.; Stanisci, A.; Carranza, M.L. Not just a sandy beach. The multi-service value of Mediterranean coastal dunes. Sci. Total Environ. 2019, 668, 1139–1155. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Marinier, M.; Cavalier, M.; Driancourt, T.; Blanfuné, A.; Perret-Boudouresque, M.; Thibaut, T. The perception of Posidonia oceanica banquettes by beachgoers in the French Riviera. In Proceedings of the 7th Mediterranean Symposium on Marine Vegetation, Genoa, Italy, 19–20 September 2022; RAC/SPA: Tunis, Tunisia, 2022; pp. 37–42. [Google Scholar]
- Bovina, G. Conservazione e restauro delle praterie di Posidonia oceanica. In Il ripristino Degli Ecosistemi Marino-Costieri e la Difesa Delle Coste Sabbiose Nelle Aree Protette; Onori, L., Ed.; ISPRA: Roma, Italy, 2009; pp. 309–339. [Google Scholar]
- Brenner, J.; Jiménez, J.A.; Sardá, R.; Garola, A. An assessment of the non-market value of the ecosystem services provided by the Catalan coastal zone, Spain. Ocean Coast. Manag. 2010, 53, 27–38. [Google Scholar] [CrossRef]
- Gordon, B.M. The Mediterranean as a tourist destination from classical Antiquity to Club Med. Mediterr. Stud. 2003, 12, 203–226. [Google Scholar]
- Otero, M.M.; Simeone, S.; Lindqvist, E.; Agaoglou, C.; Palombo, L.; Hadjioannou, L.; Savvides, P.; Petrou, N. A Manual for conserving Mediterranean Beaches with Posidonia oceanica and Assessing Progress of Management Actions; POSBEMED2 Interreg Med Project; IUCN: Gland, Switzerlan, 2022; pp. 1–106 + Annexes. [Google Scholar]
- Ranieri, F.; D’onghia, G.; Uricchio, A.F.; Ranieri, A.C.; Lopopolo, L.; Ranieri, E. Sustainable tourism in the Tremiti Islands (South Italy). Sci. Rep. 2024, 14, 10021. [Google Scholar] [CrossRef] [PubMed]
- Hobson, J.P.; Dietrich, U.C. Tourism, health and quality of life: Challenging the responsibility of using the traditional tenets of sun, sea, sand, and sex in tourism marketing. J. Trav. Tour. Market. 1995, 3, 21–38. [Google Scholar] [CrossRef]
- Aguiló, E.; Alegre, J.; Sard, M. The persistence of the sun and sand tourism model. Tour. Manag. 2005, 26, 219–231. [Google Scholar] [CrossRef]
- Merckelbagh, A. Et si le Littoral Allait Jusqu’à la mer! La Politique du Littoral sous la Vème République; Éditions Quae: Paris, France, 2009. [Google Scholar]
- Melin, H. How do the inhabitants relate to the outer-urban paths in Balagne (Corsica): Walking to re-invent the city. Envir. Urb./Urb. Environ. 2015, 9, 1–24. [Google Scholar]
- Paskoff, R. Les littoraux. Impact des Aménagements sur leur Evolution; Masson: Paris, France, 1985; pp. 1–188. [Google Scholar]
- Paskoff, R. Côtes en Danger; Masson: Paris, France, 1993; pp. 1–250. [Google Scholar]
- Bacchetta, G.; Bordigoni, A.; Cinti, M.F.; Frau, F.; Lentini, L.; Liggi, M.G.; Meloni, F.; Orrù, M.; Podda, L.; Sanna, A. Handbook of Good Practices and Guidelines for the Correct Enjoyment and Management of Natural Habitats in the Beach System; Project Life + Res Maris; 2008; pp. 1–86. Available online: https://www.researchgate.net/publication/333079917_Handbook_of_good_practices_and_guidelines_for_the_correct_enjoyment_and_management_of_natural_habitats_in_the_beach_system_LIFE_13_NATIT000433_RES_MARIS (accessed on 10 January 2025).
