Preparation of High-Belite Calcium Sulfoaluminate Cement and Calcium Sulfoaluminate Cement from Industrial Solid Waste: A Review
Abstract
1. Introduction
2. Preparation Process and Hydration Characteristics of Industrial Solid Waste-Based Calcium Sulphoaluminate Cements
2.1. Preparation of HBCSA/CSA Cements
2.1.1. Replaceability of Natural Raw Materials
2.1.2. Formulation Design Principles
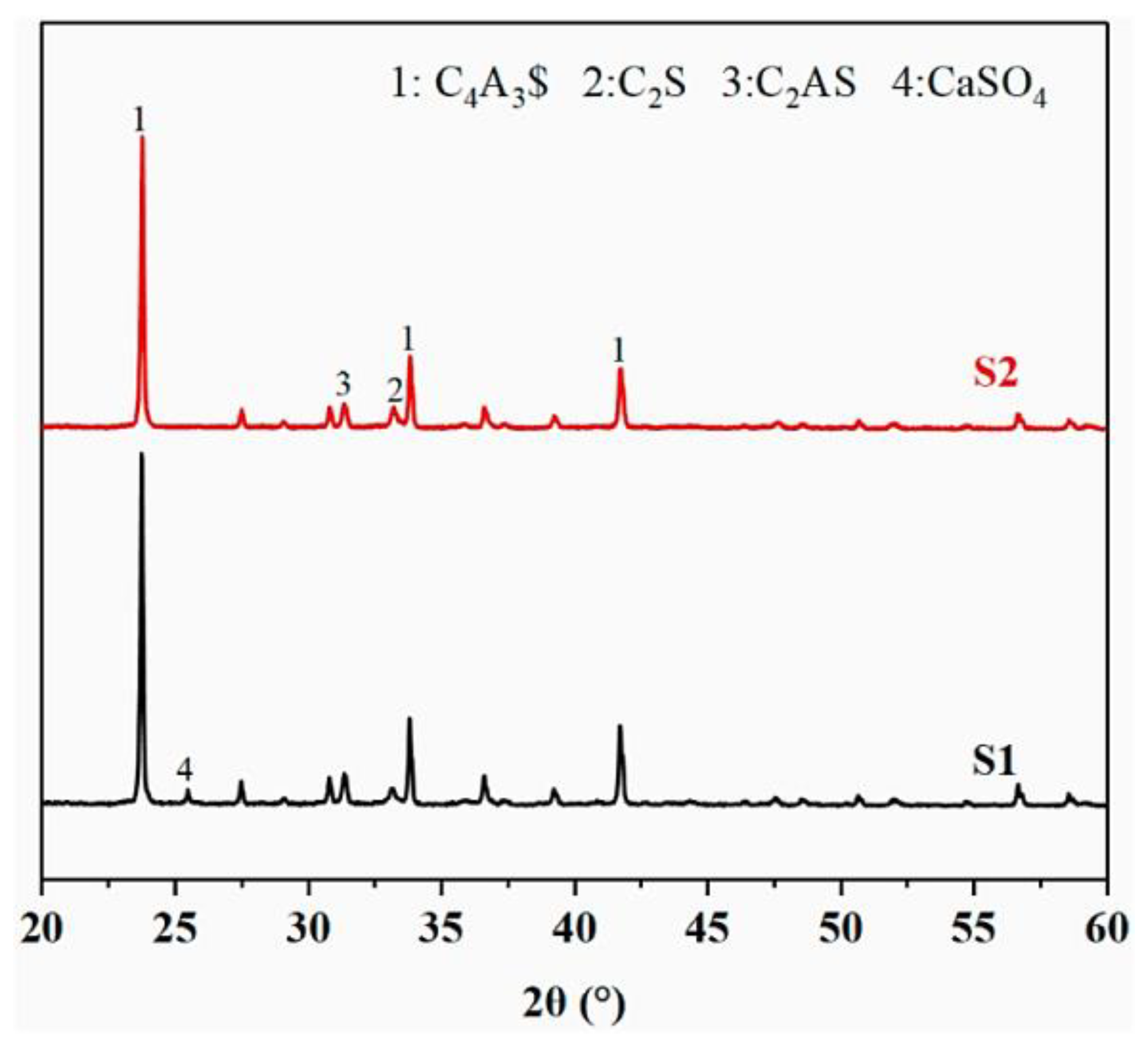
2.1.3. Determination of Calcination Regime
2.2. Hydration Characteristics
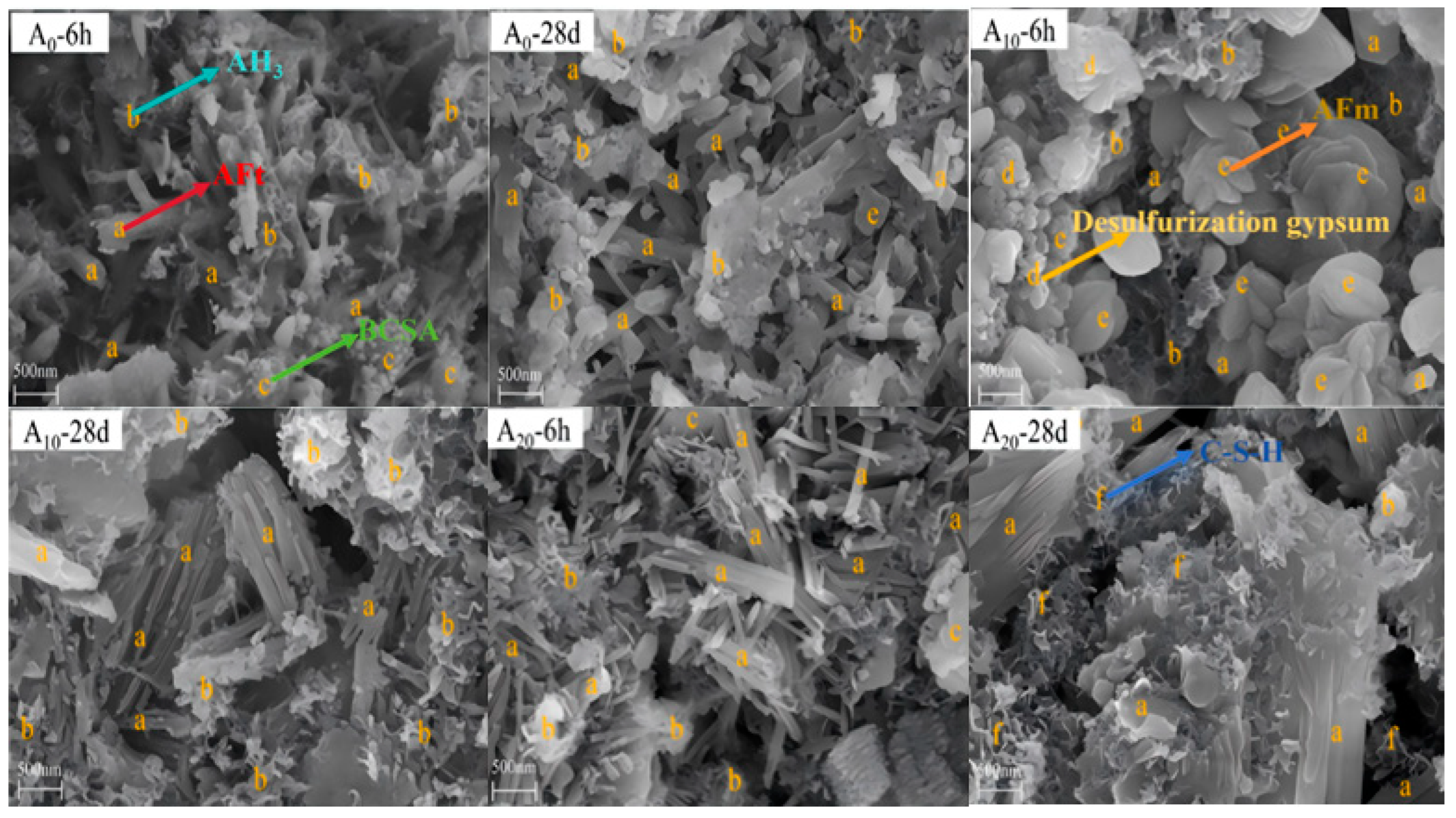
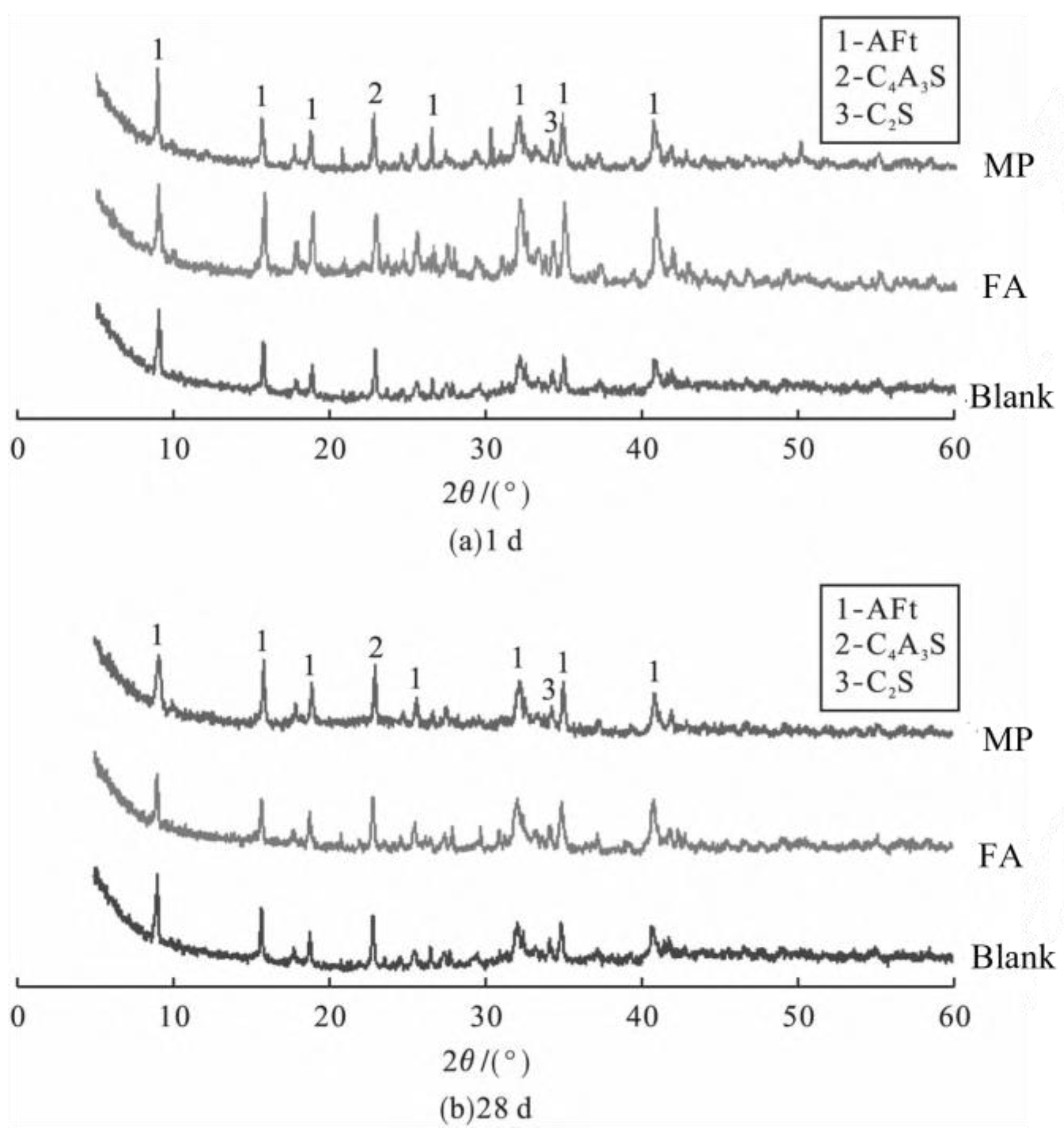
2.2.1. Hydration Minerals
2.2.2. Hydration Products
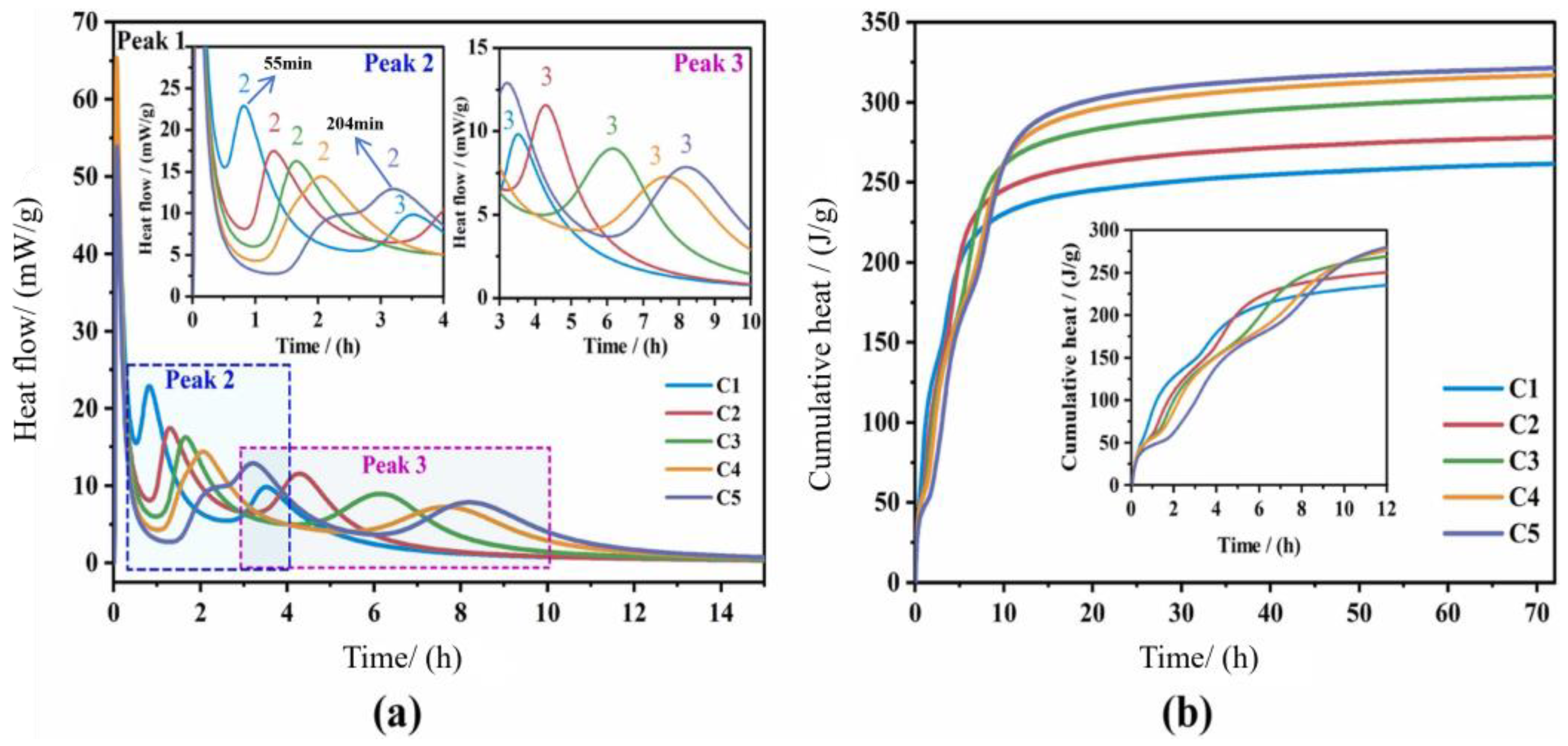
2.2.3. Hydration Exothermicity
| Mineral Composition | Post-Admixture | W/C | Industrial Solid Waste | Ref. |
|---|---|---|---|---|
| 29.13% C4A3S−, 52.99% C2S | Gypsum 8% | 0.35 | Aluminum tailings | Chen et al. [39] |
| 35% C4A3S−, 42.13% C2S | Gypsum 10% | 0.3 | Electrolytic manganese residue, Barium slag | He et al. [71] |
| 50% C4A3S−, 50%C2S | Gypsum 5% | 0.3 (W/B) | Phosphogypsum | Shen et al. [70] |
| 50% C4A3S−, 35% C2S | / | 0.6 | Fly ash, Phosphogypsum | Tao et al. [68] |
| 38.9% C4A3S−, 33.8% C2S, 17.8% CaSO4 | / | 0.5 | Phosphogypsum | Shen et al. [69] |
| 58% C4A3S−, 12% C2S | Gypsum 18% | 0.5 | / | Yeung et al. [73] |
3. Impact of Solid Waste on HBCSA and CSA Cement Performance
