Spatio-Temporal Dynamics of Fish Community and Influencing Factors in an Urban River (Haihe River), China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Site Selection
2.2. Fish Sampling
2.3. Environmental Variables and Urbanization Indicators
2.4. Data Analysis
2.4.1. Fish Community Metrics
2.4.2. Statistical Analysis
3. Results
3.1. Spatial and Seasonal Variations in Fish Community Composition
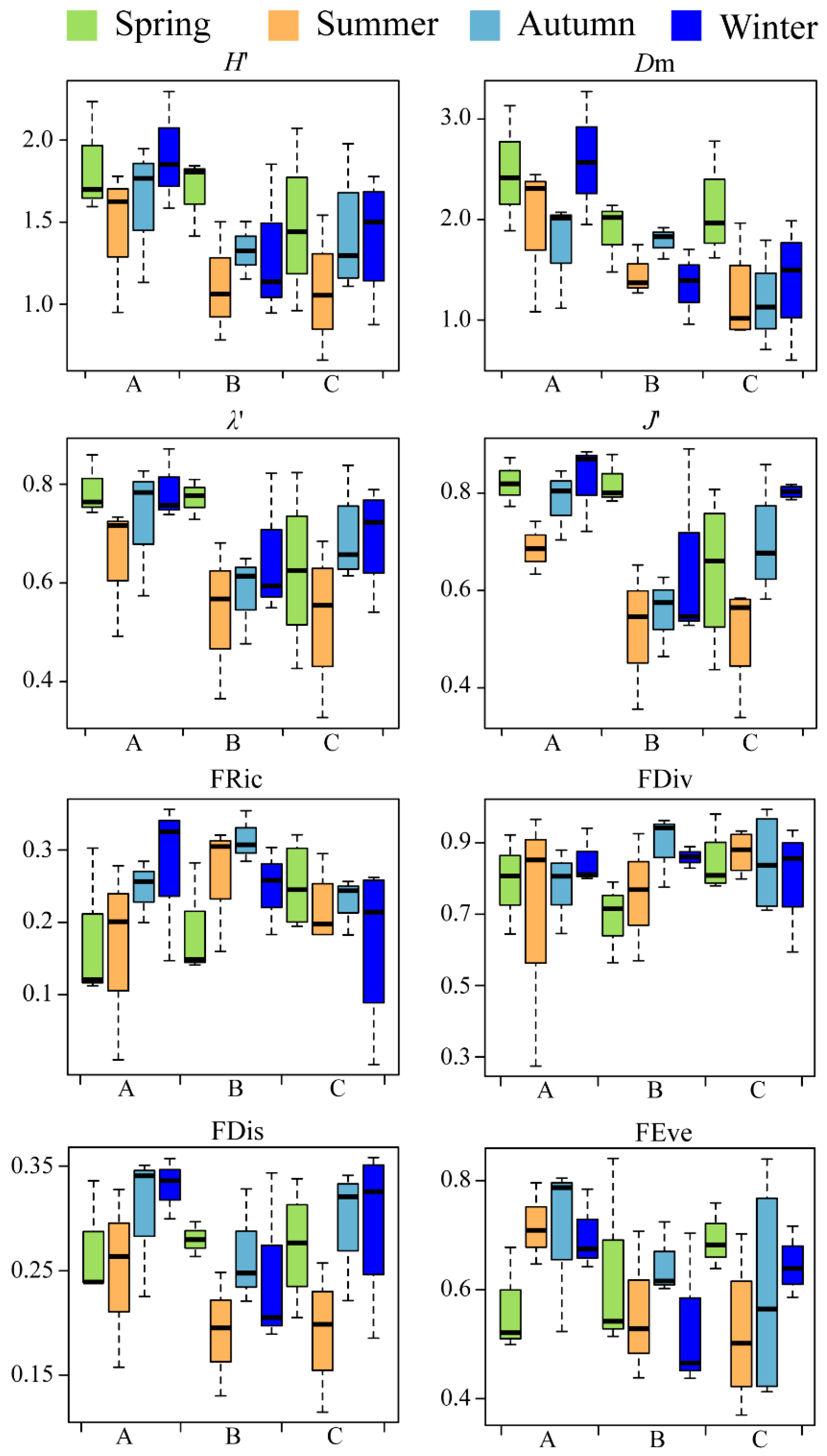
3.2. Species Diversity and Functional Diversity
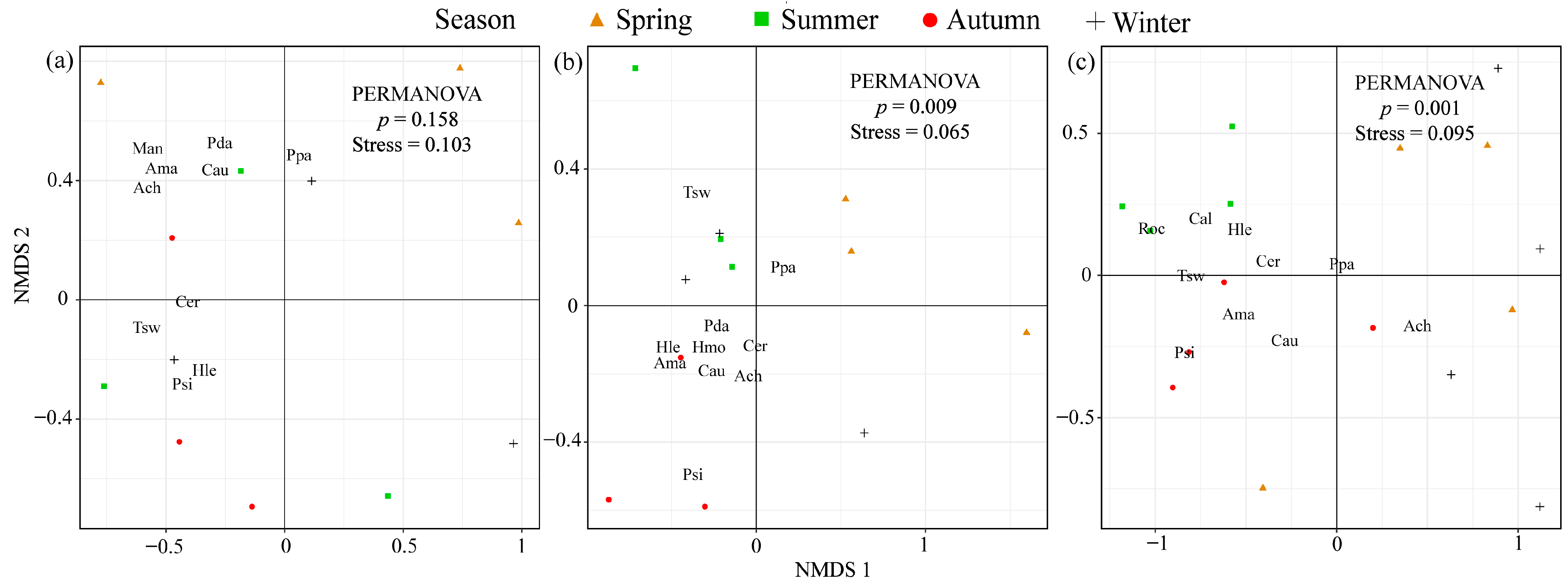
3.3. Fish Community Composition Analysis
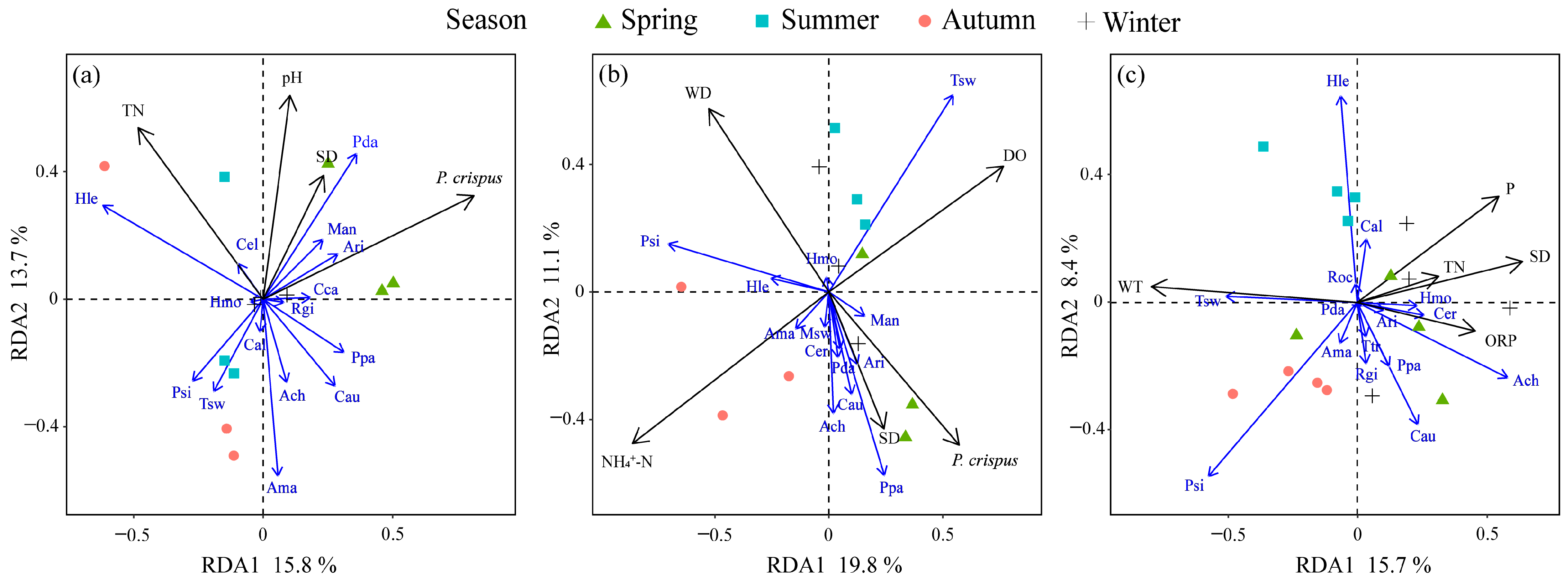
3.4. Environmental Factors Influencing Fish Communities
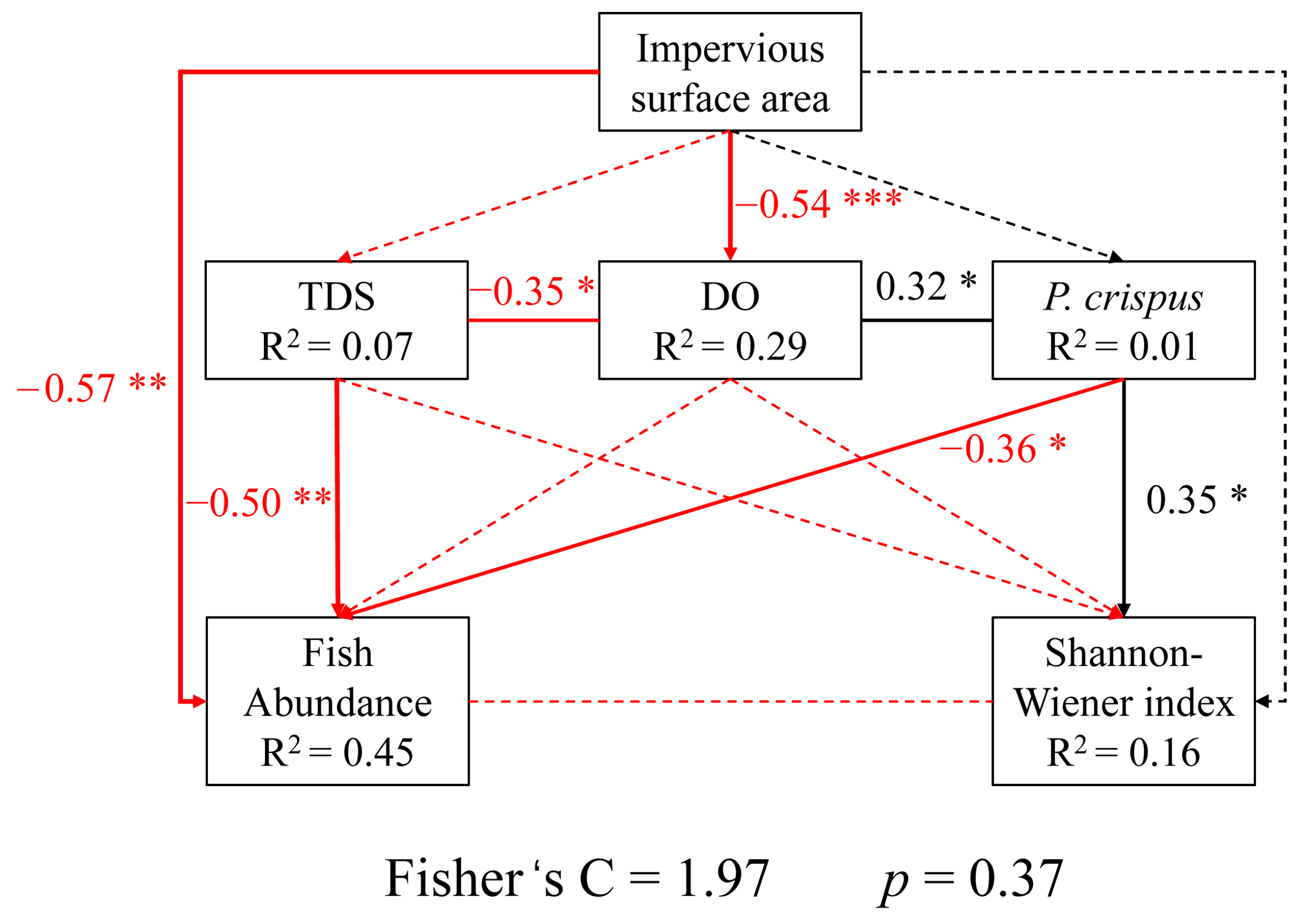
3.5. Relationships Between Urbanization Indicators, Environmental Variables, and Fish Communities
4. Discussion