- Simeone, S.; De Falco, G.; Quatrocchi, G.; Cucco, A. Morphological changes of a Mediterranean beach over one year (San Giovanni Sinis, western Mediterranean). J. Coast. Res. 2014, 70, 217–222. [Google Scholar] [CrossRef]
- Balzan, M.V.; Hassoun, A.E.R.; Aroua, A.; Baldy, V.; Bou Dagher, M.; Branquinho, C.; Dutay, J.C.; El Bour, M.; Médail, F.; Motjahid, M.; et al. Ecosystems. In Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future. First Mediterranean Assessment Report; Cramer, W., Guiot, J., Marini, K., Eds.; Union for the Mediterranean, Plan Bleu; UNEP/MAP: Marseille, France, 2020; pp. 1–151. [Google Scholar]
- Jones, A.R.; Schlacher, T.A.; Schoeman, D.S.; Dugan, J.E.; Defeo, O.; Scapini, F.; Lastra, M.; McLachlan, A. Sandy-beach ecosystems: Their health, resilience and management. Trav. Inst. Sci. Rabat Sér. Gén. 2011, 6, 125–126. [Google Scholar]
- Serra-Raventós, J. Coastal erosion: Causes and actions for its recovery. In The Mediterranean Sea: An Overview of Its Present State and Plans for Future Protection; Rodríguez-Prieto, C., Pardini, G., Eds.; Servei de Publicacions de la Universitat de Girona: Girona, Spain, 2003; pp. 131–145. [Google Scholar]
- Vu, M.T. Une Approche Numérique Pour la Conception D’ouvrages de Protection Côtière au Tombolo Oriental de la Presqu’île de Giens. Ph.D. Thesis, Université de Toulon, La Valette-du-Var, France, 2018. [Google Scholar]
- Maiolo, M.; Mel, R.A.; Sinopoli, S. A stepwise approach to beach restoration at Calabria beach. Water 2020, 12, 2677. [Google Scholar] [CrossRef]
- Malavasi, M.; Santoro, R.; Cutini, M.; Acosta, A.T.R.; Carranza, M.L. The impact of human pressure on landscape patterns and plant species richness in Mediterranean coastal dunes. Plant Biosyst. 2016, 150, 73–82. [Google Scholar] [CrossRef]
- Rodil, I.F.; Bessa, F.; Baeta, A.; Arenas, F. Global drifters: The ecological role of non-native macroalgae as beach wrack subsidies. Estuar. Coast. Shelf Sci. 2025, 319, 109289. [Google Scholar] [CrossRef]
- Comor, V.; Orgeas, J.; Ponel, P.; Rolando, C.; Delettre, Y.R. Impact of anthropogenic disturbances on beetle communities of French Mediterranean coastal dunes. Biodiv. Conserv. 2008, 17, 18–37. [Google Scholar] [CrossRef]
- Simeone, S.; De Falco, G. Posidonia oceanica banquettes removal: Sedimentological, geomorphological and ecological implications. J. Coast. Res. 2013, 65, 1045–1050. [Google Scholar]
- Ciccarelli, D.; Pinna, M.S.; Alquini, F.; Cogoni, D.; Ruocco, M.; Bacchetta, G.; Sarti, G.; Fenu, G. Development of a coastal dune vulnerability index for Mediterranean ecosystems: A useful tool for coastal managers? Estuar. Coast. Shelf Sci. 2017, 187, 84–95. [Google Scholar] [CrossRef]
- Šilc, U.; Küzmič, F.; Caković, D.; Stešević, D. Beach litter along various sand dune habitats in the southern Adriatic (E Mediterranean). Mar. Pollut. Bull. 2018, 128, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Montesinos, F.; Anfuso, G.; Randerson, P.; Williams, A.T. Seasonal comparison of beach litter on Mediterranean coastal sites (Alicante, SE Spain). Ocean Coast. Manag. 2019, 181, 104914. [Google Scholar] [CrossRef]
- Cantasano, N. Deposition dynamics of Posidonia oceanica ‘banquettes’ on Calabrian sandy beaches (Southern Italy). Coasts 2021, 1, 25–30. [Google Scholar] [CrossRef]
- Cnudde, S.; Cancemi, G.; Calendini, S.; Marengo, M.; Fontaine, Q. Recensement des Plages du Littoral Corse Caractérisées par la Présence de Banquettes de Posidonie et Concernées par des Conflits D’intérêt Entre Usagers—Rapport Final; Contrat STARESO/OEC. E11-21; OEC: Corti, France, 2021; pp. 1–80. [Google Scholar]
- Bergthold, V. POSBEMED, Gestion des Banquettes de Posidonie en Méditerranée Française. Master’s Thesis, Aix-Marseille Université, Marseille, France, 2017. [Google Scholar]
