Cascade Hydroponics as a Means to Increase the Sustainability of Cropping Systems: Evaluation of Functional, Growth, and Fruit Quality Traits of Melons
Abstract
1. Introduction
2. Material and Methods
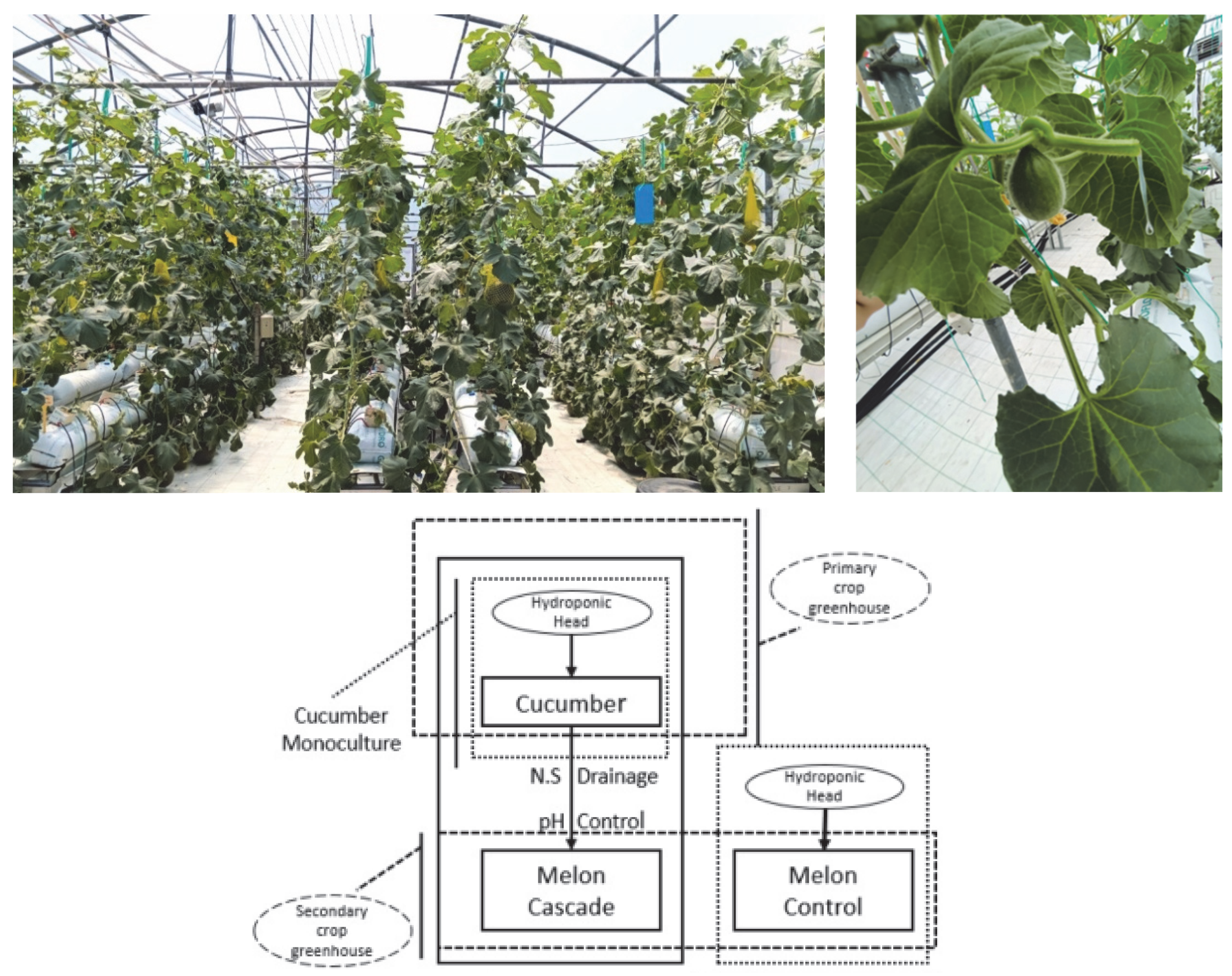
2.1. Experimental Setup
- (a)
- Control: a freshly prepared standard hydroponic solution;
- (b)
- Cascade: the drainage solution from the primary crop (cucumber), corrected for pH.
