High Performance Ru-CNx/CeO2 Catalyst for Catalytic Wet Oxidation of N-Methyldiethanolamine in Water
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Wet Oxidation (CWO) of MDEA
3. Results and Discussion
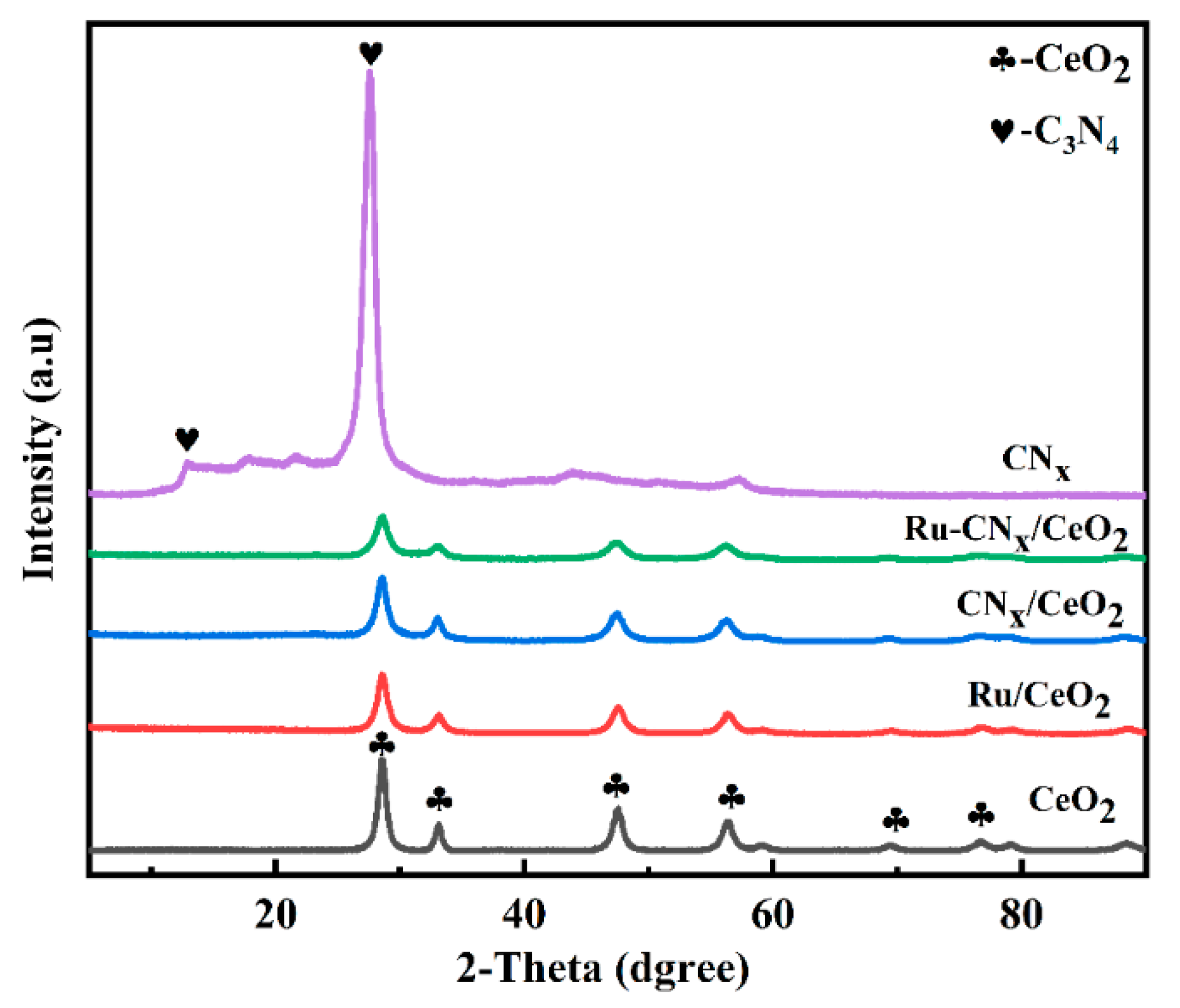
3.1. XRD Results
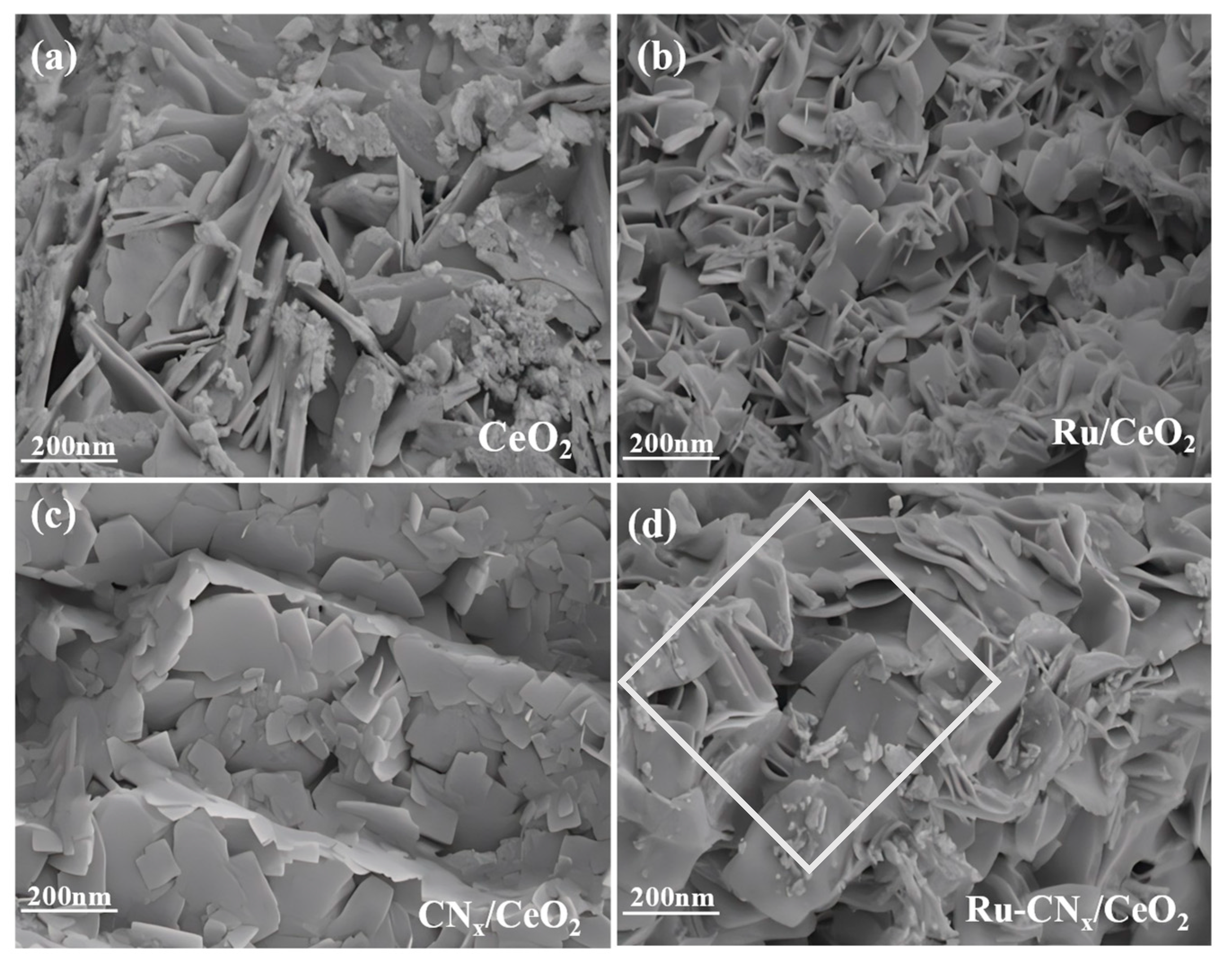
3.2. SEM and BET Surface Area (SA) Results
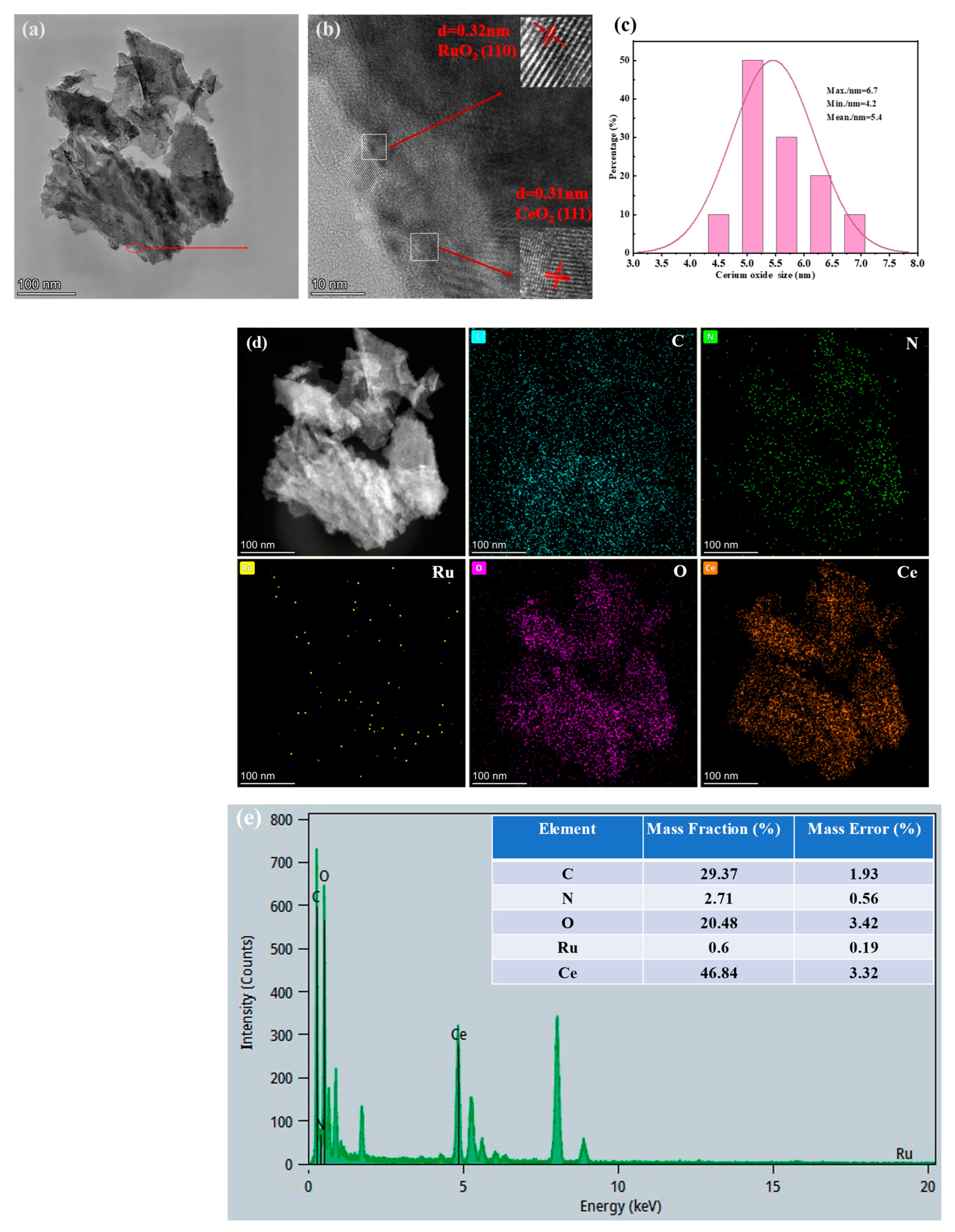
3.3. TEM and Mapping Results
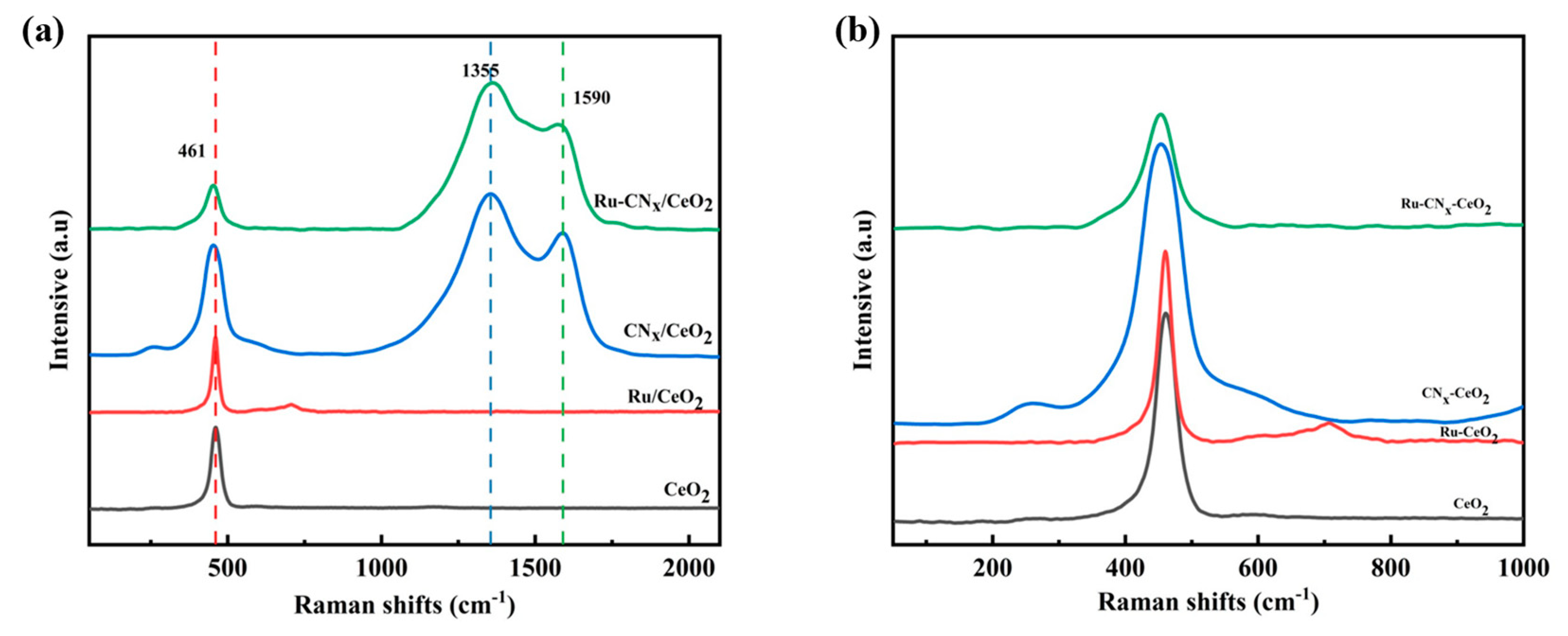
3.4. UV Raman Results
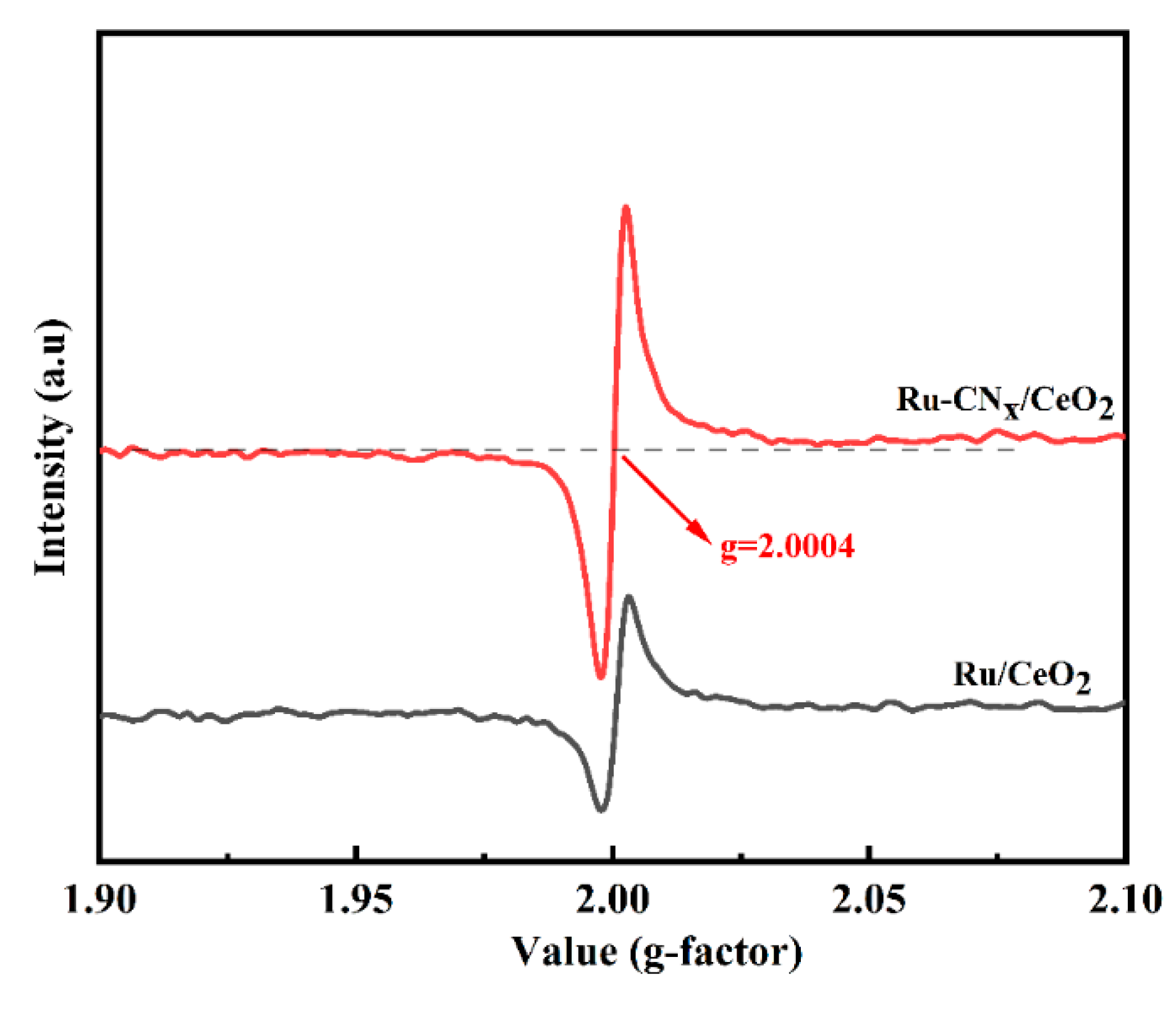
3.5. EPR Results
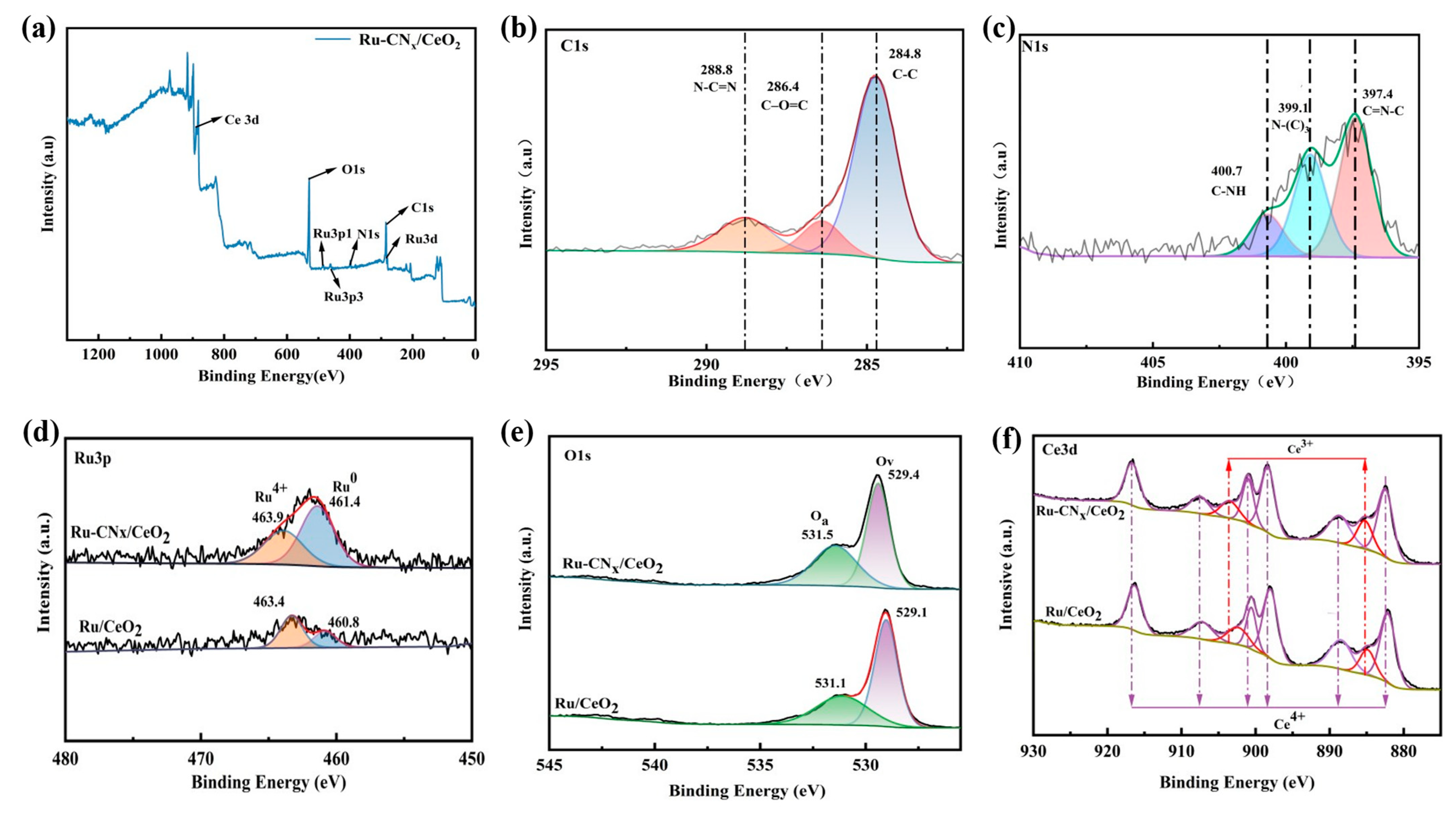
3.6. XPS Results
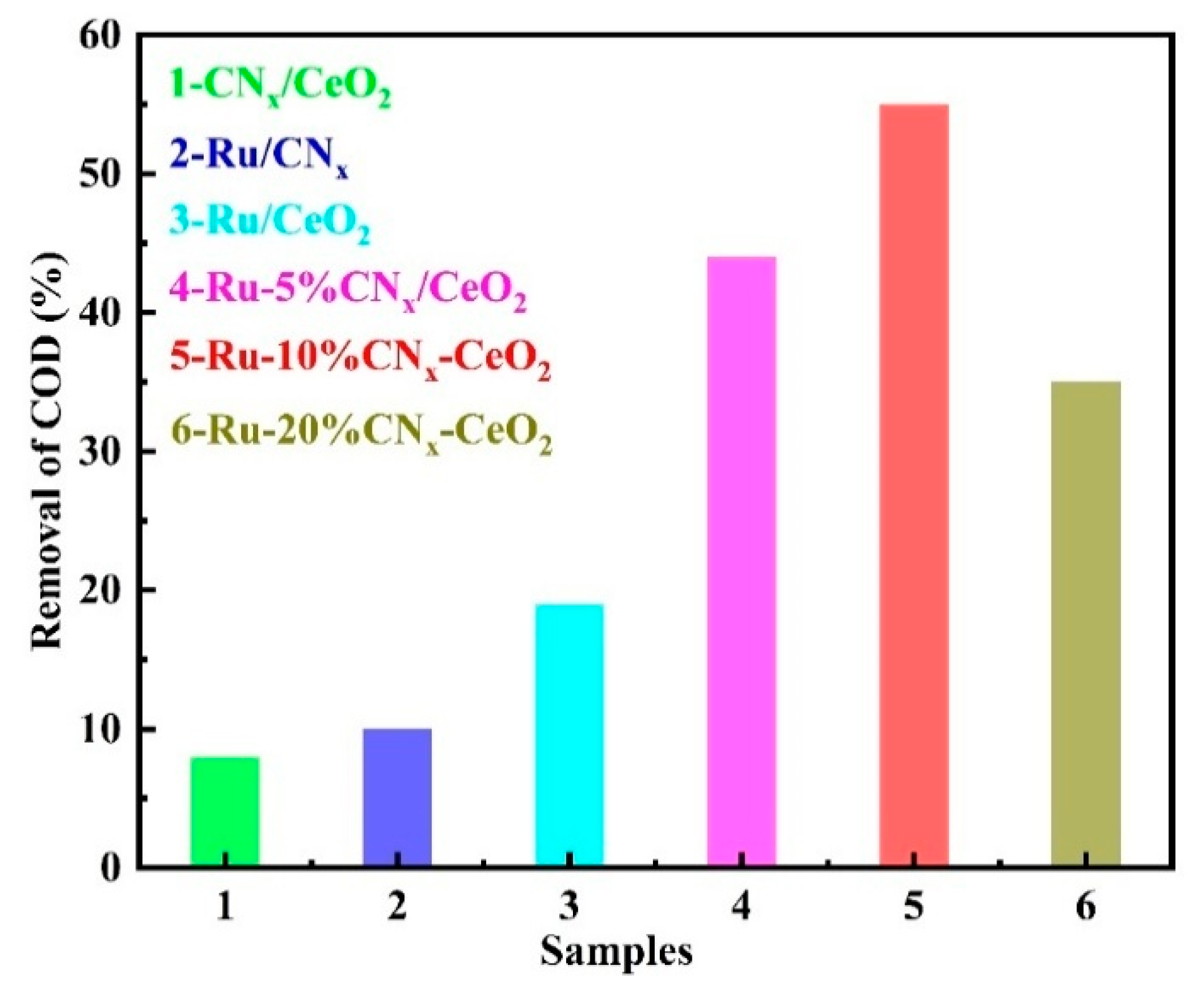
3.7. Catalytic Performances of the Prepared Catalysts
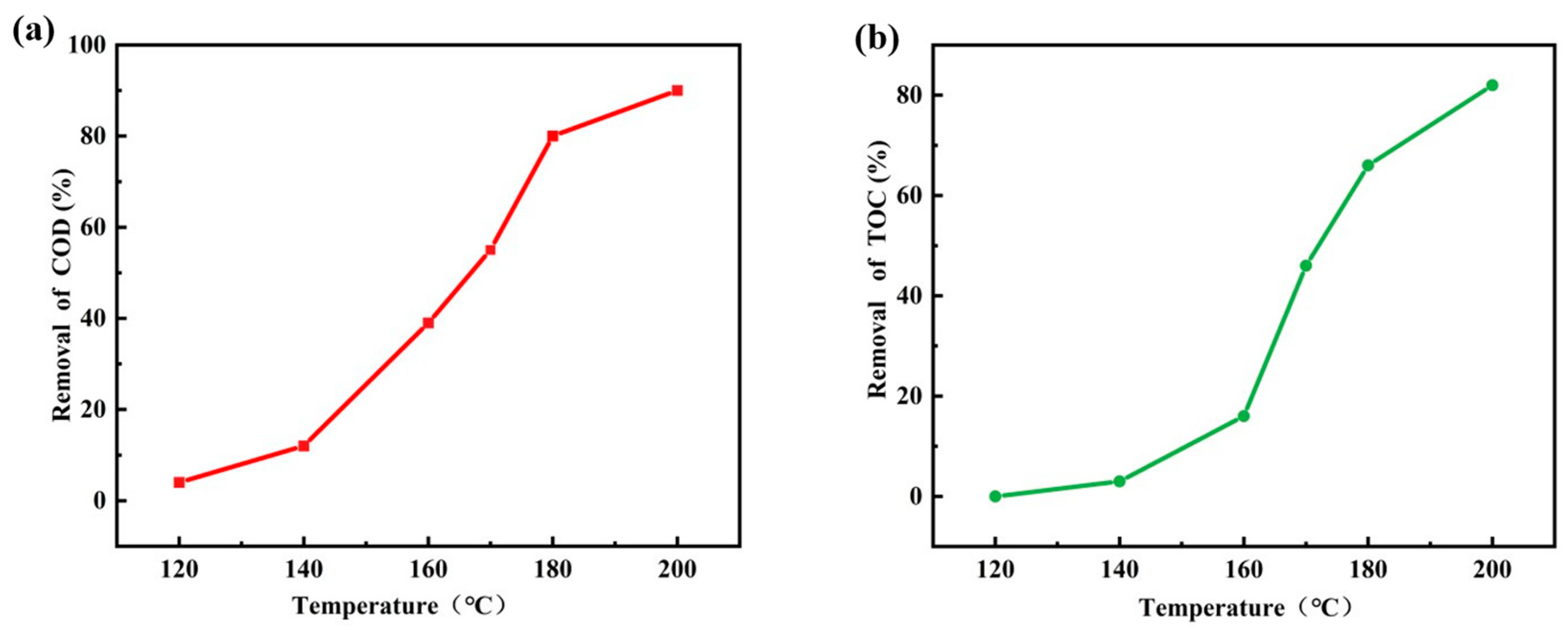
3.8. Effect of Reaction Temperature
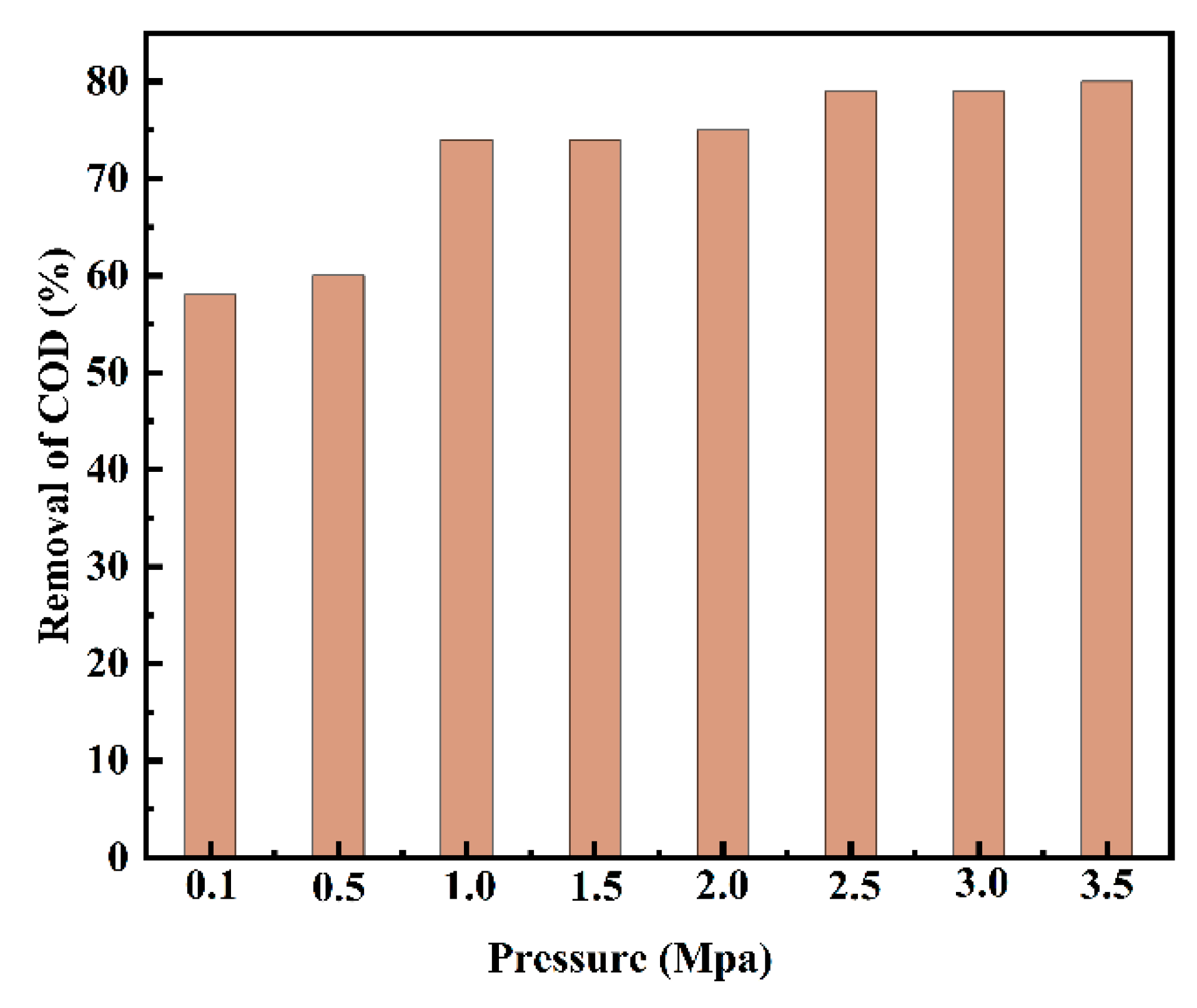
3.9. Effect of Reaction Pressure
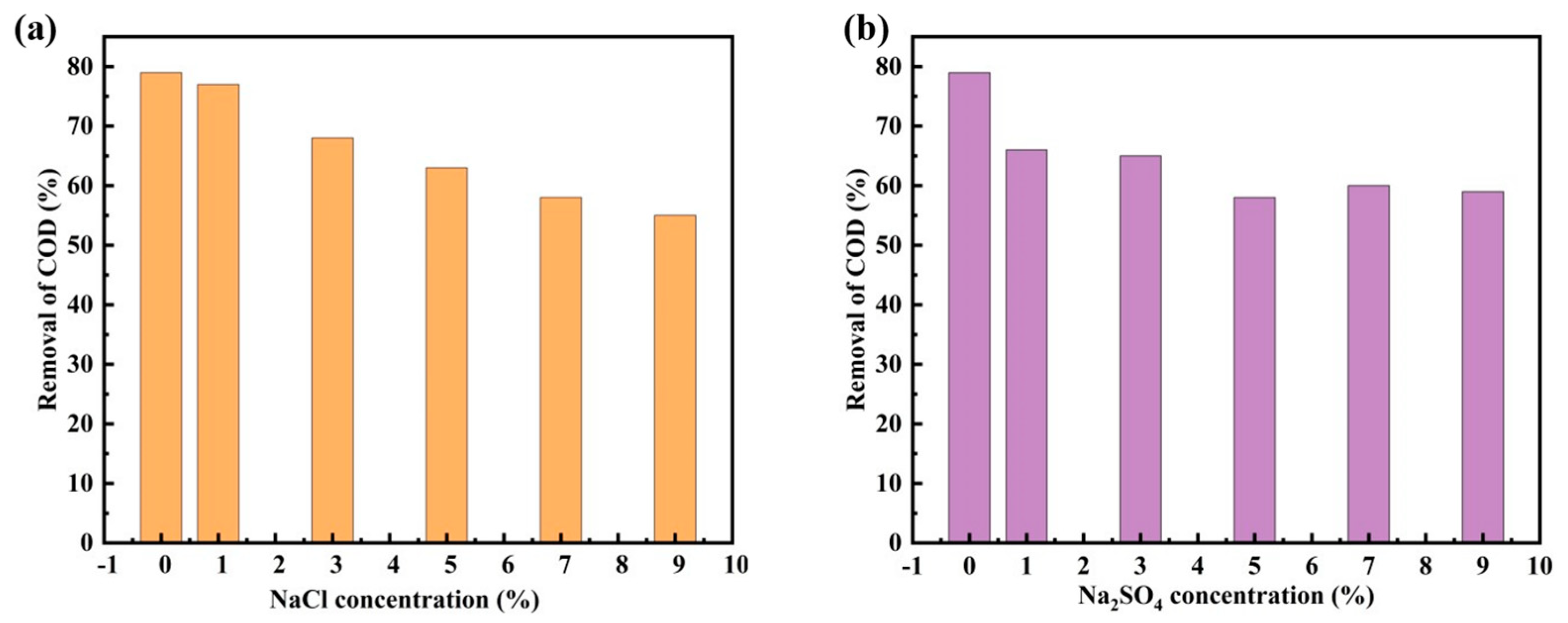
3.10. Effect of Inorganic Salt
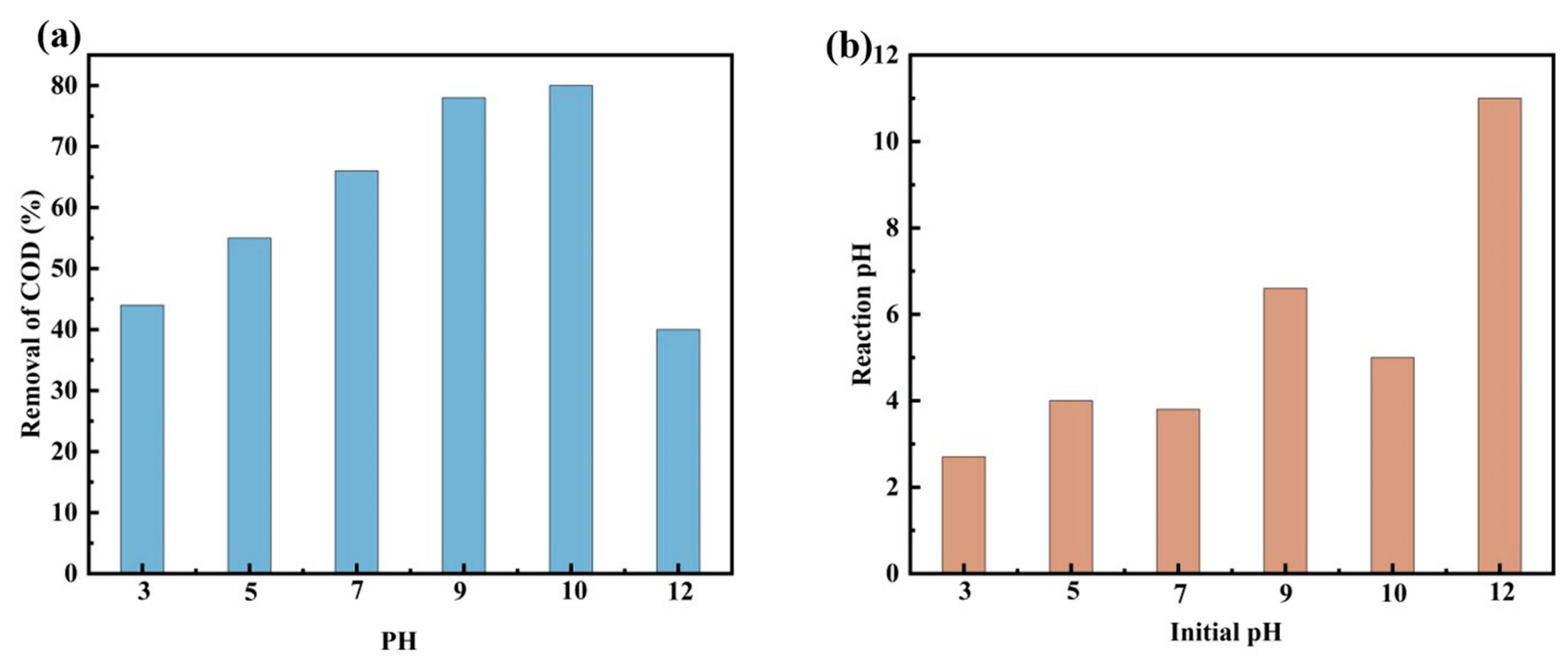
3.11. Effect of pH
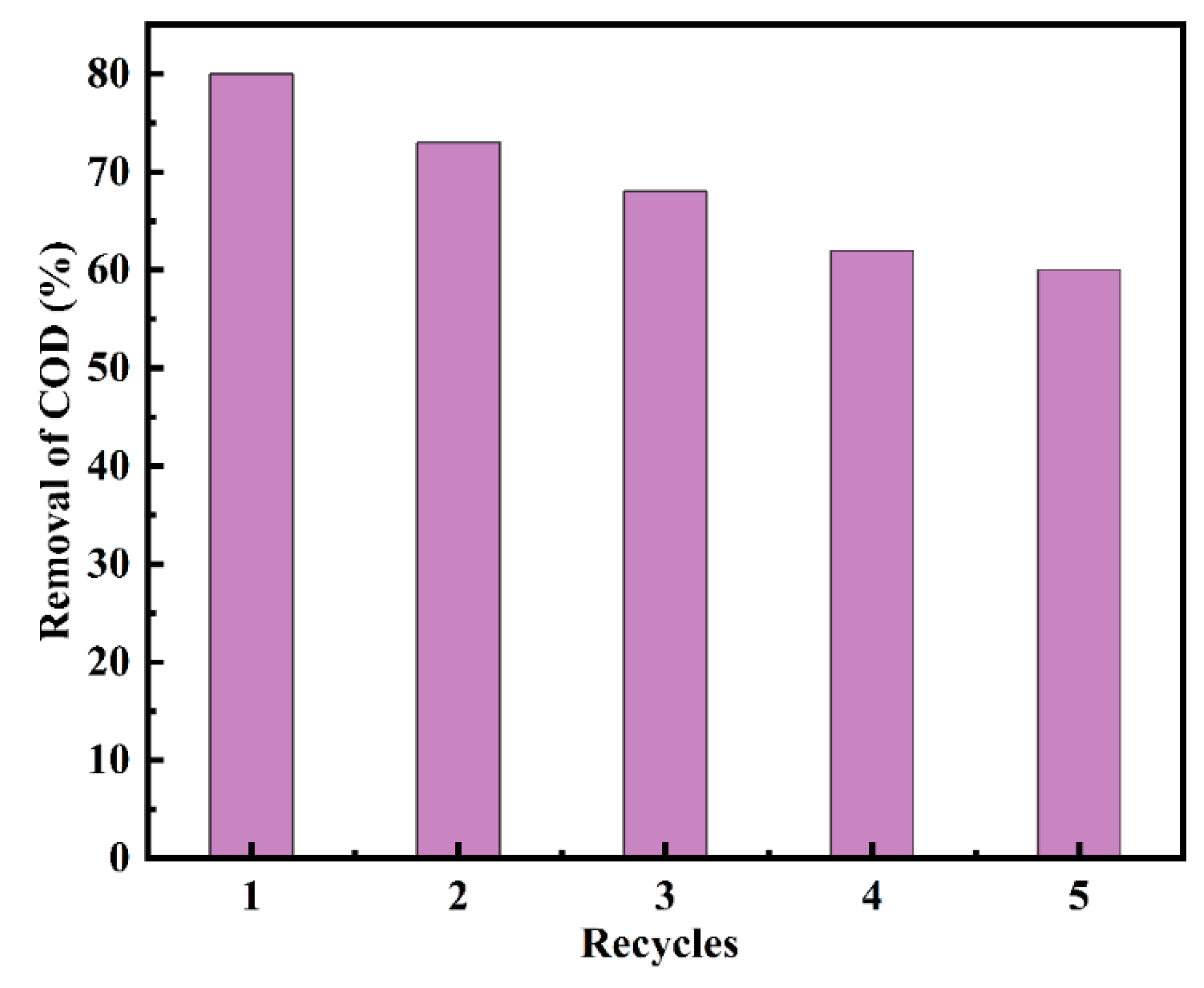
3.12. Ru-CNx/CeO2 Reusability and Stability
3.13. Discussion
3.13.1. The Promoted Effect of the CNx
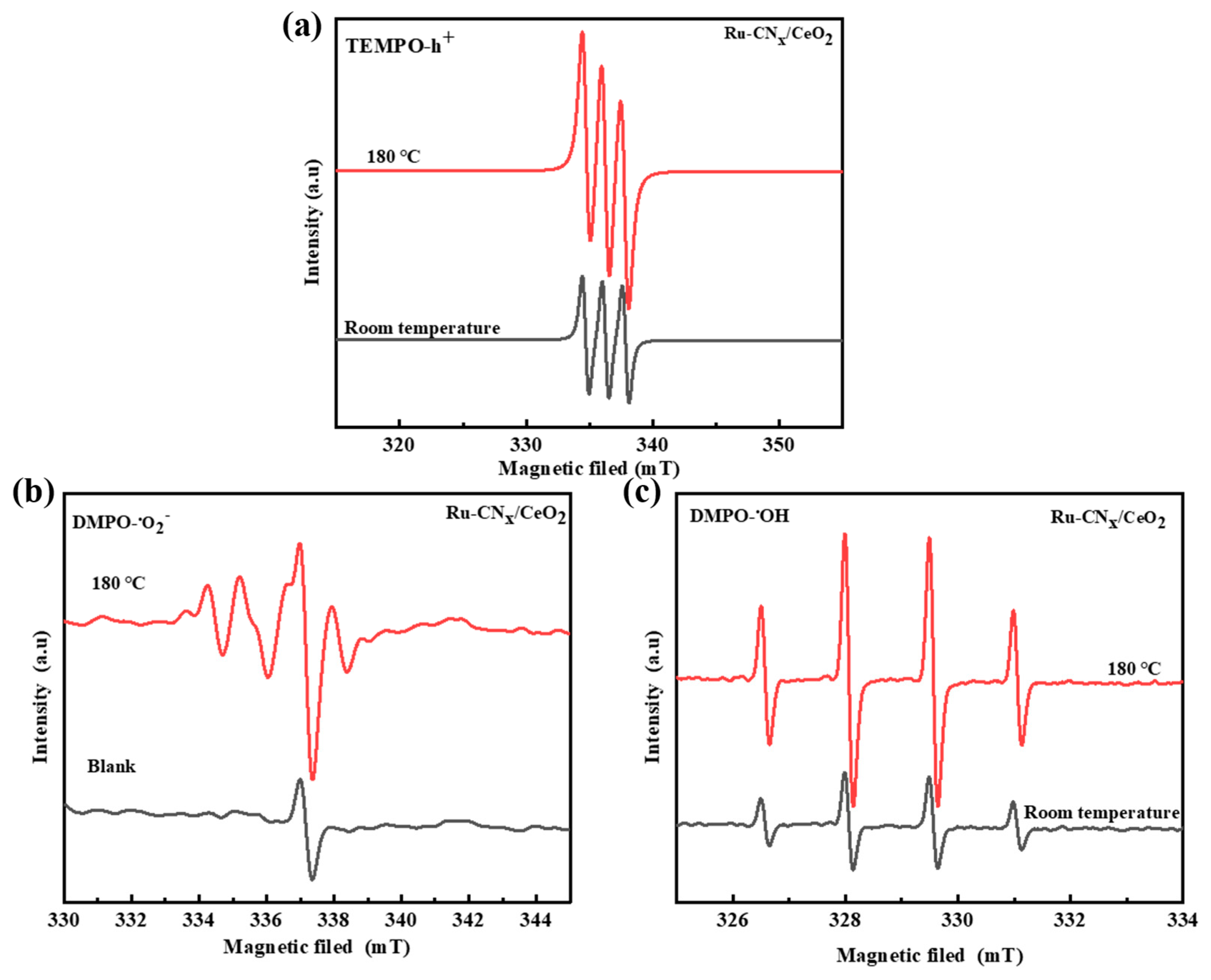
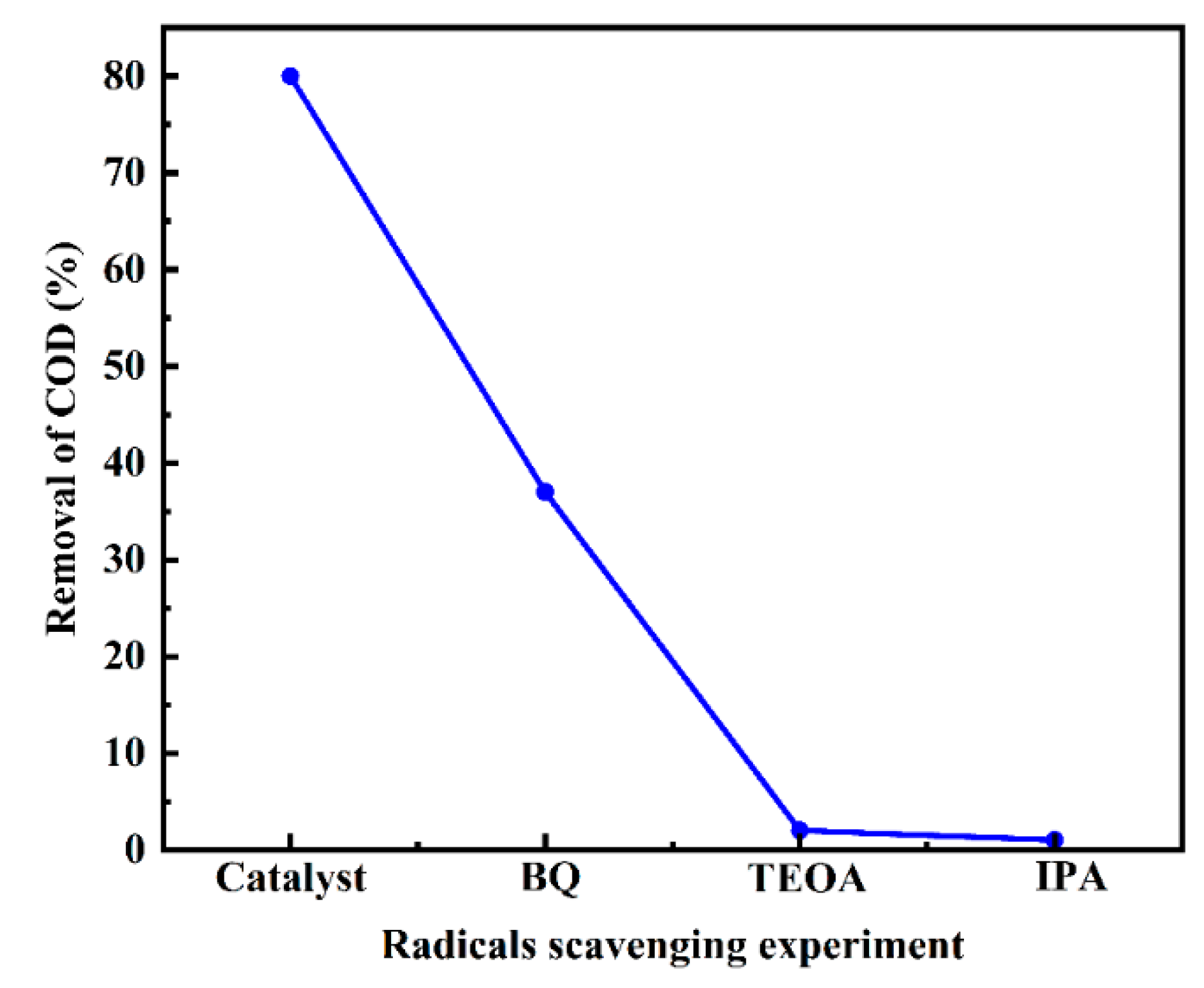
3.13.2. The Active Species on the Ru-CNx/CeO2 Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, P.; AbuKashabeh, A.; Al-Asheh, S.; Banat, F. Role of aqueous methyldiethanolamine (MDEA) as solvent in natural gas sweetening unit and process contaminants with probable reaction pathway. J. Nat. Gas. Sci. Eng. 2015, 24, 124–131. [Google Scholar] [CrossRef]
- Ghanbarabadi, H.; Gohari, F.K.Z. Optimization of MDEA concentration in flow of input solvent to the absorption tower and its effect on the performance of other processing facilities of gas treatment unit in Sarakhs refinery. J. Nat. Gas. Sci. Eng. 2014, 20, 208–213. [Google Scholar] [CrossRef]
- Randall, T.L.; Knopp, P.V. Detoxification of specific organic substances by wet oxidation. Water Pollut. Control. Fed. 1980, 52, 2117–2130. [Google Scholar]
- Yang, L.; Chen, Z.; Zhang, D.; Liu, Y.; Han, Y.; Shen, J. Adsorption of dimethylamine from aqueous solution by manganese dioxide. Water Sci. Technol. 2011, 63, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Fürhacker, M.; Pressl, A.; Allabashi, R. Aerobic biodegradability of methyldiethanolamine (MDEA) used in natural gas sweetening plants in batch tests and continuous flow experiments. Chemosphere 2003, 52, 1743–1748. [Google Scholar] [CrossRef]
- Samira Molareza, G.; Ahmadi, M.; Zinati Zadeh, A.A. Photochemical oxidation of methyldiethanolamine (MDEA) in aqueous solution by UV/K2S2O8 process. Int. J. Ind. Chem. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Wang, B.; Tian, K.; Xiong, X.; Ren, H. Treatment of overhaul wastewater containing N-methyldiethanolamine (MDEA) through modified Fe–C microelectrolysis-configured ozonation: Investigation on process optimization and degradation mechanisms. J. Hazard. Mater. 2019, 369, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yue, D.; Wang, B.; Ren, H. Degradation of MDEA in aqueous solution in the thermally activated persulfate system. Environ. Technol. 2017, 38, 730–736. [Google Scholar] [CrossRef]
