Sustainable Bioelectricity: Transformation of Chicha de Jora Waste into Renewable Energy
Abstract
1. Introduction
2. Materials and Methods
- a.
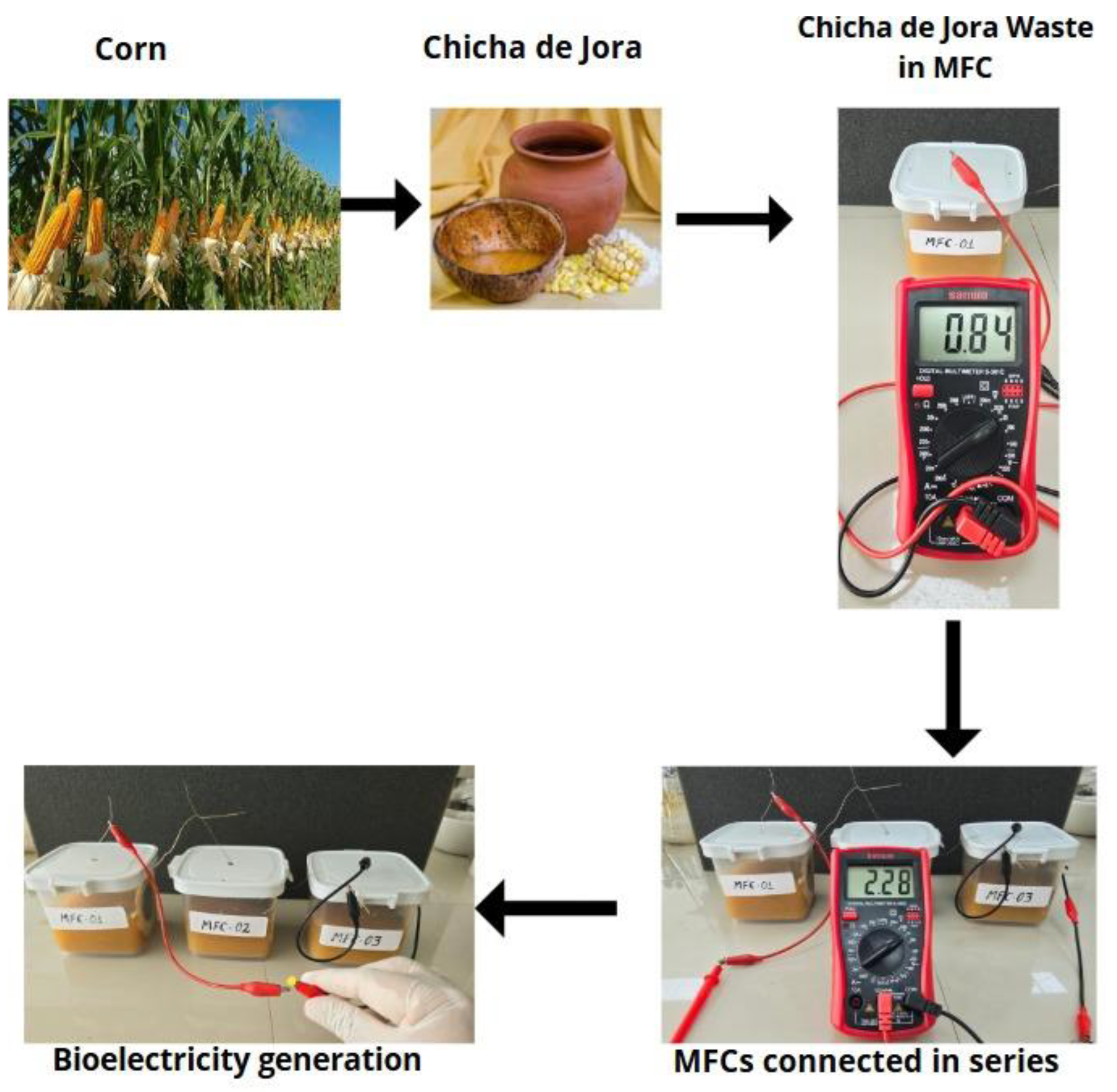
- Design and assembly of the MFCs
- b.
- Operationalization of the MFCs
- c.
