From Water Buffalo (Bubalus bubalis) Manure to Vermicompost: Testing a Sustainable Approach for Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Pre-Treatment of Buffalo Manure
2.2. Vermicomposting Process
2.3. Plant Cultivation
2.4. Morphological, Color, and Leaf Chlorophyll Measurements
2.5. Chemicals and Reagents
2.6. Starch and Soluble Carbohydrates Content
2.7. Protein and Free Amino Acid Contents
2.8. Polyphenols, Hydrogen Peroxide and ABTS Activity
2.9. Statistical Analysis
3. Results
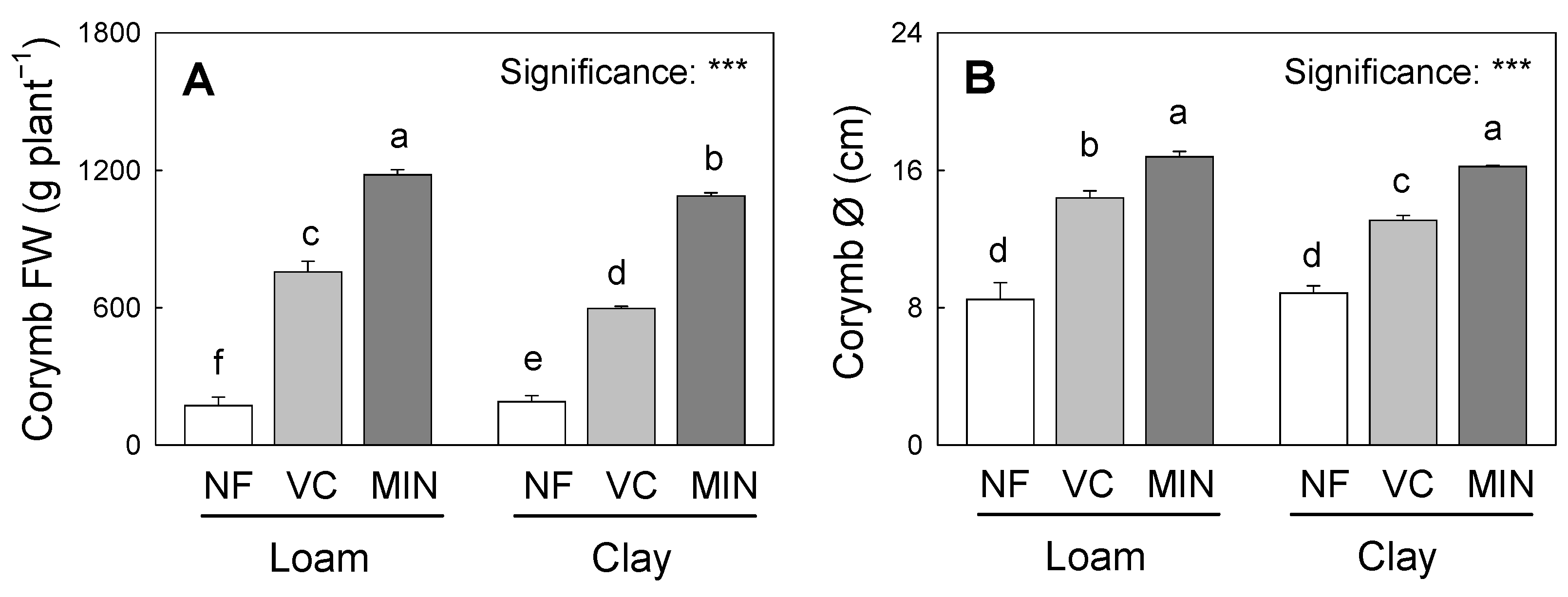
3.1. Fertilization Predominantly Shapes Yield and Morphometry
3.2. Fertilization Enhances SPAD Index, Chlorophyll, and Colorimetry
3.3. Fertilization Influences Antioxidant Compounds and Oxidative Stress Markers
3.4. Mineral Fertilization and Loam Soils Boost Nitrogen Assimilation and Amino Acid Profiles
3.5. Soil Type and Fertilization Were the Main Drivers of PCA Clustering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU. Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions—A Thematic Strategy on the Sustainable Use of Pesticides {COM(2006) 373 Final} {SEC(2006) 894} {SEC(2006) 895} {SEC(2006) 914}. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52006DC0372 (accessed on 4 May 2025).