- Ali, R.; ABU, B.W.; Mislan, S.S.; Sharifuddin, M.A. Photodegradation of n-methyldiethanolamine over ZnO/SnO2 coupled photocatalysts. Sci. Iran 2010, 17, 124–130. [Google Scholar]
- Jiang, Y.; Li, S.; Fan, X.; Tang, Z. Recent advances on aerobic photocatalytic methane conversion under mild conditions. Nano Res. 2023, 16, 12558–12571. [Google Scholar] [CrossRef]
- Petrović, M.; Rančev, S.; Đorđević, M.P.; Najdanović, S.; Velinov, N.; Vučić, M.R.; Bojić, A. Electrochemically synthesized Molybdenum oxides for enhancement of atmospheric pressure non-thermal pulsating corona plasma induced degradation of an organic compound. Chem. Eng. Sci. 2021, 230, 116209. [Google Scholar] [CrossRef]
- Sun, W.; Wei, H.; Yang An, L.; Jin, C.; Wu, H.; Xiong, Z.; Pu, C.; Sun, C. Oxygen vacancy mediated La1-xCexFeO3-δ perovskite oxides as efficient catalysts for CWAO of acrylic acid by A-site Ce doping. Appl. Catal. B Environ. 2019, 245, 20–28. [Google Scholar] [CrossRef]
- Silva, J.A. Advanced Oxidation Process in the Sustainable Treatment of Refractory Wastewater: A Systematic Literature Review. Sustainability 2025, 17, 3439. [Google Scholar] [CrossRef]
- Le Phuong, T.; Besson, M. Catalytic wet air oxidation using supported Pt and Ru catalysts for treatment of distillery wastewater (cognac and sugarcane vinasses). Energies 2019, 12, 3974. [Google Scholar] [CrossRef]
- Luck, F. Wet air oxidation: Past, present and future. Catal. Today 1999, 53, 81–91. [Google Scholar] [CrossRef]
- Luck, F. A review of industrial catalytic wet air oxidation processes. Catal. Today 1996, 27, 195–202. [Google Scholar] [CrossRef]
- Kim, K.; Ihm, S. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review. J. Hazard. Mater. 2011, 186, 16–34. [Google Scholar] [CrossRef]
- Rabelo, A.C.; Rosario, A.V.; Trapp, M.A.; Forim, M.; Pereira, E.C. Catalytic wet air oxidation of Methyl Orange onto Pt and Pt–TiO2. J. Nanosci. Nanotechnol. 2016, 16, 10040–10047. [Google Scholar] [CrossRef]
- Yang, S.; Besson, M.; Descorme, C. Catalytic wet air oxidation of succinic acid over Ru and Pt catalysts supported on CexZr1−xO2 mixed oxides. Appl. Catal. B Environ. 2015, 165, 1–9. [Google Scholar] [CrossRef]
- Bernardi, M.; Le Du, M.; Dodouche, I.; Descorme, C.; Deleris, S.; Blanchet, E.; Besson, M. Selective removal of the ammonium-nitrogen in ammonium acetate aqueous solutions by catalytic wet air oxidation over supported Pt catalysts. Appl. Catal. B Environ. 2012, 128, 64–71. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.; Zhang, Y.; Yu, D.; Piao, W.; Wei, H.; Liu, X.; Sun, C. Effect of inorganic salt on the removal of typical pollutants in wastewater by RuO2/TiO2 via catalytic wet air oxidation. Chemosphere 2023, 312, 137194. [Google Scholar] [CrossRef] [PubMed]