2.2. Measurements
2.2.1. Growth and Morphoanatomical Parameters
2.2.2. Physiological Parameters
2.2.3. Fruit Quality Determination
2.3. Statistical Analysis
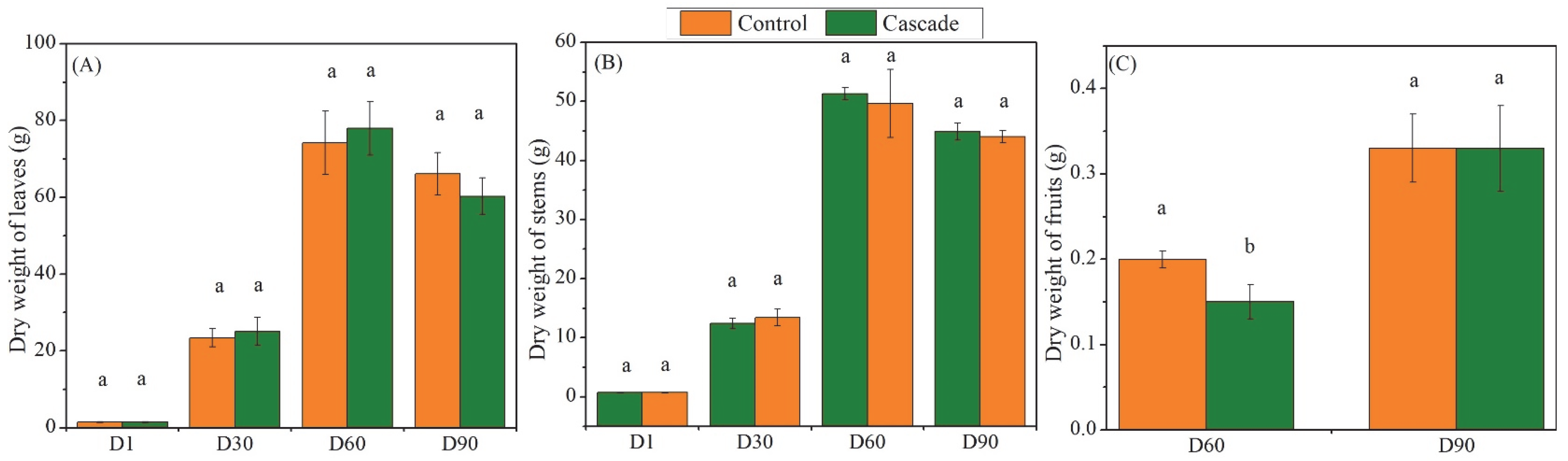
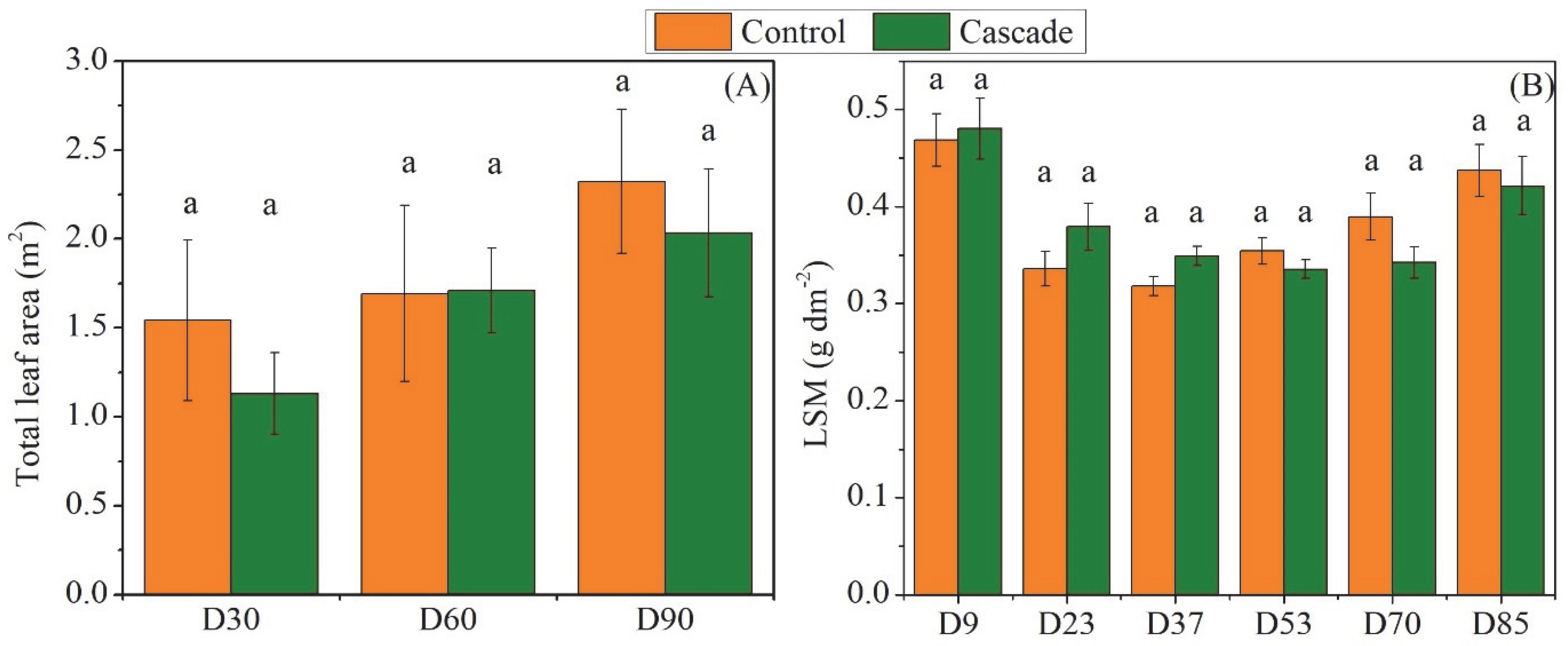
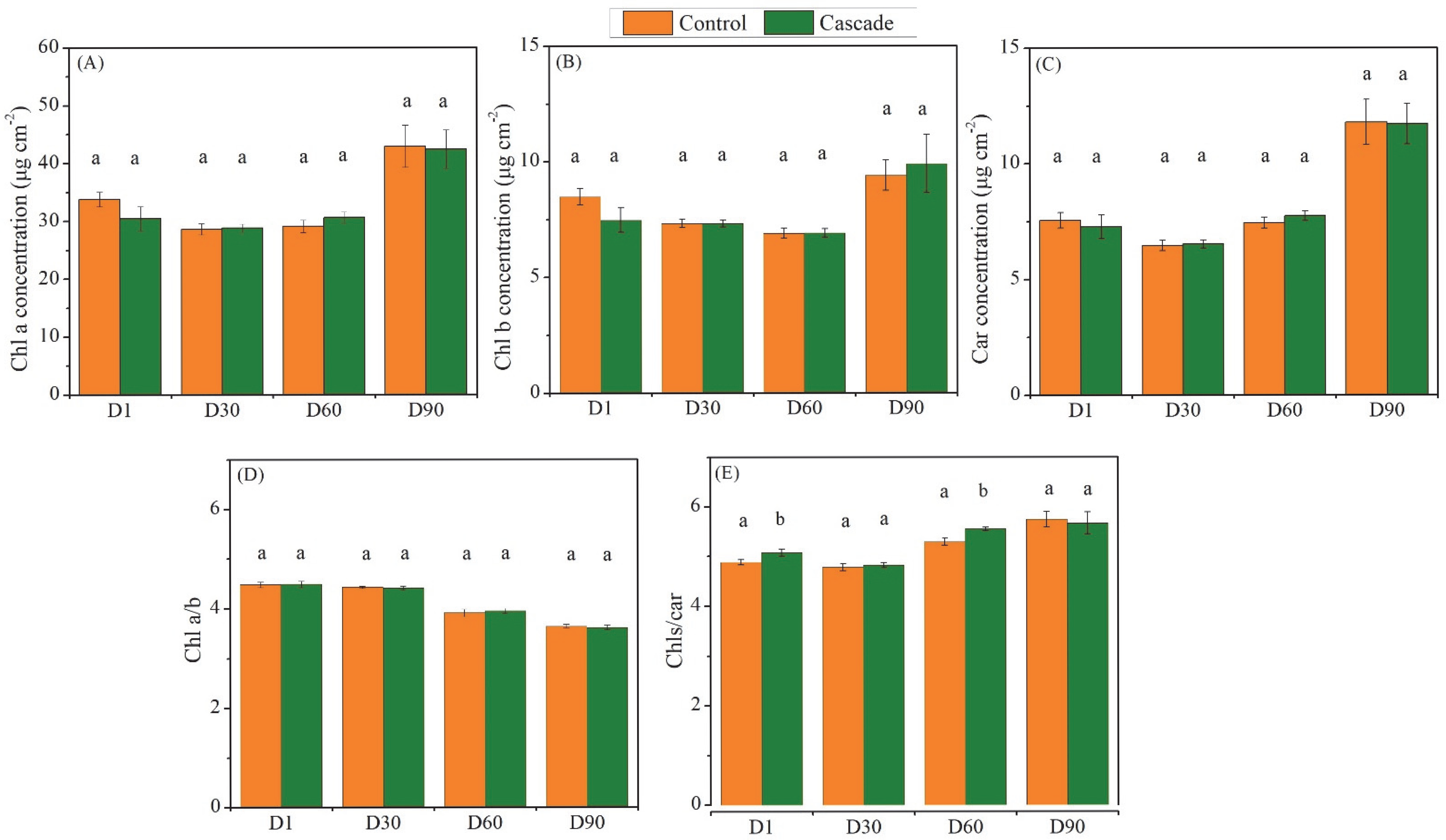
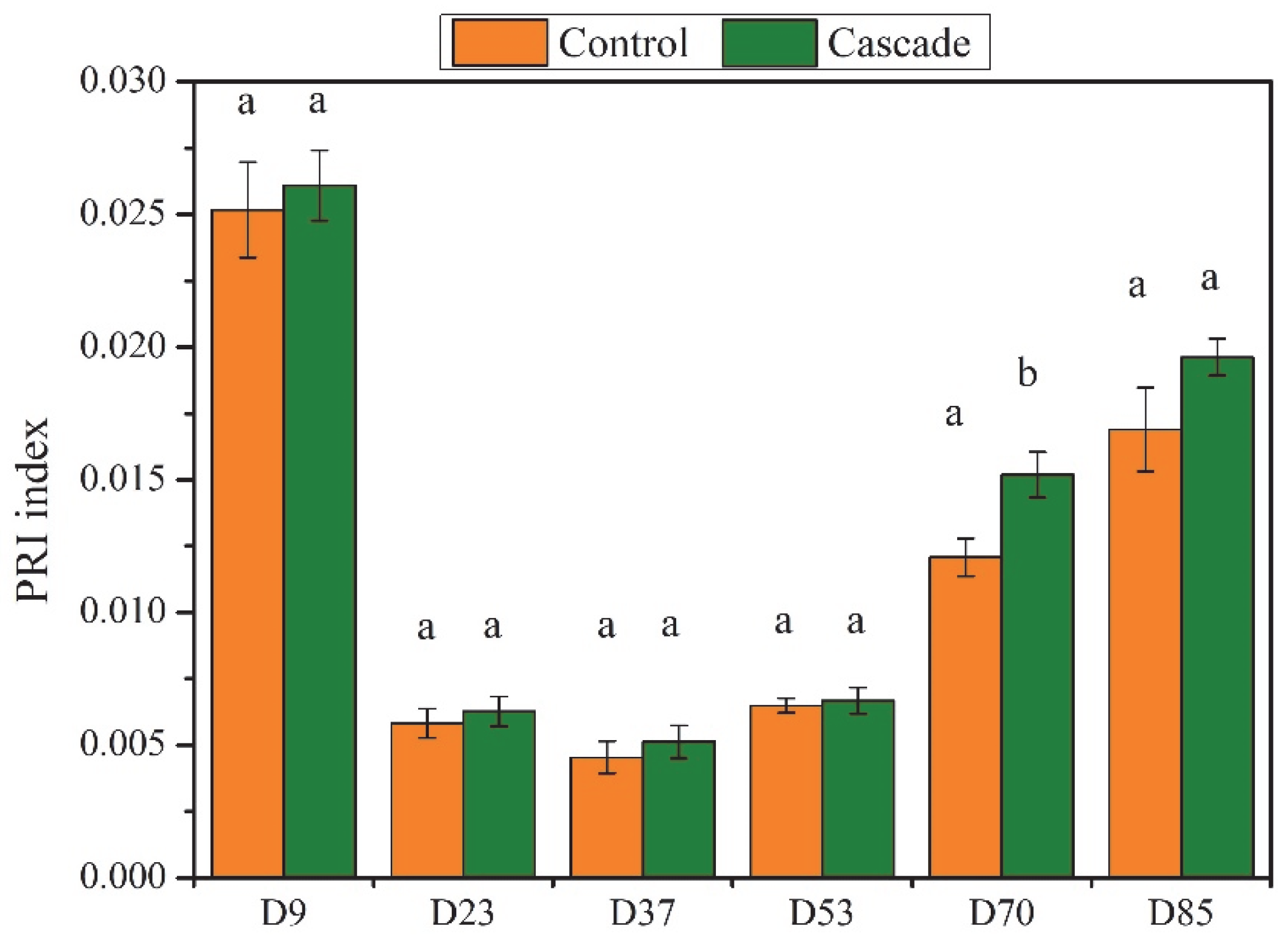
3. Results
4. Discussion
4.1. Growth and Morphoanatomical Responses
4.2. Functional Responses
4.3. Fruit Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savvas, D.; Gruda, N. Application of Soilless Culture Technologies in the Modern Greenhouse Industry—A Review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing Water and Nutrient Losses from Soilless Cropping in Southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent Advances and Perspectives in the Treatment of Hydroponic Wastewater: A Review. Rev. Environ. Sci. Biotechnol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Mielcarek, A.; Kłobukowska, K.; Rodziewicz, J.; Janczukowicz, W.; Bryszewski, K.Ł. Water Nutrient Management in Soilless Plant Cultivation versus Sustainability. Sustainability 2023, 16, 152. [Google Scholar] [CrossRef]
- Gruda, N. Do Soilless Culture Systems Have an Influence on Product Quality of Vegetables? J. Appl. Bot. Food Qual. 2009, 82, 141–147. [Google Scholar]
- Elvanidi, A.; Benitez Reascos, C.M.; Gourzoulidou, E.; Kunze, A.; Max, J.F.J.; Katsoulas, N. Implementation of the Circular Economy Concept in Greenhouse Hydroponics for Ultimate Use of Water and Nutrients. Horticulturae 2020, 6, 83. [Google Scholar] [CrossRef]