- FAO. Greenhouse Gas Emissions from Agrifood Systems: Global, Regional, and Country Trends (2000–2022). Food and Agriculture Organization of the United Nations. 2024. Available online: https://openknowledge.fao.org/items/74bfebdb-3272-4e6a-98f4-ee36c7146d44 (accessed on 4 March 2025).
- FAO. The Share of Food Systems in Total Greenhouse Gas Emissions. Global, Regional and Country Trends, 1990–2019. FAOSTAT Analytical Brief Series No. 31. Rome. 2021. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/ffb21ed0-05dd-46b1-b16c-50c9d47a6676/content (accessed on 19 January 2025).
- The European Green Deal. Striving to Be the First Climate-Neutral Continent. 2019. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 18 December 2023).
- A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN (accessed on 20 December 2023).
- VetInfo. Buffalo Population in the Campania Region as of 31/12/2024. Veterinary Information System, Statistics. 2024. Available online: https://www.vetinfo.it/j6_statistiche/#/report-pbi/1 (accessed on 4 May 2025).
- EUR-Lex Council Directive of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources (91/676/EEC). Consolidated Text. 1991. Available online: http://data.europa.eu/eli/dir/1991/676/2008-12-11 (accessed on 19 January 2025).
- Regione Campania. Delibera di Giunta Regionale n. 500 del 30 agosto 2023: Aggiornamento del Programma d’Azione per le Zone Vulnerabili ai Nitrati. 2023. Available online: https://agricoltura.regione.campania.it/reflui/pdf/DGR_500-30-08-23.pdf (accessed on 19 January 2025).
- Usta, A.N.; Guven, H. Vermicomposting organic waste with Eisenia fetida using a continuous flow-through reactor: Investigating five distinct waste mixtures. J. Environ. Chem. Eng. 2024, 12, 114384. [Google Scholar] [CrossRef]
- Nasiru, A.; Ismail, N.; Ibrahim, M.H. Vermicomposting: Tool for Sustainable Ruminant Manure Management. J. Waste Manag. 2013, 2013, 732759. [Google Scholar] [CrossRef]
- Mohite, D.D.; Chavan, S.S.; Jadhav, V.S.; Kanase, T.; Kadam, M.A.; Singh, A.S. Vermicomposting: A holistic approach for sustainable crop production, nutrient-rich bio fertilizer, and environmental restoration. Discov. Sustain. 2024, 5, 60. [Google Scholar] [CrossRef]
- Ferraz Ramos, R.; Almeida Santana, N.; de Andrade, N.; Scheffer Romagna, I.; Tirloni, B.; de Oliveira Silveira, A.; Domínguez, J.; Josemar Seminoti Jacques, R. Vermicomposting of cow manure: Effect of time on earthworm biomass and chemical, physical, and biological properties of vermicompost. Bioresour. Technol. 2022, 345, 126572. [Google Scholar] [CrossRef]
- Enebe, M.C.; Erasmus, M. Vermicomposting technology—A perspective on vermicompost production technologies, limitations and prospects. J. Environ. Manag. 2023, 345, 118585. [Google Scholar] [CrossRef]
- Tao, W.Q.; Wu, Q.Q.; Zhang, J.; Chang, T.T.; Liu, X.N. Effects of Applying Organic Amendments on Soil Aggregate Structure and Tomato Yield in Facility Agriculture. Plants 2024, 13, 3064. [Google Scholar] [CrossRef]
- Oyege, I.; Balaji Bhaskar, M.S. Effects of Vermicompost on Soil and Plant Health and Promoting Sustainable Agriculture. Soil Syst. 2023, 7, 101. [Google Scholar] [CrossRef]
- ANSA. Cavoli, Broccoli & C.: Per l’Italia un Business da 805 Milioni. 2024. Available online: https://www.ansa.it/campania/notizie/ambiente_territorio/2024/11/08/cavoli-broccolic.-per-litalia-un-business-da-805-milioni_b035e600-37cf-4ee7-8319-9c0453a0470d.html (accessed on 26 January 2025).