- Bensouilah, R.; Hammedi, T.; Ouakouak, A.; Ghorbel, A.; Ksibi, Z. Comparative study of the efficiency of different noble metals supported on zirconium oxide in the catalytic wet air oxidation of bisphenol-A solution. Chem. Phys. Lett. 2020, 761, 138022. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Yang, S.; Wang, W.; Zhou, Y. Catalytic wet air oxidation of phenol with pelletized ruthenium catalysts. Appl. Catal. B Environ. 2008, 78, 30–37. [Google Scholar] [CrossRef]
- Oliviero, L.; Barbier Jr, J.; Duprez, D.; Wahyu, H.; Ponton, J.W.; Metcalfe, I.S.; Mantzavinos, D. Wet air oxidation of aqueous solutions of maleic acid over Ru/CeO2 catalysts. Appl. Catal. B Environl 2001, 35, 1–12. [Google Scholar] [CrossRef]
- Roy, S.; Barman, P. Unraveling the Structure-Dependent Activity of Ru/CeO2 Catalysts for Oxidative Degradation of Plastic Chemical Bisphenol A by Catalytic Wet Oxidation. Res. Sq. 2024, 1–28. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Shi, T.; Cui, W.; Zhang, X.; Dong, F.; Cheng, J.; Fan, J.; Lv, K. Carbon vacancy in C3N4 nanotube: Electronic structure, photocatalysis mechanism and highly enhanced activity. Appl. Catal. B Environ. 2020, 262, 118281. [Google Scholar] [CrossRef]
- Dong, G.; Jacobs, D.L.; Zang, L.; Wang, C. Carbon vacancy regulated photoreduction of NO to N2 over ultrathin g-C3N4 nanosheets. Appl. Catal. B Environ. 2017, 218, 515–524. [Google Scholar] [CrossRef]
- Rajendran, R.; Vignesh, S.; Sasireka, A.; Suganthi, S.; Raj, V.; Baskaran, P.; Shkir, M.; AlFaify, S. Designing Ag2O modified g-C3N4/TiO2 ternary nanocomposites for photocatalytic organic pollutants degradation performance under visible light: Synergistic mechanism insight. Colloid. Surf. A Physicochem. Eng. Asp. 2021, 629, 127472. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Priya, V.S.; Balakumar, V.; Prabavathi, S.L.; Muthuraj, V. Noble metal nanoparticles (Mx= Ag, Au, Pd) decorated graphitic carbon nitride nanosheets for ultrafast catalytic reduction of anthropogenic pollutant, 4-nitrophenol. Environ. Res. 2022, 212, 113185. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, Y.; Liu, Z.; Zhang, L.; Cui, Q.; Wang, H. Study on heterogeneous catalytic wet air oxidation process of high concentration MDEA-containing wastewater. Chem. Eng. Process 2022, 171, 108744. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, J.; Li, J.; Li, X.; Ma, C.; Huo, P.; Yan, Y. Fabricated g-C3N4/Ag/m-CeO2 composite photocatalyst for enhanced photoconversion of CO2. Appl. Surf. Sci. 2020, 506, 144931. [Google Scholar] [CrossRef]