- Prokaryotic community structure in chicha de jora waste residues by 16S RNA gene analysis
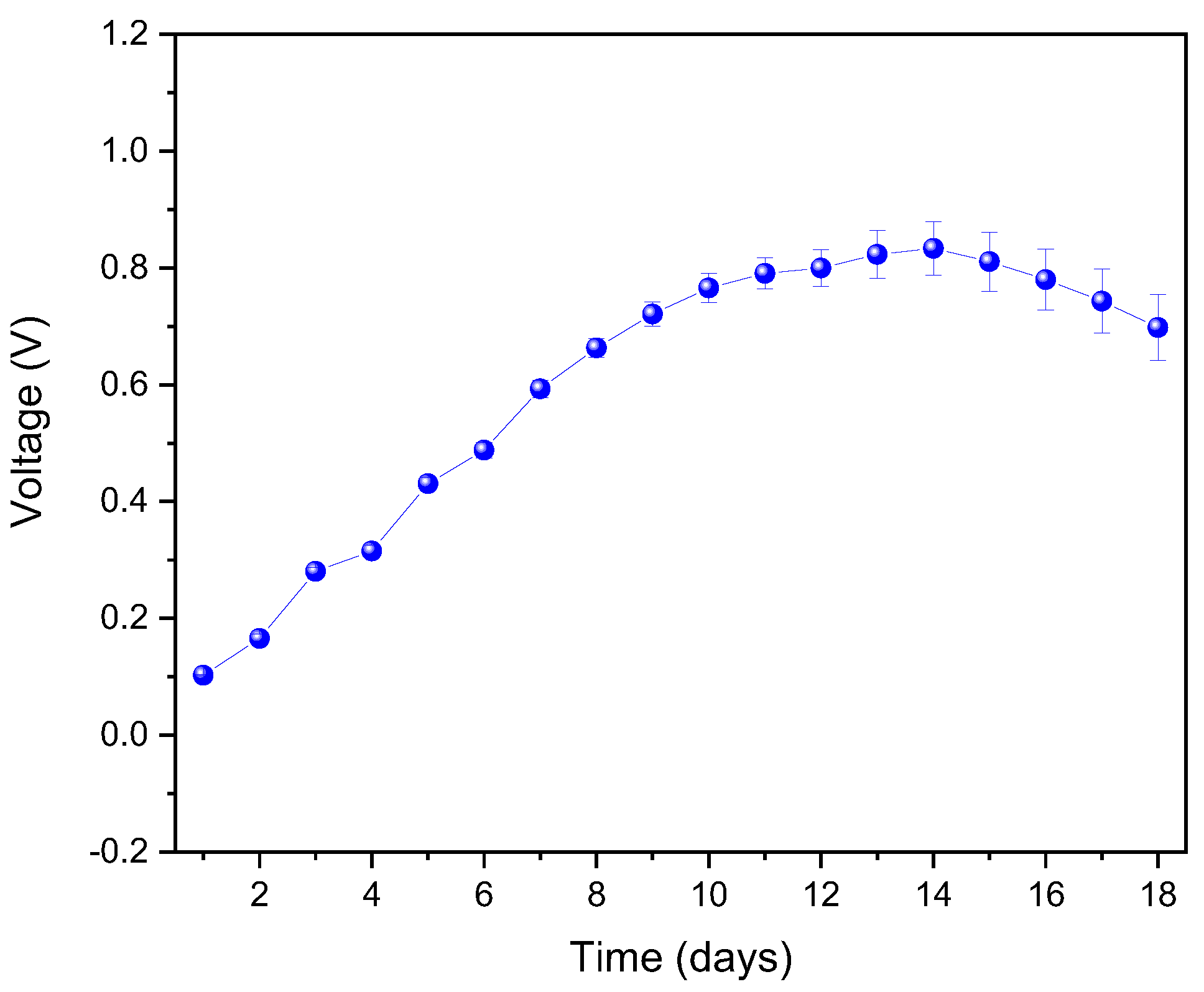
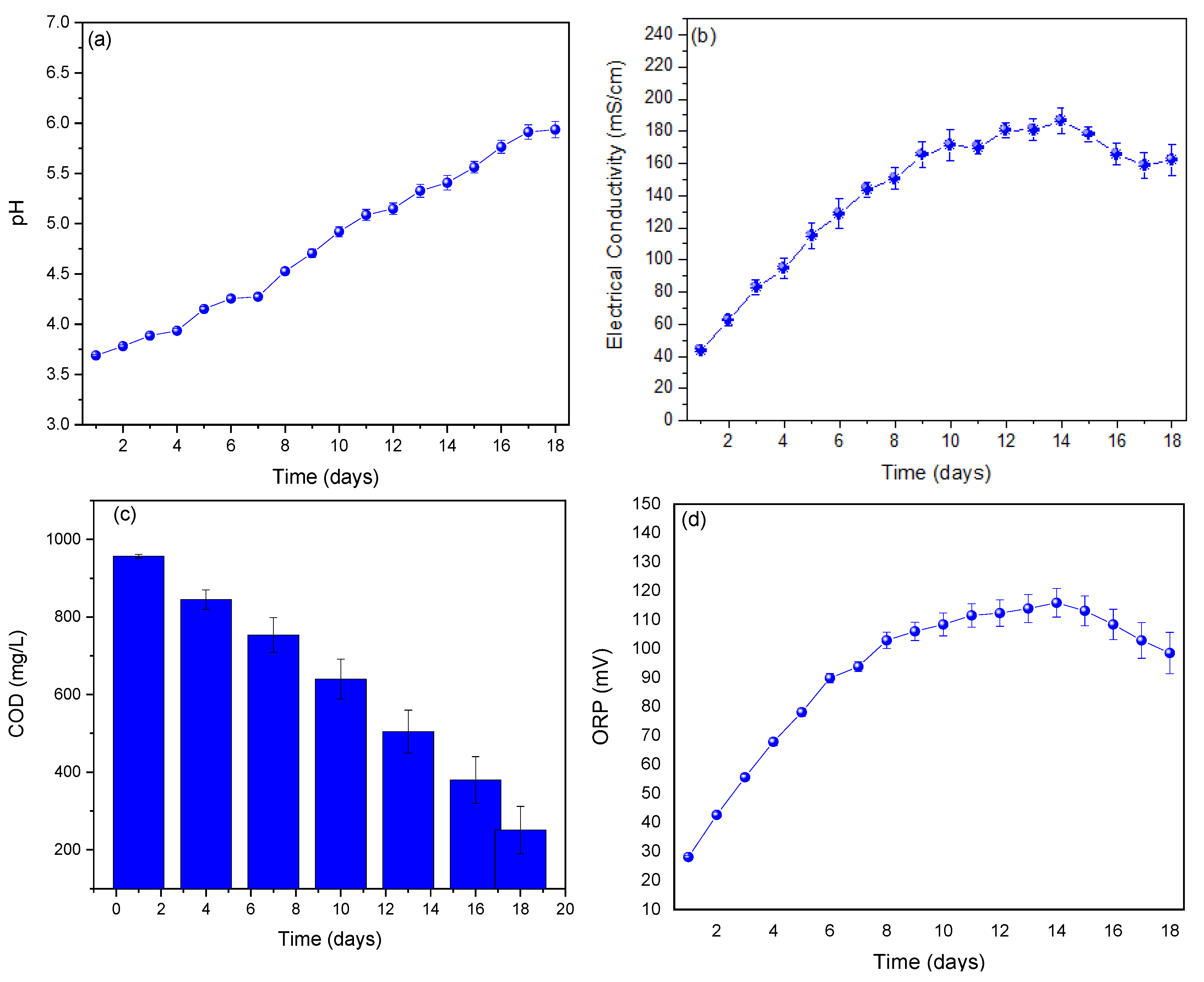
3. Results and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chong, T.Y.; Law, M.C.; Chan, Y.S. The potentials of corn waste lignocellulosic fibre as an improved reinforced bioplastic composites. J. Polym. Environ. 2021, 29, 363–381. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, W.; Ren, K.; Zhu, E.; Lu, J.; Chen, J.; Yin, P.; Yang, L.; Guan, X.; Wang, G. Electrochemical performance of corn waste derived carbon electrodes based on the intrinsic biomass properties. Materials 2023, 16, 5022. [Google Scholar] [CrossRef] [PubMed]
- Castrillón, H.D.C.; Aguilar, C.M.G.; Álvarez, B.E.A. Circular economy strategies: Use of corn waste to develop biomaterials. Sustainability 2021, 13, 8356. [Google Scholar] [CrossRef]
- Ahmad, A.M.; Ali, A.; Afzal, A.; Tanveer, N.; Mustafa, A. Corn Waste a Sustainable Solution for Plastic Pollution: A Mini Review. NUST J. Eng. Sci. 2024, 17, 17–27. [Google Scholar] [CrossRef]
- Fernandez-Fuentes, M.H.; Eras-Almeida, A.A.; Egido-Aguilera, M.A. Characterization of technological innovations in photovoltaic rural electrification, based on the experiences of Bolivia, Peru, and Argentina: Third generation solar home systems. Sustainability 2021, 13, 3032. [Google Scholar] [CrossRef]
- Campodónico, H.; Carrera, C. Energy transition and renewable energies: Challenges for Peru. Energy Policy 2022, 171, 113261. [Google Scholar] [CrossRef]
- Navarro, C.E.B.; Álvarez-Quiroz, V.J.; Sampi, J.; Sánchez, A.A.A. Does economic growth promote electric power consumption? Implications for electricity conservation, expansive, and security policies. Electr. J. 2023, 36, 107235. [Google Scholar] [CrossRef]
- Iftikhar, H.; Gonzales, S.M.; Zywiołek, J.; López-Gonzales, J.L. Electricity demand forecasting using a novel time series ensemble technique. IEEE Access 2024, 12, 88963–88975. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, P.; Dong, S.; Yu, Y.; Chen, H.; Xiao, J. Using watermelon rind and nitrite-containing wastewater for electricity production in a membraneless biocathode microbial fuel cell. J. Clean. Prod. 2021, 307, 127306. [Google Scholar] [CrossRef]
