Abstract
Asphalt binder flue gas emits irritating odors and poses health and environmental hazards. To promote the sustainable development of asphalt pavement construction, this study investigates the flue gas odor-reducing effectiveness and performance impacts of deodorizing materials on asphalt binders. A specialized asphalt binder flue gas collection device was designed, coupled with an evaluation protocol tailored for asphalt deodorants, to systematically evaluate the odor-reducing effectiveness of materials B and C on both 70# pure asphalt binder and SBS-modified asphalt binder. Finally, the impacts of two kinds of deodorizing materials on the high-temperature and low-temperature performance of different asphalt binders were discussed. The results show that the odor-reducing effectiveness of tert-butyl-co-aldehyde-acting material C on odorous volatile organic compounds (VOCs), hydrogen sulfide (H2S), and carbon monoxide (CO) in 70# pure asphalt binder flue gas is the best, reaching 66.7%, 49.1%, and 44.0%, respectively. The odor-reducing effectiveness of conjugated double bonds and aldehyde group synergistic material B on odorous VOCs, H2S, and CO in SBS-modified asphalt binder is the best, reaching 78.7%, 52.9%, and 51.0%, respectively. Both materials B and C can improve the high-temperature deformation resistance of 70# pure asphalt binder and SBS-modified asphalt binder. The anti-cracking properties of SBS-modified asphalt binder at low temperatures were improved to some extent by materials B and C, but the anti-cracking properties of 70# pure asphalt binder at low temperatures were not good. These asphalt binders all meet the specification requirements of a stiffness modulus not more than 300 MPa and a creep rate not less than 0.3.
1. Introduction
Asphalt pavement is widely adopted in domestic highways and urban road construction owing to its excellent flatness and driving comfort [1]. During the production, mixing, and paving processes, asphalt flue gas is generated under high temperatures due to the volatilization of light components, the decomposition of macromolecular substances, and interactions between certain molecules. Furthermore, operational factors such as temperature, mixing rate, and duration significantly influence the release of asphalt flue gas.
Due to the complexity of asphalt binder composition, the resulting asphalt binder flue gas is a mixed flue gas containing VOCs, nitrogen oxides, sulfides, and solid particles. VOCs in asphalt binder flue gas can be classified into alkanes, alkenes, benzenes, and so on. Low-molecular-weight saturated fractions in asphalt binders readily volatilize during high-temperature processing operations, generating alkanes. Olefins are produced by oxidation or the pyrolysis of alkanes in asphalt binder saturates and the decomposition of unsaturated structures in resins at high temperatures. Benzenes are alkane-substituted benzene or thick aromatic derivatives, which mainly come from the pyrolysis or oxidation reactions of asphalt binder aromatics. Aldehydes are mainly formed by oxidation of unsaturated aliphatic acids or oxygen-containing heteroatomic compounds in resins or asphaltenes. Phenols are derived from the joint action of modifier and asphalt binder resins. Esters are mainly derived from the cracking of resins or the use of additives. Alcohols mainly come from the oxidation products of saturated alkanes or the products after hydrolysis of the esters in resins.
Inorganic components such as H2S and CO are detectable within asphalt binder flue gas emissions. Mercaptan and disulfide in asphalt resins and thiophene heterocyclic sulfide in asphaltene can generate H2S under high-temperature operations. The long-chain alkanes in the saturates of asphalt binders and the polyaromatic hydrocarbons in aromatics are partially oxidized under the conditions of high temperatures and anoxic gas, so as to produce CO. The incorporation of SBS modifiers induces the adsorption of partial resins in asphalt onto SBS phase domains, thereby promoting the thermal decomposition of sulfur-containing compounds and hydrocarbons enriched at the resin/SBS interfaces. At the same time, the pyrolysis product of the butadiene chain segment in the SBS polymer at high temperature accelerates the pyrolysis of sulfur-containing compounds and the oxidation of hydrocarbons, thus promoting the generation of H2S and CO [2,3,4,5].
Asphalt binder flue gas has a strong pungent odor, which causes nausea, headaches, and other symptoms through the nasal cavity. Long-term exposure can lead to physical impairment and, more seriously, cancer [6,7]. At the same time, asphalt binder flue gas can pollute the environment, such as air, water, and soil, thus aggravating ecological imbalance and environmental problems. Based on the sustainable development strategy, the state has gradually increased its attention to pollution prevention and control.
In order to inhibit the release of flue gas from the source, scholars at home and abroad have developed different flue gas suppressors or flavor cleansers to reduce or eliminate the odors and harmful gas components in asphalt binder flue gas. At present, commonly used flue gas suppressants are adsorption materials, flame retardant materials, nanomaterials, and negative ion materials. Wang et al. concluded that the combination of diatomite and modified attapulgite could reduce the total volatile organic compound release in the flue gas of 70# pure asphalt binder by 81.5%, improving its high-temperature performance but slightly reducing its fatigue resistance [8]. Li et al. concluded that the mixture of kaolin and sepiolite can reduce the VOC concentration in rubberized asphalt binder flue gas by 42.26% and the H2S concentration by 65.28%, improving its deformation resistance while reducing its fatigue resistance [9]. Sharma et al. significantly inhibited the emission of volatile organic compounds by preparing calcium hydroxide–zeolite nanocomposite flue gas suppressor [10]. Xie et al. reduced the average concentrations of benzothiazole, cyclohexanethiol, and H2S in rubber-powder-modified asphalt binder flue gas by 60.4%, 58.3%, and 44.5%, respectively, through biological desulfurization of waste rubber [11]. Guo et al. showed that the combination of graphene and tourmaline can reduce the flue gas of 70# pure asphalt binder by 83% [12]. Chen et al. combined activated carbon and surfactant to effectively inhibit the release of VOCs in the flue gas of 70# pure asphalt binder and improve the deformation resistance of asphalt binder at the same time, but this has a certain negative impact on the low-temperature performance of asphalt binder [13]. The use of some flue gas suppressors has a certain negative impact on asphalt binder properties.
