Abstract
To address the high carbon emissions and resource dependency associated with conventional ordinary Portland cement (OPC) production, this study systematically investigated the preparation processes, hydration mechanisms, and chemical properties of high-belite calcium sulfoaluminate (HBCSA) and calcium sulfoaluminate (CSA) cements based from industrial solid wastes. The results demonstrate that substituting natural raw materials (e.g., limestone and gypsum) with industrial solid wastes—including fly ash, phosphogypsum, steel slag, and red mud—not only reduces raw material costs but also mitigates land occupation and pollution caused by waste accumulation. Under optimized calcination regimes, clinkers containing key mineral phases (C4A3S− and C2S) were successfully synthesized. Hydration products, such as ettringite (AFt), aluminum hydroxide (AH3), and C-S-H gel, were identified, where AFt crystals form a three-dimensional framework through disordered growth, whereas AH3 and C-S-H fill the matrix to create a dense interfacial transition zone (ITZ), thereby increasing the mechanical strength. The incorporation of steel slag and granulated blast furnace slag was found to increase the setting time, with low reactivity contributing to reduced strength development in the hardened paste. In contrast, Solid-waste gypsum did not significantly differ from natural gypsum in stabilizing ettringite (AFt). Furthermore, this study clarified key roles of components in HBCSA/CSA systems; Fe2O3 serves as a flux but substitutes some Al2O3, reducing C4A3S− content. CaSO4 retards hydration while stabilizing strength via sustained AFt formation. CaCO3 provides nucleation sites and CaO but risks AFt expansion, degrading strength. These insights enable optimized clinker designs balancing reactivity, stability, and strength.
1. Introduction
Since the inception of the Industrial Revolution in the 1760s, human society has undergone exponential growth in productivity and technological advancement. While this explosive development has generated unprecedented material wealth, it has also entrenched structural contradictions between human activities and natural systems [1]. From a systemic perspective, industrialization incurs significant ecological costs: the predatory exploitation of mineral resources, the disorderly expansion of industrial land use, and excessive emissions of industrial tri-waste (waste gas, wastewater, and solid residues) collectively drive persistent transgressions of the carrying capacity thresholds of ecosystems [2,3]. Analyzed through the lens of sustainable development, the conventional paradigm of technological application remains trapped in cognitive fallacies—treating natural systems with infinite resource reservoirs and pollution sinks while gravely neglecting the fundamental principles of natural cycles and their regenerative capacity [4,5].
This development paradigm has not only exacerbated regional ecological degradation but also accelerated global greenhouse effects through the persistent release of heat-trapping gases, such as CO2, from industrial activities. To address this crisis, 147 nations worldwide established carbon neutrality targets as of 2023, collectively covering 88% of global carbon emissions [6]. As a key energy-intensive sector, the building industry accounts for 30% of global energy consumption and will contribute 26% of lifecycle carbon emissions by 2025 [7]. As illustrated in Figure 1, direct carbon emissions from building operations show no declining trend, with decarbonization progress lagging behind the 2030 carbon peaking constraints. From 1990 to 2019, carbon emissions from building-related sectors increased by nearly 50%, reaching approximately 30% of global emissions by 2024 [8]. Over the past five years, direct CO2 emissions from cement production have remained stagnant, and China, as the world’s largest cement producer, has dominated the global output [9]. The cement industry contributes 22% of CO2 emissions from key carbon-regulated industries, ranking second only to the power sector (32%) and surpassing steel production (21%) [10], while its global greenhouse gas footprint accounts for 5–7% [11].
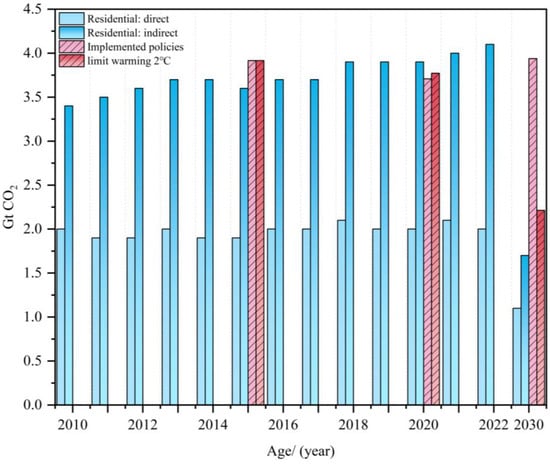
Figure 1.
Global CO2 emissions from the operation of buildings and net global CO2 emissions [7,8]. Note: actual CO2 emissions from implemented policies (policies to reduce emissions) and limit warming 2 °C (carbon emissions that limit the rate of global warming to less than 2 °C) are 10 times higher in the figure.
The adoption of low-carbon raw materials, enhanced energy conversion efficiency, utilization of low-carbon fuels, and improved material utilization rates can collectively reduce CO2 emissions during cement production [7]. Compared with ordinary Portland cement (OPC), low-carbon cement not only mitigates carbon emissions but also offers advantages such as reduced demand for natural minerals and lower production costs [12]. The definition of low-carbon cement encompasses two approaches [13]: (a) Technological innovation: Implementing novel methods to minimize process-related emissions, such as optimizing calcination regimes (e.g., lower-temperature sintering or staged combustion). (b) Material Substitution: Reducing reliance on carbon-intensive raw materials—for instance, directly utilizing industrial solid wastes (e.g., phosphogypsum) as CaO sources to replace limestone.
The circular utilization of solid wastes alleviates environmental burdens, conserves natural resources (e.g., gypsum preservation), and fosters sustainable economic growth through industrial symbiosis. Nevertheless, constrained by underdeveloped technological foundations, imperfect environmental policy enforcement mechanisms, and insufficient public awareness cultivation, developing countries predominantly rely on terminal disposal approaches—particularly landfilling—in their solid-waste management systems, achieving only 40% efficiency in waste-to-resource conversion. This stands in stark contrast to the 70–90% operational efficiency attained by developed countries through systematic implementation of waste-to-energy incineration technologies and circular resource utilization frameworks, supported by advanced industrial infrastructures, rigorous policy implementation, and sustained environmental education systems [14,15,16,17,18,19,20]. For example, China’s Solid Waste Pollution Prevention and Control Law enacted in 2020 requires a construction waste recycling rate of ≥35% in key cities, while Germany’s Circular Economy Law enacted in 2012 had already set a municipal waste recycling target of ≥65%.
The persistently low utilization rate of industrial solid wastes poses a multifaceted threat to ecological security, sustainable economic development, and public health (Table 1). Against the backdrop of accelerating green industrialization, establishing a full lifecycle management framework—encompassing source reduction, process control, and end-of-pipe resource recovery—has emerged as a critical strategic priority for the development of an ecological civilization [21,22,23,24].

Table 1.
Types, Sources, Quantities, and Environmental impacts of diverse solid wastes.
Industrial solid wastes demonstrate significant technical and economic feasibility in the preparation of novel low-carbon cements. Typical wastes, such as flue gas desulfurization (FGD) gypsum, red mud (RM), fly ash (FA), and kaolin tailings (KTs), are enriched with key oxide components (e.g., CaSO4, Al2O3, SiO2) compatible with the mineral system of high-belite calcium sulphoaluminate cement (HBCSA), providing a scientific foundation for replacing conventional natural raw materials. For example, (a) FA and FGD can be utilized to synthesize HBCSA at approximately 1250 °C, yielding hydration performance comparable to that of ordinary Portland cement (OPC) [26]. These wastes may constitute up to 40% of the raw material blend. (b) FGD and RM are co-convertible into clinkers dominated by dicalcium silicate (C2S) and calcium sulphoaluminate (C4A3S−). Here, FGD serves as the sulfur source and partial calcium supply, whereas RM contributes silica and alumina, collectively accounting for 70–90% of the raw mix [27]. (c) Kaolin tailings and marble waste can fully replace limestone and clay as calcium and silica sources in HBCSA production. Clinkers with excellent mechanical properties are synthesized at 1200–1250 °C—a 150–200 °C reduction compared with OPC clinker production—with these wastes comprising 80% of the raw materials [28].
The synergistic utilization of industrial solid wastes in the cement industry is catalyzing the emergence of cross-sectoral circular economy models. Through industrial symbiosis, the traditional linear production paradigm (resource extraction → production → disposal) is transformed into a multi-industry integrated system (Figure 2). Calcium sulphoaluminate cement (CSA) and high-belite calcium sulphoaluminate cement (HBCSA), distinguished by their unique mineral compositions and high chemical compatibility with waste-derived components, have become pivotal platforms for valorizing industrial solid wastes into high-value products.
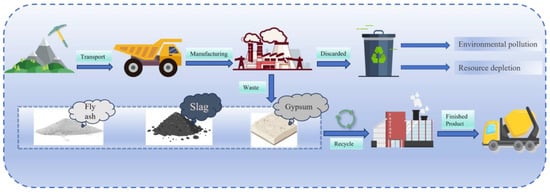
Figure 2.
A multi-industry integrated system.
Nevertheless, the industrial byproducts utilized in current experimental research—such as fly ash, steel slag, granulated blast furnace slag, desulfurization gypsum, and red mud—remain challenging to valorize due to their suboptimal quality and high processing costs. Incorporating these materials into cement formulations significantly reduces storage expenses and environmental contamination risks. However, the inherent compositional heterogeneity and inconsistent physicochemical properties of such solid wastes hinder the industrial-scale production of waste-based high-belite calcium sulfoaluminate (HBCSA) and calcium sulfoaluminate (CSA) cements. To address these limitations, future investigations must prioritize standardized preparation protocols to unify waste feedstock pretreatment and enhance the controllability of clinker phase evolution.
This study systematically analyzes the process-composition-performance relationships of solid-waste-derived HBCSA/CSA cements and elucidates the impacts of (a) calcination regimes (e.g., temperature, holding times) on clinker reactivity, (b) mineral phases (C2S, C4A3S−) on hydration kinetics and mechanical strength evolution, (c) waste types (steel slag, granulated blast furnace slag, phosphogypsum) on rheological and durability properties, and (d) chemical components (Fe2O3, CaSO4, CaCO3) on phase stability and microstructure development. By establishing a theoretical framework for optimizing calcination regimes, tailoring waste-to-clinker formulations, and leveraging the intrinsic properties of waste-derived components, this work advances the large-scale, low-carbon application of industrial solid wastes in cement production, bridging the gap between laboratory innovation and industrial implementation.
2. Preparation Process and Hydration Characteristics of Industrial Solid Waste-Based Calcium Sulphoaluminate Cements
The cement industry faces significant constraints due to its substantial CO2 emissions (0.73–0.99 tons per ton of cement produced) and heavy consumption of natural mineral resources [29,30]. Utilizing industrial solid wastes for HBCSA/CSA cement production presents a dual environmental advantage by reducing pollution through waste repurposing while decreasing dependence on virgin materials, with additional economic benefits. Many industrial solid wastes possess cementitious properties that enable their substitution for conventional raw materials. This approach simultaneously addresses resource conservation challenges and achieves secondary utilization of industrial byproducts, effectively reducing land use and pollution associated with waste disposal.
2.1. Preparation of HBCSA/CSA Cements
2.1.1. Replaceability of Natural Raw Materials
The formation of ye’elimite (C4A3S−), a key mineral phase in HBCSA/CSA cements, requires CaO, Al2O3, and CaSO4, which are traditionally sourced from limestone (CaO), bauxite (Al2O3), and natural gypsum (CaSO4) [31]. Current industrial standards mandate bauxite with over 60% Al2O3 content for HBCSA/CSA production, a stringent requirement that increases costs and limits scalability. To address these constraints, industrial solid wastes have emerged as viable alternatives: (a) Fly ash (FA) partially replaces bauxite and clay via its synergistic Al2O3-SiO2 chemistry [23]. (b) Phosphogypsum and other solid-waste gypsum provide CaSO4 and free CaO, substituting for natural gypsum and limestone [32].
In practical production, this multicomponent solid-waste co-utilization strategy achieves large-scale substitution of natural minerals while reducing the production costs of HBCSA/CSA cement systems compared with conventional processes. This approach enhances economic efficiency and drives the low-carbon transformation of the building materials industry. The cement clinker produced from various solid wastes is summarized in Table 2. Semi-waste-based preparation methods are employed to partially replace natural minerals, such as red mud to replace bauxite and desulfurization gypsum (FGD), flue gas desulfurization gypsum, or phosphogypsum to replace natural gypsum. Additionally, full-waste-based materials completely replace natural raw materials.

Table 2.
Preparation of raw materials from industrial solid waste.
The sintering temperature range for high-volume solid-waste-based cement is 1250–1330 °C, which reduces the energy consumption and carbon emissions compared with the 1300–1450 °C range required for Portland cement. However, owing to the chemical variability of solid-waste feedstocks, three key process controls are required to regulate the performance of cement clinker: (a) Post-addition of gypsum technology: the equilibrium sulfate ion concentration in the cement matrix is adjusted through the addition of calcium sulfate in gypsum. This promotes the formation of anhydrous calcium sulfoaluminate (C4A3S−) and stabilizes ettringite (AFt), thereby reducing fluctuations in compressive strength. (b) Optimization of holding time: insufficient holding time prevents stable crystallization of the target phases. Prolonging the holding time appropriately ensures and maintains the crystallinity of the target mineral C4A3S−. (c) Implementation of secondary calcination: while ensuring high-temperature crystallization of primary mineral phases, reduced calcination temperatures preserve beneficial intermediate phases and effectively mitigate interference from residues in solid wastes during sulfoaluminate mineral formation.
2.1.2. Formulation Design Principles
In the design of raw mixtures for waste-based HBCSA and waste-based CSA cements, the key parameters are the alkalinity coefficient (Cm), aluminum-to-sulfur ratio (P), and aluminum-to-silicon ratio (N). The alkalinity coefficient Cm reflects the effectiveness of CaO in reacting with three oxides (Al2O3, Fe2O3, and SiO2) to form C4A3S−, C4AF, and C2S, as expressed in Equation (4) [42].
In formulation design, Cm is generally approximately maintained. If Cm < 1, the CaO content in the clinker is insufficient for the synthesis of target minerals. Conversely, if Cm > 1, excessive free lime (f-CaO) forms, accelerating early mineral formation and leading to cracking, expansion, and strength degradation in the hardened cement paste [43]. However, a moderate amount of f-CaO may provide certain benefits for HBCSA and CSA cements (discussed further in Section 4).
The alkalinity coefficient Cm affects the dosage of gypsum. Since gypsum participates in calcination as a raw material, the calcium sulfate derived from it also acts as a calcium source during reactions. Therefore, Cm must be recalculated on the basis of this consideration [44], as shown in Equation (7).
