Aerated Compost Tea Did Not Promote Cu Downward Transfer but Increased Cu Phytoavailability in a Vineyard Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Aerated Compost Tea
2.3. Preparation and Addition of 65Cu-Enriched Solution to the Soil
2.4. Experimental Setup
2.5. Plant Sampling and Analyses
2.6. Soil Extraction and Analyses
2.7. Copper Isotope Measurement
2.8. Data Treatment and Statistical Analyses
3. Results
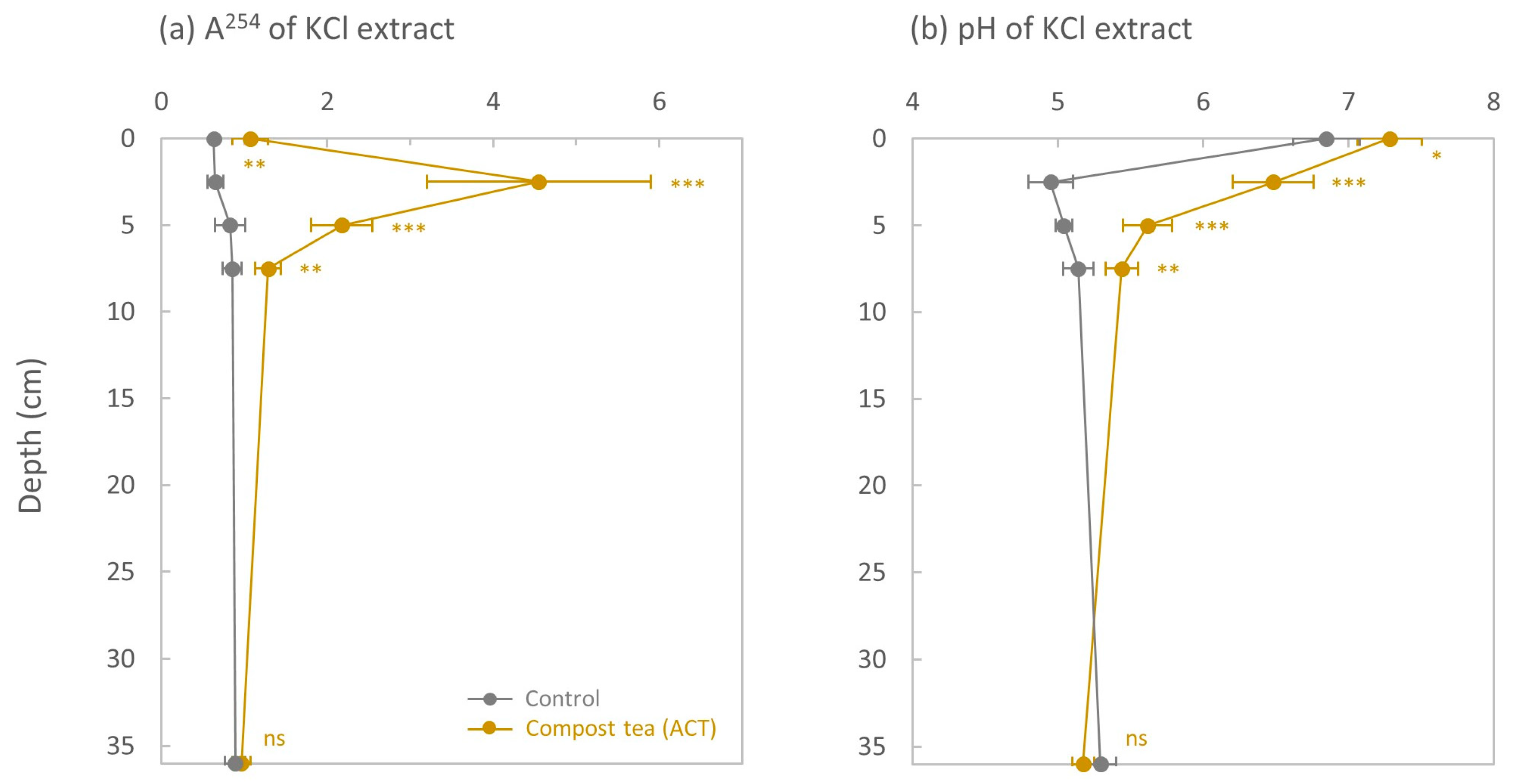
3.1. Absorbance at 254 nm and pH of the KCl Extract
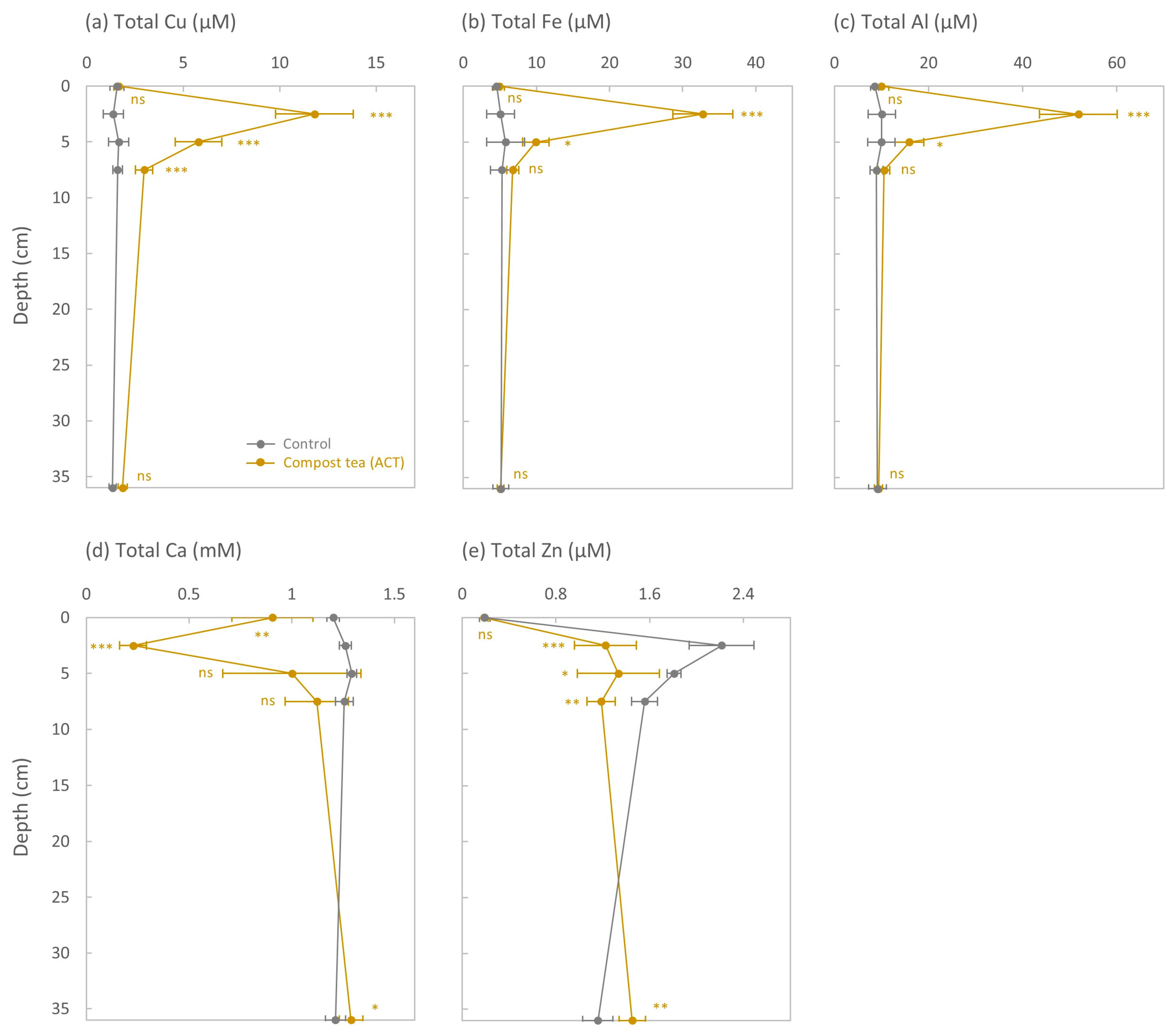
3.2. Total Concentrations of Cu, Fe, and Al in the KCl Extract
3.3. Total Concentrations of Ca and Zn in the KCl Extract
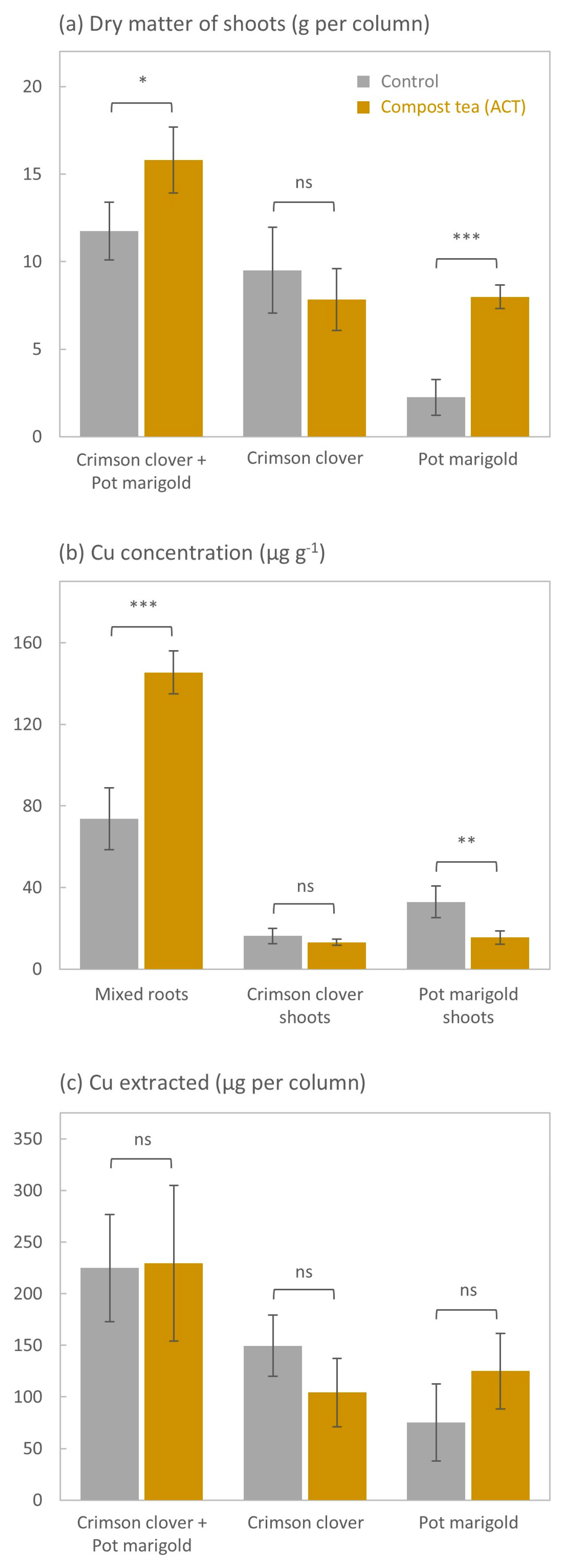
3.4. Plant Growth
3.5. Plant Elemental Composition
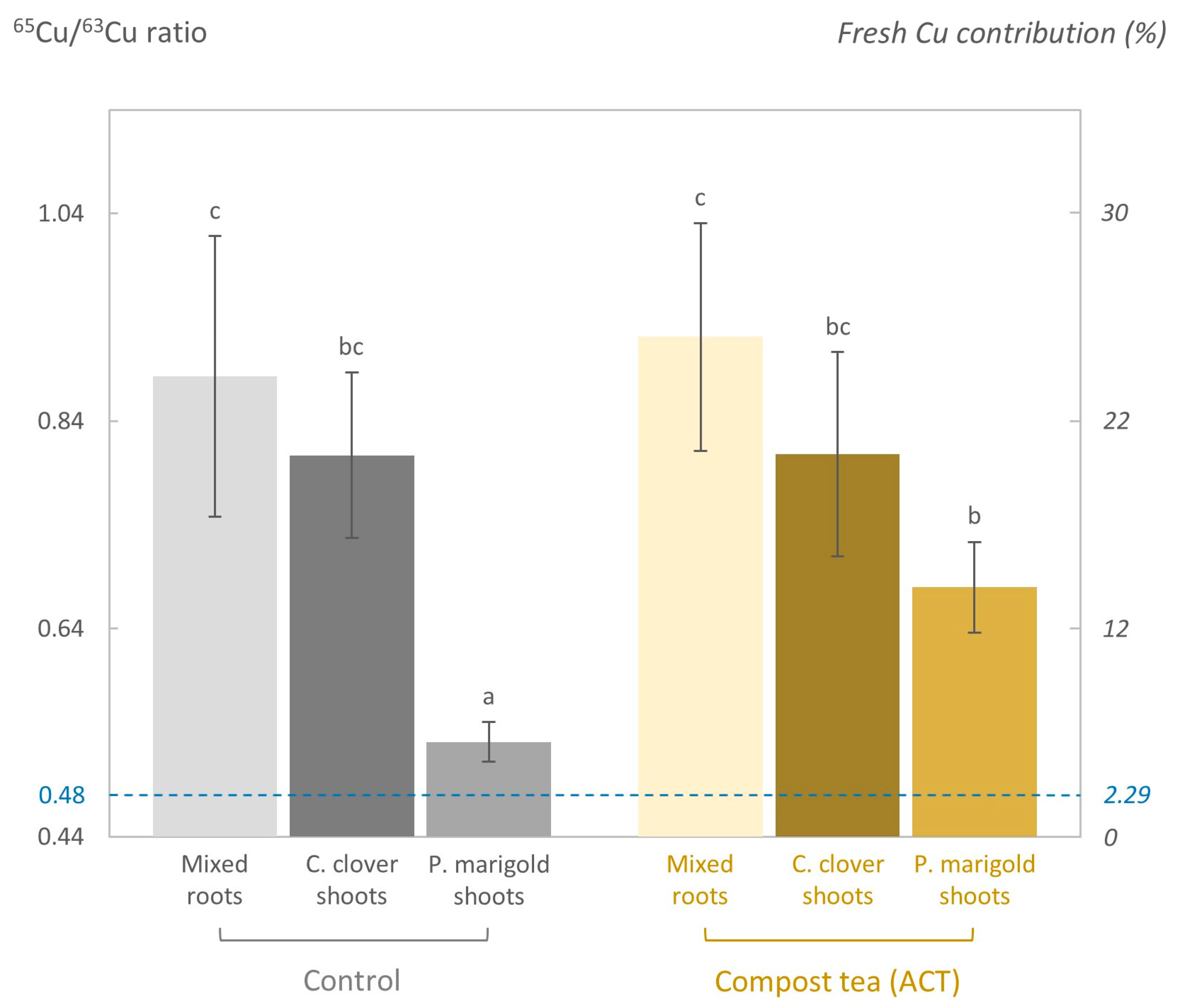
3.6. 65Cu/63Cu Isotopic Ratio and Concentration of Fresh Cu in the KCl Extract
3.7. 65Cu/63Cu Isotopic Ratio in Plants
4. Discussion
4.1. The Effect of ACT on the Downward Transfer of Cu Was Limited to the Top 7.5 cm
4.2. ACT Had No Effect on Cu Phytoextraction but Enhanced the Growth of Pot Marigold and the Accumulation of Cu in Roots
4.3. Fresh Cu Was Barely Transferred Downward and Was Slightly More Sensitive than Aged Cu to the Addition of ACT
4.4. Fresh Cu Was More Available to Plants than Aged Cu Due to Its Preferential Accumulation in the Topsoil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | Aerated compost tea |
| SHS | Soluble humic substances |
| DOM | Dissolved organic matter |
| CEC | Cation exchange capacity |
| WHC | Water holding capacity |
| A254 | Absorbance at 254 nm |
| DW | Dry weight |