- Rios, R.; Duarte, S. Selection of ideal sites for the development of large-scale solar photovoltaic projects through Analytical Hierarchical Process–Geographic information systems (AHP-GIS) in Peru. Renew. Sustain. Energy Rev. 2021, 149, 111310. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Bioelectricity generation by microbial degradation of banana peel waste biomass in a dual-chamber S. cerevisiae-based microbial fuel cell. Biomass Bioenergy 2023, 168, 106677. [Google Scholar] [CrossRef]
- Mulyono, T.; Misto, M.; Cahyono, B.E.; Fahmidia, N.H. The impact of adding vegetable waste on the functioning of microbial fuel cell. AIP Conf. Proc. 2023, 2663, 020008. [Google Scholar]
- Liu, S.; Wang, M.; Deng, Y.; Feng, X.; Pyo, S.H. Individual and competitive removal of Cr (VI) and Cu (II) by microbial fuel cell constructed wetlands. J. Environ. Chem. Eng. 2025, 13, 115917. [Google Scholar] [CrossRef]
- Cañari Sutta, J.; Pumayali, D.V. Evaluación del Contenido de Metanol y Control de Calidad Fisicoquímica y Microbiológica de la Chicha de Jora Comercializada en el Distrito de Cusco. Master’s Thesis, Universidad Nacional de San Antonio Abad del Cusco, Cusco, Peru, 2024. [Google Scholar]
- Abanto Ruiz, A.S.; Luján Valverde, A.M. Aislamiento e Identificación de Lactobacillus spp. de Chicha de Jora y Caracterización del Potencial Probiótico In Vitro. Bachelor’s Thesis, Universidad Nacional de Trujillo, Trujillo, Peru, 2024. [Google Scholar]
- Saltos, G.J.S.; Campozano, M.R.V.; Pincay, I.R. La cosmovisión en torno al consumo de la chicha de maíz en Ecuador. Rev. Científica Multidiscip. SAPIENTIAE 2024, 7, 111–127. [Google Scholar] [CrossRef]
- Afrin, A.; Swamy, P.C.A. Symphony of light: AIE and MFC in carbazole-based cyanostilbenes. J. Mater. Chem. C 2024, 12, 1923–1944. [Google Scholar] [CrossRef]
- Idris, M.O.; Noh, N.A.M.; Ibrahim, M.N.M.; Yaqoob, A.A.; Almeer, R.; Umar, K.; Guerrero-Barajas, C. Oxidation of vegetable waste and organic pollutant degradation to generate energy through microbial fuel cell. Biomass Convers. Biorefin. 2024, 1–19. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ibrahim, M.N.M.; Umar, K.; Yaakop, A.S. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar] [CrossRef]
- Halim, M.A.; Rahman, M.O.; Eti, I.A.; Shefa, N.R.; Ibrahim, M.; Alam, M.J. Electricity generation in different cell connections with optimized anodic materials in microbial fuel cells. Energy Sources Part A Recovery Util. Environ. Eff. 2025, 47, 3330–3342. [Google Scholar] [CrossRef]
- Satpathy, S.S.; Ojha, P.C.; Ojha, R.; Dash, J.; Pradhan, D. Recent Modifications of Anode Materials and Performance Evaluation of Microbial Fuel Cells: A Brief Review. J. Energy Eng. 2025, 151, 03125001. [Google Scholar] [CrossRef]