The parameter “M” is commonly used to calculate the gypsum dosage and is defined as the molar ratio of CaSO4 to C4A3S−. When “M” ranges from 0 to 1.5, the cement clinker rapidly hardens and quickly sets, as observed in the CSA and HBCSA clinkers. For “M” values of 1.5–2.5, the clinker develops significant self-stress, enabling prestressing effects on the steel reinforcement. When “M” exceeds 2.5–6, pronounced expansion occurs, leading to cracking in the cementitious matrix [45]. The molar ratio “M” (CaSO4/C4A3S−) determines the gypsum content. A higher “M” indicates a greater CaSO4 proportion. During the hydration of HBCSA/CSA clinkers, sufficient gypsum promotes the formation and stabilization of ettringite (AFt), as shown in Equation (1). Conversely, a lower “M” reduces gypsum availability. AFt continues to form (Equation (1)) until gypsum is depleted. Under gypsum-deficient conditions, monosulfoaluminate (AFm) forms (Equation (2)), and existing AFt destabilizes into AFm (Equation (3)). Maintaining “M” within an appropriate range to ensure adequate but not excessive gypsum is critical for HBCSA/CSA hydration.
The alkalinity coefficient Cm reflects the efficiency of CaSO4, Al2O3, and CaO in forming C4A3S−, whereas “M” governs whether the hydration of C4A3S− with CaSO4 sustains AFt formation. Although both parameters are related to the gypsum content in the cement matrix, they characterize distinct hydration mechanisms (Figure 3). Therefore, Cm and “M” should not be calculated independently in formulation design.
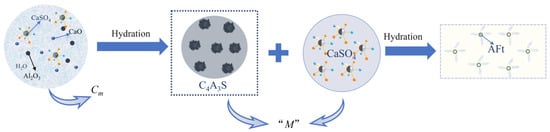
Figure 3.
Different hydration nodes for Cm and “M” control.
The aluminum-to-sulfur ratio P, defined as Al2O3/SO3 (Equation (5)) [41], governs the quantity of C4A3S− formed, with P typically required to be ≤3.82. The aluminum-to-silicon ratio N, expressed as Al2O3/SiO2 (Equation (6)) [46], reflects the proportion between C4A3S− and C2S. For HBCSA cement, N is generally unrestricted since C2S dominates over C4A3S−, resulting in the SiO2 demand for mineral formation exceeding that of Al2O3.
The three parameters (Cm, P, N) collectively determine the pre-hydration mineral availability, whereas “M” regulates the continuity of hydration reactions (post-hydration). This synergistic control ensures the sequential progression of clinker hydration.
2.1.3. Determination of Calcination Regime
The calcination process is critical for the formation of cement clinker minerals. Comparative studies (Figure 4) reveal distinct sintering characteristics between solid-waste-based HBCSA/CSA cements and ordinary Portland cement (OPC): the sintering temperature range for solid-waste-based HBCSA/CSA cements is concentrated at 1250–1300 °C. The lower decomposition temperature of Solid-waste gypsum (compared with that of natural gypsum) shifts the process temperature downward, although the optimal control temperature remains at 1300 °C. This low-temperature sintering (relative to the range of 1300–1450 °C for OPC) contributes to energy conservation and carbon emission reduction.
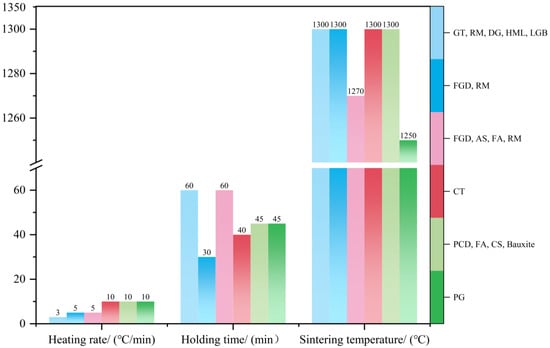
Figure 4.
Calcination regimes for different solid-waste materials [27,35,36,37,38,40].
As the core stage of clinker mineral formation, the calcination regime is governed by three synergistic parameters that regulate the crystallinity and stability of the target phases (C4A3S−, C2S), as demonstrated in Figure 4 and Figure 5: (a) Holding time (30–60 min): adjusted on the basis of the degree of formation of the target minerals. Excessive holding time leads to clinker agglomeration and phase decomposition, whereas insufficient time results in a loose clinker structure and poor crystallinity. (b) Heating rate (3–10 °C/min): balances energy efficiency with phase formation. A rate of 10 °C/min optimally promotes primary-phase crystallization. Excessive heating rates (>10 °C/min) reduce C4A3S− and C2S crystallinity, whereas slower rates increase fossil fuel consumption. (c) Peak temperature control: XRD evolution (Figure 5) indicates that C4A3S− and C2S nucleation initiates at 1200 °C, with maximum characteristic peak intensities observed in the 1300–1350 °C range. At 1350 °C, the diffraction peaks of C4A3S− and C2S decrease, whereas those of CaO and CaSO4 increase, indicating phase decomposition. This increases the liquid phase content, causing densified sintering and reduced hydration activity. Below optimal temperatures, insufficient C4A3S− and C2S formation occurs, accompanied by intermediate phases such as calcium sulfosilicate (2C2S·CaSO4), which exhibit inferior hydration activity. This temperature-phase correlation necessitates precise thermal balancing between mineral synthesis thresholds and decomposition thresholds.
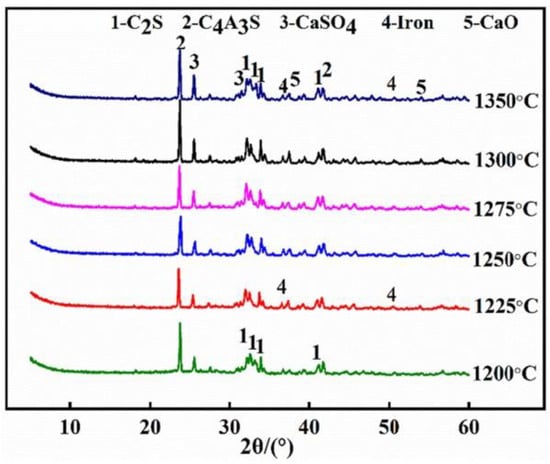
Figure 5.
XRD patterns of HBCSA clinker sintered at 1200–1350 °C [35].
The holding time influences the formation of clinker mineral phases. XRD analysis (Figure 6) revealed the following results: (a) At 30 min, distinct diffraction peaks of C2S and C4A3S− emerged, indicating initial crystallization of the primary phases. (b) Extending the holding time to 60 min increased the peak intensity, confirming improved crystallinity and stabilization. (c) Beyond 90 min, C4A3S− crystallinity markedly decreases, accompanied by the appearance of CaO and CaSO4 peaks (not labeled in the figure), attributed to C4A3S− decomposition at elevated temperatures. Thus, the holding time must balance two critical thresholds: (a) Insufficient retention (<30 min): solid-state reactions remain incomplete, reducing target phase formation. (b) Excessive retention (>90 min): sintering phenomena occur, coarsening clinker particles. This increases grinding energy consumption and delays hydration due to densified surface layer formation.
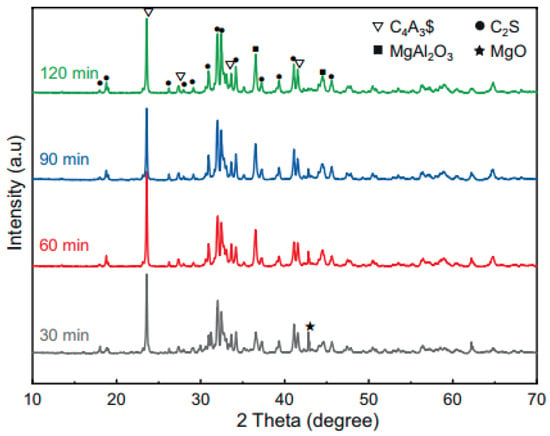
Figure 6.
XRD patterns of the clinkers after different holding times [36].
Optimal Calcination Regime: (a) Temperature: dynamically controlled at 1300 ± 50 °C to promote phase formation while mitigating decomposition. (b) Holding time: approximately 45 min, adjusted via real-time XRD monitoring of phase evolution. (c) Heating rate: 10 °C/min, balancing crystallinity stability and fossil energy conservation.
2.2. Hydration Characteristics
2.2.1. Hydration Minerals
The hydration mechanism of high-belite calcium sulfoaluminate (HBCSA) aligns with that of calcium sulfoaluminate cement (CSA). Solid-waste-based sulfoaluminate cements also exhibit hydration behaviors analogous to those of conventional CSA systems [47,48]. Thus, the hydration process is discussed primarily within the HBCSA/CSA framework.
The core hydration process in sulfoaluminate cements systems originates from calcium sulfoaluminate minerals (chemical formula: 3CaO⋅3Al2O3⋅CaSO4, abbreviated as C4A3S−) [49], whose discovery underpinned the development of CSA cements [50,51,52]. Unlike ordinary Portland cement (OPC), which is dominated by C3S, C2S, C3A, and C4AF, the CSA/HBCSA system primarily comprises C4A3S−, C2S, and CaSO4 [53]. The hydration process involves synergistic reactions among C4A3S−, C2S, and CaSO4 (providing calcium and sulfate ions), generating ettringite (AFt), C-S-H gel, and aluminum hydroxide (AH3) to form a dense structure that provides strength (Figure 7).
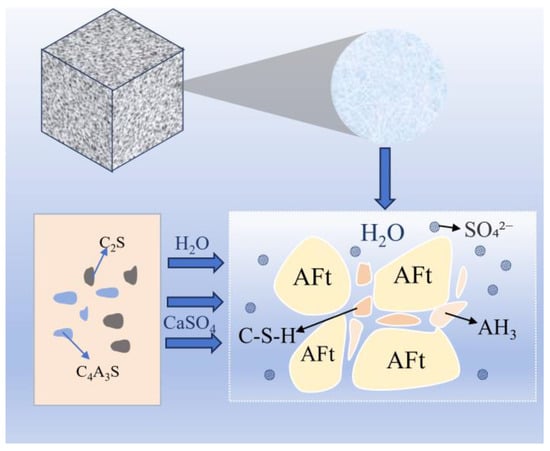
Figure 7.
HBCSA/CSA clinker hydration process.
The fast setting and hardening characteristics of CSA/HBCSA cements stem from two microstructural advantages: (a) Nanoscale dimensions: C4A3S− and C2S exhibit smaller particle sizes (<5 μm) than approximately 50 μm C3S grains in OPC (Figure 8). (b) Defect-rich interfacial structures: the C4A3S−-C2S composite forms interfaces abundant in defects (Figure 8c,d). These features increase the specific surface area and retain the intraparticle porosity after clinker grinding, significantly enhancing the hydration activity [54]. The porous network facilitates sustained water infiltration, maintaining hydration reactions while avoiding diffusion-limiting effects caused by dense hydration layers in OPC systems [55].

Figure 8.
Microscopic topography of the cement minerals (a–d) HBCSA, (e) CSA, and (f) OPC [55].
In HBCSA/CSA cements, C4A3S− (ye’elimite) and C2S govern early-age and long-term strength development, respectively, with distinct formation conditions and hydration behaviors. For C4A3S−, the cubic polymorph (C-Ye’elimite) exhibited low hydration activity due to structural stability, whereas the orthorhombic polymorph (O-Ye’elimite) demonstrated rapid hydration kinetics [49]. The exothermic behavior of hydration, a critical indicator of strength evolution, reveals the following: (a) Under sufficient gypsum (CaSO4) supply, both polymorphs exhibit similar cumulative heat release. (b) Under gypsum-deficient conditions, O-Ye’elimite generates a sharp exothermic peak within 1 h, whereas the peak of C-Ye’elimite is delayed to approximately 7 h [56]. Thus, process optimization is required in the formulation design to suppress C-Ye’elimite formation.
Unlike OPC systems dominated by highly reactive C3S, HBCSA/CSA cements utilize C4A3S− as the early-strength phase and slower-reacting C2S for long-term strength. This mineralogical reconfiguration results in subdued late-age strength growth compared with rapid early gains. However, optimizing C2S polymorphs can balance strength evolution. C2S exists in five polymorphs: α-, αH’-, αL’-, β-, and γ-C2S, with phase transition temperatures shown in Figure 9 [57].
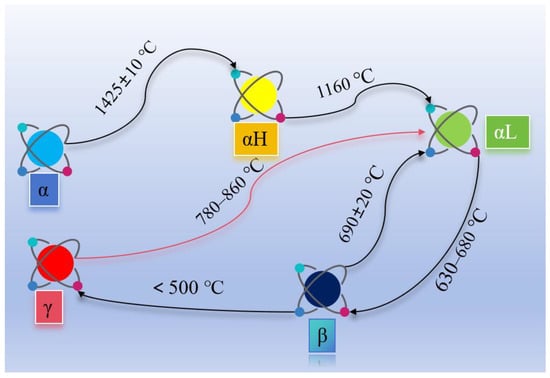
Figure 9.
C2S transition temperatures for different crystal types.
α-C2S and αH’-C2S are metastable under ambient conditions and tend to transform into β- or γ-C₂S. γ-C2S has a loose structure, negligible hydration activity, and low cementitious strength [58,59]. To stabilize α-C2S/αH’-C2S and prevent γ-C2S formation, rapid cooling is applied post-calcination [60]. Insufficient calcination temperatures or slow cooling rates promote the β-C2S to γ-C2S transition, accompanied by 11% volumetric expansion, inducing internal stresses that cause clinker dust [61].
The hydration reaction rates of the C2S polymorphs followed the order α-C2S > αH’-C2S > αL’-C2S > β-C2S > γ-C2S. While γ-C2S exhibits negligible hydraulic activity, the α-, αH’-, αL’-, and β-C2S phases possess limited reactivity characterized by slow hydration rates and low exothermicity, leading to reduced early-age strength [61]. Studies have demonstrated that lattice doping of C2S with trace elements (e.g., Ba, Fe, and Al) significantly enhances its hydration properties. Among these, Ba2+ induces exceptional solid-solution effects in β-C2S because of its unique ionic radius and charge characteristics [50]. Ba2+ doping promotes lattice distortion in β-C2S, optimizing its microstructure to accelerate hydration kinetics and markedly improve early-age reaction rates [61,62]. Natural barite (BaSO4 content > 85%), which serves as an ideal BaO source, concurrently provides SO3 and SiO2, enabling its dual substitution for traditional gypsum (CaSO4·2H2O) and clay (SiO2) in raw material formulations.
