Eco-Friendly Conversion of Waste Zeolite Dust into Dual Oil/Water Affinity Sorbents via HPGR-Based Agglomeration–Deagglomeration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chemical and Mineral Analysis
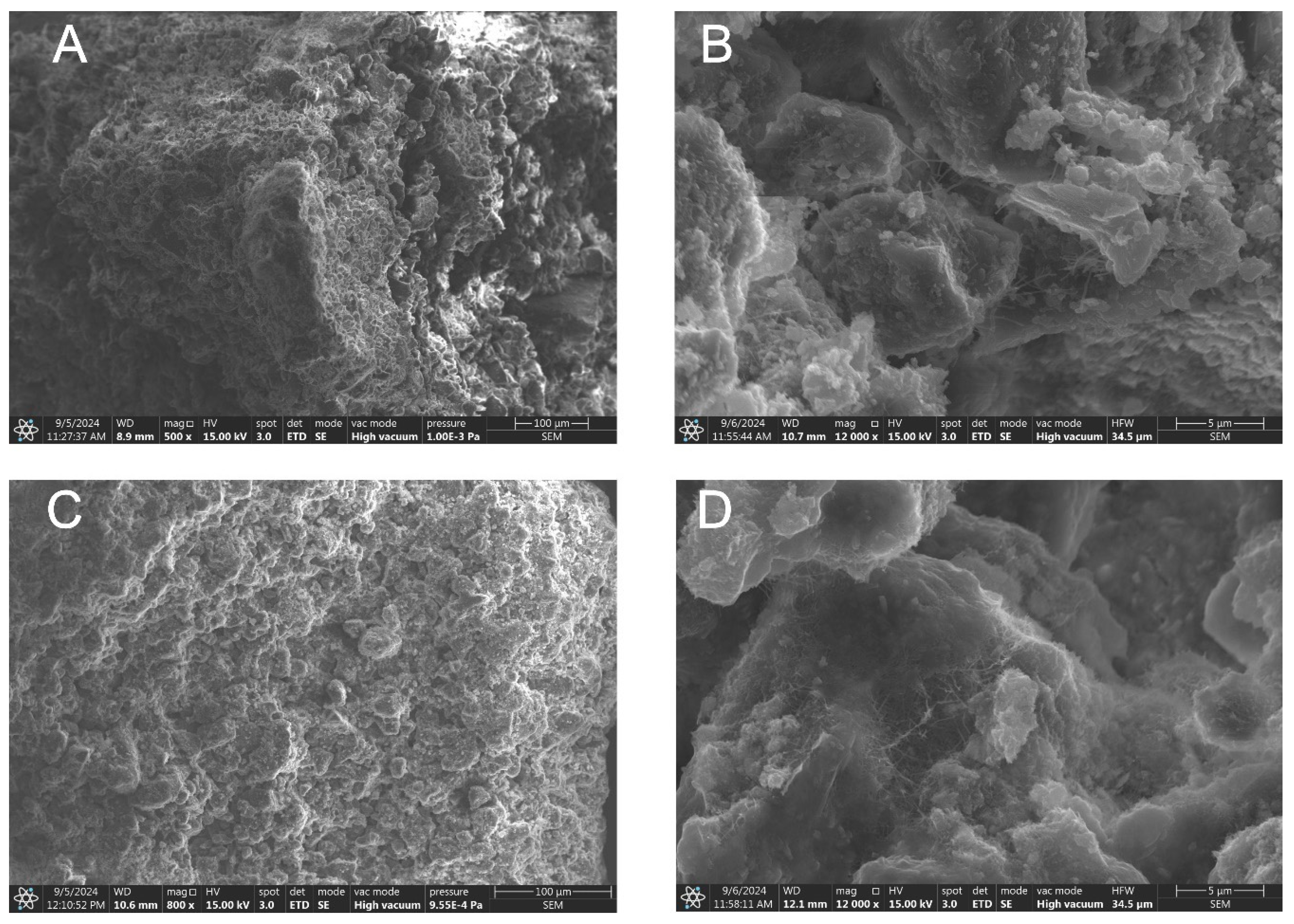
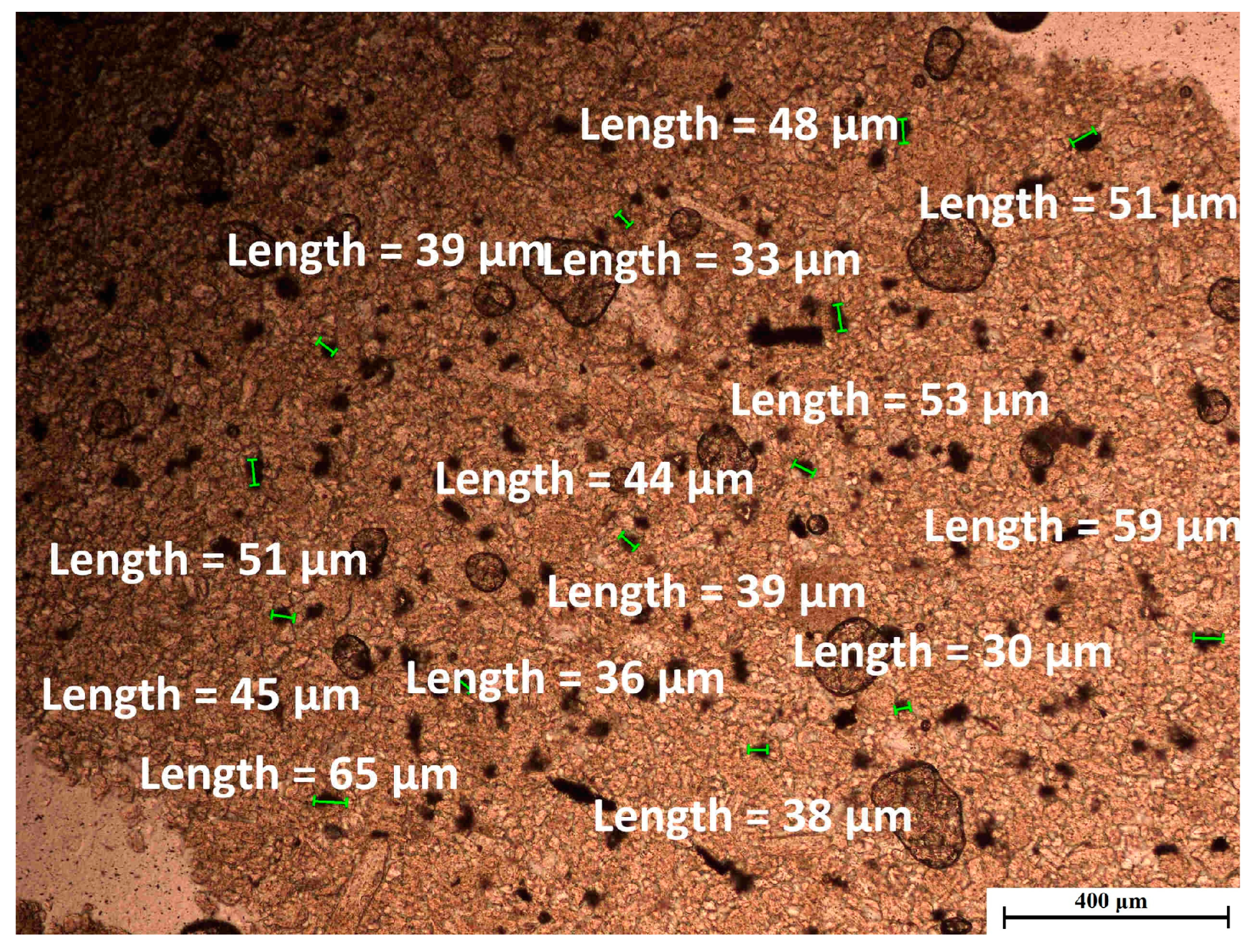
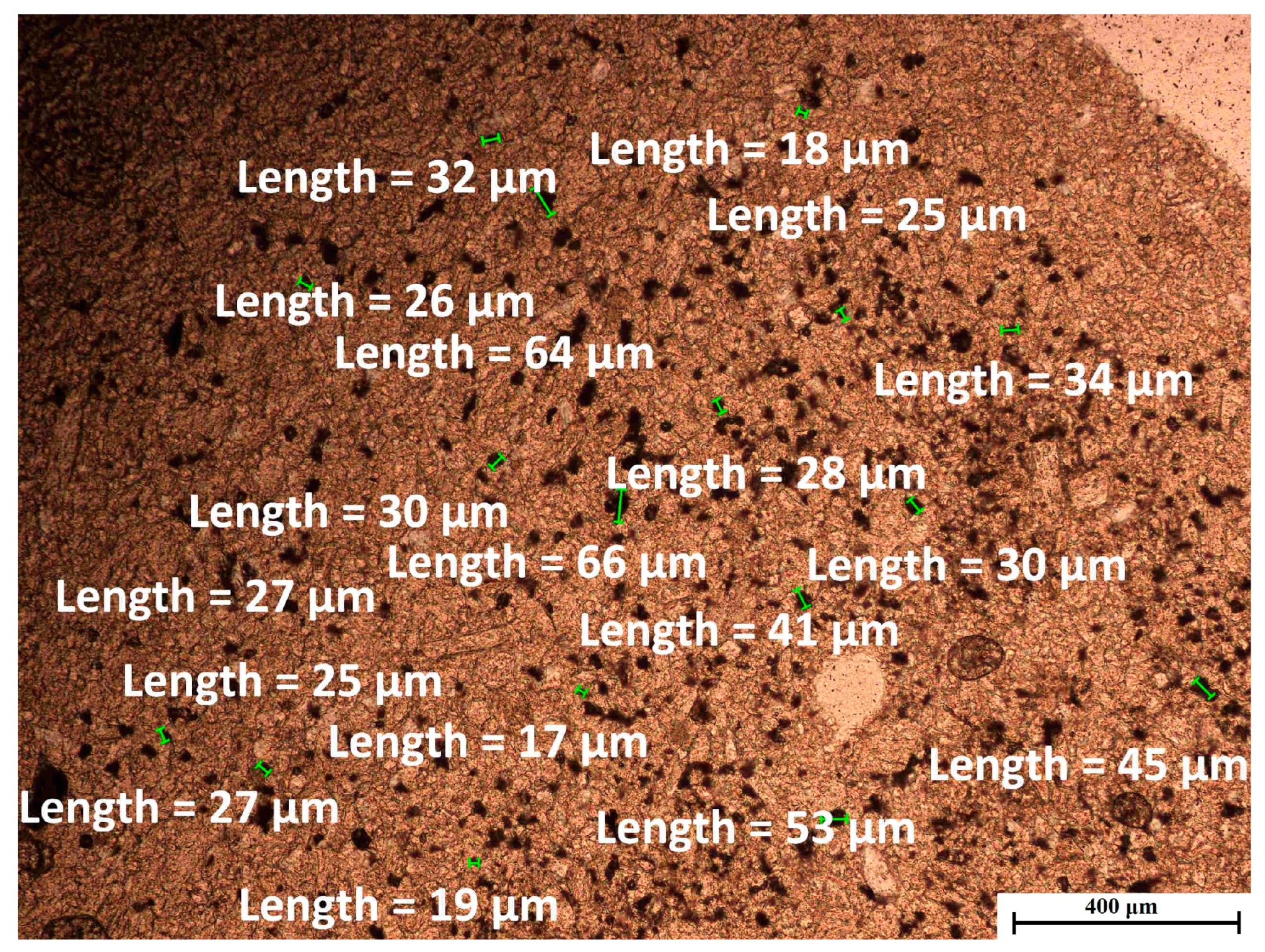
2.2.2. Morphology and Structural Analysis
- Scanning electron microscope (SEM)—Thermo Scientific Quattro ESEM (ThermoFisher Scientific, Waltham, MA, USA )operated in EBSD mode at 20 kV and ~10 mm working distance. Samples were coated with a ~20 nm carbon layer.