| DGT | Diffusive gradients in thin-films |
References
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Pesce, S.; Mamy, L.; Sanchez, W.; Artigas, J.; Bérard, A.; Betoulle, S.; Chaumot, A.; Coutellec, M.-A.; Crouzet, O.; Faburé, J.; et al. The use of copper as plant protection product contributes to environmental contamination and resulting impacts on terrestrial and aquatic biodiversity and ecosystem functions. Environ. Sci. Pollut. Res. 2024, 32, 2830–2846. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.F.; Wang, Y.J.; Li, C.C.; Sun, R.J.; Yu, Y.C.; Zhou, D.M. Subacute toxicity of copper and glyphosate and their interaction to earthworm (Eisenia fetida). Environ. Pollut. 2013, 180, 71–77. [Google Scholar] [CrossRef] [PubMed]
- De Conti, L.; Cesco, S.; Mimmo, T.; Pii, Y.; Valentinuzzi, F.; Melo, G.W.B.; Ceretta, C.A.; Trentin, E.; Marques, A.C.R.; Brunetto, G. Iron fertilization to enhance tolerance mechanisms to copper toxicity of ryegrass plants used as cover crop in vineyards. Chemosphere 2020, 243, 125298. [Google Scholar] [CrossRef]
- Brunetto, G.; Bastos de Melo, G.W.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef]
- Tsimnadis, K.; Kyriakopoulos, G.L. Investigating the role of municipal waste treatment within the European Union through a novel created common sustainability point system. Recycling 2024, 9, 42. [Google Scholar] [CrossRef]
- De Corato, U. Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: A review under the perspective of a circular economy. Sci. Total Environ. 2020, 738, 139840. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Paull, R.E. Biochemical properties of compost tea associated with compost quality and effects on pak choi growth. Sci. Hortic. 2012, 148, 138–146. [Google Scholar] [CrossRef]