- Hussain, F.; Al-Zaqri, N.; Adnan, A.B.M.; Hussin, M.H.; Oh, S.E.; Umar, K. Impact of bakery waste as an organic substrate on microbial fuel cell performance. Sustain. Energy Technol. Assess. 2022, 53, 102713. [Google Scholar] [CrossRef]
- Rokhim, D.A.; Vitarisma, I.Y.; Sumari, S.; Utomo, Y.; Asrori, M.R. Optimizing Household Wastes (Rice, Vegetables, and Fruit) as an Environmentally Friendly Electricity Generator. J. Renew. Mater. 2024, 12, 275–284. [Google Scholar] [CrossRef]
- Yolanda, Y.D.; Kim, S.; Sohn, W.; Shon, H.K.; Yang, E.; Lee, S. Simultaneous nutrient-abundant hydroponic wastewater treatment, direct carbon capture, and bioenergy harvesting using microalgae–microbial fuel cells. Desalination Water Treat. 2025, 321, 100941. [Google Scholar] [CrossRef]
- Noor, N.N.M.; Kim, K.; Kim, K. Utilization of tubular bamboo biochar anode with different lengths in sediment microbial fuel cells. Fuel 2025, 381, 133371. [Google Scholar] [CrossRef]
- Fadzli, F.S.; Rashid, M.; Yaqoob, A.A.; Ibrahim, M.N.M. Electricity generation and heavy metal remediation by utilizing yam (Dioscorea alata) waste in benthic microbial fuel cells (BMFCs). Biochem. Eng. J. 2021, 172, 108067. [Google Scholar] [CrossRef]
- Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G.; Tremouli, A. Effect of food waste condensate concentration on the performance of microbial fuel cells with different cathode assemblies. Sustainability 2022, 14, 2625. [Google Scholar] [CrossRef]
- Burns, M.; Qin, M. Ammonia recovery from organic nitrogen in synthetic dairy manure with a microbial fuel cell. Chemosphere 2023, 325, 138388. [Google Scholar] [CrossRef]
- Paucar, N.E.; Sato, C. Coupling microbial fuel cell and hydroponic system for electricity generation, organic removal, and nutrient recovery via plant production from wastewater. Energies 2022, 15, 9211. [Google Scholar] [CrossRef]
- Korojdeh, M.S.; Hadavifar, M.; Birjandi, N.; Mehrkhah, R.; Li, Q. Enhanced bioenergy production through dual-chamber microbial fuel cells: Utilizing citric acid factory wastewater and grape waste as substrates. J. Environ. Manag. 2024, 370, 122739. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, M.G.; Pandis, P.K.; Mamma, D.; Sourkouni, G.; Argirusis, C. Organic waste substrates for bioenergy production via microbial fuel cells: A key point review. Energies 2022, 15, 5616. [Google Scholar] [CrossRef]