The deodorizing material can achieve the effect of degradation through a chemical reaction with the asphalt binder flue gas or reduce the odor emission by physical concealment. By combining aldehydes with carboxylate compounds and an active agent, Guo et al. showed that high-boiling-point ester compounds could reduce VOCs and benzene homologues in SBS-modified asphalt binder flue gas by 41.7% and 50%, respectively, while magnesium silica compounds could reduce VOCs and benzene homologue emissions in SBS-modified asphalt binder flue gas by 36% and 42%, respectively [14]. The European company Shell has developed an active additive as a deodorizing material to add to asphalt binders, and the chemical reaction with the asphalt binder flue gas effectively degrades the asphalt binder flue gas, inhibits the production of odor gas, and has no impact on the performance of the asphalt binder. Cao et al. used alcohol deodorants to inhibit the release of benzene series substances and H2S in the flue gas modified by waste rubber powder [15]. Li et al. developed a deodorant containing terpinol and nitrogen-containing organic matter, which can effectively reduce the H2S emission concentration in the flue gas of rubber-modified asphalt binder [16]. Yeong et al. reduced VOC emissions in asphalt binder flue gas by 34% by adding recycled plastics [17]. Lv et al. concluded that the combination of liquid deodorant and powder deodorant could reduce the total concentration of VOCs in the flue gas of rubber-modified asphalt binder by 46.7% [18]. Therefore, the preparation and application of flavor cleanser can not only reduce the harm to the health of workers and residents but also reduce the environmental load and pollution, which is of great significance for the sustainable development of road construction and maintenance.
Although domestic research on asphalt binder flue gas has made some progress, the existing research mainly focuses on the odor analysis and control of the flue gas components of one kind of asphalt binder, and there is a lack of in-depth research on odor analysis of the organic and inorganic components in different types of asphalt binder flue gas. At present, there are few test methods that can simultaneously detect and analyze the organic and inorganic components and their content in asphalt binder flue gas. There is a lack of methods to evaluate the odor-reducing effectiveness of flavor cleanser on different types of asphalt binder flue gas, and the odor-reducing effectiveness of flavor cleanser on asphalt binder properties should be considered.
Therefore, this paper first proposed a bituminous flue gas collection device combining an enrichment detection method and direct detection method to analyze the composition of different bituminous flue gas. The organic components in the flue gas were collected by cyclohexane solution, and the composition and relative contents of VOCs were analyzed by gas chromatography–mass spectrometry (GC-MS). The inorganic components and average release concentration were analyzed by six-in-one gas detection. Then, the odor-reducing effectiveness of the two kinds of deodorizing materials on the odorous VOCs and inorganic components in asphalt binder flue gas was evaluated by the change in the relative content of twenty-two kinds of main odorous VOCs and the change in the average concentration of H2S and CO and compared with the finished deodorizing materials on the market. Finally, the impact of two kinds of deodorizing materials on the properties of different asphalt binders were discussed.
2. Materials and Methods
2.1. Materials
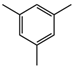
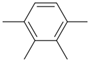
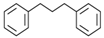
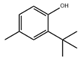
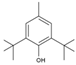
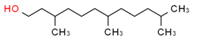
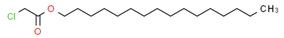
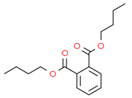
Material A is a light yellow oily chemical flavor cleanser on the market, material B and material C are chemical substances containing a benzene ring and aldehyde group, and their aroma can effectively cover the irritating odor of asphalt binder flue gas. At the same time, the carbonyl group (C=O) has strong electrophilicity and can react with asphalt binder flue gas to reduce the volatilization of irritating odor components. Conjugated double bonds exist in material B, while material C contains tert-butyl groups. The basic indicators are shown in Table 1.

Table 1.
Basic indexes of deodorizing materials.
Two asphalt binders selected in this study were 70# pure asphalt binder and SBS-modified asphalt binder (denoted as 70#, SBS), and their basic indexes are shown in Table 2.

Table 2.
Basic performance index of asphalt binder.
2.2. Preparation of Deodorized Asphalt Binder
Place 150 g of asphalt binder into a beaker and perform the following steps:
(1) Put it into a constant temperature oven (temperature for 70# pure asphalt binder is 145 °C and for SBS-modified asphalt binder is 165 °C) and heat it to a flow state.
(2) Perform oil bath heating (temperature for 70# pure asphalt binder is 165 °C; SBS-modified asphalt binder was prepared at 175 °C) with a mass ratio of 0.06% to add the deodorizing material and stir it at a rate of 800 r/min for 60 min.
(3) After constant temperature development at 135 °C for 30 min, the bubbles can be removed.
2.3. Test Method
2.3.1. Asphalt Binder Flue Gas Collection Method
The collection of asphalt binder flue gas is a combination of the enrichment detection method and direct detection method. The organic components in asphalt binder flue gas are easily dissolved in organic solvents to collect VOC components [8,9,15], and the inorganic components are collected by six-in-one gas detection. Based on the integration of multiple gas sensors, the six-in-one gas detection can detect the gas concentration in real time [19,20]. The measuring range of detectable inorganic components is shown in Table 3. Before conducting the asphalt fume collection test, 70# pure asphalt binder and SBS-modified asphalt binder had undergone the same heating and mixing processes as the deodorized asphalt binders.

Table 3.
Range of six-in-one gas detection.
The self-made asphalt binder flue gas collection device, as shown in Figure 1, consists of a magnetic stirrer, a PTFE filter membrane (0.3 µm), a three-neck flask, a sampling pump, six-in-one gas detection, a silicone plug, and a glass catheter. Since the test needs to be heated to 180 °C, glass catheters and silicone plugs are used. At the same time, glass tubes and silicone plugs are used to connect each part to ensure good air tightness. The organic solvent was collected by cyclohexane, the magnetic stirring speed was 100 r/min, the sampling pump flow rate was 2.5 L/min, and the flue gas collection time was 1 h. In order to ensure the accuracy and stability of the asphalt binder flue gas collection device, three identical tests were carried out on the same asphalt binder, and the difference in concentration values of each component measured by six-in-one gas detection was less than 5%.
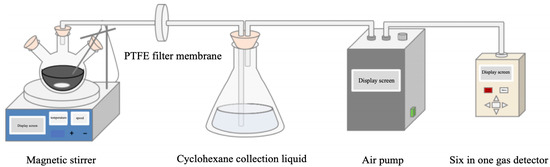
Figure 1.
Asphalt binder flue gas collection device.
2.3.2. Gas Chromatography–Mass Spectrometry
GC-MS is an instrument that combines the characteristics of gas chromatography and mass spectrometry. By using GCMS-QP2010SE manufactured by Shimadzu Corporation (Kyoto, Japan) to detect flue gas samples collected from cyclohexane solution, the influence of different deodorizing materials on organic components in asphalt binder flue gas can be analyzed [21,22,23,24,25].