- Martin, A. Analyse Socio-Economique de la Gestion des Plages: Cas des Banquettes de Posidonies sur les Communes du Littoral Méditerranéen Français; Mémoire Ingénieur Agronome, Supagro; Montpellier University: Montpellier, France, 2017. [Google Scholar]
- Mossone, P.; Guala, I.; Simeone, S. Posidonia banquettes on the Mediterranean beaches: To what extent do local administrators’ and users’ perceptions correspond? In Planning, Nature and Ecosystem Services; Gargiulo, C., Zoppi, C., Eds.; FedOAPress: Naples, Italy, 2019; pp. 225–234. [Google Scholar]
- Serantoni, É. La gestion des dépôts marins sur les plages sur l’île de Porquerolles, située en cœur du Parc national de Port-Cros (Provence, France). Sci. Rep. Port-Cros Natl. Park 2015, 29, 223–235. [Google Scholar]
- Borrello, P.; Chiesa, S.; Maltese, S.; Silvestri, C.; Scarpato, A. Management of Posidonia oceanica beached accumulations and the ‘ecological beach’ model. Rapp. Comm. Intl., Mer Méditerr. 2019, 42, 218. [Google Scholar]
- Astier, J.M.; Boudouresque, C.F.; Pergent, G.; Pergent-Martini, C. Non-removal of the Posidonia oceanica ‘banquette’ on a beach very popular with tourists: Lessons from Tunisia. Sci. Rep. Port-Cros Natl. Park 2020, 34, 15–21. [Google Scholar]
- Rotini, A.; Chiesa, S.; Manfra, L.; Borello, P.; Piermarini, R.; Silvestri, C.; Cappucci, S.; Parlagreco, L.; Devoti, S.; Pisapia, M.; et al. Effectiveness of the ‘ecological beach’ model: Beneficial management of Posidonia beach casts and banquette. Water 2020, 12, 3238. [Google Scholar] [CrossRef]
- Scarpato, A.; Borrello, P.; Chiesa, S.; Devoti, S.; Magaletti, E.; Manfra, L.; Mugnai, C.; Parlagreco, L.; Piermarini, R.; Rotini, A.; et al. La spiaggia Ecologica: Gestione Sostenible Della banquette di Posidonia oceanica sugli arenili del Lazio; ISPRA: Roma, Italy, 2020; pp. 1–52. [Google Scholar]
- Gaglioti, M. The double face of Posidonia oceanica coin and the beached remains in the Circeo Man and Biosphere Reserve and in the Aegadian Island MPA: From uncomfortable waste to valuable resource. Examines Mar. Biol. Oceanogr. 2023, 6, 1–4. [Google Scholar] [CrossRef]
- Manfra, L.; Chiesa, S.; Simeone, S.; Borrello, P.; Piermarini, R.; Agaoglou, C.; Elbour, M.; Zaaboub, N.; Vandarakis, D.; Kourliaftis, I.; et al. Towards sustainable management of beach-cast seagrass in Mediterranean coastal areas. Sustainability 2024, 16, 756. [Google Scholar] [CrossRef]
- Nudge, M.; Bustamente, B.; Badaroux, J.M. Rapport D’activité POSBEMED2. Comment Favoriser L’acceptabilité Sociale des Banquettes de Posidonie; Nudge Me: Lyon, France, 2022; pp. 1–50 + Annexes. [Google Scholar]
- Oudin, S.; Boudouresque, C.F. Let’s preserve the Mediterranean identity of our beaches! Charter of Commitment for beaches with Posidonia oceanica banquettes. Sci. Rep. Port-Cros Natl. Park 2023, 37, 313–334. [Google Scholar]
- Médail, F.; Affre, L.; Suehs, C. Carpobrotus sp., C. edulis (L.) N.E. Br. & C. aff. acinaciformis (L.) L. Bolus. Les griffes de sorcière. In Plantes Invasives de France; Muller, S., Ed.; Muséum National d’Histoire Naturelle: Paris, France, 2004; Volume 62, pp. 52–55. [Google Scholar]
- Boudouresque, C.F.; Astruch, P.; Bănaru, D.; Blanfuné, A.; Belloni, B.; Changeux, T.; Chevaldonné, P.; Fernandez, C.; Harmelin, J.G.; Perez, T.; et al. Ecosystem-based quality indices: Valuable tools for environment management. Vie Milieu—Life Environ. 2020, 70, 2–15. [Google Scholar]
- Rastorgueff, P.A.; Bellan-Santini, D.; Bianchi, C.N.; Bussotti, S.; Chevaldonné, P.; Guidetti, P.; Harmelin, J.G.; Montefalcone, M.; Morri, C.; Perez, T.; et al. An ecosystem-based approach to evaluate the ecological quality of Mediterranean undersea caves. Ecol. Indic. 2015, 54, 137–152. [Google Scholar] [CrossRef]