- Annunziata, M.G.; Carillo, P.; Fuggi, A.; Troccoli, A.; Woodrow, P. Metabolic profiling of cauliflower under traditional and reduced tillage systems. Aust. J. Crop Sci. 2013, 7, 1317–1323. [Google Scholar]
- Dominguez, J.; Edwards, C. Biology and Ecology of Earthworm Species Used for Vermicomposting. In Vermiculture Technology: Earthworms, Organic Wastes, and Environmental Management; Edwards, C., Arancon, A., Sherman, N.Q., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 27–40. [Google Scholar]
- Reinecke, A.J.; Viljoen, S.A. A comparison of the biology of Eisenia fetida and Eisenia andrei (Oligochaeta). Biol. Fertil. Soils 1991, 11, 295–300. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis Part 3 Chemical Methods; Sparks, D., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, Number 9 in Series Agronomy; American Society of Agronomy Inc.: Madison, WI, USA, 1965; p. 1149-Hach. [Google Scholar]
- Water Analysis Handbook, 3rd ed.; Hach Company: Loveland, CO, USA, 1997.
- Fusco, G.M.; Burato, A.; Pentangelo, A.; Cardarelli, M.; Nicastro, R.; Carillo, P.; Parisi, M. Can Microbial Consortium Applications Affect Yield and Quality of Conventionally Managed Processing Tomato? Plants 2022, 12, 14. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Iacuzzi, N.; Tortorici, N.; Ida, D.M.; Alaimo, F.; Cozzolino, E.; Sarno, M.; Mori, M.; Tuttolomondo, T. Biodegradable mulching films affect soil temperature and agronomic performance of open field eggplant in hot-arid environments. Ital. J. Agron. 2024, 19, 100025. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ducci, D.; Della Morte, R.; Mottola, A.; Onorati, G.; Pugliano, G. Nitrate trends in groundwater of the Campania region (southern Italy). Environ. Sci. Pollut. Res. Int. 2019, 26, 2120–2131. [Google Scholar] [CrossRef]
- Regione Campania. Deliberazione di Giunta Regionale della Campania n. 762 del 05/12/2017. Bollettino Ufficiale della Regione Campania No. 89, 11 December 2017. 2017. Available online: https://www.agricoltura.regione.campania.it/reflui/pdf/DGR_762-05-12-17.pdf (accessed on 26 January 2025).
- Regione Campania. Programma d’Azione della Campania (DGR n. 585 del 16.12.2020). Bollettino Ufficiale della Regione Campania No. 247, 21 December 2020. 2020. Available online: https://agricoltura.regione.campania.it/reflui/zone-vulnerabili-nitrati.html (accessed on 26 January 2025).
- Arancon, N.Q.; Edwards, C.A.; Bierman, P. Influences of vermicomposts on field strawberries: Part 2. Effects on soil microbiological and chemical properties. Bioresour. Technol. 2006, 97, 831–840. [Google Scholar] [CrossRef]
- Geisseler, D.; Smith, R.; Cahn, M.; Muramoto, J. Nitrogen mineralization from organic fertilizers and composts: Literature survey and model fitting. J. Environ. Qual. 2021, 50, 1325–1338. [Google Scholar] [CrossRef]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. SpringerPlus 2012, 1, 26. [Google Scholar] [CrossRef]
- Zaller, J.G. Vermicompost as a substitute for peat in potting media: Effects on germination, biomass allocation, yields and fruit quality of three tomato varieties. Sci. Hortic. 2007, 112, 191–199. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Herencia, J.F.; García-Galavís, P.A.; Dorado, J.A.R.; Maqueda, C. Comparison of nutritional quality of the crops grown in an organic and conventional fertilized soil. Sci. Hortic. 2011, 129, 882–888. [Google Scholar] [CrossRef]
- Bulmaga, A.; Lazureanu, A.; Alexa, E.; Monica, N. Study Regarding Nitrate and Nitrite Content in Cauliflower, from Agro-Food Markets in Timisoara. Analele Universitatii din Oradea, Fascicula Biologie. TOM XV. 2008. Available online: https://bioresearch.ro/2008/022-023%20-%20Bulmaga%20Alina.pdf (accessed on 4 May 2025).