- Polarizing microscope (transmitted and reflected polarized light on thin sections).Nikon Eclipse LV 100 POL (Nikon GmbH, Essen, Germany)
- Stereoscopic microscope—Nikon SMZ 1000 (Nikon GmbH, Essen, Germany)
2.2.3. Textural Analysis
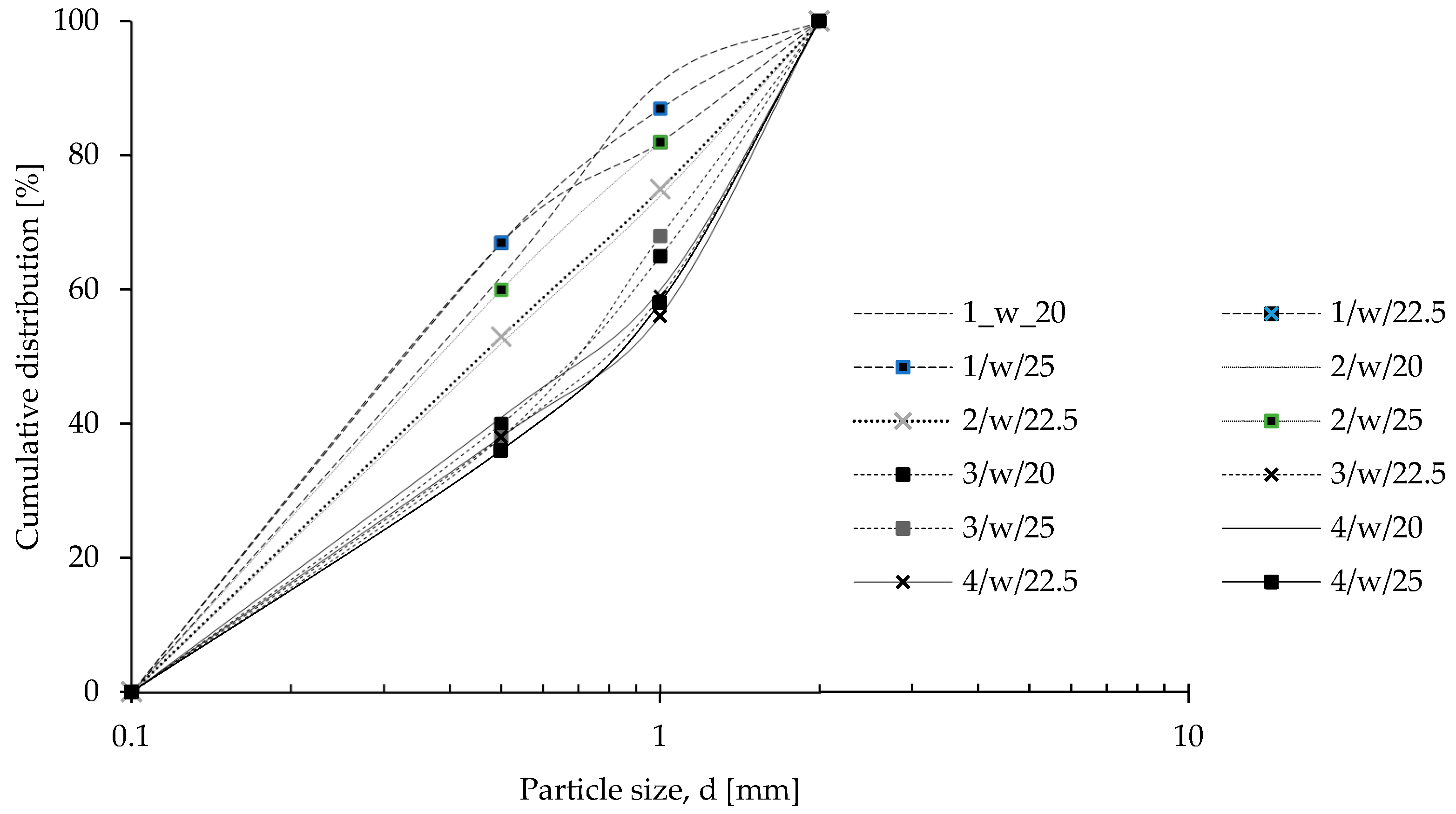
2.2.4. Particle Size Distribution
2.2.5. Basic Bulk Properties
2.2.6. Mechanical Strength Measurement
2.2.7. Sorption Measurements
- Sorption capacity—Westinghouse test
- Maximum Sorption Capacity—Droplet Experiment
2.2.8. Agglomeration Process of Natural Zeolite Dust
3. Results and Discussion