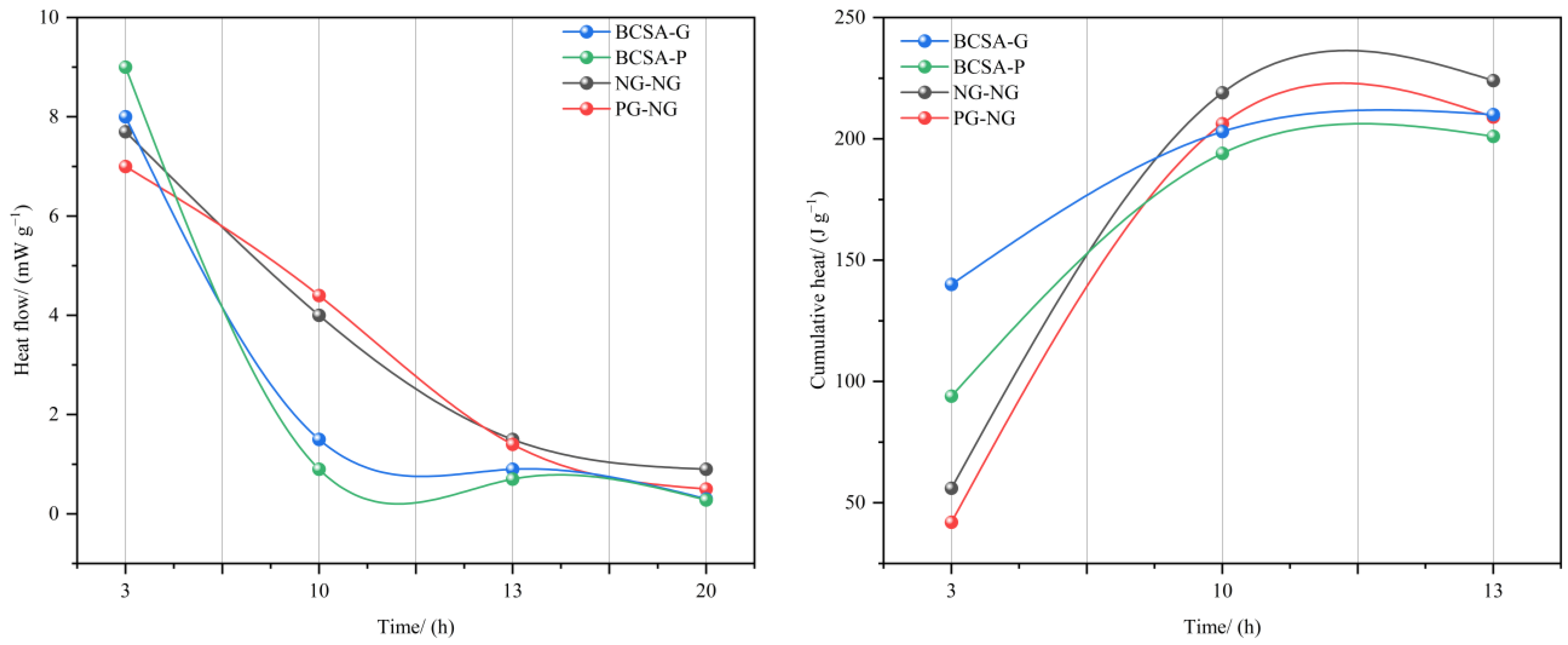
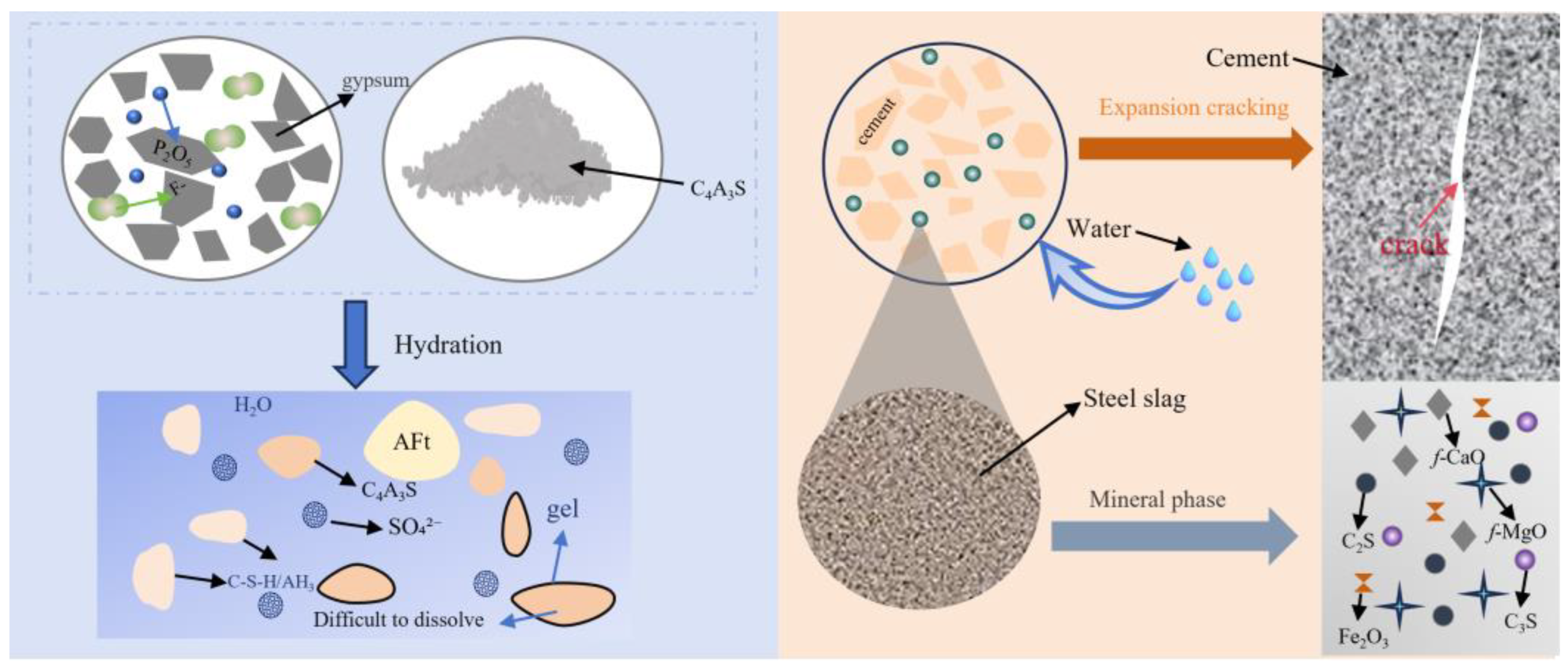
3.1. Solid-Waste Gypsum
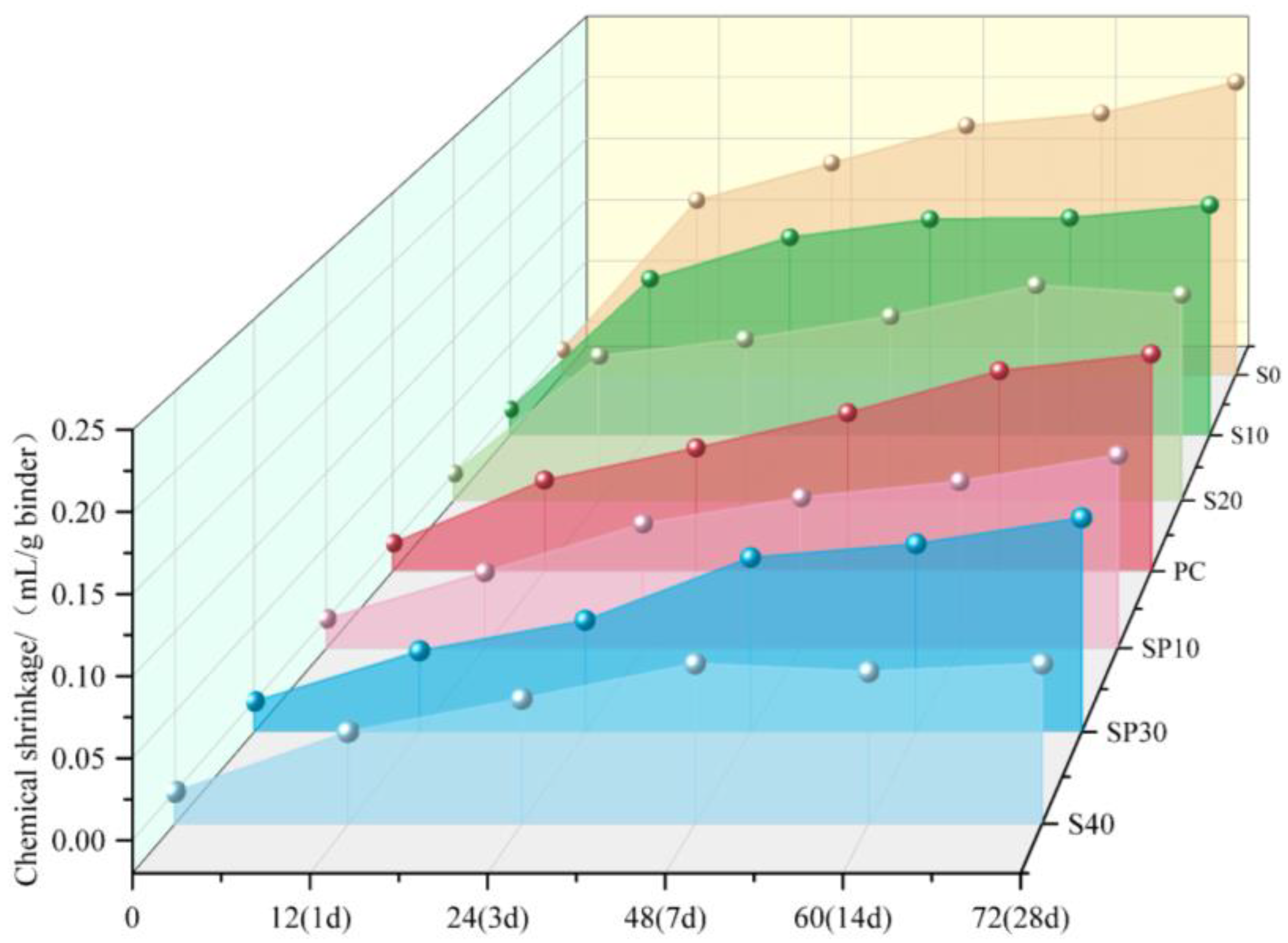
3.2. Steel Slag and Blast Furnace Slag
- (1)
- Phosphogypsum: soluble P2O5 and fluoride impurities retard C4A3S− hydration when substituting natural gypsum, reducing early strength and prolonging setting time.
- (2)
- Steel slag: Fe2O3 lowers the clinker calcination temperature and stabilizes ettringite (AFt) while suppressing chemical shrinkage, but its low reactivity delays hydration and compromises early strength at high substitution rates.
- (3)
- Blast furnace slag: pozzolanic activity necessitates alkaline activation, which conflicts with low-alkalinity CSA systems, while excessive Al2O3 content induces expansion and microcracking despite pore structure refinement.
- (a)
- Uncontrolled Impurities and Inferior Composition: solid wastes exhibit inherently variable impurity levels (e.g., P2O5 and F⁻ in phosphogypsum; free MgO and free CaO in steel slag) and lower chemical consistency compared to natural minerals.
- (b)
- Lack of Industrial Standardization: beyond laboratory settings, industrial-scale production faces inconsistencies in pretreatment, classification, processing protocols (even for identical feedstocks), and performance testing standards.
- (c)
- Performance Uncertainty: can cement properties remain stable when impurities exceed thresholds in real-world production?
- (d)
- Cost–Production Dilemma: laboratory studies predominantly use pre-treated solid wastes, but industrial procurement of such refined materials raises costs.
- (e)
- Quantification Barriers: absent unified performance standards, the properties of waste-based HBCSA/CSA cements cannot be objectively quantified due to variability in raw materials, processing methods, and chemical compositions.
4. Characteristics of the Chemical Components in HBCSA and CSA Cements
4.1. Role of Fe2O3
4.2. Role of CaSO4
4.3. Roles of CaCO3 and f-CaO in Cement Systems
5. Methodology
6. Conclusions and Future Perspectives
- (1)
- Region-specific material selection: prioritize locally abundant solid waste to ensure that feedstock composition is as consistent as possible and to reduce logistical complexity.
- (2)
- Gradual integration: Give up the pursuit of 100% waste substitution. Instead, we will first partially integrate waste, establish a sound production baseline, and then gradually realize the full utilization of waste.
- (3)
- Preparation for industrial production: establish protocols for the cost-effectiveness of waste pre-processing, the long-term environmental impact of cement, and potential risks (e.g., whether there are health risks or hazards associated with the use of such cement).