2.2.2. Hydration Products
The hydration process of sulfoaluminate cements-based materials involves the following: aluminum-rich C4A3S− reacts predominantly with calcium sulfate, forming ettringite (AFt) and aluminum hydroxide (AH3) gel through dissolution-precipitation. Simultaneously, C2S hydrolysis generates C-S-H gel and calcium hydroxide (CH), as detailed in Equations (1) and (8).
The microstructure evolution of HBCSA/CSA hardened pastes exhibits distinct hierarchical features: (a) Primary framework: needle-like/rod-shaped AFt crystals grow randomly and interweave into a three-dimensional skeletal structure. (b) Pore infilling: AH3 and C-S-H gels, as secondary hydration products, gradually fill the interstitial spaces within the AFt framework, ultimately forming a dense composite.
The sulfate content critically governs hydration product evolution. Insufficient gypsum leads to low sulfate ion concentrations in the liquid phase, destabilizing the AFt formation equilibrium [63,64]. Under such conditions, C4A3S− hydration is incomplete. Excessive gypsum, while supplementing with CaO and SO3, accelerates C4A3S− and C2S hydration, causing rapid product generation. This leaves insufficient time for AFt skeletal structure development [65,66]. The resulting hydration products encapsulate clinker particles, slowing subsequent reactions—a phenomenon exacerbated by higher gypsum dosages. These phase transformations significantly alter the pore structure and impair the development of mechanical performance.
As shown in Figure 10, the gypsum dosage significantly influences AFt generation in samples A and C. With increasing gypsum content, the AFt levels in both samples initially increase. However, at 6 h, the AFt content in A10 (10% gypsum) was lower than that in A0 (0% gypsum), indicating that gypsum temporarily suppressed AFt formation. This inhibition occurs as hydration products encapsulate clinker particles, delaying further reactions. Under water pressure, these encapsulating layers rupture, allowing hydration to resume. Consequently, A10 surpasses A0 in AFt generation shortly afterward, demonstrating that gypsum ultimately benefits the overall hydration process despite early-stage retardation.
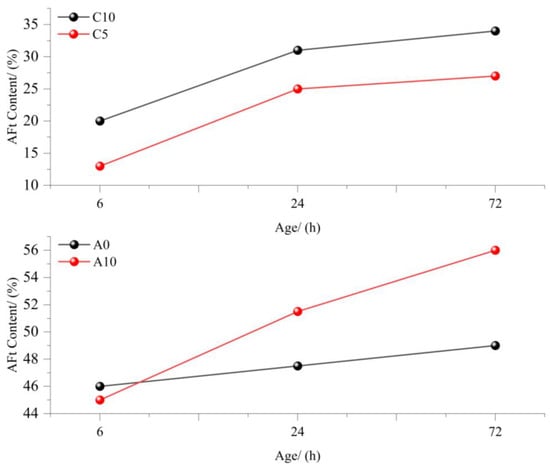
Figure 10.
Effects of different gypsum contents on AFt formation [63,64].
The combined analysis of Figure 11 and Figure 12 reveals the influence of gypsum on the AFt and ye’elimite (C4A3S−) contents in samples A and C. C0 (0% gypsum) and C15 (15% gypsum): (a) At 0 d, the C4A3S− content in C15 exceeded that in C0 due to gypsum supplementation. (b) From 1 d to 3 d, gypsum and C4A3S− in C15 gradually decrease, whereas AFt increases, which is consistent with Equation (1).
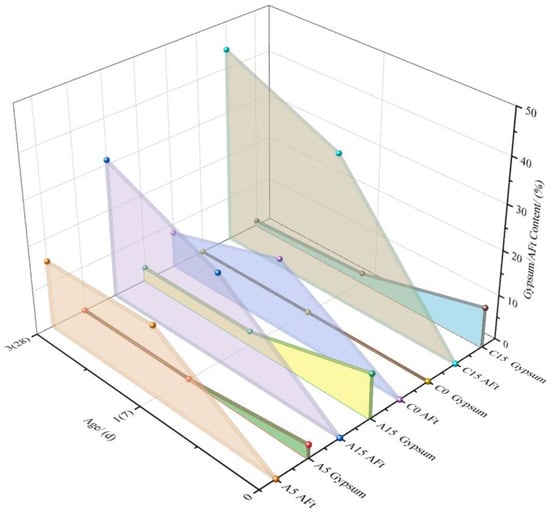
Figure 11.
Effect of gypsum content on AFt formation [64,65].
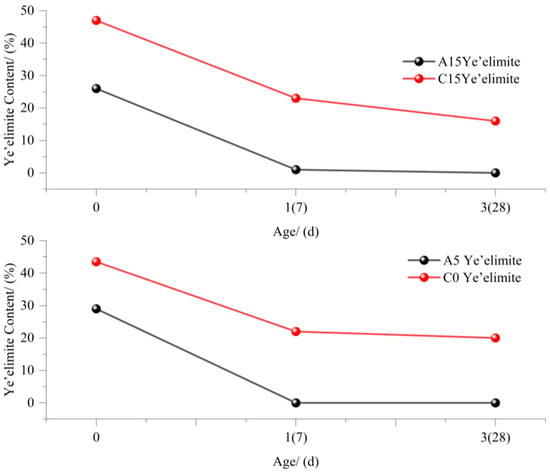
Figure 12.
Amount of change in Ye’elimite [64,65].
As shown in Figure 11 and Figure 12, the addition of 5% and 15% gypsum to sample A alters the trends of the AFt and C4A3S− contents at 0 d, 7 d, and 28 d: (a) At 15% gypsum, the early C4A3S− content decreases, which is consistent with prior observations: excessive gypsum slows initial hydration. However, the CaSO4/C4A3S− molar ratio remains balanced, allowing AFt generation to increase with increasing gypsum dosage. (b) For A5 (5% gypsum) and A15 (15% gypsum), the AFt content increased until 7 d, after which A5 decreased. As gypsum is depleted, insufficient sulfate ions destabilize AFt, leading to its decomposition into AFm (Equation (3)).
As shown in Figure 13 (additionally, owing to the distinct cement compositions of [64,66], this figure primarily compares their respective AFt (ettringite) loss on ignition (LOI) under varying gypsum contents), the mass loss of AFt within the 100–150 °C range in the cement clinker varies with the gypsum dosage and hydration duration: (a) For the BSA samples (0%, 5%, and 10% gypsum), the AFt mass loss increases progressively over 72 h, with higher gypsum dosages resulting in more pronounced losses. (b) In the GF-HBCSA clinkers (10%, 15%, and 20% gypsum), the AFt mass loss intensifies from 1 d to 7 d. At 3–7 d, the samples with 10–15% gypsum exhibit comparable mass losses, whereas the 20% gypsum sample shows significantly greater mass losses. This disparity arises because the 10% and 15% gypsum dosages are insufficient to sustain complete hydration of the cement matrix, leading to reduced AFt formation after gypsum depletion. By 28 d, all samples displayed reduced AFt mass loss, particularly in the 10–15% gypsum systems. This reduction reflects AFt destabilization and conversion to AFm (monosulfoaluminate) after gypsum exhaustion.
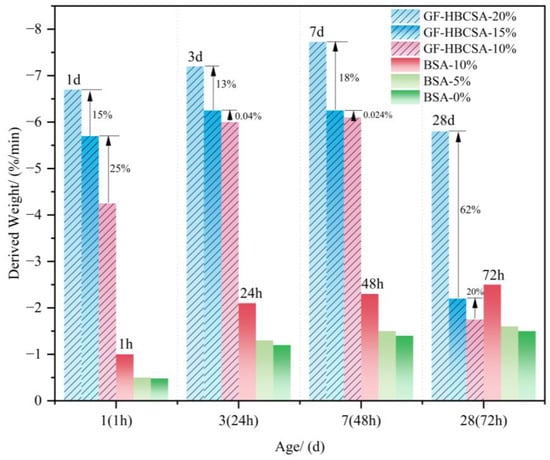
Figure 13.
The amount of change in weight loss of an Aft [64,66]. Note: the derived weights at 1 day, 3 days, 7 days, and 28 days correspond to GF-HBCSA samples, while those at 1 h, 24 h, 48 h, and 72 h correspond to BSA samples.
As shown in Figure 14, the SEM results reveal the following: (a) A0 (0% gypsum): AFt and AH3 form as early as 6 h, with minimal additional hydration products by 28 d. (b) A10 (10% gypsum): at 6 h, the hydration products include AFt, AH3, petal-like AFm, and unreacted gypsum. AFm formation (Equation (2)) is attributed to insufficient sulfate ions, which hinder AFt formation or trigger its decomposition. By 28 d, residual gypsum hydrates fully, supplying sulfate ions to convert AFm into AFt (Equation (9)), resulting in dense columnar AFt aggregates. (c) A20 (20% gypsum): needle-like/rod-shaped AFt forms rapidly at 6 h, consuming CH (Equation (10)) and accelerating C2S hydration to regenerate CH.
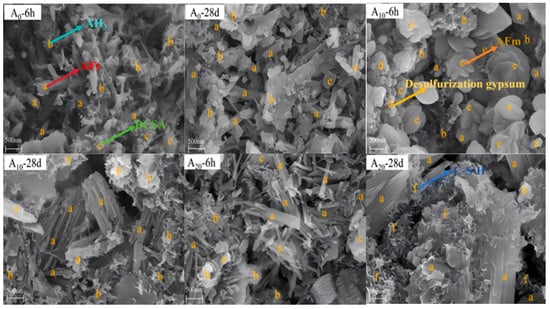
Figure 14.
Microscopic morphology of hydrated samples [63].
In sulfoaluminate cements, the hydration products are dominated by AFt and gel-like phases. While AFt is the primary strength contributor in HBCSA/CSA systems, excessive AFt content does not guarantee optimal performance. Achieving balanced formation of AFt and hydrated gels is critical to ensuring the mechanical strength of hardened cement paste.
2.2.3. Hydration Exothermicity
Hydration heat release directly reflects the hydration rate and reactivity of target minerals in cement clinker after water interaction, serving as a proxy for analyzing mechanical performance [35]. The hydration process is fundamentally governed by chemical reactions between active mineral phases and water [67]. During hydration, bonds in cement minerals (e.g., siloxane bonds (Si–O) in C3S and C2S) rupture, requiring energy absorption. Concurrently, water molecules combine with ions or atomic groups from minerals to form new bonds (e.g., C-S-H and CH), releasing energy. When the energy released from new bond formation exceeds that absorbed by bond breaking, the overall reaction is exothermic, generating hydration heat (Figure 15).
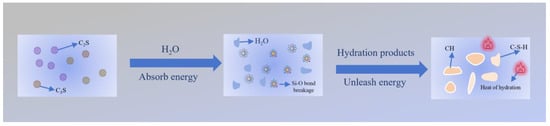
Figure 15.
Hydration exothermic reaction.
The hydration heat release behavior of calcium sulfoaluminate cement (CSA) and high-belite calcium sulfoaluminate cement (HBCSA) is closely related to their compositional characteristics. Studies indicate that the pronounced early hydration reactions in these systems arise from synergistic effects of particle size distribution and active components [55]: (a) HBCSA/CSA cements exhibit finer particle sizes than OPC cements do, increasing the specific surface area and accelerating hydration kinetics. (b) Calcium sulfate (CaSO4⋅2H2O) and calcium sulfoaluminate (C4A3S−) directly regulate the formation of hydration products.
Replacing HBCSA with iron-rich belite calcium sulfoaluminate cement (IRCSA) alters hydration exothermicity (Figure 16). IRCSA particles are coarser than HBCSA particles. As substitution increases, the exothermic peaks shift to later times (e.g., Peak 2 appears at 55 min for C1 vs. 204 min for C5), with reduced initial heat release. The subsequent hydration stages involve reactions of the remaining clinker phases, which release additional heat.
Comparing the strength development (Figure 17) and hydration heat release (Figure 16) of C1 and C5, (a) C1 exhibited intense early heat release, reflecting rapid initial hydration. (b) C5 outperforms C1 in terms of cumulative heat release after the third exothermic peak, accompanied by a significantly greater 3–28 d strength. This finding demonstrates that the hydration heat release directly reflects the strength evolution of the cement matrix.
When the gypsum dosage and C4A3S− content reach hydration equilibrium (i.e., the CaSO4/C4A3S− molar ratio), rapid reactions occur during hydration. Sufficient ettringite (AFt) and aluminum hydroxide (AH3) form in the early stage, dominating the intensity and duration of the initial exothermic peak. The regulation of hydration heat release involves multifactorial coupling: (a) Gypsum dosage: adjusts the sulfate ion concentration in the liquid phase, influencing C4A3S− dissolution and AFt crystallinity. (b) C4A3S− content: governs aluminum phase availability, requiring a molar ratio to gypsum that ensures AFt stability [44].
Deviations from the optimal CaSO4/C4A3S− ratio trigger Aft-to-AFm conversion, accompanied by altered heat release rates and peak shifts. The mechanical properties of hydrated cement depend on hydration products, supplementary gypsum dosage, and the water-to-cement ratio (w/c): (a) HBCSA/CSA systems: C4A3S− and C2S contents dictate AFt, C-S-H, and AH3 formation. (b) Supplementary gypsum: compensating for sulfate ion depletion to stabilize AFt. (c) Water demand: (i) Insufficient water: hinders complete mineral hydration. (ii) Excessive water increases porosity and shrinkage, degrading strength. Balancing these factors ensures optimal microstructure development and mechanical performance.
As shown in Table 3 and Figure 17, the compressive strengths of solid-waste-based CSA and conventional CSA cements with varying mineral compositions, gypsum dosages, and W/C ratios were evaluated at 3 d, 14 d, and 28 d. For clinkers without added gypsum [68,69] the compressive strength is governed by the C4A3S− content. When the primary mineral phases (C4A3S− and C2S) are comparable [68,70] the addition of 5% gypsum significantly enhances the compressive strength, indicating that gypsum drives strength development. However, excessive gypsum accelerates hydration reactions, generating porous structures that degrade strength despite early gains.
From the mineral compositions (Table 3) and strength evolution (Figure 17), the clinkers with high C4A3S− and moderate gypsum contents exhibit pronounced early strength. Clinkers with high C2S contents show the most significant strength increases from 7 to 28 d, confirming the role of C2S in long-term performance. Notably, in solid-waste-based CSA cements, increasing w/c [39,68,69,70,71] reduces early strength, likely due to increased porosity from excessive water.