4.1. Spatial and Seasonal Dynamics of Fish Communities
4.2. Influence of Environmental Factors
4.3. Implications for Urban River Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M. The urban stream syndrome: Current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.B.; Roy, A.H.; Smith, B.; Capps, K.A. Global perspectives on the urban stream syndrome. Freshw. Sci. 2016, 35, 412–420. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Wang, L.; Lyons, J.; Kanehl, P.; Bannerman, R. Impacts of urbanization on stream habitat and fish across multiple spatial scales. Environ. Manag. 2001, 28, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Freeman, M.; Leigh, D.; Freeman, B.J.; Pringle, C.M. Urbanization effects on fishes and habitat quality in a southern Piedmont River basin. Am. Fish. Soc. Symp. 2005, 47, 69–85. [Google Scholar]
- Leitão, R.P.; Zuanon, J.; Mouillot, D.; Leal, C.G.; Hughes, R.M.; Kaufmann, P.R.; Villéger, S.; Pompeu, P.S. Disentangling the pathways of land use impacts on the functional structure of fish assemblages in Amazon streams. Ecography 2018, 41, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z. Encyclopedia of Chinese Rivers and Lakes: Haihe River Volume; China Water Power Press: Beijing, China, 2013. [Google Scholar]
- Ding, Y.; Shan, B.; Zhao, Y. Assessment of river habitat quality in the Hai River Basin, Northern China. Int. J. Environ. Res. Public Health 2015, 12, 11699–11717. [Google Scholar] [CrossRef]
- Wang, L.; Mo, K.; Chen, Q.; Zhang, J.; Xia, J.; Lin, Y. Estimating ecological flows for fish overwintering in plain rivers using a method based on water temperature and critical water depth. Ecohydrology 2019, 12, e2098. [Google Scholar] [CrossRef]
- Brown, L.R.; Cuffney, T.F.; Coles, J.F.; Fitzpatrick, F.; McMahon, G.; Steuer, J.; Bell, A.H.; May, J.T. Urban streams across the USA: Lessons learned from studies in 9 metropolitan areas. J. N. Am. Benthol. Soc. 2009, 28, 1051–1069. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, H.; Chen, X.; Ren, Y.; Ouyang, Z. Relationships between river water quality and landscape factors in Haihe River Basin, China: Implications for environmental management. Chin. Geogr. Sci. 2016, 26, 197–207. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X. The 30 m annual land cover dataset and its dynamics in China from 1990 to 2019. Earth Syst. Sci. Data 2021, 13, 3907–3925. [Google Scholar] [CrossRef]
- Bright, E.A.; Rose, A.N.; Urban, M.L. LandScan Global Population Database. Available online: https://landscan.ornl.gov/ (accessed on 22 May 2024).