- (4)
- Other materials: for example, construction, and demolition waste as supplementary material (fine grinding) that can be better adapted to construction work.
- (5)
- Standards for durability indicators: develop a set of test standards for resistance to freezing and thawing, corrosion, and carbonation, e.g., for OPC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- O’Hara, P.A. Political economy of climate change, ecological destruction and uneven development. Ecol. Econ. 2009, 69, 223–234. [Google Scholar] [CrossRef]
- Nworie, O.E.; Lin, C. Seasonal variation in tissue-borne heavy Metal(loid)s in herbaceous plants growing in contaminated soils developed from industrial wastes of industrial revolution age. Env. Adv. 2021, 5, 100113. [Google Scholar] [CrossRef]
- Ma, L.; Konter, J.; Herndon, E.; Jin, L.; Steinhoefel, G.; Sanchez, D.; Brantley, S. Quantifying an early signature of the industrial revolution from lead concentrations and isotopes in soils of Pennsylvania, USA. Anthropocene 2014, 7, 16–29. [Google Scholar] [CrossRef]
- Xu, H.; Chen, G.; Sarwar, B.; Shahzad, I. Sustainable development and mineral resource extraction in China: Exploring the role of mineral resources, energy efficiency and renewable energy. Resour. Policy 2024, 90, 104703. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Energy & Climate Intelligence Unit ECIU. Net Zero Emission Race. 2024. Available online: https://eciu.net/netzerotracker (accessed on 30 December 2024).
- IEA. Global CO2 Emissions from the Operation of Buildings in the Net Zero Scenario, 2010–2030; IEA: Paris, France, 2023. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Mitigation of climate change. In Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; Volume 1454, p. 147. Available online: https://ipcc-browser.ipcc-data.org/browser/dataset/8460/0 (accessed on 30 December 2024).
- Clark, G.; Davis, M.; Kumar, A. Assessment of fuel switching as a decarbonization strategy in the cement sector. Energy Convers. Manag. 2024, 312, 118585. [Google Scholar] [CrossRef]
- Liu, H.X.; Wu, L.M.; Hu, Y.J.; Wu, H.Y.; Xue, J.C.; Zhang, R.Y.; Cui, Y.; Shan, S.K.; Wang, L. Research on the characteristics of CO2 emission and treatment technology in cement industry. Cement 2023, 10, 10–15. [Google Scholar]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Hua, X.; Liu, J.; Sun, G. Urban industrial solid waste metabolism based on ecological network analysis: A case study of Tianjin. Clean. Responsib. Consum. 2023, 9, 100117. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Shen, F.; Zhou, M.; Du, W. Conceptual design and life-cycle environmental and economic assessment of low-carbon cement manufacturing processes. J. Clean. Prod. 2024, 471, 143349. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, W.; Zhang, C.; Li, J.; Wu, S.; Wang, X.; Xu, Y.; Wang, W.; Feng, M. Preparation of solid-waste-based pervious concrete for pavement: A two-stage utilization approach of coal gangue. Constr. Build. Mater. 2022, 319, 125962. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, T.; Zhang, J.; Wang, Z.; Liu, J.; Cao, J.; Wang, C. Recycling and utilization of calcium carbide slag-current status and new opportunities. Renew. Sustain. Energy Rev. 2022, 159, 112133. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X.; Ma, R.; Sun, S.; Fang, L.; Lin, J.; Luo, J. Solid waste-based magnesium phosphate cements: Preparation, performance and solidification/stabilization mechanism. Constr. Build. Mater. 2021, 297, 123761. [Google Scholar] [CrossRef]
- Carneiro, G.; Bier, T.; Waida, S.; Dous, A.; Heinemann, S.; Herr, P.; Charitos, A. Treatment of energy from waste plant fly-ash for blast furnace slag substitution as a supplementary cementitious material. J. Clean. Prod. 2025, 490, 144693. [Google Scholar] [CrossRef]
- Ragossnig, A.M.; Jovovic, A. Waste management life cycle: Sensitisation–implementation/integration–transition–optimisation. Waste Manag. Res. 2016, 34, 813–815. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, T.; Tong, X. Assessing the carbon reduction potential of municipal solid waste management transition: Effects of incineration, technology and sorting in Chinese cities. Resour. Conserv. Recycl. 2023, 188, 106713. [Google Scholar] [CrossRef]
- Tong, X.; Yu, H.; Liu, T. Using weighted entropy to measure the recyclability of municipal solid waste in China: Exploring the geographical disparity for circular economy. J. Clean. Prod. 2021, 312, 127719. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Yan, J.; Qian, Y.; Wei, Y.; Li, D.; Guo, S. Value-added utilization of coal fly ash in polymeric composite decking boards. J. Mater. Res. Technol. 2023, 23, 4199–4210. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.; He, X.; Yang, W.; Deng, X. Utilization of carbide slag-granulated blast furnace slag system by wet grinding as low carbon cementitious materials. Constr. Build. Mater. 2020, 249, 118763. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem. Concr. Compos. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Lv, F.; Wang, L.; An, H.; Chen, S.; Shu, J.; Kong, D. Effects of hybrid fibers on properties of desulfurized gypsum-based composite cementitious materials. Constr. Build. Mater. 2023, 392, 131840. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Mao, Y.; Shen, X.D. Synthesis and characterization of high belite sulfoaluminate cement through rich alumina fly ash and desulfurization gypsum. Ceram-Silik 2013, 57, 7–13. [Google Scholar]
- Wang, W.; Wang, X.; Zhu, J.; Wang, P.; Ma, C. Experimental investigation and modeling of sulfoaluminate cement preparation using desulfurization gypsum and red mud. Ind. Eng. Chem. Res. 2013, 52, 1261–1266. [Google Scholar] [CrossRef]
- El-Alfi, E.A.; Gado, R.A. Preparation of calcium sulfoaluminate-belite cement from marble sludge waste. Constr. Build. Mater. 2016, 113, 764–772. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef]
- Sun, W.; Yao, G. Impact of mineral resource depletion on energy use: Role of energy extraction, CO2 intensity, and natural resource sustainability. Resour. Policy 2023, 86, 104175. [Google Scholar] [CrossRef]
- Roy, R.; Hertel, T.; Pilla, G.; Pontikes, Y. Revealing the hydration mechanisms in iron-rich calcium sulfoaluminate cements with elevated bauxite residue content. Constr. Build. Mater. 2024, 449, 138495. [Google Scholar] [CrossRef]