- Pilla, N.; Tranchida-Lombardo, V.; Gabrielli, P.; Aguzzi, A.; Caputo, M.; Lucarini, M.; Durazzo, A.; Zaccardelli, M. Effect of compost tea in horticulture. Horticulturae 2023, 9, 984. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Eon, P.; Ouerdane, L.; Goupil, A.; Vidal, A.; Cornu, J.-Y. Copper dynamics in vineyard topsoils as affected by the supply of aerated compost tea: Insights from a batch experiment. Environ. Pollut. 2024, 356, 124382. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wei, Y.; Liu, J. Comparative characterization of sewage sludge compost and soil: Heavy metal leaching characteristics. J. Hazard. Mat. 2016, 310, 1–10. [Google Scholar] [CrossRef]
- Kaschl, A.; Römheld, V.; Chen, Y. The influence of soluble organic matter from municipal solid waste compost on trace metal leaching in calcareous soils. Sci. Total Environ. 2002, 291, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Temminghoff, E.J.M.; Van Der Zee, S.E.A.T.M.; De Haan, F.A.M. Effects of dissolved organic matter on the mobility of copper in a contaminated sandy soil. Eur. J. Soil Sci. 1998, 49, 617–628. [Google Scholar] [CrossRef]
- Zhao, L.Y.L.; Schulin, R.; Nowack, B. Cu and Zn mobilization in soil columns percolated by different irrigation solutions. Environ. Pollut. 2009, 157, 823–833. [Google Scholar] [CrossRef]
- McBeath, T.M.; McLaughlin, M.J.; Kirby, J.K.; Degryse, F. A stable-isotope methodology for measurement of soil-applied zinc-fertilizer recovery in durum wheat (Triticum durum). J. Plant Nutr. Soil Sci. 2013, 176, 756–763. [Google Scholar] [CrossRef]