Gas chromatographic condition parameters are as follows:
- Types of chromatography columns: Agilent222-5532LTM capillary column manufactured by Agilent Technologies (Santa Clara, CA, USA).
- Carrier gas: 99.9% pure helium.
- Flow rate: 2.0 mL/min.
- Shunt ratio: 2:1.
- Inlet temperature: 250 °C.
- Injection volume: 2 µL.
- The initial temperature was 72 °C for 4 min, and then the rate rose to 280 °C at 8 °C/min for 2 min.
Mass spectrometry analysis condition parameters are as follows:
- Transmission line temperature: 250 °C.
- Ion source temperature: 230 °C.
- Solvent delay time is 2 min.
- Ionization method: electron bombardment (EI) with an ionization energy of 70 eV.
- Quality scan range (m/z): 30 to 1000 amu.
2.3.3. High-Temperature Performance Test
The complex modulus G* and phase angle δ of asphalt material were measured by the temperature sweep mode of a dynamic shear rheometer (DSR), and the high-temperature properties of different deodorized asphalt binders at different temperatures were analyzed. The SHRP specification defines G*/sinδ as the rutting factor, which is used to characterize the high-temperature deformation resistance of asphalt binder materials.
Temperature sweep test parameters are as follows:
- Temperature range: 46~82 °C.
- Heating rate: 6 °C/min.
- Frequency: 10 rad/s.
- Strain: 1.5%.
- Diameter of the fixture: 25 mm.
- Spacing of the fixture: 1 mm.
2.3.4. Low-Temperature Performance Test
Sui et al. pioneered a methodology utilizing a dynamic shear rheometer (DSR) to assess the low-temperature performance of asphalt binders, demonstrating strong linear correlations between DSR-derived parameters and bending beam rheometer (BBR) test data [26,27]. Therefore, this experiment uses the data measured by frequency sweep mode of DSR to obtain the stiffness modulus (S) and creep rate (m) through the equivalent conversion method proposed by Hajj et al., so as to study the low-temperature cracking resistance of deodorized asphalt binders [28].
Frequency sweep test parameters are as follows:
- Temperatures: −6 °C, −12 °C, and −18 °C.
- Angular frequency range: 0.2~100 rad/s.
- Strain: 0.05%.
- Diameter of the fixture: 4 mm.
- Spacing of the fixture: 2 mm.
3. Results and Discussion
3.1. Composition Analysis of Different Asphalt Binder Flue Gas
Table 4 shows the composition of VOCs in the flue gas of 70# pure asphalt binder and SBS-modified asphalt binder, where “−” indicates that no VOCs are detected. The VOCs produced by 70# pure asphalt binder and SBS-modified asphalt binder during the heating process are 30 and 36 kinds, respectively, indicating the complexity and diversity of the components of asphalt binder flue gas. Due to the use of modifiers, the composition of fumes produced by SBS-modified asphalt binder under high-temperature operations is more complex than that of 70# pure asphalt binder. In this regard, VOCs in asphalt fumes are classified into alkanes, olefins, benzenes, aldehydes, phenols, alcohols, and esters, with alkanes being the primary volatile organic compounds in the fumes of both 70# pure asphalt binder and SBS-modified asphalt binder, accounting for 69% and 68%, respectively. This is because the low-molecular-weight saturates in asphalt have low boiling points, and the binding capacity of asphaltenes and resins for light components is weak, making them prone to volatilization and forming alkanes under high-temperature conditions.

Table 4.
Total VOC composition and peak area of flue gas from 70# pure asphalt binder and SBS-modified asphalt binder (106).
The most abundant alkane organic components in asphalt binder flue gas have no obvious odor and are non-toxic. Olefin organic components have a slight special smell, and although they are non-toxic, long-term exposure will cause slight adverse reactions in the body, while benzene, aldehydes, phenols, alcohols, and esters and other organic components have a strong irritating smell and have certain toxicity, causing serious harm to human health. Therefore, twenty-two kinds of odorous VOCs were selected from the VOC components detected in the flue gas of 70# pure asphalt binder and SBS-modified asphalt binder, and their relative content changes were analyzed to evaluate the odor-reducing effectiveness of the deodorizing materials on organic components in the flue gas. The odorous VOCs are shown in Table 5 below.

Table 5.
Composition of odorous VOCs in the flue gas of asphalt binders.
At the same time, two inorganic components, H2S and CO, were mainly detected in the flue gas of 70# pure asphalt binder and SBS-modified asphalt binder. H2S has a strong smell of rotten eggs and is highly toxic. CO is colorless and odorless but is toxic to all body tissues and cells. Therefore, the average concentration changes in H2S and CO were analyzed to evaluate the odor-reducing effectiveness of the deodorizing material on the inorganic components in the asphalt binder flue gas.
3.2. Evaluation of Odor-Reducing Effectiveness
3.2.1. Organic Component Analysis
Figure 2 shows the chromatograms of VOCs in the flue gas of 70# pure asphalt binder, SBS-modified asphalt binder, and deodorized asphalt binder with materials A, B, and C, respectively. A chromatographic peak corresponds to an organic component, and the area of the chromatographic peak indicates the relative content of the organic component.

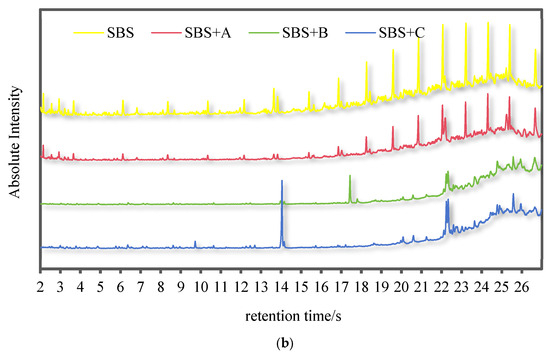
Figure 2.
Chromatograms of VOCs in the flue gas of 70# pure asphalt binder, SBS-modified asphalt binder, and deodorized asphalt binder with materials A, B, and C, respectively: (a) 70# pure asphalt binder; (b) SBS-modified asphalt binder.
As can be seen from Figure 2a, a large amount of VOCs escape from 70# pure asphalt binder during heating. After adding three kinds of deodorizing materials, the content of organic components in 70# pure asphalt binder flue gas decreased to different degrees.
As can be seen from Figure 2b, SBS-modified asphalt binder flue gas contains a large number of VOCs and more components than 70# pure asphalt binder flue gas. This is because the polymer structure of SBS-modified asphalt binder is unstable in the heating process, resulting in more organic components in the flue gas. After the addition of three deodorizing materials, the types and relative contents of organic components in SBS-modified asphalt binder flue gas were significantly reduced.