Despite their complex compositions and low critical component contents, industrial solid wastes (e.g., low-grade bauxite, phosphogypsum, low-calcium limestone) enable high natural raw material substitution rates in HBCSA/CSA cement production through synergistic utilization, significantly lowering costs. However, while such utilization demonstrates economic and environmental potential, large-scale production faces a critical challenge: standardizing protocols. Existing studies primarily focus on feasibility analyses, yet these are inherently constrained by the compositional variability of regionally sourced wastes, limiting universal applicability. Establishing standardized frameworks or controlling process variability within acceptable tolerances remains pivotal for future advancements.
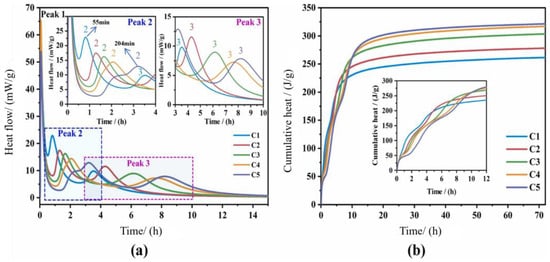
Figure 16.
(a) Heat flow evolution and (b) cumulative hydration heat of blended pastes (w/c = 0.5) [72]. Note: replacement rate (C5 > C4 > C3 > C2 > C1).

Table 3.
Target mineral content, gypsum addition amount, and W/C(W/B) of the cement clinker.
Table 3.
Target mineral content, gypsum addition amount, and W/C(W/B) of the cement clinker.
| Mineral Composition | Post-Admixture | W/C | Industrial Solid Waste | Ref. |
|---|---|---|---|---|
| 29.13% C4A3S−, 52.99% C2S | Gypsum 8% | 0.35 | Aluminum tailings | Chen et al. [39] |
| 35% C4A3S−, 42.13% C2S | Gypsum 10% | 0.3 | Electrolytic manganese residue, Barium slag | He et al. [71] |
| 50% C4A3S−, 50%C2S | Gypsum 5% | 0.3 (W/B) | Phosphogypsum | Shen et al. [70] |
| 50% C4A3S−, 35% C2S | / | 0.6 | Fly ash, Phosphogypsum | Tao et al. [68] |
| 38.9% C4A3S−, 33.8% C2S, 17.8% CaSO4 | / | 0.5 | Phosphogypsum | Shen et al. [69] |
| 58% C4A3S−, 12% C2S | Gypsum 18% | 0.5 | / | Yeung et al. [73] |
Note: [73] is CSA cement.
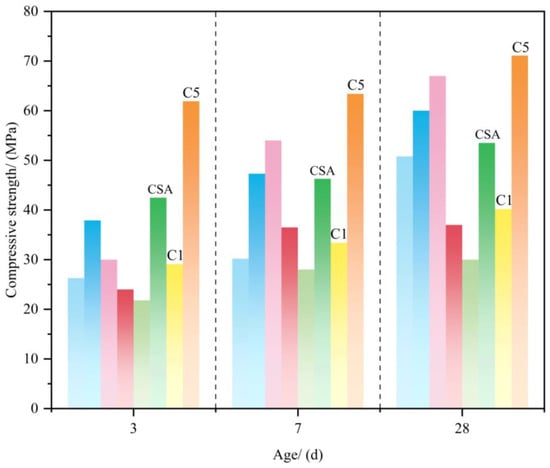
Figure 17.
Comparison of compressive strengths [39,68,69,70,71,72,73]. Note: the compressive strength data lack direct comparability due to varied materials, but data integration compares performance with CSA cement to validate feasibility.
3. Impact of Solid Waste on HBCSA and CSA Cement Performance
The previous section primarily discussed the feasibility of utilizing solid waste in cement preparation. This section will analyze the impacts of impurities in common solid-waste materials on cement performance.
Compared with natural raw materials, industrial solid wastes, when used as alternative components in cementitious materials, exhibit distinct mineralogical and hydration behaviors [74,75].
These byproducts introduce chemical impurities and unique hydration characteristics (Figure 18): phosphogypsum (PG), which contains soluble P2O5, and fluoride ions (F−), which react with aluminate phases to form low-solubility aluminum phosphate coatings (labeled “gel” in the figure), inhibits C4A3S− dissolution. Steel slag (as a siliceous feedstock) contains free MgO (f-MgO), free CaO (f-CaO), and Fe2O3, which induce volumetric expansion and microcracking in hardened pastes. Granulated blast furnace slag (GBFS): this material exhibits latent pozzolanic activity but is constrained by the alkalinity of HBCSA/CSA systems (pH 10.5–11.5). Compared with that in Portland cement systems (pH > 12.5), the Ca(OH)2 (CH) concentration in C-S-H gel systems is lower, limiting the increase in compressive strength.
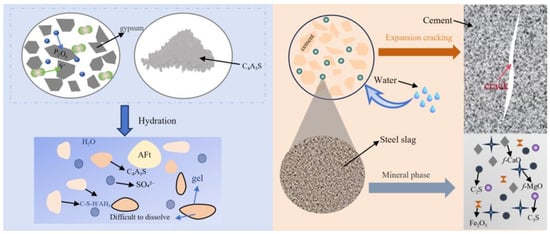
Figure 18.
Hydration characteristics of PG and steel slag.
The incorporation of industrial wastes exhibits dual effects: (1) Early strength reduction due to impurity-induced hydration inhibition. (2) Compensatory mechanisms: Steel slag provides C2S and C3S to stabilize AFt via alkalinity, enhancing long-term strength. GBFS fines act as microaggregates, mitigating drying shrinkage in hardened pastes.
3.1. Solid-Waste Gypsum
As mentioned earlier, gypsum plays a critical role in the hydration process of HBCSA/CSA cements by releasing sulfate ions to stabilize AFt (ettringite) formation. Compared to natural gypsum, Solid-waste gypsum contains inherent impurities. This subsection, therefore, systematically analyzes the effects of different types of Solid-waste gypsum on the performance of HBCSA and CSA cements.
In phosphogypsum (PG)–calcium sulfoaluminate cement (PG-CSA) systems, soluble P2O5 and fluorides (F−) in PG significantly prolong the setting time and reduce the strength/density [76]. These impurities slow PG dissolution during early hydration, limiting the availability of sulfate ions. Since C4A3S− hydration depends on gypsum reactivity [77], PG incorporation reduces ettringite (AFt) formation in PG-CSA. Soluble P2O5 forms hydration products that coat C4A3S−, further delaying hydration kinetics, macroscopically manifesting as reduced hydration heat and early-age strength [78].
XRD analysis (Figure 19) confirmed that the PG-CSA clinkers contained the primary crystalline phases C4A3S− and C2S, indicating that PG did not inhibit the formation of key mineral phases. However, PG-CSA also generates an intermediate phase, gehlenite (C2AS, 2CaO·Al2O3·SiO2), which exhibits temperature-sensitive decomposition [79] At approximately 1300 °C, C2AS decomposes into C2S and CaSO4 via solid-state reactions, participating in C4A3S− synthesis. Below optimal temperatures, undissolved C2AS accumulates at grain boundaries, degrading the crystallinity of the primary phases [72]. Thus, the presence/absence of C2AS serves as an indicator of the appropriate calcination temperature.
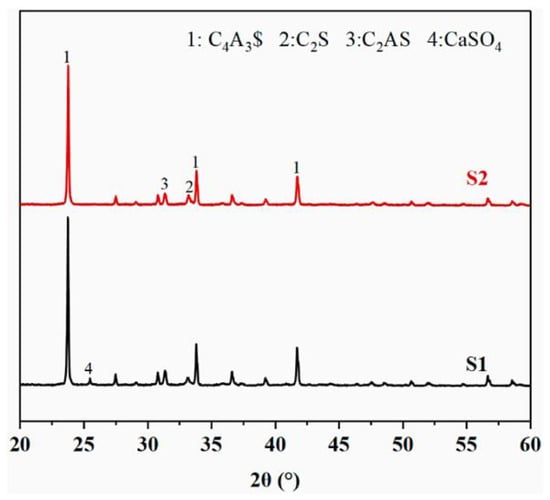
Figure 19.
XRD pattern of the PGCSA clinker [80]. Note: S1 and S2 are different batches of clinker, C4A3$=C4A3S−.
As shown in Figure 20, the compressive strengths of cements prepared with different types of gypsum are compared: (a) DSG-CSA and Desulfurization gypsum (DSG) are used as post-supplemented gypsum [75]. (b) FDG-CSA: flue gas desulfurization gypsum (FDG) replaces natural gypsum (NG) and serves as a post-added component, with the clinker calcined at 1050 °C [51]. Both FDG-CSA and DSG-CSA exhibit gradual early strength development (1–3 d) but limited later-age gains (28 d). The lower strength of FDG-CSA is attributed to insufficient formation of primary phases at 1050 °C, as FDG decomposes faster than NG at elevated temperatures. DG-CSA was prepared using coal gangue, carbide slag, and desulfurization gypsum (DG, distinct from DSG) as raw materials, with clinker calcined at 1280 °C. DG-CSA demonstrated significantly greater strength development than FDG-CSA and exceeded OPC performance, highlighting the critical role of the calcination temperature in phase formation.
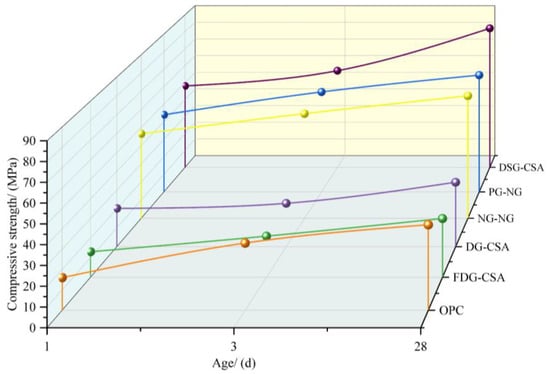
Figure 20.
Comparison of the strengths of different solid-waste-based cements and OPC cements [54,63,78,81]. Note: OPC—Ordinary Portland Cement; FDG—Flue Gas Desulfurization Gypsum; DG— Desulfogypsum; NG—Nature Gypsum; PG—Phosphogypsum; DSG (distinguished from the former)—Desulfogypsum; PG-NG—phosphogypsum as raw material, natural gypsum as post-addition material.
Figure 21 compares the hydration heat release between clinkers prepared with NG (BCSA-G, NG-NG) and PG (BCSA-P, PG-NG): BCSA-P results in greater heat release at 3 h than BCSA-G does but is surpassed by BCSA-G thereafter. The cumulative heat release confirmed the slower hydration kinetics in the PG systems due to P2O5/F− impurities.
Strength correlation: despite slower hydration, PG-NG achieves greater compressive strength than OPC does (Figure 20), demonstrating the feasibility of PG as an NG substitute in clinker production when accounting for cost-effectiveness and performance trade-offs.
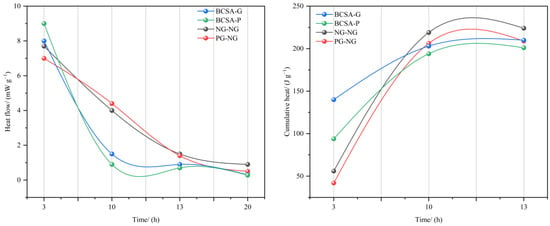
Figure 21.
Heat of hydration of cement [77,78]. Note: BCSA-G (containing natural gypsum), BCSA-P (containing phosphogypsum).
Solid-waste gypsum serves as both a calcareous material and sulfate source in cement production, fully replacing traditional limestone and natural gypsum. This substitution reduces CO2 emissions from limestone decomposition and minimizes natural gypsum extraction, achieving energy conservation, emission reduction, and resource circularity. Specifically, in clinker calcination, it decreases natural mineral consumption.
When added as post-gypsum, it optimizes setting behavior, stabilizes AFt formation, and enhances cost-effectiveness.
However, industrial application faces challenges: impurities in Solid-waste gypsum disrupt hydration, delaying early strength development and prolonging setting times. Research indicates that optimizing the gypsum dosage and calcination regime can produce clinker performance comparable to conventional materials, providing a foundation for novel low-carbon cement systems.
3.2. Steel Slag and Blast Furnace Slag
Steel slag, when incorporated as a post-addition component in HBCSA/CSA, acts as a filler and diluent in the early stages [82]. Owing to its higher density and volume than those of the CSA clinker, equivalent substitution replaces a larger quantity of CSA clinker with a smaller mass of steel slag. As substitution increases, the composite clinker particles coarsen, and the hydration reactivity decreases.
Dual effects of steel slag addition: (a) Negative impact: the low reactivity of steel slag prolongs the setting time and reduces the compressive strength at higher substitution levels, as the composite clinker requires extended periods to generate sufficient hydration products for pore filling [83]. (b) Positive impact: (i) C2S and C3S in steel slag: hydrate to produce CH, increasing the pore solution pH and stabilizing ettringite (AFt) [82,83]. (ii) Iron phases in steel slag enhance clinker burnability by promoting free lime absorption, increasing the liquid phase content, and lowering the calcination temperature [84].
Impact of Steel Slag Dosage on Setting Time and Chemical Shrinkage (Figure 22 and Figure 23): (a) Setting time: Increases with increasing steel slag content (Figure 22). This is attributed to the slag’s larger particle size, lower specific surface area, and reduced hydration activity, which limit water–clinker contact [82]. (b) Chemical shrinkage: Decreases with the addition of steel slag (S) (Figure 23). Slower slag reactivity allows the CSA clinker to hydrate fully, resulting in a denser microstructure. Reduced CSA clinker content also diminishes AFt generation, mitigating the shrinkage caused by AFt dehydration in later stages.
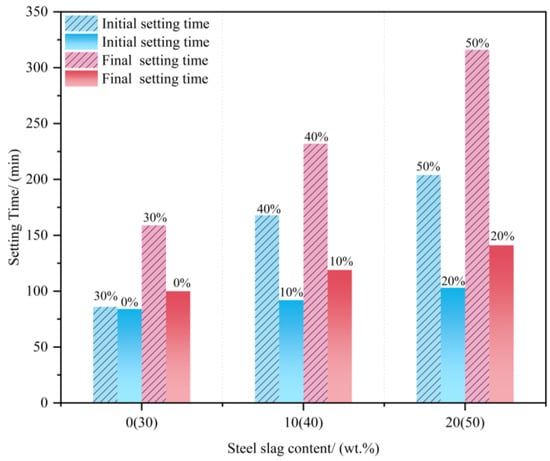
Figure 22.
Setting time of steel slag mixed with CSA cement [82,83].