- Zheng, Q.; Ren, K.; Qin, L.; Wang, X.; Ma, J. Efficient removal of piperazine by catalytic wet air oxidation using RuNiCe/γ-Al2O3-activated carbon. Environ. Sci. Water Res. Technol. 2024, 10, 1653–1665. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, J.; Gao, L.; Zang, S.; Chen, L.; Wang, L.; Mo, L. CuO/CeO2 catalysts prepared by modified impregnation method for ethyl acetate oxidation. Chem. Eng. J. 2023, 471, 144667. [Google Scholar] [CrossRef]
- Balestrieri, M.; Colis, S.; Gallart, M.; Schmerber, G.; Ziegler, M.; Gilliot, P.; Dinia, A. Photoluminescence properties of rare earth (Nd, Yb, Sm, Pr)-doped CeO2 pellets prepared by solid-state reaction. J. Mater. Chem. C 2015, 3, 7014–7021. [Google Scholar] [CrossRef]
- Wu, C. Solvothermal synthesis of N-doped CeO2 microspheres with visible light-driven photocatalytic activity. Mater. Lett. 2015, 139, 382–384. [Google Scholar] [CrossRef]
- Sharma, P.; Sasson, Y. A photoactive catalyst Ru–gC3N4 for hydrogen transfer reaction of aldehydes and ketones. Green. Chem. 2017, 19, 844–852. [Google Scholar] [CrossRef]
- Huang, Z.; Cai, X.; Zang, S.; Li, Y.; Zheng, D.; Li, F. Strong metal support effect of Pt/g-C3N4 photocatalysts for boosting photothermal synergistic degradation of benzene. Int. J. Mol. Sci. 2023, 24, 6872. [Google Scholar] [CrossRef]
- Madhavi, V.; Kumar, P.J.; Kondaiah, P.; Hussain, O.M.; Uthanna, S. Effect of molybdenum doping on the electrochromic properties of tungsten oxide thin films by RF magnetron sputtering. Ionics 2014, 20, 1737–1745. [Google Scholar] [CrossRef]
- Rao, R.; Huang, Y.; Zhang, H.; Hu, C.; Dong, X.; Fang, W.; Zhou, Q.; Chen, Z.; Fang, S.; Jin, D. A simple melamine-assisted cellulose pyrolysis synthesis of magnetic and mesoporous N-doped carbon composites with excellent adsorption of Congo red. Sep. Purif. Technol. 2024, 347, 127678. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.; Wu, L.; He, R.; Qin, Z.; Luo, S.; Yang, L.; Zeng, J. IrO2 deposited on RuO2 as core-shell structured RuO2@ IrO2 for oxygen evolution reaction in electrochemical water electrolyzer. Mol. Catal. 2023, 551, 113619. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.; Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2014, 158, 96–105. [Google Scholar] [CrossRef]
- Barathi, D.; Rajalakshmi, N.; Ranjith, R.; Sangeetha, R.; Meyvel, S. Controllable synthesis of CeO2/g-C3N4 hybrid catalysts and its structural, optical and visible light photocatalytic activity. Diam. Relat. Mater. 2021, 111, 108161. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme gC3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Chen, F.; Xiang, Y.; Yan, J.; Chu, W. Facile fabrication of hollow structured Cu-Ce binary oxides and their catalytic properties for toluene combustion. Catal. Today 2021, 376, 239–246. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer III, H.M.; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Han, L.; Jing, F.; Luo, X.; Zhong, Y.; Wang, K.; Zang, S.; Teng, D.; Liu, Y.; Chen, J.; Yang, C. Environment friendly and remarkably efficient photocatalytic hydrogen evolution based on metal organic framework derived hexagonal/cubic In2O3 phase-junction. Appl. Catal. B Environ. 2021, 282, 119602. [Google Scholar] [CrossRef]