Figure 3 shows the relative content comparison of the odorous VOCs in the flue gas of 70# pure asphalt binder and its three kinds of deodorized asphalt binders.
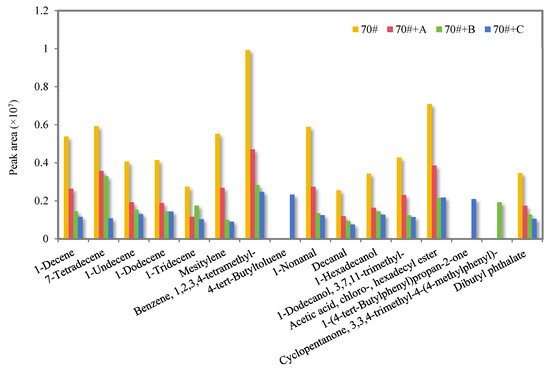
Figure 3.
The peak area of odorous VOCs in the flue gas of 70# pure asphalt binder and its three kinds of deodorized asphalt binders.
As can be seen from Figure 3, the content of most odorous VOCs in the deodorized asphalt binder flue gas with materials B and C added is less than 70# pure asphalt binder, indicating that both deodorizing materials can inhibit the release of odorous VOCs in the flue gas or transform them into other substances, and the odor-reducing effectiveness is significantly better than that of finished deodorizing material A. The odor-reducing effectiveness is particularly obvious on the odorous VOCs from the aromatics in the flue gas.
Materials B and C, which contain highly reactive aldehyde groups, can undergo substitution and other chemical reactions with unsaturated components in asphalt, especially aromatics, during thermal processing. Deodorizing material B contains conjugated double bonds, so that it has strong electrophilicity, and the additional reaction with unsaturated components in asphalt binder can effectively reduce the formation of small- and medium-sized molecules during the heating process of asphalt binders, thus inhibiting the release of organic matter in the flue gas. Deodorizing material C contains tert-butyl, which has high stability and as a three-dimensional substituent can form a significant steric hindrance, slow volatilization, and inhibit the release of some organic matter in the flue gas. At the same time, deodorizing materials B and C react with the asphalt binder flue gas components to produce a small number of new substances.
Figure 4 shows the relative content comparison of the odorous VOCs in the flue gas of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders.
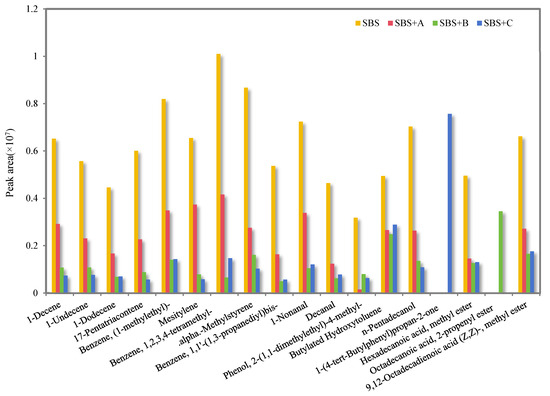
Figure 4.
The peak area of odorous VOCs in the flue gas of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders.
As can be seen from Figure 4, deodorizing materials B and C can inhibit the release of most odorous VOCs in SBS-modified asphalt binder VOC flue gas or convert them into other substances, and the odor-reducing effectiveness is significantly better than that of finished deodorizing material A. This is because the aldehyde groups in deodorizing materials B and C can combine with the styrene free radicals and butadiene free radicals generated by breaking the SBS modifier at high temperatures to form more stable compounds, and the conjugated double bonds of material B can also reduce the pyrolysis of styrene and butadiene through an additional reaction. Because of the large volume structure, the tert-butyl group of material C can reduce the contact between the SBS chain segment and oxygen, thus delaying the release of flue gas. At the same time, deodorizing materials B and C react with the asphalt binder flue gas components to produce a small number of new substances.
In order to visually analyze the odor-reducing effectiveness of the three deodorizing materials on the total release of odorous VOCs in the 70# pure asphalt binder and SBS-modified asphalt binder flue gas, the relative content of odorous VOCs is shown in Figure 5.
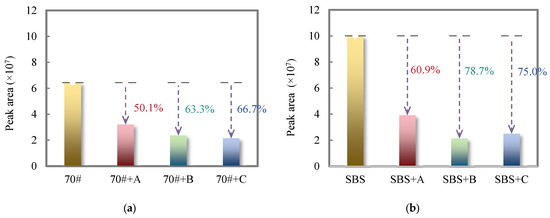
Figure 5.
Total peak area of odorous VOCs in the flue gas of 70# pure asphalt binder, SBS-modified asphalt binder, and their deodorized asphalt binders: (a) 70# pure asphalt binder; (b) SBS-modified asphalt binder.
From Figure 5a, it can be seen that the total relative content of odorous VOCs in the flue gas of the three deodorized asphalt binders is significantly reduced compared with the 70# pure asphalt binder. The total peak area of odor components in the flue gas of 70# pure asphalt binder is about 6.44 × 107, while the odor components in the flue gas of the three kinds of deodorized asphalt binder added with deodorizing materials A, B, and C are reduced by 50.1%, 63.3%, and 66.7%, respectively. These results indicated that the two deodorizing materials had significantly better inhibition effectiveness on odorous VOCs in 70# pure asphalt binder flue gas than the finished product, and the deodorizing material C had relatively good odor-reducing effectiveness.
As can be seen from Figure 5b, the two deodorizing materials B and C can inhibit the release of odorous VOCs in SBS-modified asphalt binder flue gas, and the odor-reducing effectiveness of deodorizing material B is relatively good, reaching 78.7%.
3.2.2. Inorganic Component Analysis
Figure 6 shows the change in the average release concentration of inorganic components in the flue gas of 70# pure asphalt binder and its three kinds of deodorized asphalt binders detected by six-in-one gas detection.
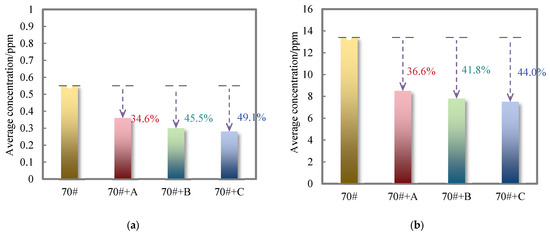
Figure 6.