- Li, M.D. The Fauna of Fishes in Tianjin; Tianjin Science and Technology Press: Tianjin, China, 2011. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Wastewater, 23rd ed.; APHA: Washington, DC, USA, 2017. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Available online: https://www.qgis.org/ (accessed on 17 October 2024).
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L. Food habits of albacore, bluefin tuna, and bonito in California waters. Calif. Fish Game Fish Bull. 1971, 152. [Google Scholar]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 17 October 2024).
- Francis, R.A.; Chadwick, M.A. Urban Ecosystems: Understanding the Human Environment; Routledge: London, UK, 2013. [Google Scholar]
- Alberti, M.; Marzluff, J.; Hunt, V.M. Urban driven phenotypic changes: Empirical observations and theoretical implications for eco-evolutionary feedback. Philos. Trans. R. Soc. B 2017, 372, 20160029. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.C. Winners and losers among stream fishes in relation to land use legacies and urban development in the southeastern US. Biol. Conserv. 2006, 127, 301–309. [Google Scholar] [CrossRef]
- Wolter, C. Temperature influence on the fish assemblage structure in a large lowland river, the lower Oder River, Germany. Ecol. Freshw. Fish 2007, 16, 493–503. [Google Scholar] [CrossRef]
- Tomasetti, S.J.; Gobler, C.J. Dissolved oxygen and pH criteria leave fisheries at risk. Science 2020, 368, 372–373. [Google Scholar] [CrossRef]
- Gücker, B.; Brauns, M.; Pusch, M.T. Effects of wastewater treatment plant discharge on ecosystem structure and function of lowland streams. J. N. Am. Benthol. Soc. 2006, 25, 313–329. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Gu, B. Urban rivers as hotspots of regional nitrogen pollution. Environ. Pollut. 2015, 205, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Xu, H.; Zhu, G.; Chen, J. Influence of Potamogeton crispus on lake water environment and phytoplankton community structure. Environ. Sci. 2020, 41, 4053–4061. [Google Scholar]
- Chapman, P.M.; McPherson, C.A. Development of a total dissolved solids (TDS) chronic effects benchmark for a northern Canadian lake. Integr. Environ. Assess. Manag. 2016, 12, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sand-Jensen, K.; Prahl, C.; Stokholm, H. Oxygen release from roots of submerged aquatic macrophytes. Oikos 1982, 38, 349–354. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, T.; Li, Z.; Liu, J. Relationships between spatial distribution of two dominant small-sized fishes and submerged macrophyte cover in Niushan Lake of China. Chin. J. Appl. Ecol. 2012, 23, 2566–2572. [Google Scholar]
- Keeler, B.L.; Hamel, P.; McPhearson, T.; Hamann, M.H. Mapping ecological and social values of urban green infrastructure: Guidance for practitioners. Sustainability 2019, 11, 1696. [Google Scholar]
- Allan, J.D.; Abell, R.; Hogan, Z.; Revenga, C.; Taylor, B.W.; Welcomme, R.L.; Winemiller, K. Overfishing of inland waters. BioScience 2005, 55, 1041–1051. [Google Scholar] [CrossRef]