- Zou, Y.; Dong, L.; Yan, S.; Liu, J.; Mu, L.; Li, L.; Hu, Y.; Qi, H.; Mao, S.; Chen, Z. Activity enhancement of Ru/CeO2 for N-alkylation of amines with alcohols through tailoring metal-support interaction. J. Catal. 2024, 429, 115241. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Kang, Z.; Jin, S.; Zhang, X.; Zheng, X.; Qi, Z.; Zhu, J.; Pan, B.; Xie, Y. Oxygen vacancy associated single-electron transfer for photofixation of CO2 to long-chain chemicals. Nat. Commun. 2019, 10, 788. [Google Scholar] [CrossRef]
- Wang, H.; Yong, D.; Chen, S.; Jiang, S.; Zhang, X.; Shao, W.; Zhang, Q.; Yan, W.; Pan, B.; Xie, Y. Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved molecular oxygen activation. J. Am. Chem. Soc. 2018, 140, 1760–1766. [Google Scholar] [CrossRef]
- Li, L.; Chen, P.; Gloyna, E.F. Generalized kinetic model for wet oxidation of organic compounds. Aiche J. 1991, 37, 1687–1697. [Google Scholar] [CrossRef]
- You, C.; Wang, C.; Cai, M.; Liu, Y.; Zhu, B.; Li, S. Improved photo-carrier transfer by an internal electric field in BiOBr/Nrich C3N5 3D/2D S-scheme heterojunction for efficiently photocatalytic micropollutant removal. Acta Phys.-Chim. Sin. 2024, 40, 2407014. [Google Scholar] [CrossRef]
- Li, G.; Chai, S.; Zhang, G.; Liu, J.; Zhang, Y.; Lv, Y.; Wang, Y.; Zhao, Y. Deactivation characteristics of Ce-modified Cu-based carbon materials for catalytic wet air oxidation of phenol wastewater. J. Environ. Chem. Eng. 2022, 10, 108228. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Li, S.; Rong, K.; Wang, X.; Shen, C.; Yang, F.; Zhang, Q. Design of carbon quantum dots/CdS/Ta3N5 S-scheme heterojunction nanofibers for efficient photocatalytic antibiotic removal. Acta Phys.-Chim. Sin. 2024, 40, 2403005. [Google Scholar] [CrossRef]
- Li, S.; You, C.; Rong, K.; Zhuang, C.; Chen, X.; Zhang, B. Chemically bonded Mn0. 5Cd0. 5S/BiOBr S-scheme photocatalyst with rich oxygen vacancies for improved photocatalytic decontamination performance. Adv. Powder Mater. 2024, 3, 100183. [Google Scholar] [CrossRef]
- Dong, K.; Shen, C.; Yan, R.; Liu, Y.; Zhuang, C.; Li, S. Integration of plasmonic effect and S-scheme heterojunction into Ag/Ag3PO4/C3N5 photocatalyst for boosted photocatalytic levofloxacin degradation. Acta Phys. Chim. Sin 2024, 40, 2310013. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, M.; Zhuzhang, H.; Zhang, G.; Anpo, M.; Wang, X. Gradient Zn-doped poly heptazine imides integrated with a van der Waals homojunction boosting visible light-driven water oxidation activities. ACS Catal. 2021, 11, 13463–13471. [Google Scholar] [CrossRef]