The average release concentration of inorganic components in the flue gas of 70# pure asphalt binder and its three kinds of deodorized asphalt binders: (a) H2S; (b) CO.
As can be seen from Figure 6, the average concentration of H2S and CO in the flue gas of 70# pure asphalt binder is 0.55 ppm and 13.4 ppm, respectively. The average concentration of H2S in the flue gas of the three deodorized asphalt binders added with odor removal materials A, B, and C is reduced by 34.6%, 45.5%, and 49.1%, respectively. At the same time, the average concentration of CO was reduced by 36.6%, 41.8%, and 44.0%, respectively, indicating that the two deodorizing materials B and C can reduce the H2S and CO release concentration in 70# pure asphalt binder flue gas, and the inhibition effectiveness is better than that of deodorizing material A.
Aldehyde groups in deodorizing materials B and C can react with sulfur-containing organic matter in the unsaturated fraction of asphalt binder to inhibit the formation of H2S, and aldehyde groups can react with alkane free radicals to inhibit the formation of CO. The conjugated double bonds in material B can react with sulfur-containing organic compounds to form more stable compounds to inhibit the formation of H2S, while the additional reaction with alkyl radicals inhibits the formation of CO. The tert-butyl group with significant spatial resistance in material C can combine with the unsaturated fraction in asphalt binder to form a dense structure, thus inhibiting the diffusion and volatilization of H2S and other small sulfur molecules and the formation of CO. Comparing the odor-reducing effectiveness of two deodorizing materials on H2S and CO concentration reduction in 70# pure asphalt binder flue gas, the odor-reducing effectiveness of deodorizing material C is relatively good.
The average release concentration of inorganic components in the flue gas of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders are shown in Figure 7.
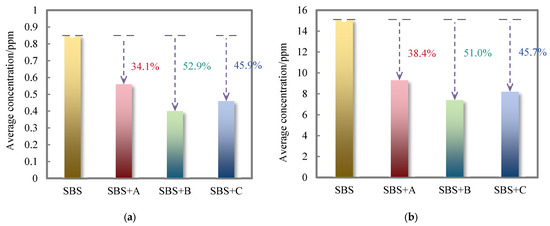
Figure 7.
The average release concentration of inorganic components in the flue gas of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders: (a) H2S; (b) CO.
As can be seen from Figure 7, both deodorizing materials B and C can reduce the release concentration of H2S and CO in SBS-modified asphalt binder flue gas, and the inhibition effectiveness is better than that of finished material A. The conjugated double bonds and tert-butyl groups in deodorizing materials B and C can coordinate the reaction of aldehyde groups with the sulfonated aldehydes and hydrocarbons at the interface of resins and SBS to inhibit the formation of H2S and react with the butadiene free radicals generated after the pyrolysis of SBS modifier to prevent further oxidation to CO. Comparing the odor-reducing effectiveness of the two deodorizing materials on H2S and CO concentration reduction in SBS-modified asphalt binder flue gas, deodorizing material B has relatively good odor-reducing effectiveness, and the reduction rate of H2S and CO reached 52.9% and 50.1%, respectively.
3.3. Performance Impact Analysis
3.3.1. High-Temperature Performance
In order to explore the effect of three deodorizing materials on the high-temperature performance of 70# pure asphalt binder, DSR was used to conduct temperature scanning tests on 70# pure asphalt binder and three deodorized asphalt binders. The complex modulus G* and phase angle δ of the four asphalt binders were obtained, and the rutting factor (G*/sinδ) was further calculated. Figure 8 shows the change in rutting factor G*/sinδ of 70# pure asphalt binder and its three kinds of deodorized asphalt binders.

Figure 8.
G*/sinδ of 70# pure asphalt binder and its three kinds of deodorized asphalt binders.
In Figure 8, G*/sinδ of the four types of asphalt binders all decrease with the increase in temperature, and the asphalt binder changes from elasticity to viscosity, gradually becomes soft, and the rutting resistance decreases. At the same time, compared with 70# pure asphalt binder, the G*/sinδ coefficients of the three kinds of deodorized asphalt binders increased, indicating that the addition of the three kinds of deodorized materials improved the high-temperature deformation resistance of 70# pure asphalt binder. This is because the aldehyde groups in the two deodorizing materials may react with active groups in asphalt binders to forming a partially cross-linked structure that inhibits the flowability of 70# pure asphalt binders at high temperatures. The increase in the G*/sinδ of the three kinds of deodorized asphalt binders gradually decreased.
It can be seen from Figure 8 that at 64 °C, the G*/sinδ of 70# pure asphalt binder is 1.27 kPa, and the G*/sinδ of 70# pure asphalt binder with deodorizing materials A, B, and C increases by 21.9%, 59.6%, and 128.7%, respectively. This shows that deodorizing material C can better improve the high-temperature rutting resistance of 70# pure asphalt binder, because the tert-butyl groups in material C, which exhibit excellent steric hindrance, synergize more effectively with the aldehyde groups to stabilize the aromatic fractions.
The G*/sinδ of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders are shown in Figure 9.
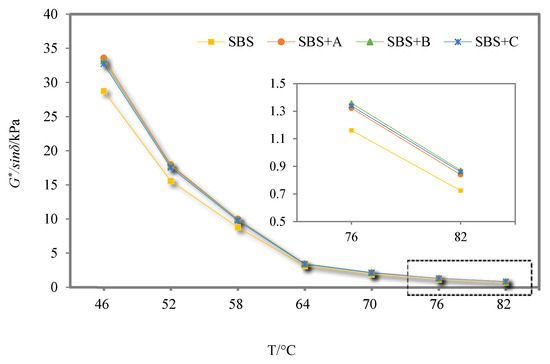
Figure 9.
G*/sinδ of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders.
In Figure 9, the G*/sinδ of the four kinds of asphalt binders all show a decreasing trend with the increase in temperature, which means that the asphalt binder may gradually increase viscosity, decrease elasticity, and decrease deformation resistance due to the increase in temperature. Compared with SBS-modified asphalt binder, the G*/sinδ coefficients of the three deodorized asphalt binders increased slightly, indicating that the addition of the three deodorizing materials improved the high-temperature deformation resistance of SBS-modified asphalt binder, but the increase in the G*/sinδ of the three deodorized asphalt binders gradually decreased with the increase in temperature.