- Astruch, P.; Orts, A.; Schohn, T.; Belloni, B.; Ballesteros, E.; Bănaru, D.; Bianchi, C.N.; Boudouresque, C.F.; Changeux, T.; Chevaldonné, P.; et al. Ecosystem-based assessment of a widespread Mediterranean marine habitat: The Coastal Detrital Bottoms, with a special focus on epibenthic assemblages. Front. Mar. Sci. 2023, 10, 1130540. [Google Scholar] [CrossRef]
- Laborel, J.; Morhange, C.; Lafont, R.; Le Campion, J.; Laborel-Deguen, F.; Sartoretto, S. Biological evidence of sea-level rise during the last 4500 years on the rocky coasts of continental southwestern France and Corsica. Mar. Geol. 1994, 120, 203–223. [Google Scholar] [CrossRef]
- Waelbroeck, C.; Labeyrie, L.; Michel, E.; Duplessy, J.C.; McManus, J.F.; Lambeck, K.; Balbon, E.; Labracherie, M. Sea-level and deep water temperature changes derived from benthic foraminifera isotopic records. Quat. Sci. Rev. 2002, 21, 295–305. [Google Scholar] [CrossRef]
- Collina-Girard, J. La transgression finiglaciaire, l’archéologie et les textes (exemple de la grotte Cosquer et du mythe de l’Atlantide). In Human Records of Recent Geological Evolution in the Mediterranean Basin—Historical and Archeological Evidence; CIESM Workshop Monographs 24; CIESM: Monte Carlo, Monaco, 2003; pp. 63–70. [Google Scholar]
- Lichter, M.; Zviely, D.; Klein, M.; Sivan, D. Sea-level changes in the Mediterranean: Past, present and future—A review. In Seaweeds and Their Role in Globally Changing Environments; Israel, A., Einav, R., Seckbach, J., Eds.; Springer: Dordrecht, Germany, 2010; pp. 5–17. [Google Scholar]
- Faivre, S.; Bakran-Petricioli, T.; Horvatinčić, N.; Sironič, A. Distinct phases of relative sea level changes in the central Adriatic during the last 1500 years—Influence of climatic variations? Palaeogeogr. Palaeoclim. Palaeoecol. 2013, 369, 163–174. [Google Scholar] [CrossRef]
- Faivre, S.; Bakran-Petricioli, T.; Kaniewski, D.; Marriner, N.; Tomljenović, B.; Sečanj, M.; Horvatić, D.; Barešić, J.; Morhange, C.; Drysdale, R.N. Driving processes of relative sea-level change in the Adriatic during the past two millennia: From local tectonic movements in the Dubrovnik archipelago (Jakljan and Šipan islands) to global mean sea level contributions (Central Mediterranean). Glob. Planet. Change 2023, 227, 104158. [Google Scholar] [CrossRef]
- Vacchi, M.; Ghilardi, M.; Spada, G.; Currás, A.; Robresco, S. New insights into the sea-level evolution in Corsica (NW Mediterranean) since the late Neolithic. J. Archaeol. Sci. Rep. 2017, 12, 782–793. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Blanfuné, A.; Pergent, G.; Thibaut, T. Restoration of seagrass meadows in the Mediterranean Sea: A critical review of effectiveness and ethical issues. Water 2021, 13, 1034. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Minne, A.; Noisette, F.; Thibaut, T. Impact of sea level rise on the Mediterranean Lithophyllum byssoides rims. Sci. Rep. 2023, 13, 10577. [Google Scholar] [CrossRef] [PubMed]
- Toomey, T.; Lira-Loarca, A.; Marcos, M.; Besio, G.; Orfila, A. Future wave climate in the Mediterranean Sea and associated uncertainty from an ensemble of 31 GCM-RCM wave simulations. Earths Future 2025, 13, e2024EF004992. [Google Scholar] [CrossRef]
- Marino, M.; Nasca, S.; Alkharoubi, A.I.K.; Cavallaro, L.; Foti, E. Efficacy of Nature-based Solutions for coastal protection under a changing climate: A modelling approach. Coast. Engin. 2025, 198, 104700. [Google Scholar] [CrossRef]













| Region or Country | Surface Area | Reference |
|---|---|---|
| Mainland Spain | ~600 km2 | Extrapolated from Telesca et al., 2015 [78] |
| Balearic Islands | 1200 km2 | Garcias-Bonet et al., 2011 [79] |
| Provence and French Riviera | 267 km2 | Andromede Oceanologie, 2014 [80] |