- Jiang, J.; Ou-yang, L.; Zhu, L.; Zheng, A.; Zou, J.; Yi, X.; Tang, H. Dependence of electronic structure of g-C3N4 on the layer number of its nanosheets: A study by Raman spectroscopy coupled with first-principles calculations. Carbon 2014, 80, 213–221. [Google Scholar] [CrossRef]
- Xiao, C.; Goh, T.; Qi, Z.; Goes, S.; Brashler, K.; Perez, C.; Huang, W. Conversion of levulinic acid to γ-valerolactone over few-layer graphene-supported ruthenium catalysts. ACS Catal. 2016, 6, 593–599. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, Y.; Zong, Z.; Zhou, S.; Cui, H.; Tan, H. In situ growth of an ultrathin Cu/gC3N4 coating over SBA-15 for catalytic wet air oxidation of pollutants under extremely mild conditions. Green. Chem. 2024, 26, 6601–6615. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, S.; Dong, M.; Wang, J.; Fan, W. Ru/CeO2 catalyst with optimized CeO2 morphology and surface facet for efficient hydrogenation of ethyl levulinate to γ-valerolactone. J. Catal. 2020, 389, 60–70. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Zha, K.; Li, H.; Shi, L.; Zhang, D. Deep insight into the structure–activity relationship of Nb modified SnO2–CeO2 catalysts for low-temperature selective catalytic reduction of NO by NH3. Catal. Sci. Technol. 2017, 7, 502–514. [Google Scholar] [CrossRef]
- Sellick, D.R.; Aranda, A.; García, T.; López, J.M.; Solsona, B.; Mastral, A.M.; Morgan, D.J.; Carley, A.F.; Taylor, S.H. Influence of the preparation method on the activity of ceria zirconia mixed oxides for naphthalene total oxidation. Appl. Catal. B Environ. 2013, 132, 98–106. [Google Scholar] [CrossRef]
- Lv, Z.; Feng, J.; Zhao, R.; Shen, J.; Yang, W. Visible-light-driven photocatalytic degradation of ibuprofen by Cu-doped tubular C3N4: Mechanisms, degradation pathway and DFT calculation. Chemosphere 2024, 358, 142106. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Li, X.; Cao, H.; Guo, Y.; Zhang, C. Mn-incorporated Co3O4 bifunctional electrocatalysts for zinc-air battery application: An experimental and DFT study. Appl. Catal. B Environ. 2022, 319, 121909. [Google Scholar] [CrossRef]
- Khawaji, M.; Chadwick, D. Au–Pd NPs immobilised on nanostructured ceria and titania: Impact of support morphology on the catalytic activity for selective oxidation. Catal. Sci. Technol. 2018, 8, 2529–2539. [Google Scholar] [CrossRef]
- Jiang, K.; Dong, L.; Shen, Q.; Wu, W.; Wu, X.; Mei, J.; Yang, S. Chlorobenzene Oxidation over Phosphotungstic-Acid-Coated Cerium Oxide: Synergistic Effect of Phosphotungstic and Cerium Oxide and Inhibition Mechanism of Sulfur Dioxide. Sustainability 2024, 16, 2245. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Brosnahan, J.T.; Zhang, S.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhan, W. Ru/CeO2 catalyst with optimized CeO2 support morphology and surface facets for propane combustion. Environ. Sci. Technol. 2019, 53, 5349–5358. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhang, X.; Wei, M.; Evans, D.G.; Duan, X. Catalytic behavior of supported Ru nanoparticles on the (100), (110), and (111) facet of CeO2. J. Catal. 2015, 329, 177–186. [Google Scholar] [CrossRef]