- Kebaili, H.; Kameche, M.; Innocent, C.; Ziane, F.Z.; Sabeur, S.A.; Sahraoui, T.; Ouis, M.; Zerrouki, A.; Charef, M.A. Treatment of fruit waste leachate using microbial fuel cell: Preservation of agricultural environment. Acta Ecol. Sin. 2021, 41, 97–105. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Nazario-Naveda, R.; Benites, S.M.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F. Use of pineapple waste as fuel in microbial fuel cell for the generation of bioelectricity. Molecules 2022, 27, 7389. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Das, B. Performance evaluation of microbial fuel cell with sewage wastewater and RO concentrate using composite anode made of Luffa aegyptiaca. Environ. Prog. Sustain. Energy 2021, 40, e13504. [Google Scholar] [CrossRef]
- La Cruz-Noriega, D.; Nazario-Naveda, R.; Benites, S.M.; Rojas-Flores, S.; Delfín-Narciso, D.; Rojas-Villacorta, W.; Diaz, F. Potential use of mango waste and microalgae Spirulina sp. for bioelectricity generation. Environ. Res. Eng. Manag. 2022, 78, 129–136. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ng, K.H.; Papadopoulos, A.M.; Le, A.T.; Kumar, S.; Hadiyanto, H. Microbial fuel cells for bioelectricity production from waste as sustainable prospect of future energy sector. Chemosphere 2022, 287, 132285. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Peleato, N.; Roberts, D. A comparison of reactor configuration using a fruit waste fed two-stage anaerobic up-flow leachate reactor microbial fuel cell and a single-stage microbial fuel cell. Bioresour. Technol. 2023, 374, 128778. [Google Scholar] [CrossRef]
- Du, H.; Shao, Z. Synergistic effects between solid potato waste and waste activated sludge for waste-to-power conversion in microbial fuel cells. Appl. Energy 2022, 314, 118994. [Google Scholar] [CrossRef]
- Verma, M.; Singh, V.; Mishra, V. Optimization of banana peel waste based microbial fuel cells by machine learning. Biomass Convers. Biorefin. 2024, 14, 22463–22478. [Google Scholar] [CrossRef]
- Yaakop, A.S.; Ahmad, A.; Hussain, F.; Oh, S.E.; Alshammari, M.B.; Chauhan, R. Domestic Organic Waste: A Potential Source to Produce the Energy via a Single-Chamber Microbial Fuel Cell. Int. J. Chem. Eng. 2023, 2023, 2425735. [Google Scholar] [CrossRef]
- Cabrera, J.; Dai, Y.; Irfan, M.; Li, Y.; Gallo, F.; Zhang, P.; Zong, Y.; Liu, X. Novel continuous up-flow MFC for treatment of produced water: Flow rate effect, microbial community, and flow simulation. Chemosphere 2022, 289, 133186. [Google Scholar] [CrossRef]