- Bernardo, G.; Telesca, A.; Valenti, G.L. A porosimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem. Concr. Res. 2006, 36, 1042–1047. [Google Scholar] [CrossRef]
- Tan, J.H.; Shi, X.L.; Zhu, K.J.; Zhao, Y.L.; Peng, J.H. Study on the preparation of high-Belite sulfoaluminate cement using low-grade bauxite and foundry waste sand. Silic. Bull. 2017, 36, 4284–4290. [Google Scholar]
- Xia, R.J.; Zhu, J.P.; Liu, S.X.; Zhu, X.J.; Feng, C.H.; Guan, X.M. Preparation of high belite sulfoaluminate cement clinker from red mud and desulfurization gypsum. Nonferrous Met. Eng. 2017, 7, 58–63+79. [Google Scholar]
- Wang, X.; Guo, M.Z.; Yue, G.; Li, Q.; Ling, T.-C. Synthesis of high belite sulfoaluminate cement with high volume of mixed solid wastes. Cem. Concr. Res. 2022, 158, 106845. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Wang, Z.Y.; Wang, S.D.; Zhao, P.Q.; Cheng, X. Synthesis of high belite sulfoaluminate cement containing magnesia-alumina spinel with solid waste. Ceram-Silik 2023, 67, 159–166. [Google Scholar] [CrossRef]
- Wu, S.; Wang, W.; Ren, C.; Yao, X.; Yao, Y.; Zhang, Q.; Li, Z. Calcination of calcium sulphoaluminate cement using flue gas desulfurization gypsum as whole calcium oxide source. Constr. Build. Mater. 2019, 228, 116676. [Google Scholar] [CrossRef]
- Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Chen, C.; Gu, X. Calcination of calcium sulphoaluminate cement using pyrite-rich cyanide tailings. Crystals 2020, 10, 971. [Google Scholar] [CrossRef]
- Chen, X.M.; Li, J.; Lu, Z.Y.; Xv, G.Y.; Zhong, W. Study on the preparation of high iron phase belite sulfoaluminate cement using aluminum tailings. Cement 2023, 3, 1–7. [Google Scholar]
- Zhang, P.; Zhang, B.; Chang, J.; Wang, T.; Zhang, J.X. Investigation of process parameters of phosphogypsum for preparing calcium sulfoaluminate cement. Buildings 2022, 12, 1774. [Google Scholar] [CrossRef]
- Tanguler-Bayramtan, M.; Turk, S.; Yaman, I.O. Hydration characteristics of calcium sulfoaluminate cements synthesized using an industrial symbiosis framework. Constr. Build. Mater. 2024, 447, 138090. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Huang, S.; Wu, F.L.; Zhu, J. Effect of Al2O3/SiO2 ratio on the chroma and phase compositions of white sulfoaluminate cement clinker. Constr. Build. Mater. 2022, 345, 128202. [Google Scholar] [CrossRef]
- Shi, H.S. Effect of free calcium oxide on the properties of cement. Cement 1992, 10, 27–31. [Google Scholar]
- Su, D.L. Basic Research on the Preparation and Application of High-Belite Sulfoaluminate Cement Based on Multiple Solid Waste Co-Disposal Technologies. Master’s Thesis, Qingdao University of Science and Technology, Qingdao, China, 2021. [Google Scholar]
- Zhang, L.; Su, M.; Wang, Y. Development of the use of sulfo- and ferroaluminate cements in China. Adv. Cem. Res. 1999, 11, 15–21. [Google Scholar] [CrossRef]
- Liu, H.M. Fundamentals of Cement Production Technology, 2nd ed.; Chemical Industry Press: Beijing, China, 2016. [Google Scholar]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Marroccoli, M.; Telesca, A.; Lothenbach, B.; Winnefeld, F. Synthesis and properties of a belite-calcium sulfoaluminate cement obtained using only waste materials. Adv. Cem. Res. 2025, 37, 78–88. [Google Scholar] [CrossRef]
- Fukuda, N. On the constitution of sulfo-aluminous clinker. Bull. Chem. Soc. Jpn. 1961, 34, 138–139. [Google Scholar] [CrossRef]
- Bye, G.C. Portland cement: Composition, production and properties. In Thomas Telford; Thomas Telford Ltd.: London, UK, 1999. [Google Scholar]
- Klein, A. Calciumaluminosulfate and expansive cements containing same. US Pat. 1964, 3, 155. [Google Scholar]
- Diao, J.J.; Xin, Z.J.; Zhang, Q.Y. Production and Application of Sulfoaluminate Cement; China Building Material Industry Press: Beijing, China, 2006. [Google Scholar]
- Zhao, X.D. Research on Fast-Setting and Fast-Hardening High Belite Sulfoaluminate Cement Clinker. Master’s Thesis, Beijing University of Technology, Beijing, China, 2017. [Google Scholar]
- Rungchet, A.; Chindaprasirt, P.; Wansom, S.; Pimraksa, K. Hydrothermal synthesis of calcium sulfoaluminate-belite cement from industrial waste materials. J. Clean. Prod. 2016, 115, 273–283. [Google Scholar] [CrossRef]
- Wang, X. Experimental Study on the Preparation of High Belite Sulfoaluminate Cement from Industrial Solid Waste. Master’s Thesis, Qingdao University of Technology, Qingdao, China, 2019. [Google Scholar]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Zhang, R. Research on Thermodynamics and Kinetics of Cement Clinker Firing. Master’s Thesis, Beijing University of Technology, Beijing, China, 2010. [Google Scholar]
- Link, T.; Bellmann, F.; Ludwig, H.; Ben Haha, M. Reactivity and phase composition of Ca2SiO4 binders made by annealing of alpha-dicalcium silicate hydrate. Cem. Concr. Res. 2015, 67, 131–137. [Google Scholar] [CrossRef]
- Bensted, J. A discussion of the paper “Idiomorphic belites in Portland cement—A genetic model” by R. Oberste-Padtberg. Cem. Concr. Res. 1987, 17, 513–514. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Tian, D.J.; Liu, Y.; Yang, C.J.; Ma, Y. Preparation of high berth silicate cement clinker from low-grade limestone and its activity enhancement. New Build. Mater. 2024, 51, 22–26. [Google Scholar]
- Chi, L. Research on Activation and Hydration Mechanism of High Belite Sulfoaluminate Cement. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2019. [Google Scholar]
- Huang, W.; Wen, Z.; Wang, M. Effect of phosphorus-sulfur composite doping on the crystalline structure of dicalcium silicate. Silic. Bull. 2018, 37, 2502–2505. [Google Scholar]
- Liu, C.; Yan, C.; Li, J.; Nie, W.; Lv, Z.; Wu, Q.; Meng, X.; Zhao, F. Effect of desulfurization gypsum on alternating-current impedance characteristics of all-solid-waste belite sulphoaluminate cement mortar. Constr. Build. Mater. 2024, 450, 138666. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, L.; Ren, X.; Ye, J.; Liu, L.; Wu, Y. Influence of free calcium sulfate levels on the early hydration of sulfate-rich belite sulfoaluminate clinkers: A comparative study with anhydrite and gypsum. Cem. Concr. Res. 2024, 181, 107522. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; da Costa, M.L.; Pöllmann, H. Waste clay from bauxite beneficiation to produce calcium sulphoaluminate eco-cements. Constr. Build. Mater. 2022, 340, 127703. [Google Scholar] [CrossRef]