- Chang, C.J.; Li, S.S.; Ko, C.M. Catalytic wet oxidations of phenol-and p-chlorophenol-contaminated waters. J. Chem. Technol. Biotechnol. 1995, 64, 245–252. [Google Scholar] [CrossRef]
- Delgado, J.J.; Chen, X.; Pérez-Omil, J.A.; Rodríguez-Izquierdo, J.M.; Cauqui, M.A. The effect of reaction conditions on the apparent deactivation of Ce–Zr mixed oxides for the catalytic wet oxidation of phenol. Catal. Today 2012, 180, 25–33. [Google Scholar] [CrossRef]
- Mao, Y.; Liang, J.; Jiang, L.; Shen, Q.; Zhang, Q.; Liu, C.; Zheng, H.; Liao, Y.; Cao, X.; Dong, H. Removal of microorganic pollutants in high salinity wastewater by comproportionation system of Fe (VI)/Fe (III): Enhancement of chloride and bicarbonate. Water Res. 2022, 214, 118182. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, S.; Song, L.; Zhu, B.; Myat, D.; Chen, J.; Gray, S.; Duke, M. UV/TiO2 photocatalytic oxidation of recalcitrant organic matter: Effect of salinity and pH. Water Sci. Technol. 2014, 70, 437–443. [Google Scholar] [CrossRef]
- Kumari, M.; Saroha, A.K. Performance of various catalysts on treatment of refractory pollutants in industrial wastewater by catalytic wet air oxidation: A review. J. Environ. Manag. 2018, 228, 169–188. [Google Scholar]
- Ao, X.; Wang, W.; Sun, W.; Lu, Z.; Li, C. Degradation and transformation of norfloxacin in medium-pressure ultraviolet/peracetic acid process: An investigation of the role of pH. Water Res. 2021, 203, 117458. [Google Scholar] [CrossRef]
- Yadav, B.R.; Garg, A. Efficacy of fresh and used supported copper-based catalysts for ferulic acid degradation by wet air oxidation process. Ind. Eng. Chem. Res. 2012, 51, 15778–15785. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, H.; Descorme, C.; Xie, Y. Phenolic compounds removal by wet air oxidation based processes. Front. Env. Sci. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Ye, Y.; Gao, L.; Xu, J.; Wang, L.; Mo, L.; Zhang, X. Effect of CuO species and oxygen vacancies over CuO/CeO2 catalysts on low-temperature oxidation of ethyl acetate. J. Rare Earth 2023, 41, 862–869. [Google Scholar] [CrossRef]
- Promhuad, P.; Sawatmongkhon, B.; Theinnoi, K.; Wongchang, T.; Chollacoop, N.; Sukjit, E.; Tunmee, S.; Tsolakis, A. Effect of Metal Oxides (CeO2, ZnO, TiO2, and Al2O3) as the Support for Silver-Supported Catalysts on the Catalytic Oxidation of Diesel Particulate Matter. ACS Omega 2024, 9, 19282–19294. [Google Scholar] [CrossRef]