- Katsoulas, N.; Savvas, D.; Kitta, E.; Bartzanas, T.; Kittas, C. Extension and Evaluation of a Model for Automatic Drainage Solution Management in Tomato Crops Grown in Semi-Closed Hydroponic Systems. Comput. Electron. Agric. 2015, 113, 61–71. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Maksimovic, I.; Lao, M.T. Cascade Cropping System with Horticultural and Ornamental Plants under Greenhouse Conditions. Water 2018, 10, 125. [Google Scholar] [CrossRef]
- Karatsivou, E.; Elvanidi, A.; Faliagka, S.; Naounoulis, I.; Katsoulas, N. Performance Evaluation of a Cascade Cropping System. Horticulturae 2023, 9, 802. [Google Scholar] [CrossRef]
- Naounoulis, I.; Faliagka, S.; Levizou, E.; Katsoulas, N. Cascade Hydroponics Enhanced Water and Nutrients Use Efficiency in a Greenhouse Cucumber-Melon Crop Combination. Sci. Hortic. 2024, 338, 113822. [Google Scholar] [CrossRef]
- Incrocci, L.; Pardossi, A.; Malorgio, F.; Maggini, R.; Campiotti, C.A. Cascade Cropping System for Greenhouse Soilless Culture. Acta Hortic. 2003, 609, 297–300. [Google Scholar] [CrossRef]
- Muñoz, P.; Paranjpe, A.; Montero, J.I.; Antón, A. Cascade Crops: An Alternative Solution for Increasing Sustainability of Greenhouse Tomato Crops in Mediterranean Zone. Acta Hortic. 2012, 927, 801–805. [Google Scholar] [CrossRef]