- Pavlovic, R.; Boskovic-Rakocevic, L. Varietal Specificities in Yield and Nitrate Content in Cauliflower. Acta Agric. Serbica 2007, 12, 69–76. [Google Scholar]
- Carillo, P.; Rouphael, Y. Nitrate Uptake and Use Efficiency: Pros and Cons of Chloride Interference in the Vegetable Crops. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Urmi, T.A.; Rahman, M.M.; Islam, M.M.; Islam, M.A.; Jahan, N.A.; Mia, M.A.; Akhter, S.; Siddiqui, M.H.; Kalaji, H.M. Integrated Nutrient Management for Rice Yield, Soil Fertility, and Carbon Sequestration. Plants 2022, 11, 138. [Google Scholar] [CrossRef]
- Nicastro, R.; El-Nakhel, C.; Geelen, D.; Fusco, G.M.; De Pascale, S.; Rouphael, Y.; Carillo, P. Exploring the potential of human urine derivatives in circular agriculture: A case study on lettuce. Front. Sustain. Food Syst. 2024, 8. [Google Scholar] [CrossRef]
- Shilpha, J.; Song, J.; Jeong, B.R. Ammonium Phytotoxicity and Tolerance: An Insight into Ammonium Nutrition to Improve Crop Productivity. Agronomy 2023, 13, 1487. [Google Scholar] [CrossRef]
- Carillo, P. GABA Shunt in Durum Wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zhang, J.; Zhao, R.; Dai, H.; Zhang, Z. Application of vermicompost improves strawberry growth and quality through increased photosynthesis rate, free radical scavenging and soil enzymatic activity. Sci. Hortic. 2018, 233, 132–140. [Google Scholar] [CrossRef]
- Irin, I.J.; Hasanuzzaman, M. Organic Amendments: Enhancing Plant Tolerance to Salinity and Metal Stress for Improved Agricultural Productivity. Stresses 2024, 4, 185–209. [Google Scholar] [CrossRef]
- Zaghloul, E.A.M.; Awad, E.A.; Mohamed, I.R.; El-Hameed, A.M.A.; Feng, D.; Desoky, E.M.; Algopishi, U.B.; Al Masoudi, L.M.; Elrys, A.S.; Mathew, B.T.; et al. Co-application of organic amendments and natural biostimulants on plants enhances wheat production and defense system under salt-alkali stress. Sci. Rep. 2024, 14, 29742. [Google Scholar] [CrossRef]
- Abate, G.G. Effects of Strains and Vermicompost Application on Growth and Yield Components of Faba Bean Under Greenhouse Condition. Am. J. Plant Biol. 2023, 8, 55–64. [Google Scholar] [CrossRef]
- El-Dakak, R.; El-Aggan, W.; Badr, G.; Helaly, A.; Tammam, A. Positive Salt Tolerance Modulation via Vermicompost Regulation of SOS1 Gene Expression and Antioxidant Homeostasis in Viciafaba Plant. Plants 2021, 10, 2477. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, R.; Bakshi, P.; Jasrotia, A.; Sinha, K.; Sharma, N.; Sharma, P.; Kumar, D.; Sood, M. Synergistic Impact of Vermicompost, Biochar and Jaggery on Antioxidants, Phenols and Flavonoids in Guava cv. L-49. BioResources 2024, 19, 8173–8187. [Google Scholar] [CrossRef]
- Gutiérrez-Miceli, F.A.; Santiago-Borraz, J.; Montes Molina, J.A.; Nafate, C.C.; Abud-Archila, M.; Oliva Llaven, M.A.; Rincón-Rosales, R.; Dendooven, L. Vermicompost as a soil supplement to improve growth, yield and fruit quality of tomato (Lycopersicum esculentum). Bioresour. Technol. 2007, 98, 2781–2786. [Google Scholar] [CrossRef]