- Su, D.; Li, Q.; Guo, Y.; Yue, G.; Wang, L. Effect of residual CaSO4 in clinker on properties of high belite sulfoaluminate cement based on solid wastes. Materials 2020, 13, 429. [Google Scholar] [CrossRef]
- Shu, X.; Jiang, Y.; Zhao, Y.; Xu, Z.; Shen, M.; Zhong, X. Superimposed hydration exothermic model of cement slurry considering different reaction rates of various active substances. Constr. Build. Mater. 2023, 372, 130783. [Google Scholar] [CrossRef]
- Tao, X.; Li, P.; Wang, G.; Li, W.; Ma, S. Preparation and hydration of Belite-Ye’elimite-Ternesite clinker based on industrial solid waste. Case Stud. Constr. Mater. 2023, 18, e01922. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Huang, Y.; Yang, D. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem. Concr. Compos. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Chai, J.; Fan, Y. Calcium sulphoaluminate cements made with phosphogypsum: Production issues and material properties. Cem. Concr. Compos. 2014, 48, 67–74. [Google Scholar] [CrossRef]
- He, W.; Li, R.; Zhang, Y.; Nie, D. Synergistic use of electrolytic manganese residue and barium slag to prepare belite-sulphoaluminate cement study. Constr. Build. Mater. 2022, 326, 126672. [Google Scholar] [CrossRef]
- Sun, D.; Wang, X.; Wang, J.; Li, J.; Mao, Y.; Hu, Z.; Li, Y.; Song, Z.; Wang, W. Properties and hydration characteristics of cementitious blends with two kinds of solid waste-based sulfoaluminate cement. Constr. Build. Mater. 2024, 411, 134482. [Google Scholar] [CrossRef]
- Yeung, J.S.K.; Yam, M.C.H.; Wong, Y.L. 1-Year development trend of concrete compressive strength using Calcium Sulfoaluminate cement blended with OPC, PFA and GGBS. Constr. Build. Mater. 2019, 198, 527–536. [Google Scholar] [CrossRef]
- Yoon, H.; Seo, J.; Kim, S.; Lee, H.; Park, S. Hydration of calcium sulfoaluminate cement blended with blast-furnace slag. Constr. Build. Mater. 2021, 268, 121214. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Cao, W.; Yi, W.; Peng, J.; Li, G.; Yin, S. Preparation of anhydrite from phosphogypsum: Influence of phosphorus and fluorine impurities on the performances. Constr. Build. Mater. 2022, 318, 126021. [Google Scholar] [CrossRef]
- El Khessaimi, Y.; Taha, Y.; Safhi, A.E.M.; Hakkou, R.; Benzaazoua, M. Synthesis of MgO-Belite calcium sulfoaluminate cement from phosphate mine waste rock and phosphogypsum. Mater. Today Proc. 2022, 58, 1081–1090. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Kang, X.; Yu, J.; Fan, Y.; Dang, Y.; Zhang, W.; Wang, S. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, D.; Wu, Y.; Yu, H. Synthesis and characterization of calcium aluminate compounds from gehlenite by high-temperature solid-state reaction. Ceram. Int. 2018, 44, 13544–13550. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, K.; Yang, Y.; Chang, J. Investigation on the preparation of low carbon cement materials from industrial solid waste phosphogypsum: Clinker preparation, cement properties, and hydration mechanism. J. Clean. Prod. 2024, 452, 142203. [Google Scholar] [CrossRef]
- Canbek, O.; Shakouri, S.; Erdoğan, S.T. Laboratory production of calcium sulfoaluminate cements with high industrial waste content. Cem. Concr. Compos. 2020, 106, 103475. [Google Scholar] [CrossRef]
- Liao, Y.S.; Jiang, G.X.; Wang, K.J.; Qunaynah, S.A.; Yuan, W.J. Effect of steel slag on the hydration and strength development of calcium sulfoaluminate cement. Constr. Build. Mater. 2020, 265, 120301. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.; Zhou, Z. Preparation of steel slag/slag composite sulfoaluminate cement with less clinker. Concrete 2011, 8, 88–90. [Google Scholar]
- Huang, Y.; Shen, X.; Ma, S.; Chen, L.; Zhong, B. Effect of Fe2O3 on the formation of calcium sulphoaluminate mineral. J. Chin. Ceram. Soc. 2007, 35, 485–488. [Google Scholar]
- Kang, X.T.; Zhou, J.; Xu, M.F.; Li, H.; Yu, Q.H.; Zhai, C.Y.; Lin, S.T.; Zhong, X.L. Research on thermal and mechanical properties of slag sulfoaluminate cement concrete. Ind. Build. 2022, 52, 158–162+185. [Google Scholar]
- Pei, T.Y.; Qi, D.Y.; Zou, D.L.; Cai, Y.H.; Wang, Z.Y.; He, L.L.; Wang, Y.Y.; Zhang, Y.; Liu, H.Y. Study on the mechanism of sulfate erosion resistance of slag-high-baylite sulfoaluminate cement. Silic. Bull. 2023, 42, 2683–2691. [Google Scholar]
- Fan, Z.Y.; Li, Q.Y.; Guo, Y.X.; Yue, G.B. Effects of mineral powder and fly ash on the hydration and strength of high belite sulfoaluminate cement. Concrete 2023, 2, 105–108+113. [Google Scholar]
- Xin, X.; Duan, G.; Huang, Y.; Li, J.; Li, C.; Hou, P. Study on the hydration of slag-belite calcium sulfoaluminate-Portland cement composite cementitious system. Constr. Build. Mater. 2024, 433, 136713. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, Z. Research on the effect of mixing material on the performance of sulfoaluminate cement. Cement Eng. 2017, 4, 8–10. [Google Scholar]
- Seo, J.; Kim, S.; Park, S.; Yoon, H.; Lee, H. Carbonation of calcium sulfoaluminate cement blended with blast furnace slag. Cem. Concr. Compos. 2021, 118, 103918. [Google Scholar] [CrossRef]
- Gao, D.Y.; Meng, Y.; Yang, L.; Tang, J.Y.; Lv, M.Y. Effect of ground granulated blast furnace slag on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 227, 116665. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Canonico, F.; Paul, G. CSA and slag: Towards CSA composite binders. Adv. Cem. Res. 2019, 31, 147–158. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Wei, J.; Ouyang, Z.; Lv, W. Effect of different calcination temperatures on clinker and hydration properties of solid waste-based calcium sulfoaluminate cement. Constr. Build. Mater. 2024, 452, 138938. [Google Scholar] [CrossRef]
- Sun, H.Q.; Lin, T.Z.; Zhuo, K.H.; Qian, J.S.; Chen, X.S.; Zhang, J.; Sun, Y.B. Sulfate optimization in ettringite rich cements by SO3/Al2O3 molar ratio: A comparative study of calcium sulfoaluminate and aluminate cements. Case Stud. Constr. Mater. 2024, 21, e03701. [Google Scholar] [CrossRef]
- Žibret, L.; Ipavec, A.; Dolenec, S. Microstructural characteristics of belite-sulfoaluminate cement clinkers with bottom ash. Constr. Build. Mater. 2022, 321, 126289. [Google Scholar] [CrossRef]