The incorporation of blast furnace slag (BFS) and fly ash (FA) into cement clinker reduces hydration heat release [85], indicating incomplete reactions with water, which increases the water-to-cement ratio (w/c), leading to fewer hydration products and lower compressive strength. Slag increases the water demand for normal consistency due to its higher specific surface area, which increases with fineness [53]. Although BFS and FA can enhance mortar mechanical properties, their reactivity requires alkaline activation. CaSO4 and Ca(OH)2 synergistically activate the hydration of Al2O3 and SiO2 in slag to form C-S-H gel and AFt, which fill pores, refine the pore structure, and densify the matrix [86]. Optimization of Slag Dosage: (a) Excessive slag: a high Al2O3 content promotes excessive AFt formation, causing expansion and microcracking at later ages. (b) Insufficient slag: fails to generate adequate AFt for strength enhancement.
XRD analysis of the HBCSA clinker with 10% mineral powder (MP, blast furnace slag powder) or fly ash (FA) (Figure 24): (a) At 1 d (Figure 24a), all the mixtures exhibited AFt, unhydrated C4A3S−, and C2S. The 10% substitution of FA or MP did not significantly reduce the amount of AFt, indicating a minimal early-stage impact on hydration. (b) At 28 d (Figure 24b), the AFt content remains stable, whereas the diffraction peaks of unhydrated C4A3S− and C2S decrease. However, in the low-alkalinity environment of HBCSA, FA and MP cannot fully hydrate, causing the conversion of AFt to AFm and reducing the number of AFt peaks [87]. Minor slag addition, unhydrated slag particles, which are smaller than clinker grains, fill pores in the cement paste, improving early strength [88].
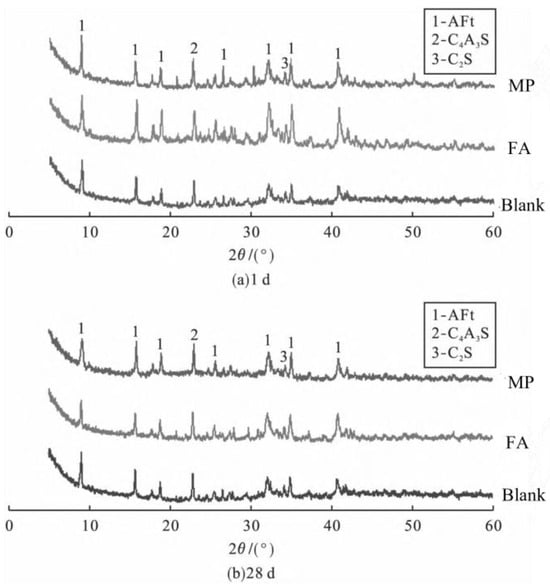
Figure 24.
XRD patterns of (a) 1 d and (b) 28 d of the 10% fly ash and mineral powder cement clinkers [87].
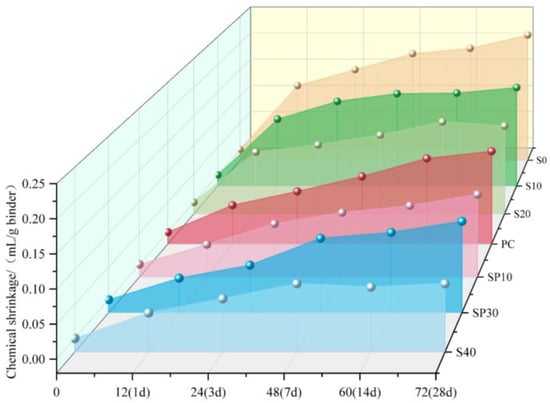
Figure 23.
Chemical shrinkage of CSA cement after blending steel slag (s) and slag powder (SP, blast furnace slag powder). Note: S0 and SP10 represent 0% and 10% dosages, respectively, of PC—Portland cement [82,88].
SEM images of the cement pastes in the blank, 10% fly ash (FA), and 10% mineral powder (MP) groups at 1 d and 28 d are shown in Figure 25. (a) Blank group: (i) At 1 d: needle-like/rod-shaped AFt dominated, with minimal C-S-H gel. (ii) At 28 d, a dense structure forms as the C-S-H gel interweaves with AFt, indicating the complete hydration of C4A3S− and C2S. (b) 10% MP addition: (i) At 1 d, the amount of flocculent C-S-H gel increased significantly compared with that in the blank group and coexisted with AFt. (ii) At 28 d, the C-S-H gel continues to grow, enveloping AFt to form a stable, interconnected framework. (c) 10% FA addition: (i) At 1 d: partial pozzolanic activation of FA increases the C-S-H gel slightly, but numerous unhydrated FA particles remain. (ii) At 28 d, the C-S-H gel content increased further but remained lower than that in the MP group. Unhydrated FA particles persist, albeit to a reduced degree.
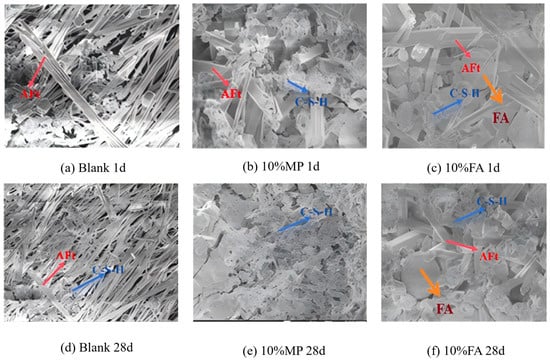
Figure 25.
SEM images of unblended, 10% mineral powder and 10% fly ash cement clinkers [87].
Hydration products: both FA and MP generate C-S-H gel akin to C2S rather than forming additional AFt, as theoretically expected. Low Alkalinity Limitation: the low Ca(OH)2 content in HBCSA/CSA systems inhibits the full activation of FA/MP, preventing complete hydration despite the presence of CaSO4. These observations validate the restricted reactivity of FA/MP in low-pH sulfoaluminate environments, emphasizing the need for alkaline activators to optimize waste utilization in such systems.
As shown in Figure 23, the incorporation of slag powder at 10% and 30% significantly reduced paste shrinkage, primarily due to its microaggregate effect. Slag particles, which are typically smaller than cement grains, effectively fill pores in the cement matrix, refine the microstructure and increase the material density [89]. However, slag addition introduces two effects [90,91,92]: (a) Dilution effect: reducing overall alkalinity, limiting pozzolanic reactions, and decreasing total hydration products. (b) Low-alkalinity constraint: this constraint slows slag hydration kinetics, leaving unreacted particles that encapsulate the clinker and hinder further hydration. Together, these effects increase porosity and cause strength loss.
While higher slag dosages suppress early ettringite (AFt) formation, mitigating expansion risks, late-age AFt dehydration shrinkage may still induce microcracking. Additionally, a reduced HBCSA/CSA clinker content delays early hydration. Although continued C-S-H gel formation partially compensates for pore filling in later stages, diminished AFt generation inevitably leads to strength degradation.
The utilization of industrial solid wastes (e.g., phosphogypsum, steel slag, blast furnace slag) in cement production enhances resource circularity and reduces carbon footprints, yet faces performance modulation challenges:
- (1)
- Phosphogypsum: soluble P2O5 and fluoride impurities retard C4A3S− hydration when substituting natural gypsum, reducing early strength and prolonging setting time.
- (2)
- Steel slag: Fe2O3 lowers the clinker calcination temperature and stabilizes ettringite (AFt) while suppressing chemical shrinkage, but its low reactivity delays hydration and compromises early strength at high substitution rates.
- (3)
- Blast furnace slag: pozzolanic activity necessitates alkaline activation, which conflicts with low-alkalinity CSA systems, while excessive Al2O3 content induces expansion and microcracking despite pore structure refinement.
Sustainable cement production utilizing solid wastes offers environmental advantages by reducing CO2 emissions through limestone substitution and conserving natural resources. However, industrial implementation faces persistent challenges:
- (a)
- Uncontrolled Impurities and Inferior Composition: solid wastes exhibit inherently variable impurity levels (e.g., P2O5 and F⁻ in phosphogypsum; free MgO and free CaO in steel slag) and lower chemical consistency compared to natural minerals.
- (b)
- Lack of Industrial Standardization: beyond laboratory settings, industrial-scale production faces inconsistencies in pretreatment, classification, processing protocols (even for identical feedstocks), and performance testing standards.
- (c)
- Performance Uncertainty: can cement properties remain stable when impurities exceed thresholds in real-world production?
- (d)
- Cost–Production Dilemma: laboratory studies predominantly use pre-treated solid wastes, but industrial procurement of such refined materials raises costs.
- (e)
- Quantification Barriers: absent unified performance standards, the properties of waste-based HBCSA/CSA cements cannot be objectively quantified due to variability in raw materials, processing methods, and chemical compositions.
Existing research remains confined to idealized laboratory conditions, focusing narrowly on feasibility experiments that verify whether cement “can be produced”—an approach insufficient to advance practical solid-waste recycling. Future research and development should focus on developing scalable, cost-effective solutions that effectively transition innovations from lab-scale research to industrial-scale applications.
4. Characteristics of the Chemical Components in HBCSA and CSA Cements
The materials discussed in previous sections—such as gypsum and steel slag—exhibit distinct characteristics when incorporated into cement, primarily attributed to the critical roles of their constituent chemical components (e.g., CaSO4, CaO, Fe2O3, CaCO3). This section therefore focuses on analyzing the three principal chemical constituents in HBCSA and CSA cements.
High-belite calcium sulfoaluminate cement (HBCSA) and calcium sulfoaluminate cement (CSA) have similar chemical compositions (SiO2, Al2O3, CaSO4, CaO, Fe2O3, and CaCO3), with calcium sulfoaluminate (C4A3S−) and belite (C2S) as the primary mineral phases [93,94,95]. While iron phases (Fe2O3) are nondominant and contribute minimally to strength, studies have shown that they optimize clinker calcination and enhance free lime (f-CaO) absorption [96,97].
In HBCSA/CSA systems, CaSO4 plays dual roles: regulating the setting time and enhancing the mechanical properties—unlike its singular function as a set retarder in ordinary Portland cement (OPC) [98,99]. Additionally, CaCO3 accelerates early hydration via nucleation effects, whereas its microfilling action reduces paste porosity. Excessive CaCO3 generates harmful f-CaO, inducing flash setting and expansion cracking; moderate f-CaO, however, is critical for rapid early hydration in HBCSA/CSA [100,101,102,103,104].
4.1. Role of Fe2O3
The use of solid wastes as raw or supplementary materials often introduces additional Fe₂O₃. For example, steel slag and fly ash contain iron phases that enter the C4A3S− lattice, forming solid solutions predominantly at the edges of C4A3S− and C2S grains. Elevated Fe2O3 concentrations create dense distributions, complicating clinker grinding [105]. Free CaSO4 (f-CaSO4) promotes partial substitution of Al2O3 by Fe2O3, forming 3CaO·3[(Al2O3)x(Fe2O3)1-x]·CaSO4, thereby reducing Al2O3 availability [106]. Excessive Fe2O3 consumption of Al2O3 to form C4AF diminishes C4A3S− and C2S formation, significantly impairing strength development [35].
Iron phases stabilize cubic ye’elimite (C-Ye’elimite) over the orthorhombic polymorph (O-Ye’elimite). Both polymorphs dissolve rapidly under sufficient gypsum to form AFt, showing no distinct hydration differences [107]. However, C-Ye’elimite consumes less gypsum and generates lower hydration heat in the early stages, subtly altering AFt or other hydrate formation rates [46]. Beneficially, Fe2O3 enhances f-CaO absorption during calcination, improving clinker burnability [84].
At varying Fe2O3 levels, the AFt morphology differs: clinker with low Fe2O3 forms needle-like AFt, whereas high Fe2O3 promotes rod-shaped AFt [68]. This indicates that Fe facilitates the decomposition of CaCO3 and CaSO4, enhancing C4A3S− and AFt formation. In Fe2O3-doped clinkers calcined at 1200–1400 °C, Fe3+ replaces Al3+ in C4A3S−, forming a solid solution C4(A0.95F0.05)6O12(SO4) [108,109,110]. Approximately 4% of the Al3+ is replaced by Fe3+, although the substitution levels remain constant regardless of the Fe2O3 content [111]. SEM-EDS Analysis at 1 d Hydration (Figure 26): (a) F0 group (0% Fe2O3): abundant needle-like AFt interweaves to provide early strength. (b) Fe2O3-doped groups (F0.7, F1.0, F1.3): Partial substitution of Al2O3 by Fe2O3 in C4A3S− causes AFt to aggregate into clusters. The amount of AFt decreases with increasing amounts of Fe2O3, transitioning from a needle morphology to a columnar morphology. (c) AFt in F0 lacks Fe3+, whereas the Fe-doped groups exhibit Fe3+ in AFt, confirming that Fe-Al substitution alters the hydrate morphology.
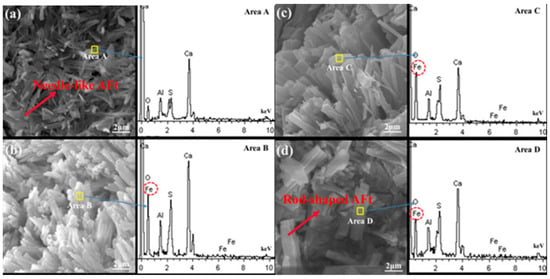
Figure 26.
SEM-EDS images of cement hydrated for 1 d: (a) F0; (b) F0.7; (c) F1.0; (d) F1.3 [111]. Note: F0, F0.7, F1.0, and F1.3 showed a gradual increase in the Fe content.
Ettringite crystals typically form needle-like/rod-shaped structures in clinkers. In concrete, these crystals aggregate into radial, annular, or spherical clusters [112,113]. Petal-like AFt clusters often develop in pores and cracks, constructing interconnected dense networks that increase the late-age compressive strength [114,115].
4.2. Role of CaSO4
This subsection summarizes the role of calcium sulfate (gypsum) in the hydration process and further elaborates on the effects of its decomposition rate and types—specifically, anhydrite (CaSO4) and dihydrate gypsum (CaSO4·2H2O)—on the cement matrix.
In ordinary Portland cement (OPC) systems, gypsum (CaSO4) serves solely as a set retarder to control the setting time. In high-belite calcium sulfoaluminate (HBCSA) and calcium sulfoaluminate (CSA) systems, CaSO4 functions dually: (a) Set regulation: delays initial hydration. (b) Sulfate supply: this supply maintains the stability of ettringite (AFt) by releasing SO42− ions and drives the conversion of monosulfoaluminate (AFm) to AFt [70]. This sulfate phase evolution mechanism dictates the hydration behavior and long-term durability of sulfoaluminate cements [68]. Excess SO42−: AFt forms via Equations (11) and (12). Deficient SO42−: AFm forms via Equation (2).