- Puccinelli, M.; Galati, D.; Carmassi, G.; Rossi, L.; Pardossi, A.; Incrocci, L. Leaf Production and Quality of Sea Beet (Beta Vulgaris Subsp. Maritima) Grown with Saline Drainage Water from Recirculating Hydroponic or Aquaculture Systems. Sci. Hortic. 2023, 322, 112416. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Parada, F.; Arcas-Pilz, V.; Petit-Boix, A.; Villalba, G.; Gabarrell, X. Closed-Loop Crop Cascade to Optimize Nutrient Flows and Grow Low-Impact Vegetables in Cities. Front. Plant Sci. 2020, 11, 596550. [Google Scholar] [CrossRef]
- Avdouli, D.; Max, J.F.J.; Katsoulas, N.; Levizou, E. Basil as Secondary Crop in Cascade Hydroponics: Exploring Salinity Tolerance Limits in Terms of Growth, Amino Acid Profile, and Nutrient Composition. Horticulturae 2021, 7, 203. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of Genetic Material and Current Technologies on Product Quality of Selected Greenhouse Vegetables—A Review. Eur. J. Hortic. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Puccinelli, M.; Carmassi, G.; Pardossi, A.; Incrocci, L. Wild Edible Plant Species Grown Hydroponically with Crop Drainage Water in a Mediterranean Climate: Crop Yield, Leaf Quality, and Use of Water and Nutrients. Agric. Water Manag. 2023, 282, 108275. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Mele, M.A.; Lee, Y.-T.; Islam, M.Z. Consumer Preference, Quality, and Safety of Organic and Conventional Fresh Fruits, Vegetables, and Cereals. Foods 2021, 10, 105. [Google Scholar] [CrossRef]
- Sonneveld, C.; Donnan, R. Nutrient Solutions for Vegetables and Flowers Grown in Water or Substrates; Glasshouse Crops Research Station: London, UK, 1999; pp. 393–403. [Google Scholar]