- Wu, S.; Cui, Y.F.; Yao, X.L.; Ren, C.Z.; Yao, Y.G.; Wang, W.L. Preparation of iron-rich sulfoaluminate cement by regulating Fe-bearing minerals. Ceram Int. 2025. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, W.; Yuan, J.; Shu, X.; Tan, X.; Wu, G.; Tian, H.; Cai, L. Study on early hydration and mechanical properties of ferrite-rich calcium sulfoaluminate cement-based grouting materials. Constr. Build. Mater. 2024, 411, 134324. [Google Scholar] [CrossRef]
- Sun, Z.; Nie, S.; Zhou, J.; Li, H.; Chen, Z.; Xu, M.; Mu, R.; Wang, Y. Hydration mechanism of calcium sulfoaluminate-activated supersulfated cement. J. Clean. Prod. 2022, 333, 130094. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Yu, B. Effect of modified phosphogypsum on the hydration properties of the phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2019, 214, 9–16. [Google Scholar] [CrossRef]
- Tsivilis, S.; Chaniotakis, E.; Batis, G.; Meletiou, C.; Kasselouri, V.; Kakali, G.; Sakellariou, A.; Pavlakis, G.; Psimadas, C. The effect of clinker and limestone quality on the gas permeability, water absorption and pore structure of limestone cement concrete. Cem. Concr. Compos. 1999, 21, 139–146. [Google Scholar] [CrossRef]
- Tsivilis, S.; Chaniotakis, E.; Badogiannis, E.; Pahoulas, G.; Ilias, A. A study on the parameters affecting the properties of Portland limestone cements. Cem. Concr. Compos. 1999, 21, 107–116. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H. A review on effects of limestone powder on the properties of concrete. Constr. Build. Mater. 2018, 192, 153–166. [Google Scholar] [CrossRef]
- Ji, G.; Chi, H.; Sun, K.; Peng, X.; Cai, Y. Effect of limestone waste on the hydration and microstructural properties of cement-based materials. Constr. Build. Mater. 2024, 443, 137784. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Lu, D.; Xu, Z.; Hooton, R. Effect of dolomite powder on the hydration and properties of calcium sulfoaluminate cements with different gypsum contents. Constr. Build. Mater. 2019, 225, 302–310. [Google Scholar] [CrossRef]
- Idrissi, M.; Diouri, A.; Damidot, D.; Greneche, J.; Talbi, M.A.; Taibi, M. Characterisation of iron inclusion during the formation of calcium sulfoaluminate phase. Cem. Concr. Res. 2010, 40, 1314–1319. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Haha, M.B. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Thaivalappil, B.; Chaunsali, P. Influence of iron content on processing conditions and early age hydration of ye’elimite. Mater Today Proc. 2023. [Google Scholar] [CrossRef]
- Cui, S.P.; Lan, M.Z.; Zhang, J.; Wang, C.Y. Role and solidification mechanism of heavy metal elements in cement clinker formation process from waste materials. J. Chin. Ceram. Soc. 2004, 32, 1264–1270. [Google Scholar]
- Huang, Y.; Pei, Y.; Qian, J.; Gao, X.; Liang, J.; Duan, G.; Zhao, P.; Lu, L.; Cheng, X. Bauxite free iron rich calcium sulfoaluminate cement: Preparation, hydration and properties. Constr. Build. Mater. 2020, 249, 118774. [Google Scholar] [CrossRef]
- Yao, X.; Yang, S.; Dong, H.; Wu, S.; Liang, X.; Wang, W. Effect of CaO content in raw material on the mineral composition of ferric-rich sulfoaluminate clinker. Constr. Build. Mater. 2020, 263, 120431. [Google Scholar] [CrossRef]
- Liang, J. Preparation of High Iron Belite-Sulfoaluminate Cement from Steel Slag. Master’s Thesis, Chongqing University, Chongqing, China, 2018. [Google Scholar]
- Qian, J.S.; Yu, J.C.; Sun, H.Q.; Ma, Y. Formation and role of ettringite. J. Silic. 2017, 11, 1569–1581. [Google Scholar]
- Lee, H.; Cody, R.; Cody, A.; Spry, P. The formation and role of ettringite in Iowa highway concrete deterioration. Cem. Concr. Res. 2005, 35, 332–343. [Google Scholar] [CrossRef]
- Tang, P.; Wen, J.; Fu, Y.; Liu, X.; Chen, W. Improving the early-age properties of eco-binder with high volume waste gypsum: Hydration process and ettringite formation. J. Build. Eng. 2024, 86, 108988. [Google Scholar] [CrossRef]
- Lv, S.H.; Ma, Y.J.; Qiu, C.C.; Sun, T.; Liu, J.J.; Zhou, Q.F. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Glasser, F.P. Advances in Sulfoaluminate Cements; The University of Aberdeen: Aberdeen, Scotland, 2002. [Google Scholar]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. Calculation of chemical water demand for hydration of calcium sulfoaluminate cement. J. Chin. Ceram. Soc. 1998, 3, 38–44. [Google Scholar]
- Xiang, B. Research on the Hydration and Hardening Mechanism of Fast-Setting and Fast-Hardening High Belite-Sulfoaluminate Cement; Beijing University of Technology: Beijing, China, 2017. [Google Scholar]
- Jia, H.X.; Lv, S.Z.; Hu, J.L.; Gao, Y.; Duan, X.Y. Influence of gypsum dosing on the properties of high Belite-sulfoaluminate cement. J. Wuhan Univ. Technol. 2014, 36, 24–28. [Google Scholar]
- Wu, S.; Yao, Y.; Yao, X.; Ren, C.; Li, J.; Xu, D.; Wang, W. Co-preparation of calcium sulfoaluminate cement and sulfuric acid through mass utilization of industrial by-product gypsum. J. Clean. Prod. 2020, 265, 121801. [Google Scholar] [CrossRef]
- Ioannou, S.; Paine, K.; Reig, L.; Quillin, K. Performance characteristics of concrete based on a ternary calcium sulfoaluminate-anhydrite-fly ash cement. Cem. Concr. Compos. 2015, 55, 196–204. [Google Scholar] [CrossRef]
- Ioannou, S.; Reig, L.; Paine, K.; Quillin, K. Properties of a ternary calcium sulfoaluminate-calcium sulfate-fly ash cement. Cem. Concr. Res. 2014, 56, 75–83. [Google Scholar] [CrossRef]
- Chen, I.A.; Hargis, C.W.; Juenger, M.C.G. Understanding expansion in calcium sulfoaluminate-belite cements. Cem. Concr. Res. 2012, 42, 51–60. [Google Scholar] [CrossRef]
- Liang, J.; Liu, N.; Liu, C.Z.; Guo, M.C.; Li, X.C.; Qian, J.S. Preparation of high belite-sulfoaluminate cement by phosphogypsum and low-grade bauxite. Nonmet. Min. 2016, 39, 17–20. [Google Scholar]
- Park, S.; Jeong, Y.; Moon, J.; Lee, N. Hydration characteristics of calcium sulfoaluminate (CSA) cement/Portland cement blended pastes. J. Build. Eng. 2021, 34, 101880. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Boehm-Courjault, E.; Zajac, M.; Ben Haha, M.; Scrivener, K. Hydration reactions and stages of clinker composed mainly of stoichiometric ye’elimite. Cem. Concr. Res. 2019, 116, 120–133. [Google Scholar] [CrossRef]