It can be seen from Figure 9 that at 76 °C, the G*/sinδ of SBS-modified asphalt binder is 1.16 kPa, and the G*/sinδ of SBS-modified asphalt binder with deodorizing materials A, B, and C increases by 1.38%, 1.70%, and 1.60%, respectively. This shows that material B can better improve the high-temperature rutting resistance of SBS-modified asphalt binder, because material B, which combines conjugated double bonds with aldehyde groups, exhibits better compatibility with the SBS modifier. The synergistic interaction between material B and the SBS modifier enhances the dispersion of resins and asphaltenes’ aggregates.
3.3.2. Low-Temperature Performance
The values of stiffness modulus (S) and creep rate (m) represent the deformation resistance and relaxation ability of asphalt binder, respectively. The higher the S value is, the greater the stress caused by pavement temperature contraction and the more cracks are generated. The lower the m value, the more likely the asphalt binder is to crack at low temperature. The SHRP specification requires S value to be no more than 300 MPa and m value to be no less than 0.3. The S and m values of 70# pure asphalt binder and its three kinds of deodorized asphalt binders are shown in Figure 10.
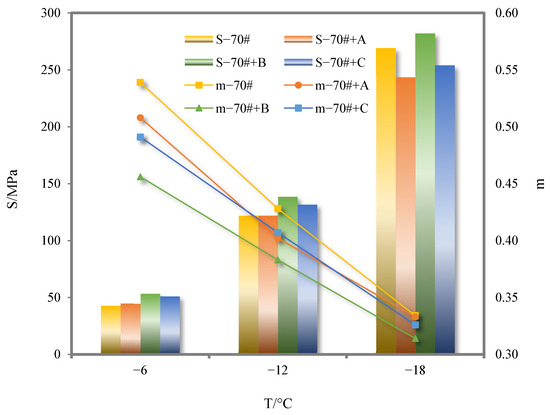
Figure 10.
S and m values of 70# pure asphalt binder and its three kinds of deodorized asphalt binders.
As can be seen from Figure 10, when the temperature drops from −6 °C to −18 °C, the S and m values of 70# pure asphalt binder and its three deodorized asphalt binders always meet the requirements of the specification. With the decrease in temperature, the S and m value of 70# pure asphalt binder and its three deodorized asphalt binders gradually increase and decrease, and the ductility of the asphalt binder decreases, which leads to the decrease in the low-temperature cracking resistance of the asphalt binder. After the addition of the three deodorizing materials, the S value of 70# pure asphalt binder is slightly increased, and the m value is decreased. Only at −18 °C, the S value of the deodorized asphalt binder with the addition of pure flavor material C is 5.62% lower than that of 70# pure asphalt binder, indicating that the three deodorizing materials all reduce the ductility of 70# pure asphalt binder. The low-temperature cracking resistance of 70# pure asphalt binder is adversely affected.
In order to make a more reasonable evaluation, Liu et al. proposed using the low-temperature index ratio m/S to evaluate the low-temperature performance of the asphalt binder [29]. The greater the m/S value, the better the low-temperature performance of thee asphalt binder. The m/S values of 70# pure asphalt binder and its three kinds of deodorized asphalt binders are shown in Figure 11.
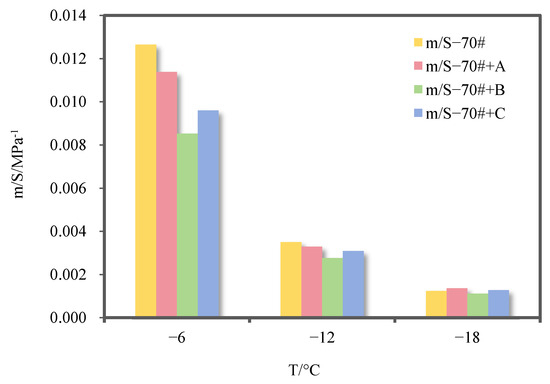
Figure 11.
m/S values of 70# pure asphalt binder and its three kinds of deodorized asphalt binders.
As can be seen from Figure 11, deodorizing material B has the most serious negative impact on the low-temperature cracking resistance of 70# pure asphalt binder. Deodorizing material C and finished material A decrease the m/S value of 70# pure asphalt binder at −6 °C and −12 °C, thus adversely affecting its low-temperature performance, while it increases slightly at −18 °C. This is because the partially cross-linked structure formed by the reaction between aldehyde groups in the two deodorizing materials and active groups in asphalt binders restricts the flexibility of molecular chains, reducing stress relaxation capacity at low temperatures and increasing brittleness. Taking the temperature of −12 °C as an example, the m/S values of 70# pure asphalt binder with materials A, B, and C added decreased by 6.1%, 21.2%, and 11.9%, respectively. Finished material A had the least negative impact on the low-temperature cracking resistance of 70# pure asphalt binder, followed by material C.
The S and m values of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders are shown in Figure 12.
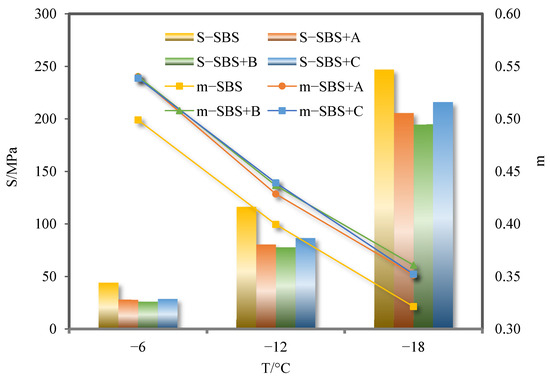
Figure 12.
S and m values of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders.
As can be seen from Figure 12, when the temperature drops from −6 °C to −18 °C, the S and m values of SBS-modified asphalt binder and its three deodorized asphalt binders always meet the requirements of the specification. As can be seen from Figure 12, with the decrease in temperature, the S value of SBS-modified asphalt binder increases while the m value decreases, asphalt binder ductility decreases, and anti-cracking performance at low temperature deteriorates. After the addition of three deodorizing materials, the S value of SBS-modified asphalt binder decreased and the m value increased, which indicates that the three deodorizing materials can improve the low-temperature cracking resistance of SBS-modified asphalt binder.
The m/S values of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders at different temperatures are shown in Figure 13.