- Rodriguez, J.C.; Shaw, N.L.; Cantliffe, D.J. Influence of Plant Density on Yield and Fruit Quality of Greenhouse-Grown Galia Muskmelons. HortTechnology 2007, 17, 580–585. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Papaioannou, C.; Katsoulas, N.; Maletsika, P.; Siomos, A.; Kittas, C. Effects of a UV-Absorbing Greenhouse Covering Film on Tomato Yield and Quality. Span. J. Agric. Res. 2012, 10, 959–966. [Google Scholar] [CrossRef]
- Claro, A.M.; Fonseca, A.; Fraga, H.; Santos, J.A. Future Agricultural Water Availability in Mediterranean Countries under Climate Change: A Systematic Review. Water 2024, 16, 2484. [Google Scholar] [CrossRef]
- Santos, M.G.; Moreira, G.S.; Pereira, R.; Carvalho, S.M.P. Assessing the Potential Use of Drainage from Open Soilless Production Systems: A Case Study from an Agronomic and Ecotoxicological Perspective. Agric. Water Manag. 2022, 273, 107906. [Google Scholar] [CrossRef]
- Colla, G.; Roupahel, Y.; Cardarelli, M.; Rea, E. Effect of Salinity on Yield, Fruit Quality, Leaf Gas Exchange, and Mineral Composition of Grafted Watermelon Plants. HortScience 2006, 41, 622–627. [Google Scholar] [CrossRef]
- Sarabi, B.; Ghashghaie, J. Evaluating the Physiological and Biochemical Responses of Melon Plants to NaCl Salinity Stress Using Supervised and Unsupervised Statistical Analysis. Plant Stress 2022, 4, 100067. [Google Scholar] [CrossRef]