- Seneviratne, G.; Weerasekara, C.; Tennakoon, N.; Kulasooriya, S.A. Quality improvement of organic vegetables through effective nutrient management. Trop. Agric. Res. 2009, 21, 1–9. [Google Scholar] [CrossRef]
- Aldanondo-Ochoa, A.M.; Almansa-Sáez, C. The private provision of public environment: Consumer preferences for organic production systems. Land Use Policy 2009, 26, 669–682. [Google Scholar] [CrossRef]




| Soil Parameters | O.M. % | N-Kjeldhal % | NO3-N ppm | NH4-N ppm | ||||
|---|---|---|---|---|---|---|---|---|
| Start | End | Start | End | Start | End | Start | End | |
| Loam NF | 2.2 | 2.3 | 0.118 | 0.145 | 28.9 | 40.7 | 10.0 | 12.4 |
| Loam MIN | 2.2 | 2.6 | 0.118 | 0.150 | 28.9 | 85.8 | 10.0 | 12.0 |
| Loam VC | 2.2 | 2.7 | 0.118 | 0.150 | 28.9 | 84.1 | 10.0 | 9.1 |
| Clay NF | 1.9 | 1.7 | 0.122 | 0.106 | 17.0 | 17.4 | 7.7 | 5.6 |
| Clay MIN | 1.9 | 2.4 | 0.122 | 0.138 | 17.0 | 96.5 | 7.7 | 12.1 |
| Clay VC | 1.9 | 2.6 | 0.122 | 0.150 | 17.0 | 30.4 | 7.7 | 6.1 |
| Source of Variance | Total FW (g) | IRHI | Corymb Diameter (cm) | Corymb Height (cm) |
|---|---|---|---|---|
| Soil (S) | ||||
| Loam | 1095 ± 495 | 35.8 ± 8.8 | 13.23 ± 3.74 | 9.84 ± 1.80 |
| Clayey | 1080 ± 529 | 34.6 ± 4.8 | 12.73 ± 3.21 | 9.80 ± 1.60 |
| ns | ns | ns | ns | |
| Fertilization (F) | ||||
| Control (NF) | 498 ± 34 c | 26.8 ± 3.4 c | 8.68 ± 0.69 c | 7.73 ± 0.91 c |
| Vermicompost (VC) | 1089 ± 16 b | 38.3 ± 4.1 b | 13.75 ± 0.77 b | 10.45 ± 0.37 b |
| Mineral (MIN) | 1674 ± 320 a | 40.5 ± 2.2 a | 16.52 ± 1.70 a | 11.28 ± 0.63 a |
| *** | ** | *** | *** | |
| S × F | ||||
| Loam × NF | 532 ± 34 c | 24.5 ± 3.2 d | 8.50 ± 0.95 e | 7.67 ± 1.19 |
| Loam × VC | 1080 ± 12 b | 41.3 ± 3.5 a | 14.40 ± 0.40 c | 10.47 ± 0.40 |
| Loam × MIN | 1672 ± 126 a | 41.5 ± 2.1 a | 16.80 ± 0.30 a | 11.40 ± 0.10 |
| Clayey × NF | 463 ± 28 d | 29.1 ± 1.5 c | 8.87 ± 0.40 e | 7.80 ± 0.80 |
| Clayey × VC | 1099 ± 95 b | 35.3 ± 1.6 b | 13.10 ± 0.26 d | 10.43 ± 0.42 |
| Clayey × MIN | 1677 ± 120 a | 39.4 ± 2.1 a | 16.23 ± 0.06 b | 11.17 ± 0.21 |
| * | ** | * | ns |
| Source of Variance | L* | a* | b* | SPAD Index | Total Chlorophylls (mg g−1 FW) |
|---|---|---|---|---|---|
| Soil (S) | |||||
| Loam | 83.6 ± 5.62 | −0.91 ± 1.96 | 18.11 ± 3.13 | 36.52 ± 7.38 | 0.80 ± 0.12 |
| Clayey | 83.7 ± 4.76 | −0.92 ± 1.26 | 17.10 ± 2.11 | 33.39 ± 8.57 | 0.73 ± 0.09 |
| ns | ns | ns | ns | ns | |
| Fertilization (F) | |||||
| Control (NF) | 79.4 ± 2.51 b | −2.47 ± 0.50 c | 20.93 ± 1.42 a | 25.52 ± 2.58 c | 0.67 ± 0.07 c |
| Vermicompost (VC) | 81.5 ± 2.13 c | −1.36 ± 0.29 b | 16.57 ± 0.57 b | 35.65 ± 2.49 b | 0.76 ± 0.07 b |
| Mineral (MIN) | 89.9 ± 5.23 a | 1.08 ± 1.38 a | 15.32 ± 0.92 c | 43.70 ± 4.74 a | 0.87 ± 0.04 a |
| *** | *** | *** | *** | ** | |
| S × F | |||||
| Loam × NF | 77.7 ± 2.10 e | −2.89 ± 0.29 e | 22.10 ± 0.40 a | 27.47 ± 1.06 | 0.71 ± 0.04 |
| Loam × VC | 82.8 ± 1.67 b | −1.34 ± 0.12 b | 16.93 ± 0.47 b | 37.87 ± 0.75 | 0.77 ± 0.10 |