- Huang, J.; Gao, K.; Yang, L.; Lu, Y. Successional action of Bacteroidota and Firmicutes in decomposing straw polymers in a paddy soil. Environ. Microbiome 2023, 18, 76. [Google Scholar] [CrossRef]
- Martins, G.L.; de Souza, A.J.; Mendes, L.W.; Gontijo, J.B.; Rodrigues, M.M.; Coscione, A.R.; Oliveira, F.C.; Regitano, J.B. Physicochemical and bacterial changes during composting of vegetable and animal-derived agro-industrial wastes. Bioresour. Technol. 2023, 376, 128842. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, L.; Yu, J.; Yao, Z.; Feng, J.; Wang, H.; Shen, R.; Luo, J. Novel insights from agricultural waste to acetic acid through regulated high-solids anaerobic digestion: Focus on the key microbial communities. Ind. Crops Prod. 2024, 221, 119339. [Google Scholar] [CrossRef]
- Ahmed, M.; Saini, P.; Iqbal, U. Production, Optimization, and Characterization of Bio-cellulose Produced from Komagataeibacter (Acetobacter aceti MTCC 3347) Usage of Food Sources as Media. Recent Adv. Food Nutr. Agric. 2024, 15, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Li, H.; Chen, S.; Yuan, X.; Chen, Y.; Huang, Y.; Zhou, Q.; Guan, H. Microbiome and response surface methodology analyses reveal Acetobacter pasteurianus as the core bacteria responsible for aerobic spoilage of corn silage (Zea mays) in hot and humid areas. Front. Microbiol. 2024, 15, 1473238. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segundo, R.-F.; Luis, C.-C.; Otiniano, N.M.; De La Cruz-Noriega, M. Sustainable Bioelectricity: Transformation of Chicha de Jora Waste into Renewable Energy. Sustainability 2025, 17, 4499. https://doi.org/10.3390/su17104499
Segundo R-F, Luis C-C, Otiniano NM, De La Cruz-Noriega M. Sustainable Bioelectricity: Transformation of Chicha de Jora Waste into Renewable Energy. Sustainability. 2025; 17(10):4499. https://doi.org/10.3390/su17104499
Chicago/Turabian StyleSegundo, Rojas-Flores, Cabanillas-Chirinos Luis, Nélida Milly Otiniano, and Magaly De La Cruz-Noriega. 2025. "Sustainable Bioelectricity: Transformation of Chicha de Jora Waste into Renewable Energy" Sustainability 17, no. 10: 4499. https://doi.org/10.3390/su17104499
APA StyleSegundo, R.-F., Luis, C.-C., Otiniano, N. M., & De La Cruz-Noriega, M. (2025). Sustainable Bioelectricity: Transformation of Chicha de Jora Waste into Renewable Energy. Sustainability, 17(10), 4499. https://doi.org/10.3390/su17104499