3.1. Characteristics of Raw Material Prior to Agglomeration Process
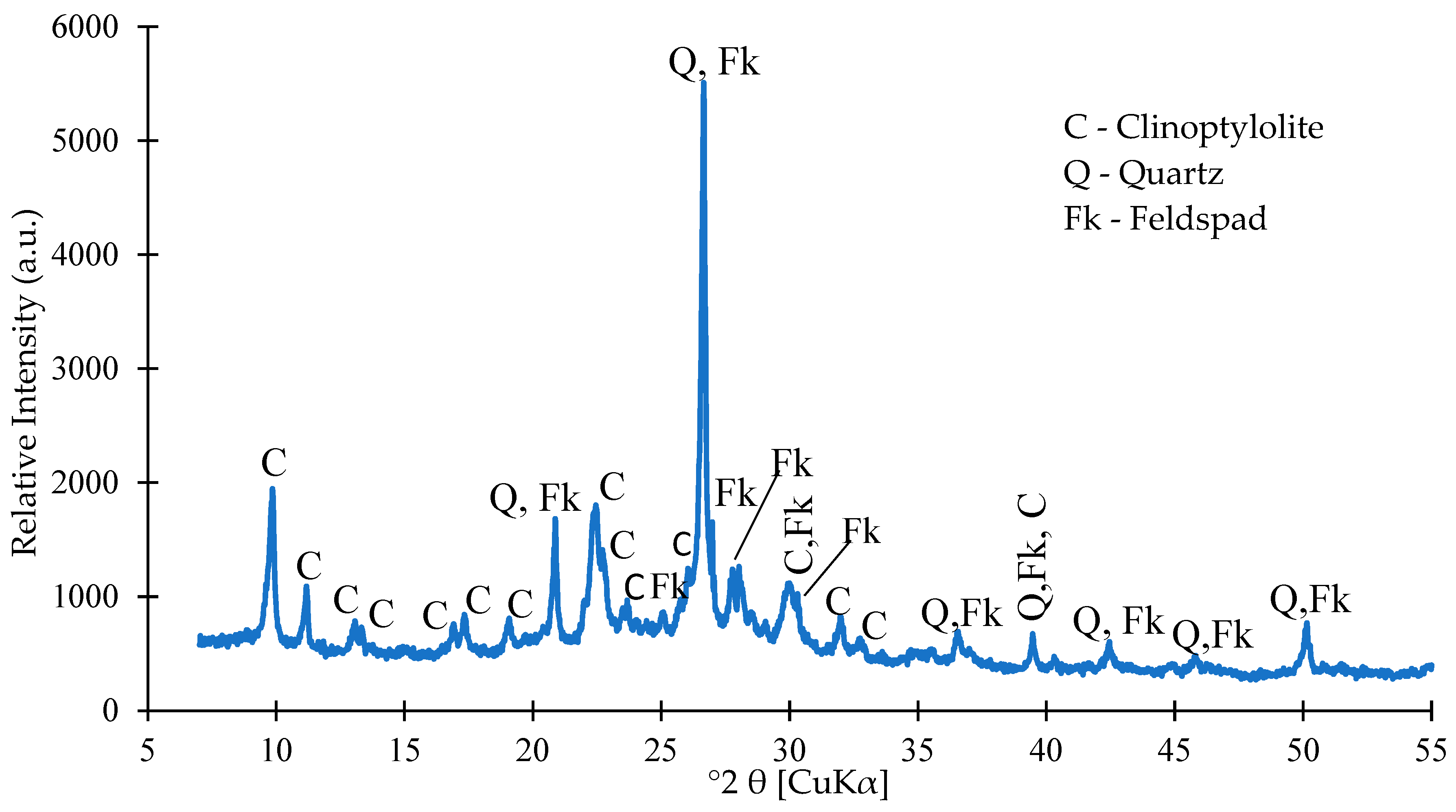
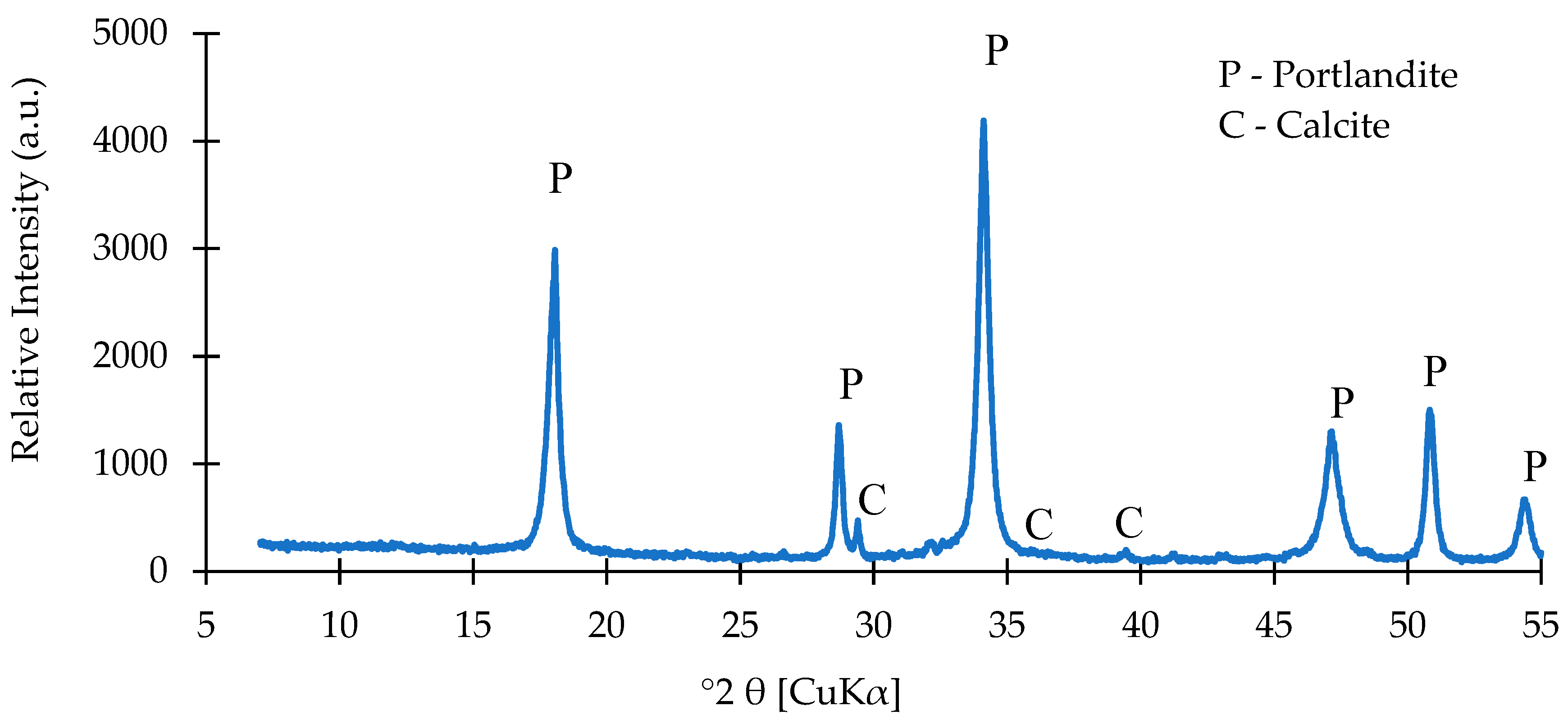
3.1.1. Chemical and Mineralogical Composition
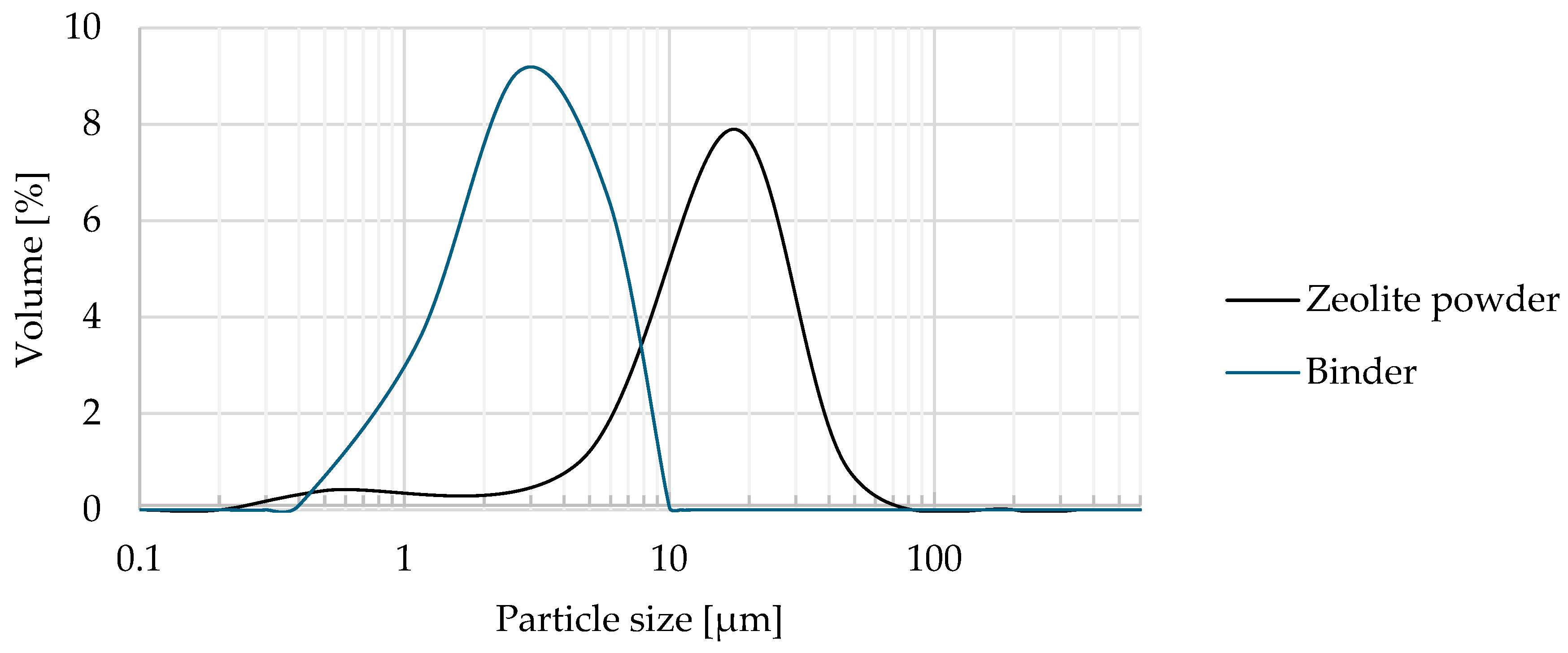
3.1.2. Particle Size Distribution and Basic Bulk Properties
3.1.3. Structural and Textural Characterization
3.2. Characterization of Products from the Agglomeration Process
3.2.1. Basic Properties of Ribbons
3.2.2. Physico-Mechanical Properties of Produced Zeolite-Based Agglomerates
3.2.3. Sorption Properties of Produced Zeolite-Based Agglomerates