The molar ratio of CaSO4 to C4A3S− governs the AFt/AFm ratio in the hydration products, with higher CaSO4 dosages favoring AFt [116]. Maximum AFt formation occurs at approximately 30% CaSO4 addition, corresponding to the highest water demand for complete hydration [117,118]. While increased CaSO4 enhances strength, it elevates the normal consistency water demand and hydration heat, prolonging the setting time [119].
At a C4A3S/CaSO4 molar ratio of 0.5, CaSO4 is fully consumed, but this does not indicate complete cement hydration [45]. Post depletion, AFt destabilizes, compromising mechanical performance. This dual functionality underscores the necessity of a precise CaSO4 dosage to balance hydration kinetics, microstructure, and durability in sulfoaluminate systems.
In HBCSA and CSA cements, calcium sulfate (CaSO4) acts as both a retarder and a strength promoter. To ensure balanced early- and late-age strength development, the CaSO4 content must be carefully controlled. Excessive CaSO4 accelerates hydration rates beyond the diffusion capacity of hydration products, leading to concentrated heat release and expansive AFt formation, which causes late-age strength regression (Equations (13) and (14)). While expansive AFt contributes to early strength, its generation consumes excess CaSO4, aluminum hydroxide (AH3), calcium hydroxide (CH), and water, limiting normal AFt formation (Equations (7) and (8)) and impairing long-term strength development.
The CaSO4 in HBCSA/CSA cements is generally derived from dihydrate gypsum and anhydrite. Dihydrate gypsum (CaSO4⋅2H2O): rapid dissolution in water but low solubility, providing quick sulfate and calcium release. However, its limited solubility restricts late-age strength growth. Anhydrite (CaSO4): slower dissolution but higher solubility, which is commonly used in HBCSA/CSA systems to balance strength development and retarding needs [53]. Increasing the CaSO4 dosage prolongs the setting time and initially enhances the strength of both HBCSA cements and CSA cements. Beyond the optimal “M” value, however, excessive CaSO4 delays setting further while sharply reducing the strength. This occurs because (a) early hydration products (e.g., AFt) encapsulate clinker particles, hindering further reactions. (b) Overlapping AFt crystals create unoccupied pores due to insufficient C-S-H and AH3 gel infilling. (c) Premature depletion of reactive phases limits late-age AFt generation, inducing strength regression [120]. For HBCSA/CSA cements, the ideal anhydrite dosage is typically 9% to balance setting control and mechanical performance [121].
When gypsum or industrial Solid-waste gypsum is utilized as a source of CaSO₄ and CaO, the decomposition efficiency of gypsum warrants attention. Two factors significantly increase the decomposition rate of gypsum: (a) Calcination temperature: the decomposition temperature varies depending on the gypsum type (e.g., natural vs. industrial Solid-waste gypsum). (b) Oxides: the presence of Al2O3 or Al2O3 + SiO2 during calcination promotes the decomposition of gypsum into CaSO4 [122]. The ratios of CaSO4 to C₂S and C4A3S− profoundly influence the hydration process of cement clinker [116]. By adjusting these ratios, CSA cement can exhibit tailored properties such as rapid hardening, high early strength, shrinkage compensation [32], and self-stress characteristics [123,124,125]. Notably, the conversion of dihydrate gypsum (CaSO4·2H2O) to anhydrite (CaSO4) at elevated temperatures does not adversely affect the early mechanical performance of high-belite calcium sulfoaluminate cement (HBCSA) [126].
In most studies, the early strength of HBCSA and CSA cements relies primarily on the framework formed by ettringite (AFt), alongside contributions from amorphous C-S-H and AH3 [127,128,129,130]. AFt formation depends critically on the availability of CaSO4 to react with C4A3S−. Thus, ensuring sufficient CaSO4 content is essential for optimizing the hydration kinetics and mechanical properties of HBCSA and CSA cements [131,132].
4.3. Roles of CaCO3 and f-CaO in Cement Systems
Limestone (CaCO3) exhibits multiscale regulatory effects on the hydration behavior and microstructure evolution of cementitious materials: (a) Physical effects: (i) Limestone particles adsorb onto cement clinker surfaces, acting as nucleation sites to accelerate early hydration kinetics [133]. (ii) Their physical filling effect reduces the porosity of hardened cement paste [134]. (b) Chemical effects: (i) In HBCSA/CSA systems, limestone incorporation intensifies hydration exothermicity [135]. (ii) Limestone reacts with C3A and C4AF to form needle-like calcium carbonate crystals. These crystals stabilize ettringite (AFt) and enhance matrix densification and early strength via a three-dimensional interlocking network [136,137]. (iii) Limestone powder induces the formation of strätlingite (a layered calcium aluminate silicate hydrate), further refining the pore structure and improving compactness [135].
For ordinary Portland cement (OPC), the free lime (f-CaO) generated from limestone decomposition during high-temperature calcination reacts with water to form Ca(OH)₂. If this reaction occurs before cement hardening, minimal adverse effects arise. However, post-hardening reactions of residual f-CaO generate localized expansive stresses within the hardened matrix, significantly compromising the strength of the concrete [43].
In HBCSA/CSA cements, excessive free lime (f-CaO) induces flash setting, whereas moderate f-CaO levels supplement CaO to facilitate the formation of C4A3S− during clinker synthesis. After calcination, appropriate f-CaO provides a calcium source, where its hydration generates Ca(OH)2 (CH), which reacts with CaSO4 to form C4A3S−. The subsequent hydration of C4A3S− produces ettringite (AFt) (Equation (10)), explaining why excessive f-CaO triggers rapid setting in CSA cements [53].
Excessive f-CaO prematurely depletes CaSO4, which is critical for enhancing early C2S hydration. This depletion reduces late-stage C-S-H gel formation and compromises strength development. Moreover, rapid AFt formation (including expansive AFt variants) under high f-CaO conditions limits early strength attainment [35].
In contrast to ordinary Portland cement (OPC), where high calcination temperatures (approximately 1400 °C) induce dead-burned f-CaO (which is inert during early hydration), the HBCSA clinker calcined at lower temperatures (approximately 1300 °C) retains reactive f-CaO. This reactive f-CaO participates in early hydration, contributing to initial strength enhancement without significant adverse effects on long-term performance [132].
The incorporation of limestone (CaCO3) increases the calcium hydroxide (CH) content in HBCSA/CSA cement systems. In the absence of CH, non-expansive ettringite (AFt) forms, whereas CH promotes the generation of expansive AFt (Equation (10)) [138]. Excessive AFt formation accelerates CaSO₄ depletion, leading to AFt instability and subsequent conversion to less reactive AFm phases (Equation (3)).
In HBCSA cement, the high content of slow-hydrating C2S (>40%) and reduced proportion of fast-hydrating C4A3S− (approximately 25%) mitigate rapid expansion and cracking despite its overall rapid hydration [25]. Notably, even in the absence of gypsum, calcium carbonate (CaCO3) enhances AFt formation and stabilization [139] (Table 4). While ettringite formed during clinker hydration without supplementary minerals (e.g., CaCO3 or CaSO4) has poor stability, the presence of CaCO3 or CaSO4 effectively stabilizes AFt and inhibits its transformation to AFm. The reaction between CaCO3 and C4A3S− to form AFt (Equation (14)) parallels the mechanism described in Equation (10), where CaCO3 decomposition yields C-S-H and CH, which subsequently react with C4A3S−. However, AFt formed via CH participation may exhibit expansion tendencies. Although AFt constitutes the primary framework of hydrated cement, pore-filling contributions arise from C-S-H and AH3 gels generated by the hydration of C4A3S− and C2S.

Table 4.
Quantitative XRD analysis of the CSA Sample [140].
5. Methodology
Approximately one-third of the cited literature originates from articles reviewed during my graduate studies, primarily sourced from the China Knowledge Network (CNKI) database (covering Peking University Core-, CSSCI-, and CSCD-indexed publications) and the Web of Science Core Collection (including SCI-, SSCI-, and AHCI-indexed journals). Initial searches employed the following search terms: “High-Belite Calcium Sulphoaluminate Cement”, “Calcium Sulphoaluminate Cement”, and “Industrial Solid Wastes”, among others. To systematically synthesize knowledge and strengthen theoretical foundations, supplementary literature was subsequently retrieved through the same platforms using expanded search terms: “Hydration Reaction”, “Fly Ash”, “Steel Slag”, and “Gypsum”, among others.
Due to the specificity of the search terms (“High-Belite Sulphoaluminate Cement” and “Calcium Sulphoaluminate Cement”), only a limited number of irrelevant articles were identified. These were manually excluded through disciplinary categorization, abstract screening, and content evaluation processes. Supplementary literature addressing secondary themes (e.g., carbon emissions) underwent identical screening protocols to ensure relevance.
Significant challenges arose in establishing inclusion criteria due to substantial variations in experimental protocols across studies. Although the macroscopic research direction was well-defined, inconsistencies in cement preparation methods and raw material compositions among researchers hindered direct comparison of experimental data. Nevertheless, given that most existing studies focus on demonstrating fabrication feasibility—even those labeled as “HBCSA/CSA” exhibit divergent compressive strength, setting time, and hydration heat characteristics—this study systematically compiled these parameters to identify overarching trends, thereby validating the feasibility of solid-waste-based cement production.
For instance, Section 2.2.2 analyzes gypsum dosage effects on AFt formation (Figure 10). Despite material incomparability between studies, these data were included to substantiate the fundamental premise that gypsum modulates AFt formation. Similarly, Section 2.2.3 (Figure 17) presents compressive strength values of cement samples. As with the preceding analysis, direct comparability is constrained by disparities in raw materials and fabrication methods. To contextualize performance, CSA cement produced via standard industrial processes was incorporated into the figure as a reference benchmark.
The manuscript structure was ultimately organized to address four core objectives: establishing standardized calcination protocols under diverse thermal regimes; elucidating hydration mechanisms of HBCSA/CSA cement; demonstrating fabrication feasibility through compiled solid-waste-based cement data; decoupling functional roles of chemical components during hydration. This framework aims to bridge laboratory-scale innovations with industrial-scale implementation.
6. Conclusions and Future Perspectives
This study systematically analyzes the calcination parameters, primary mineral phases, and impacts of various solid wastes on the performance of solid-waste-based cement clinkers, elucidating the roles of chemical components in cement matrices. The key conclusions are as follows:
High-belite calcium sulfoaluminate cement (HBCSA) and calcium sulfoaluminate cement (CSA) can be synthesized by partially or fully replacing natural raw materials with industrial solid wastes. The primary mineral phases in solid-waste-based HBCSA/CSA systems, including C2S, C4A3S−, C-S-H, and AH3, are successfully formed during calcination. Upon hydration, these phases generate an interlaced ettringite (AFt) network filled with AH3 and C-S-H colloids, which enhances the mechanical strength of the cement matrix.
Calcination conditions critically govern the crystallinity and stability of the target mineral phases (C4A3S− and C2S), directly determining the hydration reactivity of the clinkers. The hydration process, accompanied by characteristic heat release, reflects reaction kinetics modulated by the crystal structures of C2S and C4A3S−, as well as gypsum content.
However, the substitution of natural materials with industrial solid waste has been demonstrated to result in alterations to mineral composition and hydration behavior. For instance, steel slag and fly ash have been observed to cause delayed setting time, reduced early compressive strength, and inhibited hydration reactions (e.g., reduced shrinkage and decreased hydration products). Additionally, research has demonstrated that solid-waste gypsum can result in a delayed setting time and compromised strength.
The performance of HBCSA/CSA cement stems from synergistic interactions among chemical components. These components optimize calcination processes to stabilize key mineral phases, enhance hydration kinetics to promote AFt formation, and regulate phase evolution at distinct stages, collectively enabling controllable cement property development.
Current research predominantly remains confined to the preliminary “feasibility demonstration” phase. Although all studies ostensibly aim to achieve “solid waste recycling”, the absence of standardized preparation protocols and scalable manufacturing processes renders industrial implementation impractical—constituting the primary technical bottleneck. Beyond this stage, durability assessments (e.g., freeze–thaw resistance, corrosion resistance, carbonation resistance) face analogous challenges to compressive strength evaluation: divergent raw materials and fabrication methods yield incomparable durability metrics under fragmented evaluation frameworks, which provide limited guidance for industrial production.
Even if industrialization becomes technically viable, economic sustainability concerns emerge. Laboratory studies typically employ pre-treated solid wastes, whereas industrialized production procurement of such processed materials would escalate costs. Alternatively, utilizing raw industrial wastes necessitates additional expenditures for on-site pretreatment, safety, and environmental compliance—factors that critically undermine economic viability.
To overcome these limitations, I offer the following recommendations:
- (1)
- Region-specific material selection: prioritize locally abundant solid waste to ensure that feedstock composition is as consistent as possible and to reduce logistical complexity.
- (2)
- Gradual integration: Give up the pursuit of 100% waste substitution. Instead, we will first partially integrate waste, establish a sound production baseline, and then gradually realize the full utilization of waste.
- (3)
- Preparation for industrial production: establish protocols for the cost-effectiveness of waste pre-processing, the long-term environmental impact of cement, and potential risks (e.g., whether there are health risks or hazards associated with the use of such cement).
- (4)
- Other materials: for example, construction, and demolition waste as supplementary material (fine grinding) that can be better adapted to construction work.
- (5)
- Standards for durability indicators: develop a set of test standards for resistance to freezing and thawing, corrosion, and carbonation, e.g., for OPC.
Author Contributions
H.L.: investigation, conceptualization, writing—original draft. C.L.: data curation, formal analysis, writing—original draft. J.W. and Y.G.: software. J.S., C.W., W.Z., Q.Y. and Y.L.: data curation. T.S. and F.C.: conceptualization, funding acquisition, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Nature Science Foundation of China (52368024), China Postdoctoral Science Foundation (2022M723687), Natural Science Foundation of Inner Mongolia Autonomous Region (2022LHMS05011), State-level Innovation and Entrepreneurship Training Program for College Students (202410433053, 202410433004), and Provincial Student Innovation and Entrepreneurship Training Program (S202410433084).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Hara, P.A. Political economy of climate change, ecological destruction and uneven development. Ecol. Econ. 2009, 69, 223–234. [Google Scholar] [CrossRef]
- Nworie, O.E.; Lin, C. Seasonal variation in tissue-borne heavy Metal(loid)s in herbaceous plants growing in contaminated soils developed from industrial wastes of industrial revolution age. Env. Adv. 2021, 5, 100113. [Google Scholar] [CrossRef]
- Ma, L.; Konter, J.; Herndon, E.; Jin, L.; Steinhoefel, G.; Sanchez, D.; Brantley, S. Quantifying an early signature of the industrial revolution from lead concentrations and isotopes in soils of Pennsylvania, USA. Anthropocene 2014, 7, 16–29. [Google Scholar] [CrossRef]
- Xu, H.; Chen, G.; Sarwar, B.; Shahzad, I. Sustainable development and mineral resource extraction in China: Exploring the role of mineral resources, energy efficiency and renewable energy. Resour. Policy 2024, 90, 104703. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Energy & Climate Intelligence Unit ECIU. Net Zero Emission Race. 2024. Available online: https://eciu.net/netzerotracker (accessed on 30 December 2024).