- Yan, B.F.; Nguyen, C.; Pokrovsky, O.S.; Candaudap, F.; Coriou, C.; Bussiere, S.; Robert, T.; Cornu, J.-Y. Cadmium allocation to grains in durum wheat exposed to low Cd concentrations in hydroponics. Ecotox. Environ. Safe. 2019, 184, 109592. [Google Scholar] [CrossRef]
- Wiggenhauser, M.; Moore, R.E.T.; Wang, P.; Bienert, G.P.; Laursen, K.H.; Blotevogel, S. Stable isotope fractionation of metals and metalloids in plants: A review. Front. Plant Sci. 2022, 13, 840941. [Google Scholar] [CrossRef]
- Zheng, X.; Han, G.; Song, Z.; Liang, B.; Yang, X.; Yu, C.; Guan, D.X. Biogeochemical cycle and isotope fractionation of copper in plant–soil systems: A review. Rev. Environ. Sci. Bio. 2024, 23, 21–41. [Google Scholar] [CrossRef]
- Ostermann, A.; He, Y.; Siemens, J.; Welp, G.; Heuser, A.; Wombacher, F.; Münker, C.; Xue, Q.; Lin, X.; Amelung, W. Tracing copper derived from pig manure in calcareous soils and soil leachates by 65Cu labeling. Environ. Sci. Technol. 2015, 49, 4609–4617. [Google Scholar] [CrossRef]
- Wiggenhauser, M.; Illmer, D.; Spiess, E.; Holzkämper, A.; Prasuhn, V.; Liebisch, F. Cadmium, zinc, and copper leaching rates determined in large monolith lysimeters. Sci. Total Environ. 2024, 926, 171482. [Google Scholar] [CrossRef] [PubMed]
- Revaillot, S.; Pouget, C.; Alvarez, G.; Fontaine, S. Mesurer la capacité de rétention en eau d’un sol par centrifugation: Une méthode fiable, facile et rapide à mettre en œuvre dans un laboratoire. Cahier Techniques l’INRA 2021, 107, 1–27. [Google Scholar]