- Sorption Capacity of Petrochemical Compounds—Westinghouse test
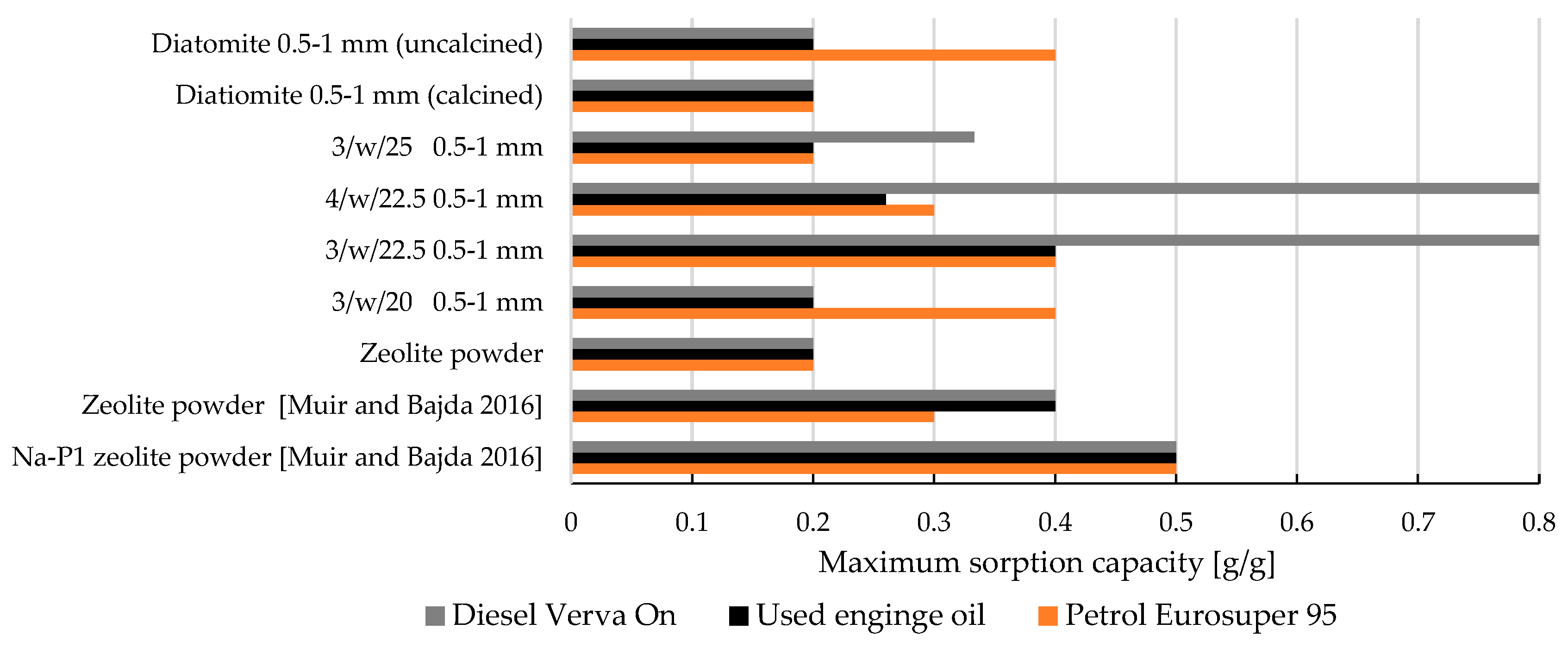
- Maximum sorption capacity—droplet experiment
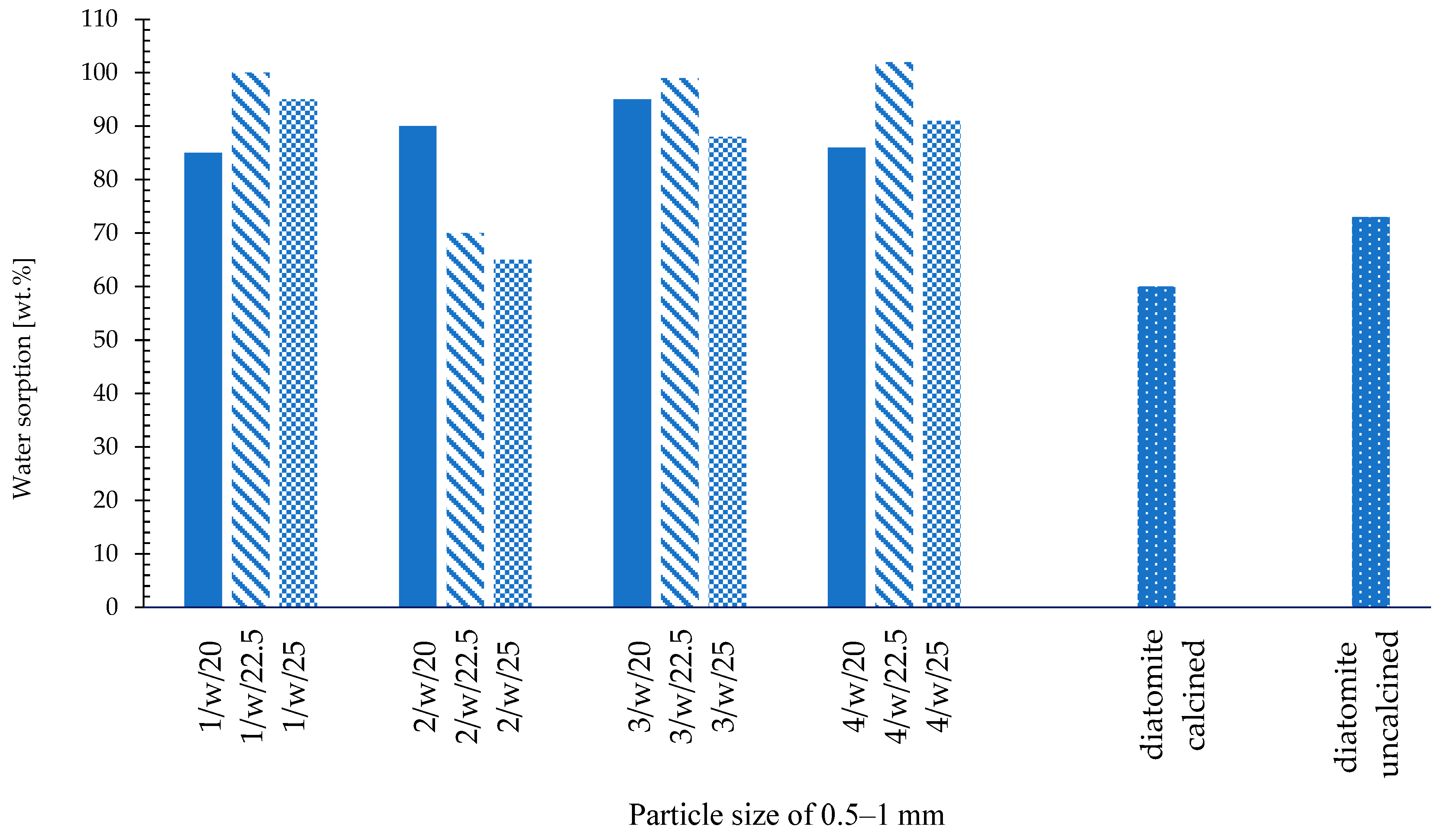
- Water sorption—Westinghouse test
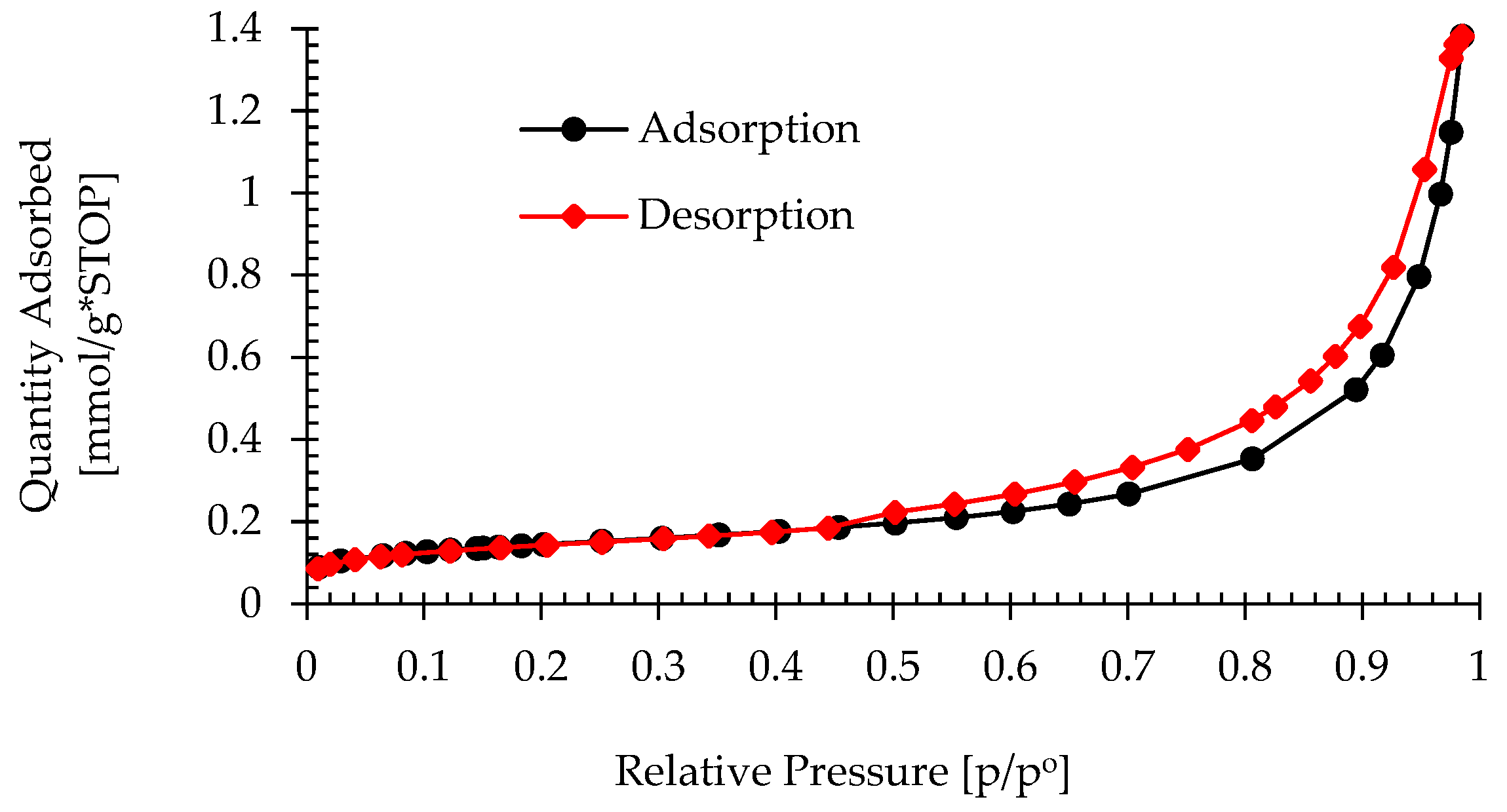
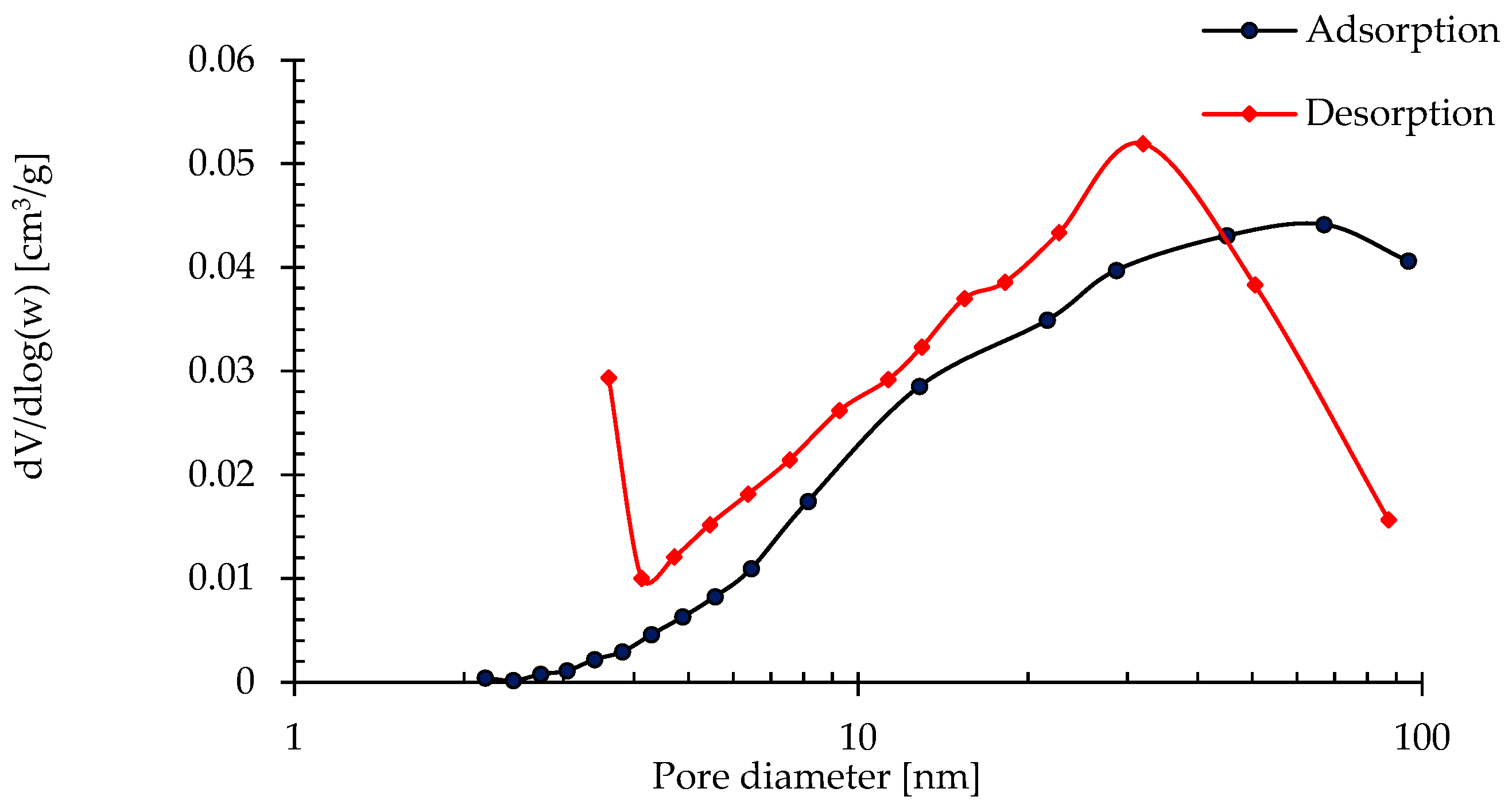
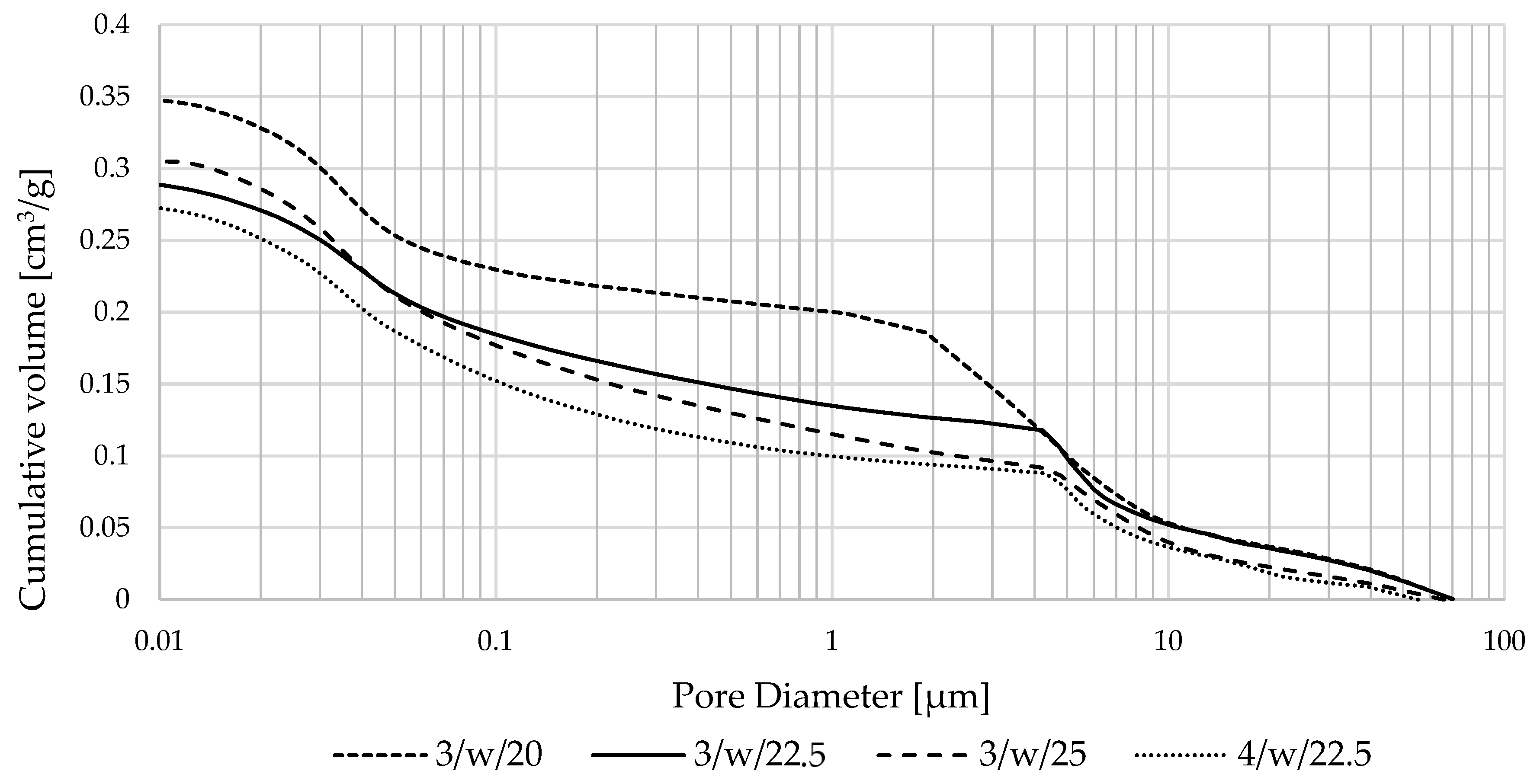
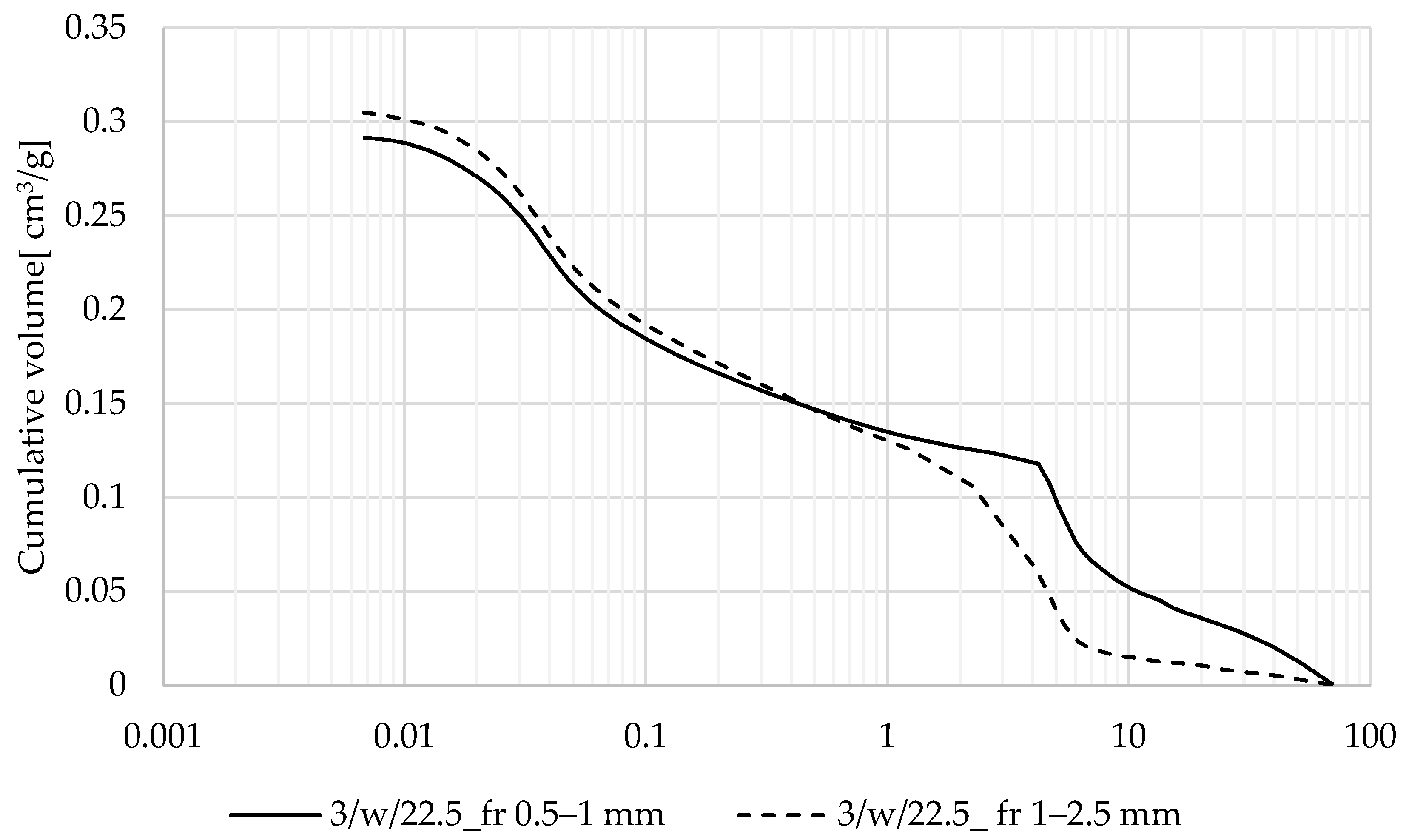
3.2.4. Textural Characteristics of Produced Zeolite-Based Agglomerates and Their Relationship with Sorption Performance
3.3. Interaction of Liquid Sorbate (Petroleum-Derived Compounds and Water) and Produced Zeolite-Based Agglomerates