- Wenger, S.J.; Roy, A.H.; Jackson, C.R.; Bernhardt, E.S.; Carter, T.L.; Filoso, S.; Gibson, C.A.; Hession, W.C.; Kaushal, S.S.; Martí, E.; et al. Twenty-six key research questions in urban stream ecology: An assessment of the state of the science. J. N. Am. Benthol. Soc. 2009, 28, 1080–1098. [Google Scholar] [CrossRef]

| Species (Coding) | Feeding Habit | Section A | Section B | Section C |
|---|---|---|---|---|
| Cypriniformes | ||||
| Cyprinidae | ||||
| Carassius auratus (Cau) | Omnivorous | + | + | + |
| Cyprinus carpio (Cca) | Omnivorous | + | + | + |
| Pseudorasbora parva (Ppa) | Planktivorous | + | + | + |
| Toxabramis swinhonis (Tsw) | Omnivorous | + | + | + |
| Abbottina rivularis (Ari) | Planktivorous | + | + | + |
| Hemiculter bleekeri (Hbl) | Omnivorous | + | + | − |
| Hemiculter leucisculus (Hle) | Omnivorous | + | + | + |
| Acheilognathus macropterus (Ama) | Detritivorous | + | + | + |
| Pseudobrama simoni (Psi) | Detritivorous | + | + | + |
| Acanthorhodeus chankaensis (Ach) | Detritivorous | + | + | + |
| Xenocypris argentea (Xar) | Detritivorous | + | − | − |
| Culter alburnus (Cal) | Piscivorous | + | + | + |
| Culterichthys erythropter (Cer) | Piscivorous | + | + | + |
| Hemibarbus maculatus (Hem) | Zoobenthivorous | + | − | − |
| Hypophthalmichthys molitrix (Hmo) | Planktivorous | + | + | + |
| Squalidus argentatus (Sar) | Omnivorous | + | − | − |
| Aristichys nobilis (Ano) | Planktivorous | − | + | − |
| Rhodeus ocellatus (Roc) | Detritivorous | + | − | + |
| Ctenopharyngodon idella (Cid) | Herbivorous | − | + | − |
| Cobitidae | ||||
| Misgurnus anguillicaudatus (Man) | Omnivorous | + | + | + |
| Paramisgurnus dabryanus (Pda) | Omnivorous | + | + | + |
| Perciformes | ||||
| Channidae | ||||
| Channa argus (Car) | Piscivorous | − | − | + |
| Odontobutidae | ||||
| Micropercops swinhonis (Msw) | Piscivorous | − | + | − |
| Osphronemidae | ||||
| Macropodus chinensis (Mch) | Piscivorous | − | − | + |
| Gobiidae | ||||
| Tridentiger trigonocephalus (Ttr) | Piscivorous | − | − | + |
| Rhinogobius giurinus (Rgi) | Piscivorous | + | + | + |
| Siluriformes | ||||
| Bagridae | ||||
| Pelteobagrus fulvidraco (Pfu) | Zoobenthivorous | + | − | + |
| Siluridae | ||||
| Silurus asotus (Sas) | Piscivorous | + | − | − |
| Mugiliformes | ||||
| Mugilidae | ||||
| Liza haematocheila (Lha) | Omnivorous | − | − | + |
| Beloniformes | ||||
| Hemiramphidae | ||||
| Hyporhamphus intermedius (Hin) | Planktivorous | + | − | + |
| Species | N (ind.) | N (%) | B (g) | B (%) | Occurrence | IRI | |
|---|---|---|---|---|---|---|---|
| Section A | Spring | ||||||
| Carassius auratus | 33 | 13% | 2235.26 | 30% | 67% | 2857.36 | |
| Paramisgurnus dabryanus | 41 | 16% | 900.86 | 12% | 83% | 2317.17 | |
| Cyprinus carpio | 7 | 3% | 1699.05 | 23% | 83% | 2141.9 | |
| Acheilognathus macropterus | 59 | 22% | 912.11 | 12% | 50% | 1740.14 | |
| Misgurnus anguillicaudatus | 31 | 12% | 359.75 | 5% | 67% | 1111.05 | |
| Summer | |||||||
| Hemiculter leucisculus | 77 | 34% | 1567.98 | 38% | 100% | 7236.65 | |
| Toxabramis swinhonis | 48 | 21% | 401.5 | 10% | 67% | 2065.99 | |
| Autumn | |||||||