- Cornu, J.-Y.; Denaix, L.; Schneider, A.; Pellerin, S. Temporal evolution of redox processes and free Cd dynamics in a metal-contaminated soil after rewetting. Chemosphere 2007, 70, 306–314. [Google Scholar] [CrossRef]
- Eon, P.; Deogratias, J.-M.; Robert, T.; Coriou, C.; Bussiere, S.; Sappin-Didier, V.; Denaix, L.; Cornu, J.-Y. Ability of aerated compost tea to increase the mobility and phytoextraction of copper in vineyard soil. J. Environ. Manag. 2023, 325, 116560. [Google Scholar] [CrossRef] [PubMed]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA: Madison, WI, USA, 1996; pp. 1018–1020. [Google Scholar]
- Goswami, S.; Das, S. Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotox. Environ. Safe. 2016, 126, 211–218. [Google Scholar] [CrossRef]
- Kemper, R.; Bublitz, T.A.; Müller, P.; Kautz, T.; Döring, T.F.; Athmann, M. Vertical root distribution of different cover crops determined with the profile wall method. Agriculture 2020, 10, 503. [Google Scholar] [CrossRef]
- Ouédraogo, F.; Cornu, J.-Y.; Janot, N.; Nguyen, C.; Sourzac, M.; Parlanti, E.; Denaix, L. Do DOM optical parameters improve the prediction of copper availability in vineyard soils? Environ. Sci. Pollut. Res. 2022, 29, 29268–29284. [Google Scholar] [CrossRef]
- Rodríguez-Cea, A.; de la Campa, M.D.F.; Alonso, J.I.G.; Sanz-Medel, A. The use of enriched 111Cd as tracer to study de novo cadmium accumulation and quantitative speciation in Anguilla anguilla tissues. J. Anal. At. Spectrom. 2006, 21, 270–278. [Google Scholar] [CrossRef]
- Jardine, P.M.; Weber, N.L.; McCarthy, J.F. Mechanisms of dissolved organic carbon adsorption on soil. Soil Sci. 1989, 53, 1378–1385. [Google Scholar] [CrossRef]