4. Conclusions
- The proposed novel agglomeration approach enhances secondary porosity and sorption capacities of zeolite-based sorbents compared to traditional wet granulation methods.
- This agglomeration method significantly increases the specific surface area of zeolite products, representing a notable improvement over conventional techniques.
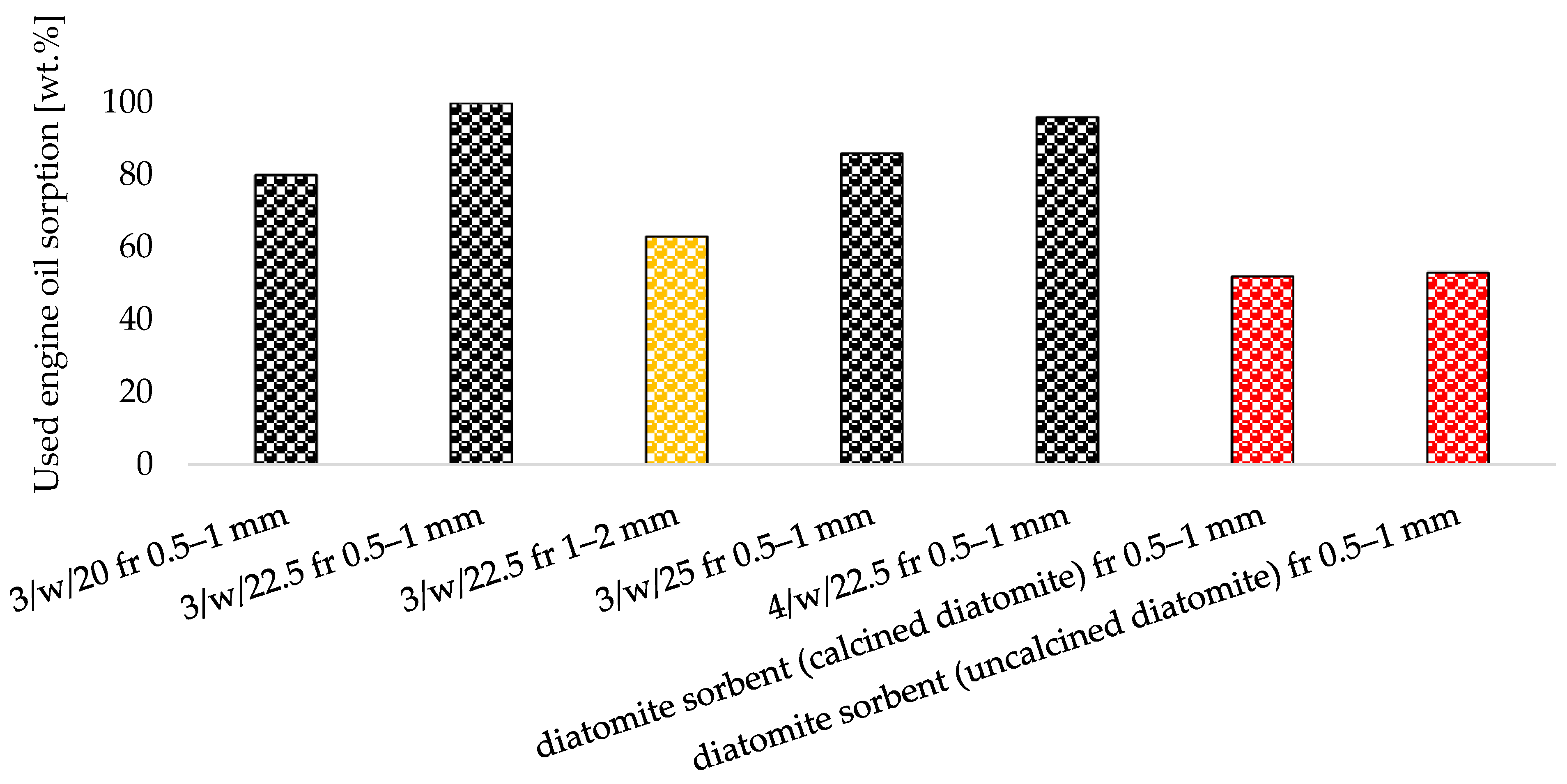
- All produced zeolite-based agglomerates (0.5–1 mm) exceeded the 50 wt.% oil sorption threshold required for petroleum spill clean-up, demonstrating superior affinity for petrochemical compounds.