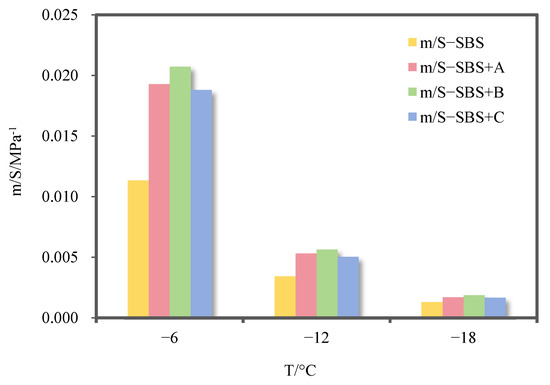
Figure 13.
m/S values of SBS-modified asphalt binder and its three kinds of deodorized asphalt binders.
It can be seen from Figure 13 that the m/S value of SBS-modified asphalt binder is increased after the addition of three deodorizing materials, which indicates that the three deodorizing materials can improve the low-temperature cracking resistance of SBS-modified asphalt binder. This is because the aldehyde groups and aromatic ring structures in the two deodorizing materials can enhance the compatibility between the SBS modifier and aromatics in the asphalt binder while reducing the ability of the SBS network to capture saturated components. Taking −12 °C as an example, deodorizing materials A, B, and C increased the m/S value of SBS-modified asphalt binder by 54.9%, 64.1%, and 46.7%, respectively, indicating that deodorizing material B can better improve the low-temperature cracking resistance of SBS-modified asphalt binder while having the best odor-reducing effectiveness.
4. Conclusions and Future Prospects
4.1. Conclusions
In this paper, organic and inorganic components in the flue gas of 70# pure asphalt binder and SBS-modified asphalt binder were investigated, and a comprehensive evaluation system for VOCs/inorganic gas was established according to odor differences. The odor-reducing effectiveness of the two deodorizing materials B and C on two kinds of asphalt binder flue gas was evaluated by the change in the relative content of the odorous VOCs and the concentration of inorganic components, and the differential odor-reducing effectiveness of two deodorizing materials on the asphalt binder properties was investigated. The results show the following:
- (1)
- The aldehyde groups in the deodorizing materials B and C can react with the asphalt binder flue gas to produce new substances to reduce the volatilization of the irritating odor components. Meanwhile, material B can adsorb free radicals through conjugated double bonds and reduce the pyrolysis, thereby inhibiting the release of organic flue gas with the aldehyde groups. The tert-butyl co-acting material C has the best odor-reducing effectiveness on odorous VOCs, H2S, and CO in 70# pure asphalt binder flue gas, which is up to 66.7%, 49.1%, and 44.0%, respectively. The conjugated double bond co-acting material B has the best odor-reducing effectiveness on odorous VOCs, H2S, and CO in SBS-modified asphalt binder. The results were 78.7%, 52.9%, and 51.0%, respectively, which were better than finished material A.
- (2)
- The two deodorizing materials improved high-temperature deformation resistance of 70# pure asphalt binder and SBS-modified asphalt binder. Deodorizing material C better improves the G*/sinδ of 70# pure asphalt binder at 64 °C by 128.7%, and deodorizing material B better improves the G*/sinδ of SBS-modified asphalt binder at 76 °C by 1.7%.
- (3)
- The two deodorizing materials improved the low-temperature cracking resistance of SBS-modified asphalt binder to a certain extent but have a negative impact on the low-temperature cracking resistance of 70# pure asphalt binder. Deodorizing material B can better improve the m/S value of SBS-modified asphalt binder at −12 °C by 64.1%. The negative impact of deodorizing material C on the low-temperature cracking resistance of 70# pure asphalt binder is relatively small, and the m/S value of 70# pure asphalt binder at −12 °C is reduced by 11.9%, but all meet the specification requirements of an S value not more than 300 MPa and an m value not less than 0.3.
This study proves that the two functional base deodorizing materials can realize the synergistic control of 70# pure asphalt and SBS-modified asphalt binder flue gas deodorized and asphalt binder properties, which provides a new choice for the development of environmentally friendly road materials. This is conducive to the sustainable development of green roads.
4.2. Future Prospects
- (1)
- The road performance of the two types of asphalt binder before and after adding deodorizing materials requires further investigation.
- (2)
- To mitigate the negative impact on the low-temperature performance of 70# pure asphalt binder, a composite design incorporating deodorizing materials B and C with other functional group materials should be considered.
- (3)
- It is necessary to combine Fourier-transform infrared (FTIR) spectroscopy tests, four-component analysis experiments, and fluorescence microscopy tests to elucidate the interaction mechanisms between the deodorizing materials and asphalt binders.
Author Contributions
All authors contributed to this study’s conception and design. M.G. (Meng Guo), L.W. and Y.F. were responsible for material preparation, data collection, and analysis; L.W. wrote the paper; M.G. (Meng Guo) supervised the research; and M.G. (Mingyang Guan) revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (U24A20198, 52478429).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the editor and anonymous reviewers for their numerous constructive comments and encouragement that have improved this paper greatly.