- Kaiser, K.; Haumaier, L.; Zech, W. The sorption of organic matter in soils as affected by the nature of soil carbon. Soil Sci. 2000, 165, 305–313. [Google Scholar] [CrossRef]
- Milne, C.J.; Kinniburgh, D.G.; Van Riemsdijk, W.H.; Tipping, E. Generic NICA-Donnan model parameters for metal-ion binding by humic substances. Environ. Sci. Technol. 2003, 37, 958–971. [Google Scholar] [CrossRef]
- Christl, I. Magnesium binding by terrestrial humic acids. Environ. Chem. 2018, 15, 317–324. [Google Scholar] [CrossRef]
- Römkens, P.F.A.M.; Dolfing, J. Effect of Ca on the solubility and molecular size distribution of DOC and Cu binding in soil solution samples. Environ. Sci. Technol. 1998, 32, 363–369. [Google Scholar] [CrossRef]
- Sauvé, S.; Hendershot, W.H.; Allen, H.E. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ. Sci. Technol. 2000, 34, 1125–1131. [Google Scholar] [CrossRef]
- Printz, B.; Lutts, S.; Hausman, J.F.; Sergeants, K. Copper trafficking in plants and its implication on cell wall dynamics. Front. Plant. Sci. 2016, 7, 601. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409. [Google Scholar] [CrossRef]
- Plénet, D.; Lemaire, G. Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil 1999, 216, 65–82. [Google Scholar] [CrossRef]
- Ortega, P.; Sánchez, E.; Gil, E.; Matamoros, V. Use of cover crops in vineyards to prevent groundwater pollution by copper and organic fungicides. Soil column studies. Chemosphere 2022, 303, 134975. [Google Scholar] [CrossRef]
- Brun, L.A.; Maillet, J.; Richarte, J.; Herrmann, P.; Remy, J.C. Relationships between extractable copper, soil properties and copper uptake by wild plants in vineyard soils. Environ. Pollut. 1998, 102, 151–161. [Google Scholar] [CrossRef]