- A high-performance zeolite-based sorbent (3/w/22.5) was developed, achieving maximum sorption capacities of 0.4 g/g for Euro-super 95 petrol, 0.8 g/g for Verva ON diesel, and 0.3 g/g for used engine oil.
- Formulations 3/w/22.5 and 4/w/22.5 outperformed commercial diatomite and synthetic Na-P1 zeolite in used engine oil sorption, reaching maximum sorption capacities of up to 0.8 g/g.
- The selective oil/water sorption is closely linked to the specific Si/Al ratio (5.2), which imparts unique amphiphilic properties to the zeolite-based sorbents by reducing hydrophilicity and enhancing hydrophobicity. This distinctive balance enables efficient sorption of both polar (e.g., water) and nonpolar (e.g., petroleum hydrocarbons) substances, significantly broadening the range of potential environmental applications.
- The sorption mechanism of petroleum-derived compounds onto the produced zeolite-based sorbents is primarily governed by physical processes, such as the diffusion of nonpolar organic molecules into mesopores and macropores, driven mainly by van der Waals interactions. Although sorption occurs predominantly on the external surface, the high mesopore volume, surface heterogeneity, and optimal pore size distribution facilitate capillary condensation and internal diffusion, thereby enhancing overall sorption efficiency.
- Increased binder content improves mechanical stability and sorption performance, with optimal binder levels identified at 7.5 wt.%.
- The relationship between binder and water content in zeolite agglomerates and their physical and sorption properties is complex and warrants further investigation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senila, M.; Cadar, O. Modification of natural zeolites and their applications for heavy metal removal from polluted environments: Challenges. recent advances. and perspectives. Heliyon 2024, 10, e25303. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, M.; Zhu, L.; Wu, Q.; Meng, X.; Xiao, F.S. Recent advances in sustainable synthesis of zeolites. Mater. Today Sustain. 2025, 29, 101065. [Google Scholar] [CrossRef]
- Pietre, M.K.; Freitas, J.C.C. Fundamental studies on zeolite–adsorbate interactions: Designing a better aluminosilicate adsorbent for pollutants’ removal. Environ. Earth Sci. 2022, 81, 17. [Google Scholar] [CrossRef]
- Payra, P.; Dutta, P.K. Zeolites: A primer. In Handbook of Zeolite Science and Technology; CRC Press: Boca Raton, FL, USA, 2003; Volume 2, pp. 1–19. [Google Scholar]
- Bingre, R.; Louis, B.; Nguyen, P. An Overview on Zeolite Shaping Technology and Solutions to Overcome Diffusion Limitations. Catalysts 2018, 8, 163. [Google Scholar] [CrossRef]
- Fungaro, D.A.; Bertolini, T.C.R. Optimization Of Pelleting Parameters For Producing Composite Pellets Using Zeolitic Material From Fly Ash. Appl. Mater. Technol. 2023, 3, 13–23. [Google Scholar] [CrossRef]
- Shams, K.; Mirmohammadi, S.J. Preparation of 5A zeolite monolith granular extrudates using kaolin: Investigation of the effect of binder on sieving/adsorption properties using a mixture of linear and branched paraffin hydrocarbons. Microporous Mesoporous Mater. 2007, 106, 268–277. [Google Scholar] [CrossRef]
- Król, M.; Mikuła, A. Synthesis of the zeolite granulate for potential sorption application. Microporous Mesoporous Mater. 2017, 243, 201–205. [Google Scholar] [CrossRef]
- Schumann, K.; Unger, B.; Brandt, A.; Scheffler, F. Investigation on the pore structure of binderless zeolite 13× shapes. Microporous Mesoporous Mater. 2012, 154, 119–123. [Google Scholar] [CrossRef]
- Muir, B.; Matusik, J.; Bajda, T. New insights into alkylammonium-functionalized clinoptilolite and Na-P1 zeolite: Structural and textural features. Appl. Surf. Sci. 2016, 361, 242–250. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Abduljawad, M.M.; Alassmy, Y.A.; Cardon, L.; Van Steenberge, P.H.M.; Sebakhy, K.O. A Comparative Review of Binder-Containing Extrusion and Alternative Shaping Techniques for Structuring of Zeolites into Different Geometrical Bodies. Catalysts 2023, 13, 656. [Google Scholar] [CrossRef]
- Michels, N.L.; Mitchell, S.; Pérez-Ramírez, J. Effects of Binders on the Performance of Shaped Hierarchical MFI Zeolites in Methanol-to-Hydrocarbons. ACS Catal. 2014, 4, 2409–2417. [Google Scholar] [CrossRef]
- Mitchell, W.J.; Moore, W.F. Bonded Molecular Sieves. U.S. Patent 2973327A, 28 February 1961. [Google Scholar]
- Haden, W.L., Jr.; Dzierzanowski, F.J. Zeolite Agglomerates and the Preparation Thereof. U.S. Patent 3287281A, 22 November 1966. [Google Scholar]
- Chu, P.; Garwood, W.E. Zeolite-Clay Composition and Uses Thereof. U.S. Patent 5079201A, 7 January 1992. [Google Scholar]
- Shaniuk, T.J.; Russo, R.V. Composite Material, Preparation and Use Thereof. U.S. Patent 6107354A, 22 August 2000. [Google Scholar]
- Jasra, R.V.; Tyagi, B.; Badheka, Y.M.; Choudary, V.N.; Bhat, T.S.G. Effect of Clay Binder on Sorption and Catalytic Properties of Zeolite Pellets. Ind. Eng. Chem. Res. 2003, 42, 3263–3272. [Google Scholar] [CrossRef]
- Cañizares, P.; Durán, A.; Dorado, F.; Carmona, F.M. The role of sodium montmorillonite on bounded zeolite-type catalysts. Appl. Clay Sci. 2000, 16, 273–287. [Google Scholar] [CrossRef]
- Fougerit, J.M.; Gnep, N.S.; Guisnet, M.; Amigues, P.; Duplan, J.L.; Hugues, F. Effect of the binder on the properties of a mordenite catalyst for the selective conversion of methanol into light olefins. Stud. Surf. Sci. Catal. 1994, 84, 1723–1730. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Devadas, P.; Kinage, A.K.; Guisnet, M. Influence of binder on the acidity and performance of H-Gallosilicate (MFI) zeolite in propane aromatization. Appl. Catal. A Gen. 1997, 162, 223–233. [Google Scholar] [CrossRef]
- Rajniak, P.; Mancinelli, C.; Chern, R.T.; Stepanek, F.; Farber, L.; Hill, B.T. Experimental study of wet granulation in fluidized bed: Impact of the binder properties on the granule morphology. Int. J. Pharm. 2007, 334, 92–102. [Google Scholar] [CrossRef]
- Fu, J.; Adams, M.J.; Reynolds, G.K.; Salman, A.D.; Hounslow, M.J. Impact deformation and rebound of wet granules. Powder Technol. 2004, 140, 248–257. [Google Scholar] [CrossRef]
- Müller, P.; Antonyuk, S.; Tomas, J. Influence of moisture content on the compression behavior of granules. Chem. Eng. Technol. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Kasture, M.W.; Niphadkar, P.S.; Bokade, V.V.; Joshi, P.N. On the catalytic performance in isopropylation of benzene over H/β zeolite catalysts: Influence of binder. Catal. Commun. 2007, 8, 1003–1008. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J. Influence of alumina binder content on catalytic performance of Ni/HZSM-5 for hydrodeoxygenation of cyclohexanone. PLoS ONE 2014, 9, 5–10. [Google Scholar] [CrossRef]
- Gleichmann, K.; Unger, B.; Brandt, A. Industrial zeolite molecular sieves. In Zeolites Useful Minerals; InTech: London, UK, 2016. [Google Scholar]
- Freiding, J.; Patcas, F.C.; Kraushaar-Czarnetzki, B. Extrusion of zeolites: Properties of catalysts with a novel aluminium phosphate sintermatrix. Appl. Catal. A Gen. 2007, 328, 210–218. [Google Scholar] [CrossRef]