- Pu, T.; Setiawan, A.; Mosevitzky Lis, B.; Zhu, M.; Ford, M.E.; Rangarajan, S.; Wachs, I.E. Nature and reactivity of oxygen species on/in silver catalysts during ethylene oxidation. ACS Catal. 2022, 12, 4375–4381. [Google Scholar] [CrossRef]
- Heydariyan, Z.; Monsef, R.; Salavati-Niasari, M. Insights into impacts of Co3O4-CeO2 nanocomposites on the electrochemical hydrogen storage performance of g-C3N4: Pechini preparation, structural design and comparative study. J. Alloy Compd. 2022, 924, 166564. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Zu, P.; Zhang, C.; Guan, J.; Song, C.; Sun, X.; Wang, S. Sonocatalytic degradation of ciprofloxacin using hydrogel beads of TiO2 incorporated biochar and chitosan. J. Hazard. Mater. 2022, 434, 128879. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Pandey, K.; Verma, N. Augmented complete mineralization of glyphosate in wastewater via microbial degradation post CWAO over supported Fe-CNF. Chem. Eng. J. 2022, 428, 132008. [Google Scholar] [CrossRef]
- Geng, K.; Wu, Y.; Jiang, G.; Liu, K.; Jiang, L. RuC@ g-C3N4 (H+)/TiO2 visible active photocatalyst: Facile fabrication and Z-scheme carrier transfer mechanism. Mol. Catal. 2018, 458, 33–42. [Google Scholar] [CrossRef]
| Catalysts | SA (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | CeO2 Crystal Size a (nm) |
|---|---|---|---|---|
| CeO2 b | 98.1 | 0.17 | 6.8 | 9.9 |
| Ru/CeO2 | 56.0 | 0.07 | 5.5 | 8.8 |
| CNx/CeO2 | 60.0 | 0.14 | 11.1 | 8.0 |
| Ru-CNx/CeO2 | 53.0 | 0.15 | 12.5 | 6.3 |
| Ru-CNx/CeO2 c | 47.2 | 0.15 | 9.6 | 8.3 |
| Sample | Surface Composition (at.%) | Atomic Concentration (at.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | N | Ru | O | Ce | Ru0/(Ru0 + Ru4+) | OA/OL | Ce3+/Ce4+ | |
| Ru/CeO2 | / | / | 2.73 | 43.63 | 13.02 | 0.38 | 0.45 | 0.17 |
| Ru-CNx/CeO2 | 43.07 | 5.95 | 3.78 | 36.80 | 10.31 | 0.72 | 0.72 | 0.25 |
| Ru-CNx/CeO2 a | 34.09 | 2.53 | 3.31 | 45.78 | 14.29 | 0.61 | 0.51 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Ye, Y.; Yu, W.; Zang, S.; Ji, L.; Li, S.; Mo, L. High Performance Ru-CNx/CeO2 Catalyst for Catalytic Wet Oxidation of N-Methyldiethanolamine in Water. Sustainability 2025, 17, 4358. https://doi.org/10.3390/su17104358
Han Y, Ye Y, Yu W, Zang S, Ji L, Li S, Mo L. High Performance Ru-CNx/CeO2 Catalyst for Catalytic Wet Oxidation of N-Methyldiethanolamine in Water. Sustainability. 2025; 17(10):4358. https://doi.org/10.3390/su17104358
Chicago/Turabian StyleHan, Yuantao, Yuchuan Ye, Wanjin Yu, Shaohong Zang, Lili Ji, Shijie Li, and Liuye Mo. 2025. "High Performance Ru-CNx/CeO2 Catalyst for Catalytic Wet Oxidation of N-Methyldiethanolamine in Water" Sustainability 17, no. 10: 4358. https://doi.org/10.3390/su17104358
APA StyleHan, Y., Ye, Y., Yu, W., Zang, S., Ji, L., Li, S., & Mo, L. (2025). High Performance Ru-CNx/CeO2 Catalyst for Catalytic Wet Oxidation of N-Methyldiethanolamine in Water. Sustainability, 17(10), 4358. https://doi.org/10.3390/su17104358