- Manceau, A.; Matynia, A. The nature of Cu bonding to natural organic matter. Geochim. Cosmochim. Acta 2010, 74, 2556–2580. [Google Scholar] [CrossRef]
- Buekers, J.; Van Laer, L.; Amery, F.; Van Buggenhout, S.; Maes, A.; Smolders, E. Role of soil constituents in fixation of soluble Zn, Cu, Ni and Cd added to soils. Eur. J. Soil Sci. 2007, 58, 1514–1524. [Google Scholar] [CrossRef]
- Zhou, S.W.; Xu, M.G.; Ma, Y.B.; Chen, S.B.; Wei, D.P. Aging mechanism of copper added to bentonite. Geoderma 2008, 147, 86–92. [Google Scholar] [CrossRef]
- Cornu, J.-Y.; Eon, P.; Candaudap, F.; Pokrovsky, O.S. Aging of copper in vineyard topsoil: Use of isotopic labeling to distinguish freshly added copper from aged copper. Pedosphere, 2025; in press. [Google Scholar] [CrossRef]
- Wyngaarden, S.L.; Gaudin, A.C.M.; Deen, W.; Martin, R.C. Expanding red clover (Trifolium pratense) usage in the corn–soy–wheat rotation. Sustainability 2015, 7, 15487–15509. [Google Scholar] [CrossRef]
- Fujii, K. Plant strategy of root system architecture and exudates for acquiring soil nutrients. Ecol. Res. 2024, 39, 623–633. [Google Scholar] [CrossRef]

| ACT | |
|---|---|
| pH | 7.52 |
| Conductivity (mS cm−1) | 78.11 |
| TC (mg L−1) | 2009 |
| TN (mg L−1) | 370 |
| A254 | 45 |
| Humic acids 1 (%) | 26 |
| Fulvic acids 1 (%) | 74 |
| Macronutrients (mM) | |
| N | 26 |
| P | 1.4 |
| S | 2.2 |
| K | 26 |
| Ca | 1.2 |
| Mg | 1.0 |
| Micronutrients and trace metals (µM) | |
| Al | 119 |
| Fe | 75 |
| Mn | 16 |
| Cu | 44 |
| Zn | 10 |
| Dry Matter | N | P | K | S | Ca | Mg | Fe | Mn | Cu | Zn | Si | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | % | mg g−1 | µg g−1 | ||||||||||