- Jansen, D.; Wolf, J.J.; Fobbe, N. The hydration of nearly pure ye’elimite with a sulfate carrier in a stoichiometric ettringite binder system. Implications for the hydration process based on in-situ XRD, 1H-TD-NMR, pore solution analysis, and thermodynamic modeling. Cem. Concr. Res. 2020, 127, 105923. [Google Scholar] [CrossRef]
- Damion, T.; Chaunsali, P. Effect of gypsum addition on acid resistance of ye’elimite rich calcium sulfoaluminate cement. Cem. Concr. Compos. 2024, 145, 105335. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Mrak, M.; Winnefeld, F.; Lothenbach, B.; Dolenec, S. The influence of calcium sulfate content on the hydration of belite-calcium sulfoaluminate cements with different clinker phase compositions. Mater. Struct. 2021, 54, 1–17. [Google Scholar] [CrossRef]
- Bonavetti, V.; Donza, H.; Rahhal, V.; Irassar, E. Influence of initial curing on the properties of concrete containing limestone blended cement. Cem. Concr. Res. 2000, 30, 703–708. [Google Scholar] [CrossRef]
- Livesey, P. Strength characteristics of Portland-limestone cements. Constr. Build. Mater. 1991, 5, 147–150. [Google Scholar] [CrossRef]
- Péra, J.; Husson, S.; Guilhot, B. Influence of finely ground limestone on cement hydration. Cem. Concr. Compos. 1999, 21, 99–105. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Ma, J.; Yu, Z.; Ni, C.; Shi, H.; Shen, X. Effects of limestone powder on the hydration and microstructure development of calcium sulphoaluminate cement under long-term curing. Constr. Build. Mater. 2019, 199, 688–695. [Google Scholar] [CrossRef]
- Péra, J.; Ambroise, J. New applications of calcium sulfoaluminate cement. Cem. Concr. Res. 2004, 34, 671–676. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, C. Improved ettringite stabilization by calcium carbonate and calcium nitrate additions in ternary PC-CSA-C$ systems. Cem. Concr. Res. 2024, 175, 107383. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
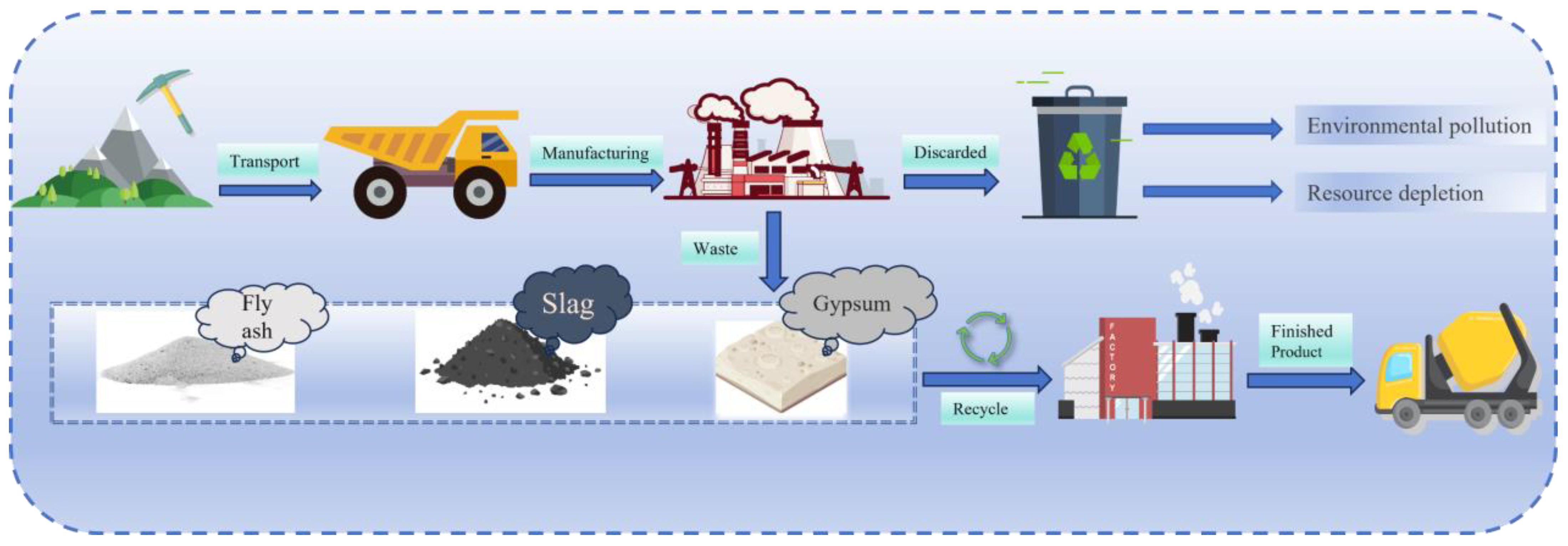


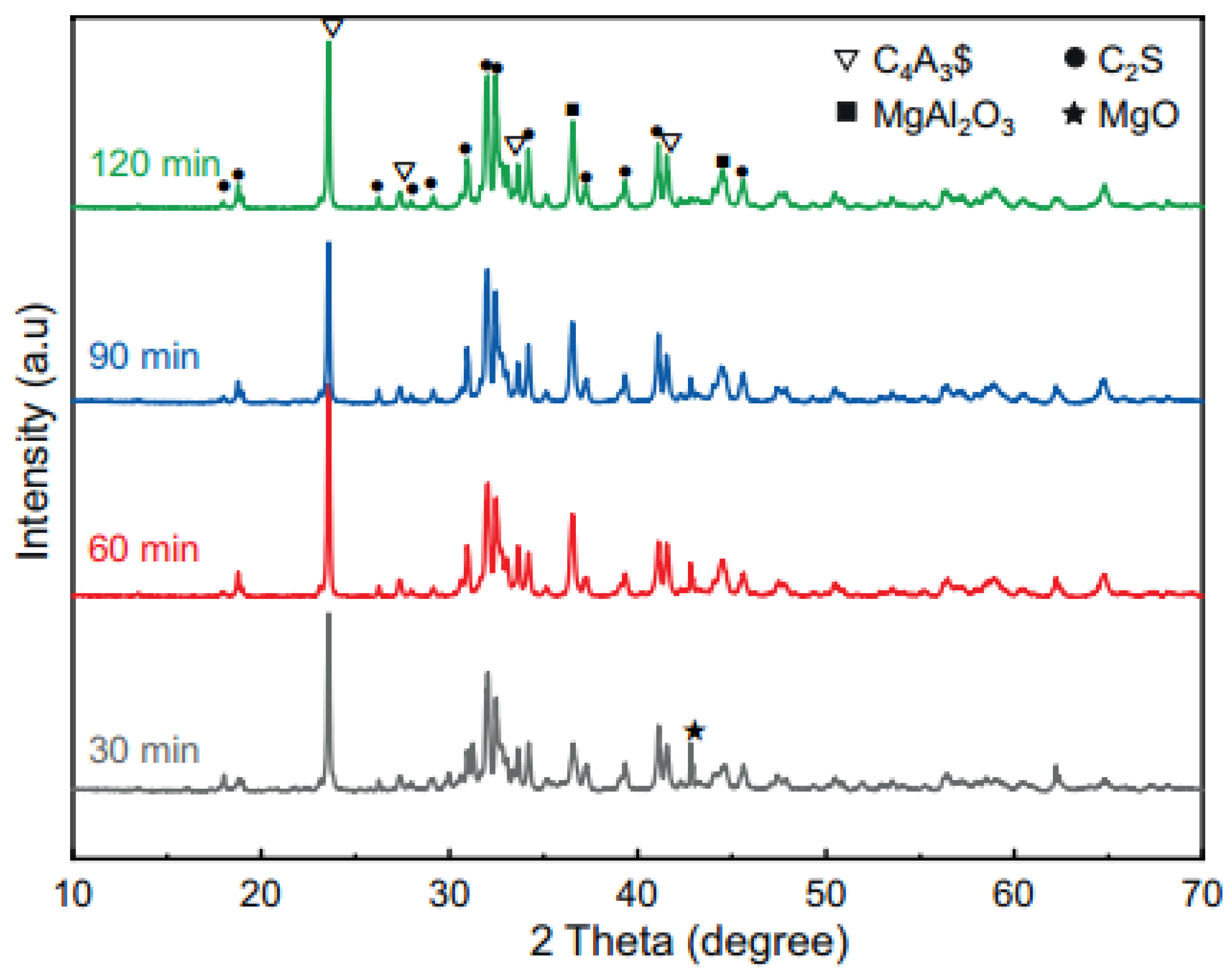
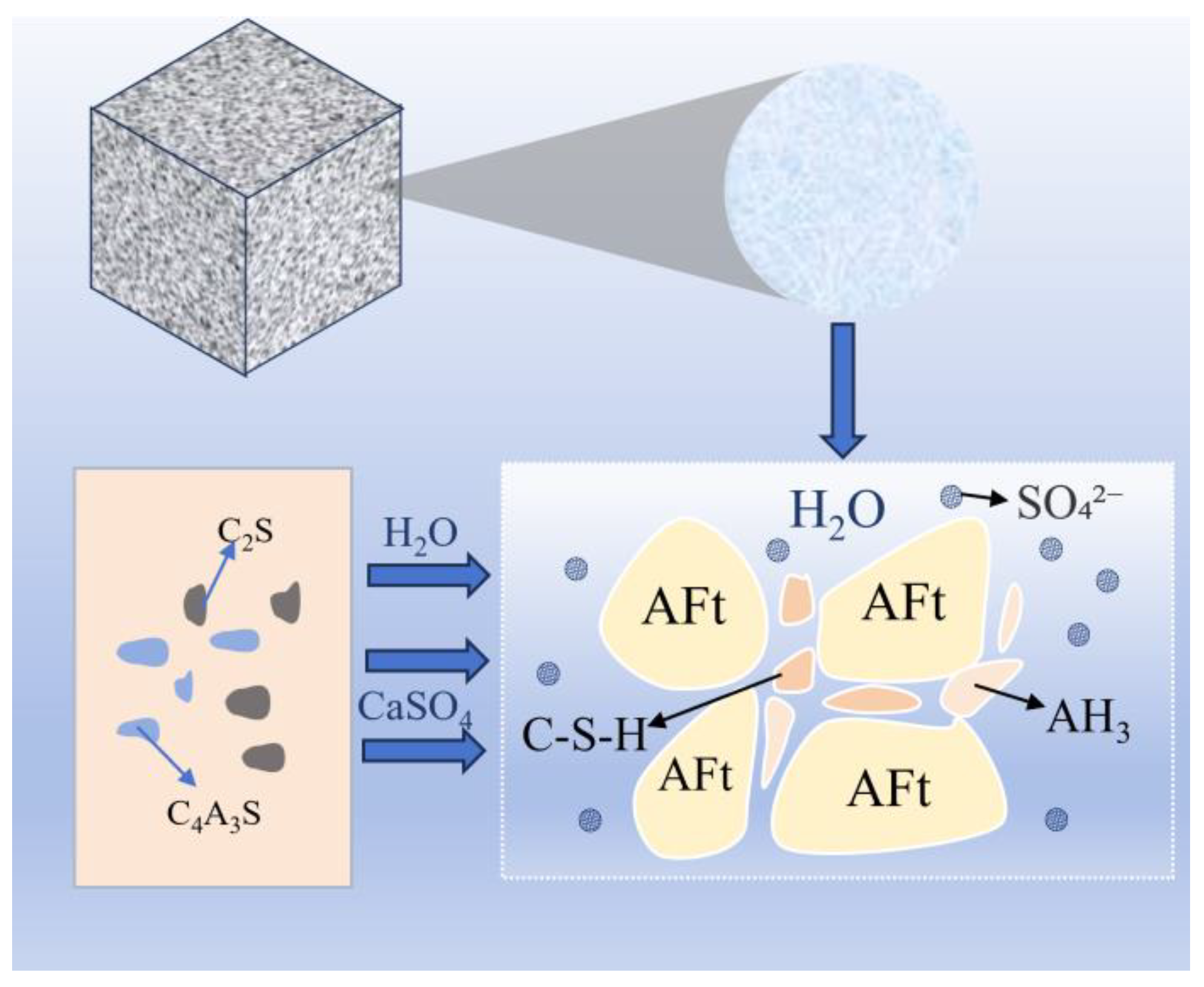


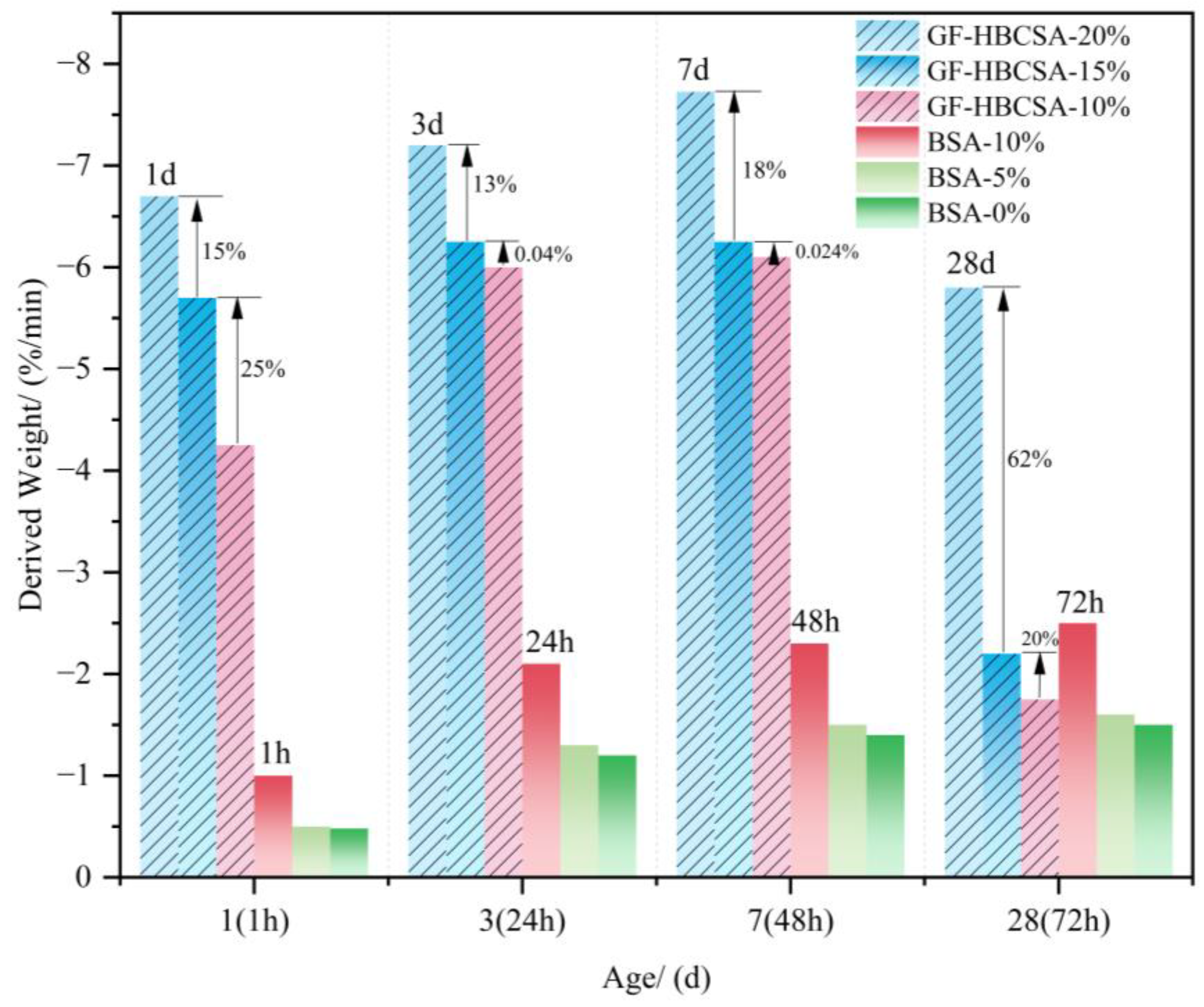




| Types | Sources | Quantities | Environmental Impacts | Ref. |
|---|---|---|---|---|
| Flue gas desulfurization gypsum (FDG) | Thermal power plants and coal-fired plants | Approximately 130 million metric tonnes. (2019) | Metal contamination, Release of acidic substances, Land occupation | Lv et al. [25] |
| Red mud (RM) | Extraction and refining processes of bauxite | Total accumulated red mud is around 600 million tonnes. (2021) | Alkalinity contamination, Metal contamination, Land occupation and groundwater contamination | Zhang et al. [21] |
| Fly ash (FA) | Coal-fired thermal power plants during coal combustion | Total accumulated Fly ash has exceeded 3 billion tonnes. (2023) | Metal contamination, Soil contamination, Airborne particulate matter (PM) pollution | Wang et al. [22] |
| Carbide slag (CS) | Process of acetylene production | In China, 56 million tonnes are produced annually. (2020) | Alkalinity and corrosivity contamination, Airborne particulate matter (PM) pollution | Zhang et al. [23] |
| Coal gangue (CG) | Coal mining and coal washing | China Total accumulated more than 500 million tons of CG, with more than 70 million tons added each year. (2022) | Metal contamination, Land occupation and groundwater contamination, Soil contamination | Wu et al. [14] |
| Industrial Solid Waste | Temperature | W/C | Post-Admixture | Holding Time | Ref. |
|---|---|---|---|---|---|
| Bauxite, Foundry waste sand (FWS) | 1300 °C | 0.35 | 10% Gypsum | 90 min | Tan et al. [33] |
| Flue gas desulfurization gypsum (FGD), Red mud (RM) | 1300 °C | 0.35 | 5% Gypsum | 30 min | Wang et al. [27] |
| Desulfurization gypsum (DG), Red mud (RM) | 1280 °C | 0.40 | Gypsum/cement ratio, 85:15 | 30 min | Xia et al. [34] |
| Petroleum coke desulfurization residue (PCD), Fly ash (FA), Carbide slag (CS), Bauxite | 1300 °C | 0.55 | 10% Gypsum | 45 min | Wang et al. [35] |
| Gold tailings (CTs), Red mud (RM), Desulfurized gypsum (DG), High-magnesium limestone (HML), Low-grade bauxite (LGB) | 1300 °C | / | / | 60 min | Yu et al. [36] |
| FGD gypsum, Aluminum slag (AS), Fly ash (FA), Red mud (RM) | 1270–1310 °C | 0.28 | Gypsum | 60–100 min | Wu et al. [37] |
| Cyanide tailings (CTs) | 1300 °C | 0.50 | 30% Gypsum | 40 min | Dong et al. [38] |
| Aluminum tailings (ATs) | 1330 °C | 0.35 | 8% Gypsum | 30 min | Chen et al. [39] |
| Phosphogypsum (PG) | 1250 °C | 0.5 | Gypsum (molar ratio of 1:1.5) | 45 min | Zhang et al. [40] |
| Serox, Ladle furnace slag (LFS), Ceramic waste (CW), Glass waste (GS) | 1250 °C | 0.5 | / | 90 min | Tanguler-Bayramtan et al. [41] |
| Sample | Age | Ye’elimite | AFt | AFm |
|---|---|---|---|---|
| C4A3S− | 28 days | 4% | 8% | 71% |
| C4A3S− _C10 | 28 days | 4% | 16% | 46% |
| C4A3S− _V10 | 28 days | 8% | 44% | 1% |
| C4A3S− _G15 | 28 days | 8% | 61% | 17% |
| C4A3S− _G15_V10 | 28 days | 5% | 66% | 2% |
| C4A3S− _G15_C10 | 28 days | 6% | 65% | 2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, C.; Wu, J.; Gao, Y.; Shao, J.; Wang, C.; Su, T.; Cao, F.; Zhang, W.; Yang, Q.; et al. Preparation of High-Belite Calcium Sulfoaluminate Cement and Calcium Sulfoaluminate Cement from Industrial Solid Waste: A Review. Sustainability 2025, 17, 4269. https://doi.org/10.3390/su17104269
Liu H, Liu C, Wu J, Gao Y, Shao J, Wang C, Su T, Cao F, Zhang W, Yang Q, et al. Preparation of High-Belite Calcium Sulfoaluminate Cement and Calcium Sulfoaluminate Cement from Industrial Solid Waste: A Review. Sustainability. 2025; 17(10):4269. https://doi.org/10.3390/su17104269
Chicago/Turabian StyleLiu, Huaiqin, Chengjian Liu, Jing Wu, Yanjiao Gao, Jianwen Shao, Chenxia Wang, Tian Su, Fubo Cao, Weishen Zhang, Qifan Yang, and et al. 2025. "Preparation of High-Belite Calcium Sulfoaluminate Cement and Calcium Sulfoaluminate Cement from Industrial Solid Waste: A Review" Sustainability 17, no. 10: 4269. https://doi.org/10.3390/su17104269
APA StyleLiu, H., Liu, C., Wu, J., Gao, Y., Shao, J., Wang, C., Su, T., Cao, F., Zhang, W., Yang, Q., & Li, Y. (2025). Preparation of High-Belite Calcium Sulfoaluminate Cement and Calcium Sulfoaluminate Cement from Industrial Solid Waste: A Review. Sustainability, 17(10), 4269. https://doi.org/10.3390/su17104269