- Whiting, G.T.; Chung, S.H.; Stosic, D.; Chowdhury, A.D.; van der Wal, L.I.; Fu, D.; Zecevic, J.; Travert, A.; Houben, K.; Baldus, M. Multiscale Mechanistic Insights of Shaped Catalyst Body Formulations and Their Impact on Catalytic Properties. ACS Catal. 2019, 9, 4792–4803. [Google Scholar] [CrossRef]
- Baldovino-Medrano, V.G.; Le, M.T.; Van Driessche, I.; Bruneel, E.; Alcázar, C.; Colomer, M.T.; Moreno, R.; Florencie, A.; Farin, B.; Gaigneaux, E.M. Role of shaping in the preparation of heterogeneous catalysts: Tableting and slip-casting of oxidation catalysts. Catal. Today 2015, 246, 81–91. [Google Scholar] [CrossRef]
- Devyatkov, S.Y.; Zinnurova, A.A.; Aho, A.; Kronlund, D.; Peltonen, J.; Kuzichkin, N.V.; Lisitsyn, N.V.; Murzin, D.Y. Shaping of Sulfated Zirconia Catalysts by Extrusion: Understanding the Role of Binders. Ind. Eng. Chem. Res. 2016, 55, 6595–6606. [Google Scholar] [CrossRef]
- Absi-Halabi, M.; Stanislaus, A.; Al-Zaid, H. Effect of acidic and basic vapors on pore size distribution of alumina under hydrothermal conditions. Appl. Catal. A Gen. 1993, 101, 117–128. [Google Scholar] [CrossRef]
- Bazer-Bachi, D.; Harbuzaru, B.; Lecolier, E. Zeolite Formed by Extrsion and Pelleting with a Hydraulic Binder Having Improved Mechanical Properties and Process and Preparing Same. U.S. Patent 20.160.288.109A1, 6 October 2016. [Google Scholar]
- Bika, D.; Tardos, G.I.; Panmai, S.; Farber, L.; Michaels, J. Strength and morphology of solid bridges in dry granules of pharmaceutical powders. Powder Technol. 2005, 150, 104–116. [Google Scholar] [CrossRef]
- Charkhi, A.; Kazemeini, M.; Ahmadi, S.J.; Kazemian, H. Fabrication of granulated NaY zeolite nanoparticles using a new method and study the adsorption properties. Powder Technol. 2012, 231, 1–6. [Google Scholar] [CrossRef]
- Li, N.; Liu, W.; Liu, L.; Gao, P.; Wu, Q.; Ma, X.; Li, G.K. Evaluation of gas adsorption and separation performance of binder-free Y zeolite particles prepared by the rotary pelletization method. Sep. Purif. Technol. 2025, 361, 131520. [Google Scholar] [CrossRef]
- Luzzi, E.; Aprea, P.; Salzano de Luna, M.; Caputo, D.; Filippone, G. Mechanically Coherent Zeolite 13X/Chitosan Aerogel Beads for Effective CO2 Capture. ACS Appl. Mater. Interfaces 2021, 13, 20728–20734. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Roelant, R.; Blom, R.; Arstad, B.; Li, Z.; Rombouts, M.; Middelkoop, V.; Borras, A.B.; Naldoni, L. Scaling up 3D printed hybrid sorbents towards (cost) effective post-combustion CO2 capture: A multiscale study. Int. J. Greenh. Gas Control. 2024, 132, 104069. [Google Scholar] [CrossRef]
- Wang, S.; Bai, P.; Sun, M.; Liu, W.; Li, D.; Wu, W.; Yan, W.; Shang, J.; Yu, J. Fabricating mechanically robust binder-free structured zeolites by 3D printing coupled with zeolite soldering: A superior configuration for CO2 capture. Adv. Sci. 2019, 6, 1901317. [Google Scholar] [CrossRef] [PubMed]
- Jivrakh, K.B.; Varghese, A.M.; Ehrling, S.; Kuppireddy, S.; Polychronopoulou, K.; Abu Al-Rub, R.K.; Alamoodi, N.; Karanikolos, G.N. 3D-printed zeolite 13X gyroid monolith adsorbents for CO2 capture. Chem. Eng. J. 2024, 497, 1385–8947. [Google Scholar] [CrossRef]
- Rianjanu, A.; Taher, T.; Desriani, F.; Delmita, R.O.; Sianturi, A.G.N.; Muhtar, S.A.; Ariwahjoedi, B.; Khamidy, N.I.; Adhika, D.R.; Arif, M.F. Examining the influence of sintering temperatures on the efficiency of 3D-printed natural zeolite for methylene blue dye adsorption. Results Surf. Interfaces 2024, 17, 2666–8459. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. J. Ger. Chemisal Soc. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Bazer-Bachi, D.; Assié, L.; Lecocq, V.; Harbuzaru, B.; Falk, V. Towards industrial use of metal-organic framework: Impact of shaping on the MOF properties. Powder Technol. 2014, 255, 52–59. [Google Scholar] [CrossRef]
- Panek, R.; Wdowin, M.; Bandura, L.; Wisła-Walsh, E.; Gara, P.; Franus, W. Changes in the Textural Parameters of Fly Ash-Derived Na-P1 Zeolite During Compaction Processes. Mineralogia 2017, 40, 3–22. [Google Scholar] [CrossRef]
- Marinko, N.; Sedlářová, I.; Römerová, S.; Gajdošová, M.; Zvoníček, V.; Zámostný, P.R. Method to determine envelope density for roller compacted ribbons by solid displacement of glass microspheres. Powder Technol. 2024, 444, 120014. [Google Scholar] [CrossRef]
- Kleinebudde, P. Improving Process Understanding in Roll Compaction. J. Pharm. Sci. 2022, 111, 552–558. [Google Scholar] [CrossRef]
- Reimer, H.L.; Kleinebudde, P. Hybrid modeling of roll compaction processes with the Styl’One Evolution. Powder Technol. 2019, 341, 66–74. [Google Scholar] [CrossRef]
- Vadaga, A.K.; Gudla, S.S.; Nareboina, G.S.K.; Gubbala, H.; Golla, B. Comprehensive review on modern techniques of granulation in pharmaceutical solid dosage forms. Intell. Pharm. 2024, 2, 609–629. [Google Scholar] [CrossRef]
- Patil, N.; Khadse, S.C.; Ige, P.P. Review on novel granulation techniques. World J. Pharm. Res. 2016, 5, 7. [Google Scholar]
- Miller, R.W. Roller Compaction Technology. In Handbook of Pharmaceutical Granulation Technology; CRC Press: Boca Raton, FL, USA, 2005; p. 32. [Google Scholar] [CrossRef]
- Teng, Y.; Qiu, Z.; Wen, H. Systematical approach of formulation and process development using roller compaction. Eur. J. Pharm. Biopharm. 2009, 73, 219–229. [Google Scholar] [CrossRef]
- García-Triñanes, P.; Morgeneyer, M.; Casares, J.J.; Bao, M. Use of organic byproducts as binders in the roll compaction of caustic magnesia. Powder Technol. 2012, 226, 173–179. [Google Scholar] [CrossRef]
- Yusof, Y.A.; Smith, A.C.; Briscoe, B.J. Roll compaction of maize powder. Chem. Eng. Sci. 2005, 60, 3919–3931. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, S.I.; Song, J.S.; Kim, K.H. A study on the pelletizing condition for roll compaction of powdered radioactive wastes. Powder Technol. 2021, 284, 554–563. [Google Scholar] [CrossRef]
- ISO 13320; Particle Size Analysis—Laser Diffraction Methods. International Organization for Standardization: Geneva, Switzerland, 2020.
- PN-EN 933-1; Examination of Geometric Properties of Aggregates—Part 1 Determination of Grain Composition. iTeh Standards: Newark, DE, USA, 2012.
- EN 1097-7; Tests for Mechanical and Physical Properties of Aggregates—Part 7: Determination of the Particle Density of Filler—Pyknometer Method. iTeh Standards: Newark, DE, USA, 2022.
- EN 1097-3; Tests for Mechanical and Physical Properties of Aggregates—Part 3: Determination of Loose Bulk Density and Voids. iTeh Standards: Newark, DE, USA, 1998.
- Muir, B.; Bajda, T. Organically Modified Zeolites in Petroleum Compounds Spill Cleanup—Production, Efficiency, Utilization. Fuel Process. Technol. 2016, 149, 153–162. [Google Scholar] [CrossRef]
- Pabiś-Mazgaj, E.; Gawenda, T.; Pichniarczyk, P.; Stempkowska, A. Mineral Composition and Structural Characterization of the Clinoptilolite Powders Obtained from Zeolite-Rich Tuffs. Minerals 2021, 11, 1030. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases. with special reference to the evaluation of surface area and pore size distribution (UPAC Technical Report). Pure Appl. Chem. 2015, 87, 9–10. [Google Scholar] [CrossRef]