| Crimson clover | Control | 9.5 | 1.8 | 2.2 | 4.3 | 1.1 | 15 | 3.9 | 88 | 109 | 16 | 88 | 30 |
| ACT | 7.8 | 1.6 | 1.9 | 45 | 2.6 | 7.5 | 1.9 | 141 | 71 | 13 | 53 | 32 | |
| Pot marigold | Control | 2.2 | 1.9 | 2.4 | 6.3 | 3.6 | 30 | 5.0 | 91 | 239 | 33 | 163 | 26 |
| ACT | 8.0 | 1.9 | 1.9 | 42 | 2.7 | 11 | 1.7 | 73 | 92 | 15 | 48 | 26 | |
| Mixed roots | Control | / | / | 1.6 | 4.5 | 1.4 | 3.7 | 1.8 | 558 | 58 | 74 | 74 | 33 |
| ACT | / | / | 1.6 | 25 | 2.5 | 3.4 | 1.1 | 806 | 65 | 145 | 47 | 25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eon, P.; Candaudap, F.; Robert, T.; Denaix, L.; Cornu, J.-Y. Aerated Compost Tea Did Not Promote Cu Downward Transfer but Increased Cu Phytoavailability in a Vineyard Soil. Sustainability 2025, 17, 4414. https://doi.org/10.3390/su17104414
Eon P, Candaudap F, Robert T, Denaix L, Cornu J-Y. Aerated Compost Tea Did Not Promote Cu Downward Transfer but Increased Cu Phytoavailability in a Vineyard Soil. Sustainability. 2025; 17(10):4414. https://doi.org/10.3390/su17104414
Chicago/Turabian StyleEon, Pierre, Frédéric Candaudap, Thierry Robert, Laurence Denaix, and Jean-Yves Cornu. 2025. "Aerated Compost Tea Did Not Promote Cu Downward Transfer but Increased Cu Phytoavailability in a Vineyard Soil" Sustainability 17, no. 10: 4414. https://doi.org/10.3390/su17104414
APA StyleEon, P., Candaudap, F., Robert, T., Denaix, L., & Cornu, J.-Y. (2025). Aerated Compost Tea Did Not Promote Cu Downward Transfer but Increased Cu Phytoavailability in a Vineyard Soil. Sustainability, 17(10), 4414. https://doi.org/10.3390/su17104414