- Soares, M.D.M.; Dantas, M.V.; Soares De Lima, G.; Oliveira, V.K.N.; Soares, L.A.D.A.; Gheyi, H.R.; Sousa, P.F.D.N.; De Andrade Silva, L.; Dantas Fernandes, P. Physiology and Yield of ‘Gaúcho’ Melon under Brackish Water and Salicylic Acid in Hydroponic Cultivation. Arid Land Res. Manag. 2023, 37, 134–153. [Google Scholar] [CrossRef]
- Hikmat, H.; Haghighi, M.; Eshghizadeh, H.R. Salinity Tolerance Screening in Iranian and Afghan Melons (Cucumis melon L.) Based on Several Associated Morphological and Physiological Traits. J. Agric. Sci. Technol. 2025, 27, 217–232. [Google Scholar] [CrossRef]
- Vanikiotis, T.; Stagakis, S.; Kyparissis, A. MODIS PRI Performance to Track Light Use Efficiency of a Mediterranean Coniferous Forest: Determinants, Restrictions and the Role of LUE Range. Agric. For. Meteorol. 2021, 307, 108518. [Google Scholar] [CrossRef]
- Zhang, C.; Filella, I.; Liu, D.; Ogaya, R.; Llusià, J.; Asensio, D.; Peñuelas, J. Photochemical Reflectance Index (PRI) for Detecting Responses of Diurnal and Seasonal Photosynthetic Activity to Experimental Drought and Warming in a Mediterranean Shrubland. Remote Sens. 2017, 9, 1189. [Google Scholar] [CrossRef]
- Park, E.; Luo, Y.; Marine, S.C.; Everts, K.L.; Micallef, S.A.; Bolten, S.; Stommel, J. Consumer Preference and Physicochemical Evaluation of Organically Grown Melons. Postharvest Biol. Technol. 2018, 141, 77–85. [Google Scholar] [CrossRef]
- Ajitama, T.F.; Susila, A.D.; Suwarno, W.B. The Effects of Watering Volume and Topping on the Fruit Quality of Two Melon Varieties in a Substrate Hydroponic System. J. Trop. Crop Sci. 2024, 11, 165–174. [Google Scholar] [CrossRef]