| Loam × MIN | 90.1 ± 1.63 a | 1.50 ± 0.54 a | 15.30 ± 0.96 e | 44.23 ± 1.19 | 0.93 ± 0.07 |
| Clayey × NF | 81.1 ± 1.67 c | −2.04 ± 0.05 d | 19.77 ± 0.90 a | 23.57 ± 2.01 | 0.62 ± 0.06 |
| Clayey × VC | 80.2 ± 1.89 d | −1.37 ± 0.44 c | 16.20 ± 0.44 c | 33.43 ± 0.47 | 0.76 ± 0.05 |
| Clayey × MIN | 89.7 ± 1.29 a | 0.66 ± 0.45 a | 15.33 ± 0.40 d | 43.17 ± 1.19 | 0.80 ± 0.05 |
| * | ** | * | ns | ns |
| Source of Variance | NO3− (mg Kg−1 FW) | N-Kjeldahl (%) | Proteins (mg g−1 DW) | Starch (mg g−1 DW) | TNSCs (mg g−1 DW) |
|---|---|---|---|---|---|
| Soil (S) | |||||
| Loam | 585.8 ± 258.5 | 2.32 ± 0.45 | 63.53 ± 9.70 | 1.49 ± 0.34 | 23.73 ± 2.40 |
| Clayey | 176.8 ± 104.6 | 2.49 ± 0.59 | 49.34 ± 10.10 | 1.79 ± 0.59 | 22.00 ± 3.11 |
| *** | ns | ** | *** | ns | |
| Fertilization (F) | |||||
| Control (NF) | 183.5 ± 124.6 c | 2.44 ± 0.38 | 55.07 ± 16.49 b | 1.69 ± 0.46 | 23.26 ± 2.05 |
| Vermicompost (VC) | 392.0 ± 284.9 b | 2.26 ± 0.41 | 51.72 ± 4.82 c | 1.70 ± 0.54 | 23.42 ± 2.99 |
| Mineral (MIN) | 568.3 ± 331.5 a | 2.52 ± 0.47 | 62.53 ± 10.14 a | 1.55 ± 0.38 | 21.91 ± 1.90 |
| * | ns | *** | ns | ns | |
| S × F | |||||
| Loam × NF | 295.0 ± 37.2 d | 2.51 ± 0.49 c | 68.24 ± 6.30 b | 1.30 ± 0.17 | 21.98 ± 1.49 |
| Loam × VC | 622.7 ± 200.1 b | 2.60 ± 0.26 b | 51.93 ± 5.35 d | 1.44 ± 0.29 | 25.30 ± 2.58 |
| Loam × MIN | 839.7 ± 15.0 a | 1.86 ± 0.05 f | 70.43 ± 1.21 a | 1.74 ± 0.45 | 23.91 ± 2.39 |
| Clayey × NF | 72.0 ± 11.14 f | 2.37 ± 0.32 d | 41.90 ± 10.94 f | 2.07 ± 0.21 | 24.54 ± 1.84 |
| Clayey × VC | 161.3 ± 57.5 e | 1.92 ± 0.06 e | 51.51 ± 5.42 e | 1.95 ± 0.67 | 21.55 ± 2.25 |
| Clayey × MIN | 297.0 ± 42.6 c | 3.18 ± 0.20 a | 54.62 ± 11.27 c | 1.36 ± 0.68 | 19.90 ± 3.70 |
| * | *** | * | ns | ns (p < 0.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, G.M.; Di Mola, I.; Mori, M.; Cozzolino, E.; Morrone, B.; Trasacco, F.; Carillo, P. From Water Buffalo (Bubalus bubalis) Manure to Vermicompost: Testing a Sustainable Approach for Agriculture. Sustainability 2025, 17, 4253. https://doi.org/10.3390/su17104253
Fusco GM, Di Mola I, Mori M, Cozzolino E, Morrone B, Trasacco F, Carillo P. From Water Buffalo (Bubalus bubalis) Manure to Vermicompost: Testing a Sustainable Approach for Agriculture. Sustainability. 2025; 17(10):4253. https://doi.org/10.3390/su17104253
Chicago/Turabian StyleFusco, Giovanna Marta, Ida Di Mola, Mauro Mori, Eugenio Cozzolino, Biagio Morrone, Fulvio Trasacco, and Petronia Carillo. 2025. "From Water Buffalo (Bubalus bubalis) Manure to Vermicompost: Testing a Sustainable Approach for Agriculture" Sustainability 17, no. 10: 4253. https://doi.org/10.3390/su17104253
APA StyleFusco, G. M., Di Mola, I., Mori, M., Cozzolino, E., Morrone, B., Trasacco, F., & Carillo, P. (2025). From Water Buffalo (Bubalus bubalis) Manure to Vermicompost: Testing a Sustainable Approach for Agriculture. Sustainability, 17(10), 4253. https://doi.org/10.3390/su17104253