- IEA. Global CO2 Emissions from the Operation of Buildings in the Net Zero Scenario, 2010–2030; IEA: Paris, France, 2023. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Mitigation of climate change. In Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; Volume 1454, p. 147. Available online: https://ipcc-browser.ipcc-data.org/browser/dataset/8460/0 (accessed on 30 December 2024).
- Clark, G.; Davis, M.; Kumar, A. Assessment of fuel switching as a decarbonization strategy in the cement sector. Energy Convers. Manag. 2024, 312, 118585. [Google Scholar] [CrossRef]
- Liu, H.X.; Wu, L.M.; Hu, Y.J.; Wu, H.Y.; Xue, J.C.; Zhang, R.Y.; Cui, Y.; Shan, S.K.; Wang, L. Research on the characteristics of CO2 emission and treatment technology in cement industry. Cement 2023, 10, 10–15. [Google Scholar]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Hua, X.; Liu, J.; Sun, G. Urban industrial solid waste metabolism based on ecological network analysis: A case study of Tianjin. Clean. Responsib. Consum. 2023, 9, 100117. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Shen, F.; Zhou, M.; Du, W. Conceptual design and life-cycle environmental and economic assessment of low-carbon cement manufacturing processes. J. Clean. Prod. 2024, 471, 143349. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, W.; Zhang, C.; Li, J.; Wu, S.; Wang, X.; Xu, Y.; Wang, W.; Feng, M. Preparation of solid-waste-based pervious concrete for pavement: A two-stage utilization approach of coal gangue. Constr. Build. Mater. 2022, 319, 125962. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, T.; Zhang, J.; Wang, Z.; Liu, J.; Cao, J.; Wang, C. Recycling and utilization of calcium carbide slag-current status and new opportunities. Renew. Sustain. Energy Rev. 2022, 159, 112133. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X.; Ma, R.; Sun, S.; Fang, L.; Lin, J.; Luo, J. Solid waste-based magnesium phosphate cements: Preparation, performance and solidification/stabilization mechanism. Constr. Build. Mater. 2021, 297, 123761. [Google Scholar] [CrossRef]
- Carneiro, G.; Bier, T.; Waida, S.; Dous, A.; Heinemann, S.; Herr, P.; Charitos, A. Treatment of energy from waste plant fly-ash for blast furnace slag substitution as a supplementary cementitious material. J. Clean. Prod. 2025, 490, 144693. [Google Scholar] [CrossRef]
- Ragossnig, A.M.; Jovovic, A. Waste management life cycle: Sensitisation–implementation/integration–transition–optimisation. Waste Manag. Res. 2016, 34, 813–815. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, T.; Tong, X. Assessing the carbon reduction potential of municipal solid waste management transition: Effects of incineration, technology and sorting in Chinese cities. Resour. Conserv. Recycl. 2023, 188, 106713. [Google Scholar] [CrossRef]
- Tong, X.; Yu, H.; Liu, T. Using weighted entropy to measure the recyclability of municipal solid waste in China: Exploring the geographical disparity for circular economy. J. Clean. Prod. 2021, 312, 127719. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Yan, J.; Qian, Y.; Wei, Y.; Li, D.; Guo, S. Value-added utilization of coal fly ash in polymeric composite decking boards. J. Mater. Res. Technol. 2023, 23, 4199–4210. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.; He, X.; Yang, W.; Deng, X. Utilization of carbide slag-granulated blast furnace slag system by wet grinding as low carbon cementitious materials. Constr. Build. Mater. 2020, 249, 118763. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem. Concr. Compos. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Lv, F.; Wang, L.; An, H.; Chen, S.; Shu, J.; Kong, D. Effects of hybrid fibers on properties of desulfurized gypsum-based composite cementitious materials. Constr. Build. Mater. 2023, 392, 131840. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Mao, Y.; Shen, X.D. Synthesis and characterization of high belite sulfoaluminate cement through rich alumina fly ash and desulfurization gypsum. Ceram-Silik 2013, 57, 7–13. [Google Scholar]
- Wang, W.; Wang, X.; Zhu, J.; Wang, P.; Ma, C. Experimental investigation and modeling of sulfoaluminate cement preparation using desulfurization gypsum and red mud. Ind. Eng. Chem. Res. 2013, 52, 1261–1266. [Google Scholar] [CrossRef]
- El-Alfi, E.A.; Gado, R.A. Preparation of calcium sulfoaluminate-belite cement from marble sludge waste. Constr. Build. Mater. 2016, 113, 764–772. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef]
- Sun, W.; Yao, G. Impact of mineral resource depletion on energy use: Role of energy extraction, CO2 intensity, and natural resource sustainability. Resour. Policy 2023, 86, 104175. [Google Scholar] [CrossRef]
- Roy, R.; Hertel, T.; Pilla, G.; Pontikes, Y. Revealing the hydration mechanisms in iron-rich calcium sulfoaluminate cements with elevated bauxite residue content. Constr. Build. Mater. 2024, 449, 138495. [Google Scholar] [CrossRef]
- Bernardo, G.; Telesca, A.; Valenti, G.L. A porosimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem. Concr. Res. 2006, 36, 1042–1047. [Google Scholar] [CrossRef]
- Tan, J.H.; Shi, X.L.; Zhu, K.J.; Zhao, Y.L.; Peng, J.H. Study on the preparation of high-Belite sulfoaluminate cement using low-grade bauxite and foundry waste sand. Silic. Bull. 2017, 36, 4284–4290. [Google Scholar]
- Xia, R.J.; Zhu, J.P.; Liu, S.X.; Zhu, X.J.; Feng, C.H.; Guan, X.M. Preparation of high belite sulfoaluminate cement clinker from red mud and desulfurization gypsum. Nonferrous Met. Eng. 2017, 7, 58–63+79. [Google Scholar]
- Wang, X.; Guo, M.Z.; Yue, G.; Li, Q.; Ling, T.-C. Synthesis of high belite sulfoaluminate cement with high volume of mixed solid wastes. Cem. Concr. Res. 2022, 158, 106845. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Wang, Z.Y.; Wang, S.D.; Zhao, P.Q.; Cheng, X. Synthesis of high belite sulfoaluminate cement containing magnesia-alumina spinel with solid waste. Ceram-Silik 2023, 67, 159–166. [Google Scholar] [CrossRef]
- Wu, S.; Wang, W.; Ren, C.; Yao, X.; Yao, Y.; Zhang, Q.; Li, Z. Calcination of calcium sulphoaluminate cement using flue gas desulfurization gypsum as whole calcium oxide source. Constr. Build. Mater. 2019, 228, 116676. [Google Scholar] [CrossRef]
- Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Chen, C.; Gu, X. Calcination of calcium sulphoaluminate cement using pyrite-rich cyanide tailings. Crystals 2020, 10, 971. [Google Scholar] [CrossRef]
- Chen, X.M.; Li, J.; Lu, Z.Y.; Xv, G.Y.; Zhong, W. Study on the preparation of high iron phase belite sulfoaluminate cement using aluminum tailings. Cement 2023, 3, 1–7. [Google Scholar]
- Zhang, P.; Zhang, B.; Chang, J.; Wang, T.; Zhang, J.X. Investigation of process parameters of phosphogypsum for preparing calcium sulfoaluminate cement. Buildings 2022, 12, 1774. [Google Scholar] [CrossRef]
- Tanguler-Bayramtan, M.; Turk, S.; Yaman, I.O. Hydration characteristics of calcium sulfoaluminate cements synthesized using an industrial symbiosis framework. Constr. Build. Mater. 2024, 447, 138090. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Huang, S.; Wu, F.L.; Zhu, J. Effect of Al2O3/SiO2 ratio on the chroma and phase compositions of white sulfoaluminate cement clinker. Constr. Build. Mater. 2022, 345, 128202. [Google Scholar] [CrossRef]
- Shi, H.S. Effect of free calcium oxide on the properties of cement. Cement 1992, 10, 27–31. [Google Scholar]
- Su, D.L. Basic Research on the Preparation and Application of High-Belite Sulfoaluminate Cement Based on Multiple Solid Waste Co-Disposal Technologies. Master’s Thesis, Qingdao University of Science and Technology, Qingdao, China, 2021. [Google Scholar]
- Zhang, L.; Su, M.; Wang, Y. Development of the use of sulfo- and ferroaluminate cements in China. Adv. Cem. Res. 1999, 11, 15–21. [Google Scholar] [CrossRef]
- Liu, H.M. Fundamentals of Cement Production Technology, 2nd ed.; Chemical Industry Press: Beijing, China, 2016. [Google Scholar]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Marroccoli, M.; Telesca, A.; Lothenbach, B.; Winnefeld, F. Synthesis and properties of a belite-calcium sulfoaluminate cement obtained using only waste materials. Adv. Cem. Res. 2025, 37, 78–88. [Google Scholar] [CrossRef]
- Fukuda, N. On the constitution of sulfo-aluminous clinker. Bull. Chem. Soc. Jpn. 1961, 34, 138–139. [Google Scholar] [CrossRef]
- Bye, G.C. Portland cement: Composition, production and properties. In Thomas Telford; Thomas Telford Ltd.: London, UK, 1999. [Google Scholar]
- Klein, A. Calciumaluminosulfate and expansive cements containing same. US Pat. 1964, 3, 155. [Google Scholar]
- Diao, J.J.; Xin, Z.J.; Zhang, Q.Y. Production and Application of Sulfoaluminate Cement; China Building Material Industry Press: Beijing, China, 2006. [Google Scholar]
- Zhao, X.D. Research on Fast-Setting and Fast-Hardening High Belite Sulfoaluminate Cement Clinker. Master’s Thesis, Beijing University of Technology, Beijing, China, 2017. [Google Scholar]
- Rungchet, A.; Chindaprasirt, P.; Wansom, S.; Pimraksa, K. Hydrothermal synthesis of calcium sulfoaluminate-belite cement from industrial waste materials. J. Clean. Prod. 2016, 115, 273–283. [Google Scholar] [CrossRef]
- Wang, X. Experimental Study on the Preparation of High Belite Sulfoaluminate Cement from Industrial Solid Waste. Master’s Thesis, Qingdao University of Technology, Qingdao, China, 2019. [Google Scholar]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Zhang, R. Research on Thermodynamics and Kinetics of Cement Clinker Firing. Master’s Thesis, Beijing University of Technology, Beijing, China, 2010. [Google Scholar]
- Link, T.; Bellmann, F.; Ludwig, H.; Ben Haha, M. Reactivity and phase composition of Ca2SiO4 binders made by annealing of alpha-dicalcium silicate hydrate. Cem. Concr. Res. 2015, 67, 131–137. [Google Scholar] [CrossRef]
- Bensted, J. A discussion of the paper “Idiomorphic belites in Portland cement—A genetic model” by R. Oberste-Padtberg. Cem. Concr. Res. 1987, 17, 513–514. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Tian, D.J.; Liu, Y.; Yang, C.J.; Ma, Y. Preparation of high berth silicate cement clinker from low-grade limestone and its activity enhancement. New Build. Mater. 2024, 51, 22–26. [Google Scholar]
- Chi, L. Research on Activation and Hydration Mechanism of High Belite Sulfoaluminate Cement. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2019. [Google Scholar]
- Huang, W.; Wen, Z.; Wang, M. Effect of phosphorus-sulfur composite doping on the crystalline structure of dicalcium silicate. Silic. Bull. 2018, 37, 2502–2505. [Google Scholar]
- Liu, C.; Yan, C.; Li, J.; Nie, W.; Lv, Z.; Wu, Q.; Meng, X.; Zhao, F. Effect of desulfurization gypsum on alternating-current impedance characteristics of all-solid-waste belite sulphoaluminate cement mortar. Constr. Build. Mater. 2024, 450, 138666. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, L.; Ren, X.; Ye, J.; Liu, L.; Wu, Y. Influence of free calcium sulfate levels on the early hydration of sulfate-rich belite sulfoaluminate clinkers: A comparative study with anhydrite and gypsum. Cem. Concr. Res. 2024, 181, 107522. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; da Costa, M.L.; Pöllmann, H. Waste clay from bauxite beneficiation to produce calcium sulphoaluminate eco-cements. Constr. Build. Mater. 2022, 340, 127703. [Google Scholar] [CrossRef]
- Su, D.; Li, Q.; Guo, Y.; Yue, G.; Wang, L. Effect of residual CaSO4 in clinker on properties of high belite sulfoaluminate cement based on solid wastes. Materials 2020, 13, 429. [Google Scholar] [CrossRef]
- Shu, X.; Jiang, Y.; Zhao, Y.; Xu, Z.; Shen, M.; Zhong, X. Superimposed hydration exothermic model of cement slurry considering different reaction rates of various active substances. Constr. Build. Mater. 2023, 372, 130783. [Google Scholar] [CrossRef]
- Tao, X.; Li, P.; Wang, G.; Li, W.; Ma, S. Preparation and hydration of Belite-Ye’elimite-Ternesite clinker based on industrial solid waste. Case Stud. Constr. Mater. 2023, 18, e01922. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Huang, Y.; Yang, D. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem. Concr. Compos. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Chai, J.; Fan, Y. Calcium sulphoaluminate cements made with phosphogypsum: Production issues and material properties. Cem. Concr. Compos. 2014, 48, 67–74. [Google Scholar] [CrossRef]
- He, W.; Li, R.; Zhang, Y.; Nie, D. Synergistic use of electrolytic manganese residue and barium slag to prepare belite-sulphoaluminate cement study. Constr. Build. Mater. 2022, 326, 126672. [Google Scholar] [CrossRef]
- Sun, D.; Wang, X.; Wang, J.; Li, J.; Mao, Y.; Hu, Z.; Li, Y.; Song, Z.; Wang, W. Properties and hydration characteristics of cementitious blends with two kinds of solid waste-based sulfoaluminate cement. Constr. Build. Mater. 2024, 411, 134482. [Google Scholar] [CrossRef]