- Journal of Laws of the Republic of Poland; No. 85, item 553; Polish Public Procurement Office: Warsaw, Poland, 2010.
- Król, M.; Rożek, P. Sorption of oil products on the synthetic zeolite granules. Mineralogia 2020, 51, 1–7. [Google Scholar] [CrossRef]
- Bandura, L.; Franus, M.; Józefaciuk, G.; Franus, W. Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Bandura, L.; Franus, M.; Panek, R.; Woszuk, A.; Franus, W. Characterization of zeolites and their use as adsorbents of petroleum substances. Przem. Chem. 2015, 3, 323–327. [Google Scholar]
- Pabiś-Mazgaj, E.; Pichniarczyk, P.; Stempkowska, A.; Gawenda, T. Possibility of Using Natural Zeolite Waste Granules Obtained by Pressure Agglomeration as a Sorbent for Petroleum Substances from Paved Surfaces. Materials 2022, 15, 6871. [Google Scholar] [CrossRef]
- Müller, P.; Russell, A.; Tomas, J. Influence of binder and moisture content on the strength of zeolite 4A granules. Chem. Eng. Sci. 2015, 126, 204–215. [Google Scholar] [CrossRef]
- García-Martínez, J.; Cazorla-Amorós, D.; Linares-Solano, A.; Lin, Y.S. Synthesis and Characterization of MFI-Type Zeolites Supported on Carbon Materials. Microporous Mesoporous Mater. 2001, 42, 255–268. [Google Scholar] [CrossRef]
- Sharma, K.; Shah, G.; Singh, H.; Bhatt, U.; Singhal, K.; Soni, V. Advancements in natural remediation management techniques for oil spills: Challenges, innovations, and future directions. Environ. Pollut. Manag. 2024, 1, 128–146. [Google Scholar] [CrossRef]
- Salamonowicz, Z.; Grudziecki, A.K.; Lipińsky, D.; Dmochowska, A. Comparative analysis of the absorbency of sorbents determined by various method for diesel oil and white spirit. Sci. Rep. Fire Univ. 2023, 1, 297–310. [Google Scholar] [CrossRef]
- Carmody, O.; Frost, R.; Xi, Y.; Kokot, S. Surface characterisation of selected sorbent materials for common hydrocarbon fuels. Surf. Sci. 2007, 601, 2066–2076. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]








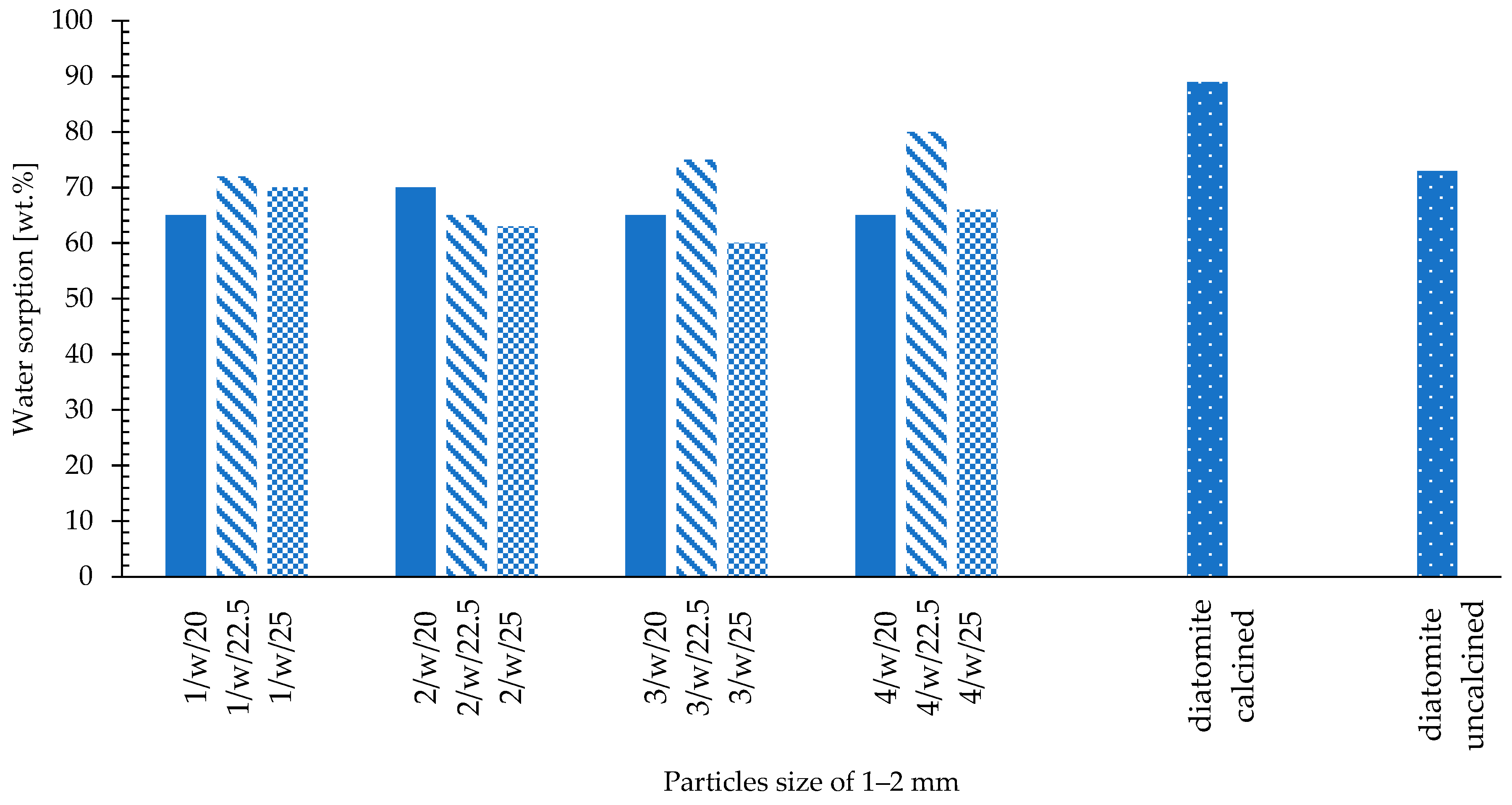
| Produced Zeolite-Based Agglomerates | Binder [%] | Zeolite Powder [%] | Water [%] |
|---|---|---|---|
| 1/w/20 | 2.5 | 97.5 | 20 |
| 2/w/20 | 5.0 | 95.0 | |
| 3/w/20 | 7.5 | 92.5 | |
| 4/w/20 | 10.0 | 90.0 | |
| 1/w/22.5 | 2.5 | 97.5 | 22.5 |
| 2/w/22.5 | 5.0 | 95.0 | |
| 3/w/22.5 | 7.5 | 92.5 | |
| 4/w/22.5 | 10.0 | 90.0 | |
| 1/w/25 | 2.5 | 97.5 | 25 |
| 2/w/25 | 5.0 | 95.0 | |
| 3/w/25 | 7.5 | 92.5 | |
| 4/w/25 | 10.0 | 90.0 |
| Oxide Composition [wt.%] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | P2O5 | TiO2 | Mn2O3 | SrO | ZnO | LOI |
| 68.76 | 11.7 | 1.98 | 2.58 | 0.72 | 0.25 | 3.28 | 1.21 | 0.02 | 0.16 | 0.01 | 0.04 | 0.01 | 9.28 |
| Powder Type | BET [m2/g] | Specific Gravity [g/cm3] | Loos Bulk Density [g/cm3] | Tapped Bulk Density [g/cm3] | Compaction [%] | Moisture Content [%] | Avg. Grain Size [µm] |
|---|---|---|---|---|---|---|---|
| Zeolite | 11.58 | 2.31 | 0.97 | 1.190 | 18.5 | 0.70 | 17.75 |
| Binder | 9.94 | 2.2 | 0.47 | 0.78 | 39.7 | 0.53 | 2,91 |
| Sample | SBET [m2/g] | Vtot 0.99 [cm3/g] | V BJHmes [cm3/g] | VmicBJH [cm3/g] | Vmac [cm3/g] | Average Pore Diameter 4V/A by BET [nm] | Average Pore width 4V/A by BJH [nm] |
|---|---|---|---|---|---|---|---|
| Zeolite powder | 11.58 | 0.048 | 0.043 | 0.001 | 0.0041 | 1.01 | 20.11 |
| Produced Zeolite-Based Agglomerates | Ribbon Moisture [%] | Ribbons Fragility | Ribbons Powdering |
|---|---|---|---|
| 1/w/20 | 17.6 | + | P |
| 2/w/20 | 16.7 | ++ | P |
| 3/w/20 | 19.5 | ++++ | PP |
| 4/w/20 | 17.6 | ++++ | PPP |
| 1/w/22.5 | 19.4 | + | P |
| 2/w/22.5 | 18.9 | +++ | P |
| 3/w/22.5 | 19.0 | +++++ | PP |
| 4/w/22.5 | 18.2 | +++++ | PPP |
| 1/w/25 | 22.0 | + | P |
| 2/w/25 | 22.9 | ++ | P |
| 3/w/25 | 23.3 | ++++ | PP |
| 4/w/25 | 23.1 | ++++ | PPP |
| Produced Zeolite-Based Agglomerates | Loose Bulk Density | Drop Strength [%] | |
|---|---|---|---|
| Granule Size [mm] | |||
| 0.5–1 | 1–2.5 | ||
| 1/w/20 | 881 | 87 | 84 |
| 2/w/20 | 860 | 95 | 93 |
| 3/w/20 | 853 | 99 | 98 |
| 4/w/20 | 824 | 98 | 98 |
| 1/w/22.5 | 875 | 88 | 86 |
| 2/w/22.5 | 850 | 95 | 93 |
| 3/w/22.5 | 921 | 98 | 97 |
| 4/w/22.5 | 854 | 99 | 98 |
| 1/w/25 | 879 | 90 | 88 |
| 2/w/25 | 865 | 95 | 95 |
| 3/w/25 | 877 | 98 | 98 |
| 4/w/25 | 850 | 98 | 98 |
| Uncalcined granular diatomite | - | 100 | 94 |
| Calcined granular diatomite | - | 100 | 100 |
| Mineral Sorbent | Diesel Oil Sorption [wt.%] (Westinghouse Test) | Reference |
|---|---|---|
| Clinoptilolite-type zeolite (natural zeolite dust) | 50 | [63] |
| Expanded glass | 70 | [63] |
| Commercial synthetic zeolite LTA | 20 | [63] |
| Synthetic zeolite Na-P1 | 91 | [64] |
| Synthetic zeolite Na-P1 | 110 | [63] |
| Synthetic zeolite Na-P1 | 118 | [59] |
| Synthetic zeolite Na-P1 | 140 | [65] |
| Commercial sorbent DAMSORB | 83 | [66] |
| Produced Zeolite-Based Agglomerates | Particle Fraction [mm] | Total volume of Intrusion [cm3/g] | Total Pore Surface [m2/g] | Average Pore Radius [2V/A] | Apparent Density [g/cm3] | Specific Surface Area [m2/g] |
|---|---|---|---|---|---|---|
| 3/w/20 | 0.5–1 | 16.1056 | 7.8984 | 4.0782 | 1.23 | 18.33 |
| 3/w/25 | 13.5428 | 7.0563 | 3.8385 | 1.17 | 19.59 | |
| 4/w/22.5 | 12.1479 | 6.788 | 3.579 | 1.31 | 18.72 | |
| 3/w/22.5 | 13.1967 | 5.6687 | 4.656 | 1.20 | 16.15 | |
| 3/w/22.5 | 1–2 | 11.952 | 6.8599 | 3.485 | 1.31 | 17.12 |
| Produced Zeolite-Based Agglomerates | Particle Fraction [mm] | Porosity | Pore Size | Mode | ||||
|---|---|---|---|---|---|---|---|---|
| 1–10 nm | 10–100 nm | 100 nm–1 µm | 1–10 µm | 10–100 µm | [µm] | |||
| Pore Content (%) | ||||||||
| 3/w/20 | 0.5–1 | 43.09 | 2.1 | 33.6 | 7.8 | 42.9 | 13.5 | 0.034 |
| 3/w/25 | 36.02 | 1.2 | 41.3 | 20.4 | 24.2 | 14.2 | 0.035 | |
| 4/w/22.5 | 36.26 | 1.5 | 39.8 | 18.8 | 23.7 | 16.2 | 4.87 | |
| 3/w/22.5 | 35.10 | 2.3 | 35.6 | 16.3 | 29.4 | 16.5 | 4.64 | |
| 3/w/22.5 | 1–2 | 40.07 | 0.7 | 35.1 | 17.5 | 39.9 | 6.8 | 4.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pabiś-Mazgaj, E.; Stempkowska, A.; Gawenda, T. Eco-Friendly Conversion of Waste Zeolite Dust into Dual Oil/Water Affinity Sorbents via HPGR-Based Agglomeration–Deagglomeration. Sustainability 2025, 17, 4359. https://doi.org/10.3390/su17104359
Pabiś-Mazgaj E, Stempkowska A, Gawenda T. Eco-Friendly Conversion of Waste Zeolite Dust into Dual Oil/Water Affinity Sorbents via HPGR-Based Agglomeration–Deagglomeration. Sustainability. 2025; 17(10):4359. https://doi.org/10.3390/su17104359
Chicago/Turabian StylePabiś-Mazgaj, Ewelina, Agata Stempkowska, and Tomasz Gawenda. 2025. "Eco-Friendly Conversion of Waste Zeolite Dust into Dual Oil/Water Affinity Sorbents via HPGR-Based Agglomeration–Deagglomeration" Sustainability 17, no. 10: 4359. https://doi.org/10.3390/su17104359
APA StylePabiś-Mazgaj, E., Stempkowska, A., & Gawenda, T. (2025). Eco-Friendly Conversion of Waste Zeolite Dust into Dual Oil/Water Affinity Sorbents via HPGR-Based Agglomeration–Deagglomeration. Sustainability, 17(10), 4359. https://doi.org/10.3390/su17104359