- Ben, G.O.; Kafkafi, U. Melon Fruit Quality as Affected by Timing, Duration and Concentration of Phosphate and Nitrogen Sources in Recycled Hydroponics System. J. Plant Nutr. 2002, 25, 1563–1583. [Google Scholar] [CrossRef]
- Senesi, E.; Di Cesare, L.F.; Prinzivalli, C.; Scalzo, R.L. Influence of Ripening Stage on Volatiles Composition, Physicochemical Indexes and Sensory Evaluation in Two Varieties of Muskmelon (Cucumis melo L Var Reticulatus Naud). J. Sci. Food Agric. 2005, 85, 1241–1251. [Google Scholar] [CrossRef]
- Guo, S.; Sun, H.; Zhang, H.; Liu, J.; Ren, Y.; Gong, G.; Jiao, C.; Zheng, Y.; Yang, W.; Fei, Z.; et al. Comparative Transcriptome Analysis of Cultivated and Wild Watermelon during Fruit Development. PLoS ONE 2015, 10, e0130267. [Google Scholar] [CrossRef]
- Martuscelli, M.; Di Mattia, C.; Stagnari, F.; Speca, S.; Pisante, M.; Mastrocola, D. Influence of Phosphorus Management on Melon (Cucumis Melo L.) Fruit Quality: Influence of Phosphorus Management on Melon Fruit Quality. J. Sci. Food Agric. 2016, 96, 2715–2722. [Google Scholar] [CrossRef]
- Pulela, B.L.; Maboko, M.M.; Soundy, P.; Amoo, S.O. Cultivar and Postharvest Storage Duration Influence Fruit Quality, Nutritional and Phytochemical Profiles of Soilless-Grown Cantaloupe and Honeydew Melons. Plants 2022, 11, 2136. [Google Scholar] [CrossRef]

| Parameter | Measurement Day | Control | Cascade |
|---|---|---|---|
| L* | 70 | 61.06 ± 0.94 a | 64.53 ± 2.05 a |
| 77 | 63.83 ± 1.29 a | 63.64 ±0.41 a | |