Conflicts of Interest
Author Mingyang Guan was employed by the company Chongqing Municipal Facilities Operation Guarantee Center. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Guo, M.; Zhang, R.; Du, X.; Liu, P. A State-of-the-Art Review on the Functionality of Ultra-Thin Overlays towards a Future Low Carbon Road Maintenance. Engineering 2024, 32, 82–98. [Google Scholar] [CrossRef]
- Espinoza, J.; Medina, C.; Calabi-Floody, A.; Sánchez-Alonso, E.; Valdés, G.; Quiroz, A. Evaluation of Reductions in Fume Emissions (VOCs and SVOCs) From Warm Mix Asphalt Incorporating Natural Zeolite and Reclaimed Asphalt Pavement for Sustainable Pavements. Sustainability 2020, 12, 9546. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Rong, H.; Meng, Y.; Liu, X.; Liu, Y.; Peng, C. Review of fume-generation mechanism, test methods, and fume suppressants of asphalt materials. J. Clean. Prod. 2022, 347, 131240. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Nian, T.; Mao, Y. An overview of studies on the hazards, component analysis and suppression of fumes in asphalt and asphalt mixtures. Constr. Build. Mater. 2021, 289, 123185. [Google Scholar] [CrossRef]
- Chang, X.; Long, Y.; Wang, C.; Xiao, Y. Chemical fingerprinting of volatile organic compounds from asphalt binder for quantitative detection. Constr. Build. Mater. 2023, 371, 130766. [Google Scholar] [CrossRef]
- Mundt, K.A.; Dell, L.D.; Crawford, L.; Sax, S.N.; Boffetta, P. Cancer risk associated with exposure to bitumen and bitumen fumes: An updated systematic review and meta-analysis. J. Occup. Environ. Med. 2017, 60, 6–54. [Google Scholar] [CrossRef]
- Kriech, A.J.; Schreiner, C.A.; Osborn, L.V.; Riley, A.J. Assessing cancer hazards of bitumen emissions—A case study for complex petroleum substances. Crit. Rev. Toxicol. 2017, 48, 121–142. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Q.; Wu, S.; Lv, Y.; Wan, P.; Gong, X.; Liu, G. Study of synergistic effect of diatomite and modified attapulgite on reducing asphalt volatile organic compounds emission. Constr. Build. Mater. 2023, 400, 132827. [Google Scholar] [CrossRef]
- Li, S.; Liu, Q.; Wang, H.; Wang, J.; He, L.; Wu, S. Effect of kaolin and sepiolite on fume emissions of rubber modified asphalt. Constr. Build. Mater. 2024, 416, 135276. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, B. A novel nanocomposite of Ca(OH)2-incorporated zeolite as an additive to reduce atmospheric emissions of PM and VOCs during asphalt production. Environ. Sci. Nano 2017, 4, 613–624. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.; Liu, Y.; Ge, D.; Wang, S.; Ding, Z.; Lv, S. Microbial treatment of waste crumb rubber: Reducing energy consumption and harmful emissions during asphalt production process. J. Clean. Prod. 2024, 464, 142778. [Google Scholar] [CrossRef]
- Guo, T.; Fu, H.; Wang, C.; Chen, H.; Chen, Q.; Wang, Q.; Chen, Y.; Li, Z.; Chen, A. Road Performance and Emission Reduction Effect of Graphene/Tourmaline-Composite-Modified Asphalt. Sustainability 2021, 13, 8932. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Li, Q.; Zhang, W.; Yan, C. The Investigation of Volatile Organic Compounds (VOCs) Emissions in Environmentally Friendly Modified Asphalt. Polymers 2022, 14, 3459. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Sun, L.; Xu, X.; Zhang, H. Characterization of fume suppression effect and performance of SBS-modified asphalt with deodorant. Processes 2024, 12, 2603. [Google Scholar] [CrossRef]
- Cao, L.; Yang, C.; Li, A.; Wang, P.; Zhang, Y.; Dong, Z. Flue gas composition of waste rubber modified asphalt (WRMA) and effect of deodorants on hazardous constituents and WRMA. J. Hazard. Mater. 2021, 403, 123814. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Zhou, T.; Cao, L.; Ding, X.; Zhang, B.; Wei, L. Performance evaluation and multiscale investigation of deodorant on mechanism of rubber modified asphalt. J. Clean. Prod. 2023, 425, 138954. [Google Scholar] [CrossRef]
- Boom, Y.J.; Enfrin, M.; Xuan, D.L.; Grist, S.; Robert, D.; Giustozzi, F. Laboratory evaluation of PAH and VOC emission from plastic-modified asphalt. J. Clean. Prod. 2022, 377, 134489. [Google Scholar] [CrossRef]
- Lv, Y.; Wua, S.; Li, N.; Liu, Q.; Yang, C.; Zou, Y.; Amirkhanian, S. Flue gas suppression and environmental evaluation of deodorizer-modified rubber asphalt based on radar method. Constr. Build. Mater. 2024, 411, 134526. [Google Scholar] [CrossRef]
- Sun, X.; Qin, X.; Liu, Z.; Yin, Y.; Jiang, S.; Wang, X. Applying feasibility analysis and catalytic purifying potential of novel modifying agent used in asphalt pavement materials. Constr. Build. Mater. 2020, 245, 118467. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, T.; Zhao, P.; Luo, G.; Deng, Y. Effect of odour-less additive on asphalt flue gas emission and performance of asphalt and asphalt mixture. Case Stud. Constr. Mater. 2024, 20, e03006. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, P.; Xu, R.; Liang, X.; Xiao, X.; Shi, L.; Lv, J. Influence of smoke suppressant on the smoke inhibition effect and properties of different types of asphalt. Front. Mater. 2023, 10, 1205050. [Google Scholar] [CrossRef]
- Meng, Y.; Fang, G.; Hu, Y.; Qin, Y.; Xu, R.; Yang, F.; Lei, J.; Zhang, C. Study on the effect of different aldehyde modifiers on the fume suppression effect, mechanism and road performance of SBS modified asphalt. Sci. Total Environ. 2024, 912, 169162. [Google Scholar] [CrossRef] [PubMed]
- Boom, Y.J.; Enfrin, M.; Grist, S.; Giustozzi, F. Recycled plastic modified bitumen: Evaluation of VOCs and PAHs from laboratory generated fumes. Sci. Total Environ. 2022, 832, 155037. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, T.; Cao, L.; Zhou, J.; Liu, Z.; Dong, Z. Characterization of emissions from rubber modified asphalt and their impact on environmental burden: Insights into composition variability and hazard assessment. J. Hazard. Mater. 2024, 477, 135336. [Google Scholar] [CrossRef]
- Janhäll, S.; Strandberg, B.; Wallqvist, V.; Rissler, J. A new method and first results for comparing emissions of fumes during construction of asphalt surfaces. Constr. Build. Mater. 2024, 422, 135736. [Google Scholar] [CrossRef]
- Sui, C.; Farrar, M.J.; Tuminello, W.H.; Turner, T.F. New Technique for Measuring Low-Temperature Properties of Asphalt Binders with Small Amounts of Material. Transp. Res. Rec. J. Transp. Res. Board 2010, 2179, 23–28. [Google Scholar] [CrossRef]
- Wang, D.; Falchetto, A.C.; Poulikakos, L.; Hofko, B.; Porot, L. RILEM TC 252-CMB report: Rheological modeling of asphalt binder under different short and long-term aging temperatures. Mater. Struct. 2019, 52, 73. [Google Scholar] [CrossRef]
- Hajj, R.; Filonzi, A.; Rahman, S.; Bhasin, A. Considerations for using the 4 mm Plate Geometry in the Dynamic Shear Rheometer for Low Temperature Evaluation of Asphalt Binders. Transp. Res. Rec. J. Transp. Res. Board 2019, 2673, 649–659. [Google Scholar] [CrossRef]
- Liu, S.T.; Cao, W.D.; Shang, S.J.; Qi, H.S.; Fang, J.G. Analysis and application of relationships between low-temperature rheological performance parameters of asphalt binders. Constr. Build. Mater. 2010, 24, 471–478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).