- Yeung, J.S.K.; Yam, M.C.H.; Wong, Y.L. 1-Year development trend of concrete compressive strength using Calcium Sulfoaluminate cement blended with OPC, PFA and GGBS. Constr. Build. Mater. 2019, 198, 527–536. [Google Scholar] [CrossRef]
- Yoon, H.; Seo, J.; Kim, S.; Lee, H.; Park, S. Hydration of calcium sulfoaluminate cement blended with blast-furnace slag. Constr. Build. Mater. 2021, 268, 121214. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Cao, W.; Yi, W.; Peng, J.; Li, G.; Yin, S. Preparation of anhydrite from phosphogypsum: Influence of phosphorus and fluorine impurities on the performances. Constr. Build. Mater. 2022, 318, 126021. [Google Scholar] [CrossRef]
- El Khessaimi, Y.; Taha, Y.; Safhi, A.E.M.; Hakkou, R.; Benzaazoua, M. Synthesis of MgO-Belite calcium sulfoaluminate cement from phosphate mine waste rock and phosphogypsum. Mater. Today Proc. 2022, 58, 1081–1090. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Kang, X.; Yu, J.; Fan, Y.; Dang, Y.; Zhang, W.; Wang, S. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, D.; Wu, Y.; Yu, H. Synthesis and characterization of calcium aluminate compounds from gehlenite by high-temperature solid-state reaction. Ceram. Int. 2018, 44, 13544–13550. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, K.; Yang, Y.; Chang, J. Investigation on the preparation of low carbon cement materials from industrial solid waste phosphogypsum: Clinker preparation, cement properties, and hydration mechanism. J. Clean. Prod. 2024, 452, 142203. [Google Scholar] [CrossRef]
- Canbek, O.; Shakouri, S.; Erdoğan, S.T. Laboratory production of calcium sulfoaluminate cements with high industrial waste content. Cem. Concr. Compos. 2020, 106, 103475. [Google Scholar] [CrossRef]
- Liao, Y.S.; Jiang, G.X.; Wang, K.J.; Qunaynah, S.A.; Yuan, W.J. Effect of steel slag on the hydration and strength development of calcium sulfoaluminate cement. Constr. Build. Mater. 2020, 265, 120301. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.; Zhou, Z. Preparation of steel slag/slag composite sulfoaluminate cement with less clinker. Concrete 2011, 8, 88–90. [Google Scholar]
- Huang, Y.; Shen, X.; Ma, S.; Chen, L.; Zhong, B. Effect of Fe2O3 on the formation of calcium sulphoaluminate mineral. J. Chin. Ceram. Soc. 2007, 35, 485–488. [Google Scholar]
- Kang, X.T.; Zhou, J.; Xu, M.F.; Li, H.; Yu, Q.H.; Zhai, C.Y.; Lin, S.T.; Zhong, X.L. Research on thermal and mechanical properties of slag sulfoaluminate cement concrete. Ind. Build. 2022, 52, 158–162+185. [Google Scholar]
- Pei, T.Y.; Qi, D.Y.; Zou, D.L.; Cai, Y.H.; Wang, Z.Y.; He, L.L.; Wang, Y.Y.; Zhang, Y.; Liu, H.Y. Study on the mechanism of sulfate erosion resistance of slag-high-baylite sulfoaluminate cement. Silic. Bull. 2023, 42, 2683–2691. [Google Scholar]
- Fan, Z.Y.; Li, Q.Y.; Guo, Y.X.; Yue, G.B. Effects of mineral powder and fly ash on the hydration and strength of high belite sulfoaluminate cement. Concrete 2023, 2, 105–108+113. [Google Scholar]
- Xin, X.; Duan, G.; Huang, Y.; Li, J.; Li, C.; Hou, P. Study on the hydration of slag-belite calcium sulfoaluminate-Portland cement composite cementitious system. Constr. Build. Mater. 2024, 433, 136713. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, Z. Research on the effect of mixing material on the performance of sulfoaluminate cement. Cement Eng. 2017, 4, 8–10. [Google Scholar]
- Seo, J.; Kim, S.; Park, S.; Yoon, H.; Lee, H. Carbonation of calcium sulfoaluminate cement blended with blast furnace slag. Cem. Concr. Compos. 2021, 118, 103918. [Google Scholar] [CrossRef]
- Gao, D.Y.; Meng, Y.; Yang, L.; Tang, J.Y.; Lv, M.Y. Effect of ground granulated blast furnace slag on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 227, 116665. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Canonico, F.; Paul, G. CSA and slag: Towards CSA composite binders. Adv. Cem. Res. 2019, 31, 147–158. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Wei, J.; Ouyang, Z.; Lv, W. Effect of different calcination temperatures on clinker and hydration properties of solid waste-based calcium sulfoaluminate cement. Constr. Build. Mater. 2024, 452, 138938. [Google Scholar] [CrossRef]
- Sun, H.Q.; Lin, T.Z.; Zhuo, K.H.; Qian, J.S.; Chen, X.S.; Zhang, J.; Sun, Y.B. Sulfate optimization in ettringite rich cements by SO3/Al2O3 molar ratio: A comparative study of calcium sulfoaluminate and aluminate cements. Case Stud. Constr. Mater. 2024, 21, e03701. [Google Scholar] [CrossRef]
- Žibret, L.; Ipavec, A.; Dolenec, S. Microstructural characteristics of belite-sulfoaluminate cement clinkers with bottom ash. Constr. Build. Mater. 2022, 321, 126289. [Google Scholar] [CrossRef]
- Wu, S.; Cui, Y.F.; Yao, X.L.; Ren, C.Z.; Yao, Y.G.; Wang, W.L. Preparation of iron-rich sulfoaluminate cement by regulating Fe-bearing minerals. Ceram Int. 2025. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, W.; Yuan, J.; Shu, X.; Tan, X.; Wu, G.; Tian, H.; Cai, L. Study on early hydration and mechanical properties of ferrite-rich calcium sulfoaluminate cement-based grouting materials. Constr. Build. Mater. 2024, 411, 134324. [Google Scholar] [CrossRef]
- Sun, Z.; Nie, S.; Zhou, J.; Li, H.; Chen, Z.; Xu, M.; Mu, R.; Wang, Y. Hydration mechanism of calcium sulfoaluminate-activated supersulfated cement. J. Clean. Prod. 2022, 333, 130094. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Yu, B. Effect of modified phosphogypsum on the hydration properties of the phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2019, 214, 9–16. [Google Scholar] [CrossRef]
- Tsivilis, S.; Chaniotakis, E.; Batis, G.; Meletiou, C.; Kasselouri, V.; Kakali, G.; Sakellariou, A.; Pavlakis, G.; Psimadas, C. The effect of clinker and limestone quality on the gas permeability, water absorption and pore structure of limestone cement concrete. Cem. Concr. Compos. 1999, 21, 139–146. [Google Scholar] [CrossRef]
- Tsivilis, S.; Chaniotakis, E.; Badogiannis, E.; Pahoulas, G.; Ilias, A. A study on the parameters affecting the properties of Portland limestone cements. Cem. Concr. Compos. 1999, 21, 107–116. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H. A review on effects of limestone powder on the properties of concrete. Constr. Build. Mater. 2018, 192, 153–166. [Google Scholar] [CrossRef]
- Ji, G.; Chi, H.; Sun, K.; Peng, X.; Cai, Y. Effect of limestone waste on the hydration and microstructural properties of cement-based materials. Constr. Build. Mater. 2024, 443, 137784. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Lu, D.; Xu, Z.; Hooton, R. Effect of dolomite powder on the hydration and properties of calcium sulfoaluminate cements with different gypsum contents. Constr. Build. Mater. 2019, 225, 302–310. [Google Scholar] [CrossRef]
- Idrissi, M.; Diouri, A.; Damidot, D.; Greneche, J.; Talbi, M.A.; Taibi, M. Characterisation of iron inclusion during the formation of calcium sulfoaluminate phase. Cem. Concr. Res. 2010, 40, 1314–1319. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Haha, M.B. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Thaivalappil, B.; Chaunsali, P. Influence of iron content on processing conditions and early age hydration of ye’elimite. Mater Today Proc. 2023. [Google Scholar] [CrossRef]
- Cui, S.P.; Lan, M.Z.; Zhang, J.; Wang, C.Y. Role and solidification mechanism of heavy metal elements in cement clinker formation process from waste materials. J. Chin. Ceram. Soc. 2004, 32, 1264–1270. [Google Scholar]
- Huang, Y.; Pei, Y.; Qian, J.; Gao, X.; Liang, J.; Duan, G.; Zhao, P.; Lu, L.; Cheng, X. Bauxite free iron rich calcium sulfoaluminate cement: Preparation, hydration and properties. Constr. Build. Mater. 2020, 249, 118774. [Google Scholar] [CrossRef]
- Yao, X.; Yang, S.; Dong, H.; Wu, S.; Liang, X.; Wang, W. Effect of CaO content in raw material on the mineral composition of ferric-rich sulfoaluminate clinker. Constr. Build. Mater. 2020, 263, 120431. [Google Scholar] [CrossRef]
- Liang, J. Preparation of High Iron Belite-Sulfoaluminate Cement from Steel Slag. Master’s Thesis, Chongqing University, Chongqing, China, 2018. [Google Scholar]
- Qian, J.S.; Yu, J.C.; Sun, H.Q.; Ma, Y. Formation and role of ettringite. J. Silic. 2017, 11, 1569–1581. [Google Scholar]
- Lee, H.; Cody, R.; Cody, A.; Spry, P. The formation and role of ettringite in Iowa highway concrete deterioration. Cem. Concr. Res. 2005, 35, 332–343. [Google Scholar] [CrossRef]
- Tang, P.; Wen, J.; Fu, Y.; Liu, X.; Chen, W. Improving the early-age properties of eco-binder with high volume waste gypsum: Hydration process and ettringite formation. J. Build. Eng. 2024, 86, 108988. [Google Scholar] [CrossRef]
- Lv, S.H.; Ma, Y.J.; Qiu, C.C.; Sun, T.; Liu, J.J.; Zhou, Q.F. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Glasser, F.P. Advances in Sulfoaluminate Cements; The University of Aberdeen: Aberdeen, Scotland, 2002. [Google Scholar]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. Calculation of chemical water demand for hydration of calcium sulfoaluminate cement. J. Chin. Ceram. Soc. 1998, 3, 38–44. [Google Scholar]
- Xiang, B. Research on the Hydration and Hardening Mechanism of Fast-Setting and Fast-Hardening High Belite-Sulfoaluminate Cement; Beijing University of Technology: Beijing, China, 2017. [Google Scholar]
- Jia, H.X.; Lv, S.Z.; Hu, J.L.; Gao, Y.; Duan, X.Y. Influence of gypsum dosing on the properties of high Belite-sulfoaluminate cement. J. Wuhan Univ. Technol. 2014, 36, 24–28. [Google Scholar]
- Wu, S.; Yao, Y.; Yao, X.; Ren, C.; Li, J.; Xu, D.; Wang, W. Co-preparation of calcium sulfoaluminate cement and sulfuric acid through mass utilization of industrial by-product gypsum. J. Clean. Prod. 2020, 265, 121801. [Google Scholar] [CrossRef]
- Ioannou, S.; Paine, K.; Reig, L.; Quillin, K. Performance characteristics of concrete based on a ternary calcium sulfoaluminate-anhydrite-fly ash cement. Cem. Concr. Compos. 2015, 55, 196–204. [Google Scholar] [CrossRef]
- Ioannou, S.; Reig, L.; Paine, K.; Quillin, K. Properties of a ternary calcium sulfoaluminate-calcium sulfate-fly ash cement. Cem. Concr. Res. 2014, 56, 75–83. [Google Scholar] [CrossRef]
- Chen, I.A.; Hargis, C.W.; Juenger, M.C.G. Understanding expansion in calcium sulfoaluminate-belite cements. Cem. Concr. Res. 2012, 42, 51–60. [Google Scholar] [CrossRef]
- Liang, J.; Liu, N.; Liu, C.Z.; Guo, M.C.; Li, X.C.; Qian, J.S. Preparation of high belite-sulfoaluminate cement by phosphogypsum and low-grade bauxite. Nonmet. Min. 2016, 39, 17–20. [Google Scholar]
- Park, S.; Jeong, Y.; Moon, J.; Lee, N. Hydration characteristics of calcium sulfoaluminate (CSA) cement/Portland cement blended pastes. J. Build. Eng. 2021, 34, 101880. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Boehm-Courjault, E.; Zajac, M.; Ben Haha, M.; Scrivener, K. Hydration reactions and stages of clinker composed mainly of stoichiometric ye’elimite. Cem. Concr. Res. 2019, 116, 120–133. [Google Scholar] [CrossRef]
- Jansen, D.; Wolf, J.J.; Fobbe, N. The hydration of nearly pure ye’elimite with a sulfate carrier in a stoichiometric ettringite binder system. Implications for the hydration process based on in-situ XRD, 1H-TD-NMR, pore solution analysis, and thermodynamic modeling. Cem. Concr. Res. 2020, 127, 105923. [Google Scholar] [CrossRef]
- Damion, T.; Chaunsali, P. Effect of gypsum addition on acid resistance of ye’elimite rich calcium sulfoaluminate cement. Cem. Concr. Compos. 2024, 145, 105335. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Mrak, M.; Winnefeld, F.; Lothenbach, B.; Dolenec, S. The influence of calcium sulfate content on the hydration of belite-calcium sulfoaluminate cements with different clinker phase compositions. Mater. Struct. 2021, 54, 1–17. [Google Scholar] [CrossRef]
- Bonavetti, V.; Donza, H.; Rahhal, V.; Irassar, E. Influence of initial curing on the properties of concrete containing limestone blended cement. Cem. Concr. Res. 2000, 30, 703–708. [Google Scholar] [CrossRef]
- Livesey, P. Strength characteristics of Portland-limestone cements. Constr. Build. Mater. 1991, 5, 147–150. [Google Scholar] [CrossRef]
- Péra, J.; Husson, S.; Guilhot, B. Influence of finely ground limestone on cement hydration. Cem. Concr. Compos. 1999, 21, 99–105. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Ma, J.; Yu, Z.; Ni, C.; Shi, H.; Shen, X. Effects of limestone powder on the hydration and microstructure development of calcium sulphoaluminate cement under long-term curing. Constr. Build. Mater. 2019, 199, 688–695. [Google Scholar] [CrossRef]
- Péra, J.; Ambroise, J. New applications of calcium sulfoaluminate cement. Cem. Concr. Res. 2004, 34, 671–676. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, C. Improved ettringite stabilization by calcium carbonate and calcium nitrate additions in ternary PC-CSA-C$ systems. Cem. Concr. Res. 2024, 175, 107383. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).