| 90 | 65.19 ± 1.44 a | 67.23 ± 0.55 a | |
| a* | 70 | −3.82 ± 0.69 a | −2.57 ± 1.33 a |
| 77 | −1.94 ± 0.38 b | −3.99 ±0.25 a | |
| 90 | 1.01 ± 0.44 a | 0.698 ± 0.47 a | |
| b* | 70 | 33.77 ± 2.23 a | 41.07 ± 3.70 a |
| 77 | 38.07 ± 2.62 a | 37.17 ± 0.60 a | |
| 90 | 41.34 ± 2.49 b | 48.29 ± 0.80 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karachaliou, Z.; Naounoulis, I.; Katsoulas, N.; Levizou, E. Cascade Hydroponics as a Means to Increase the Sustainability of Cropping Systems: Evaluation of Functional, Growth, and Fruit Quality Traits of Melons. Sustainability 2025, 17, 4527. https://doi.org/10.3390/su17104527
Karachaliou Z, Naounoulis I, Katsoulas N, Levizou E. Cascade Hydroponics as a Means to Increase the Sustainability of Cropping Systems: Evaluation of Functional, Growth, and Fruit Quality Traits of Melons. Sustainability. 2025; 17(10):4527. https://doi.org/10.3390/su17104527
Chicago/Turabian StyleKarachaliou, Zoe, Ioannis Naounoulis, Nikolaos Katsoulas, and Efi Levizou. 2025. "Cascade Hydroponics as a Means to Increase the Sustainability of Cropping Systems: Evaluation of Functional, Growth, and Fruit Quality Traits of Melons" Sustainability 17, no. 10: 4527. https://doi.org/10.3390/su17104527
APA StyleKarachaliou, Z., Naounoulis, I., Katsoulas, N., & Levizou, E. (2025). Cascade Hydroponics as a Means to Increase the Sustainability of Cropping Systems: Evaluation of Functional, Growth, and Fruit Quality Traits of Melons. Sustainability, 17(10), 4527. https://doi.org/10.3390/su17104527