| Corsica | 537 km2 | Pergent-Martini et al., 2017 [81] |
| Sardinia | 1534 km2 | In Pergent et al., 2019 [82] |
| Sicily | 760 km2 | Calvo et al., 2010 [83] |
| Tunisia (Gulf of Gabès—Libyan border to Kerkennah Islands) | 11,530 km2 | Hattour and Ben Mustapha, 2013 [84] |
| Greece | 2510 km2 | Traganos et al., 2018 [85] |
| Cyprus | 90 km2 | Kletou et al., 2020 [86] |
| Beach (Region) | Length of the Beach (m) | Volume or DM of the Banquette | Volume or DM per Metre of Shoreline | Reference |
|---|---|---|---|---|
| Nueva Tabarca (Alicante, Spain) | 357 | - | 500 kg | Mateo et al., 2003 [94] |
| Four à Chaux (Giens, Provence, France) | 65 | 126 m3 (June) | 1.9 m3 | Authors’ unpublished data |
| Petit Lequin (Porquerolles Island, Provence, France) | 45 | 143 m3 (June) | 3.1 m3 | Authors’ unpublished data |
| Macinaggio (Corsica) | 620 | 4355 m3 | 7.0 m3 | Fontaine et al., 2020 [95] |
| Pietracorbara (Corsica) | 550 | 1133 m3 | 2.1 m3 | Fontaine et al., 2020 [95] |
| Olzu (Patrimonio, Corsica) | 200 | 727 m3 | 3.6 m3 | Fontaine et al., 2020 [95] |
| Olzu (Patrimonio, Corsica) | 200 | 1348 m3 (June) | 9.6 m3 | Authors’ unpublished data |
| Stentino (Asinara, Sardinia) | 2100 | 255 t | 121 kg | Vitale and Chessa, 1998 [96] |
| Calabria (Italy) | - | - | 18 to 500 kg | Cantasano, 2011 [97] |
| Beach | Region | Dry Mass of Sand (kg DM/m3) | Reference |
|---|---|---|---|
| Padulu-Macinaggio | Corsica | 2–15 | Fontaine et al., 2020 [95] |
| Pietracorbara | Corsica | 1–294 | Fontaine et al., 2020 [95] |
| Calvi | Corsica | 2–3 * | Pergent-Martini et al., 2006 [92] |
| Region of Bonifacio | Corsica | 6–160 | Cancemi and Buron, 2008 [88] |
| Olzu | Corsica | 8–72 | Fontaine et al., 2020 [95] |
| 44 beaches | Sardinia | 93 | De Falco et al., 2008 [98] |
| Punta d’Elice | Sardinia | 43 | Chessa et al., 2000 [17] |
| Punta Negra | Sardinia | 43 | Chessa et al., 2000 [17] |
| Punta Trabuccato | Sardinia | 7 | Chessa et al., 2000 [17] |
| San Giovanni | Sardinia | 1 | Chessa et al., 2000 [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudouresque, C.-F.; Astruch, P.; Belloni, B.; Blanfuné, A.; Francesiaz, C.; Maury, M.; Médail, F.; Paradis, G.; Perret-Boudouresque, M.; Piazza, C.; et al. The Mediterranean Dune–Beach–Banquette Ecosystem, Its Pivotal Role in Land–Sea Coupling and the Functioning of Coastal Systems, and Some Related Management Issues. Sustainability 2025, 17, 4556. https://doi.org/10.3390/su17104556
Boudouresque C-F, Astruch P, Belloni B, Blanfuné A, Francesiaz C, Maury M, Médail F, Paradis G, Perret-Boudouresque M, Piazza C, et al. The Mediterranean Dune–Beach–Banquette Ecosystem, Its Pivotal Role in Land–Sea Coupling and the Functioning of Coastal Systems, and Some Related Management Issues. Sustainability. 2025; 17(10):4556. https://doi.org/10.3390/su17104556
Chicago/Turabian StyleBoudouresque, Charles-François, Patrick Astruch, Bruno Belloni, Aurélie Blanfuné, Charlotte Francesiaz, Maële Maury, Frédéric Médail, Guilhan Paradis, Michèle Perret-Boudouresque, Carole Piazza, and et al. 2025. "The Mediterranean Dune–Beach–Banquette Ecosystem, Its Pivotal Role in Land–Sea Coupling and the Functioning of Coastal Systems, and Some Related Management Issues" Sustainability 17, no. 10: 4556. https://doi.org/10.3390/su17104556
APA StyleBoudouresque, C.-F., Astruch, P., Belloni, B., Blanfuné, A., Francesiaz, C., Maury, M., Médail, F., Paradis, G., Perret-Boudouresque, M., Piazza, C., Ponel, P., Sindou, P., & Thibaut, T. (2025). The Mediterranean Dune–Beach–Banquette Ecosystem, Its Pivotal Role in Land–Sea Coupling and the Functioning of Coastal Systems, and Some Related Management Issues. Sustainability, 17(10), 4556. https://doi.org/10.3390/su17104556