| Hemiculter leucisculus | 38 | 23% | 340.3 | 7% | 100% | 2970.15 | |
| Toxabramis swinhonis | 40 | 24% | 264.2 | 5% | 100% | 2938.35 | |
| Pseudobrama simoni | 22 | 13% | 316.5 | 6% | 100% | 1958.67 | |
| Hypophthalmichthys molitrix | 2 | 1% | 2645 | 53% | 33% | 1804.52 | |
| Winter | |||||||
| Hypophthalmichthys molitrix | 13 | 8% | 19,710 | 83% | 50% | 4549.83 | |
| Hemiculter leucisculus | 54 | 31% | 482.57 | 2% | 100% | 3325.78 | |
| Carassius auratus | 15 | 9% | 810.33 | 3% | 100% | 1210.27 | |
| Section B | Spring | ||||||
| Toxabramis swinhonis | 53 | 32% | 601.49 | 19% | 83% | 4173 | |
| Hemiculter leucisculus | 39 | 23% | 674.13 | 21% | 83% | 3665.03 | |
| Carassius auratus | 18 | 11% | 984.63 | 30% | 83% | 3420.42 | |
| Summer | |||||||
| Toxabramis swinhonis | 388 | 62% | 4283.95 | 34% | 100% | 9523.93 | |
| Hemiculter leucisculus | 167 | 27% | 3161.82 | 25% | 100% | 5131.5 | |
| Hypophthalmichthys molitrix | 6 | 1% | 2337.16 | 18% | 67% | 1283.98 | |
| Autumn | |||||||
| Hemiculter leucisculus | 418 | 51% | 3882.5 | 29% | 100% | 8044.44 | |
| Pseudobrama simoni | 180 | 22% | 2163.6 | 16% | 83% | 3195.14 | |
| Carassius auratus | 52 | 6% | 2149.8 | 16% | 83% | 1879.29 | |
| Toxabramis swinhonis | 72 | 9% | 427.6 | 3% | 83% | 1003.46 | |
| Winter | |||||||
| Hemiculter leucisculus | 170 | 43% | 2583.97 | 48% | 100% | 9062.75 | |
| Toxabramis swinhonis | 167 | 42% | 1003.2 | 19% | 100% | 6049.83 | |
| Carassius auratus | 16 | 4% | 706.63 | 13% | 67% | 1142.81 | |
| Section C | Spring | ||||||
| Hemiculter leucisculus | 89 | 26% | 1691.25 | 31% | 75% | 4283.5 | |
| Toxabramis swinhonis | 111 | 33% | 844.46 | 15% | 63% | 3005.89 | |
| Carassius auratus | 13 | 4% | 975.93 | 18% | 88% | 1896.61 | |
| Acanthorhodeus chankaensis | 41 | 12% | 381.74 | 7% | 75% | 1428.13 | |
| Summer | |||||||
| Hemiculter leucisculus | 927 | 48% | 20,837.59 | 60% | 125% | 13,508.9 | |
| Toxabramis swinhonis | 737 | 38% | 5483.4 | 16% | 100% | 5386.75 | |
| Autumn | |||||||
| Pseudobrama simoni | 517 | 36% | 7788.8 | 37% | 100% | 7355.45 | |
| Hemiculter leucisculus | 351 | 25% | 4981.5 | 24% | 100% | 4846.99 | |
| Toxabramis swinhonis | 366 | 26% | 2511.9 | 12% | 88% | 3298.34 | |
| Carassius auratus | 88 | 6% | 4790.3 | 23% | 100% | 2911.11 | |
| Winter | |||||||
| Hemiculter leucisculus | 41 | 25% | 878.04 | 8% | 100% | 3300.3 | |
| Hypophthalmichthys molitrix | 6 | 4% | 6546.98 | 60% | 50% | 3166.6 | |
| Carassius auratus | 31 | 19% | 1867.74 | 17% | 75% | 2694.47 | |
| Acanthorhodeus chankaensis | 30 | 18% | 256.5 | 2% | 63% | 1289.41 | |
| Toxabramis swinhonis | 30 | 18% | 203.5 | 2% | 63% | 1259.22 |
| Diversity Index | Factors | F | p |
|---|---|---|---|
| Shannon-Wiener index | season | 2.49 | 0.08 |
| spatial | 3.59 | <0.05 | |
| season × spatial | 0.26 | 0.95 | |
| Margalef Richness index | season | 3.32 | 0.03 |
| spatial | 7.28 | <0.01 | |
| season × spatial | 1.07 | 0.4 | |
| Simper Diversity index | season | 3.23 | <0.05 |
| spatial | 2.97 | 0.068 | |
| season × spatial | 0.7 | 0.65 | |
| Pielou Evenness index | season | 6.62 | <0.01 |
| spatial | 5.75 | <0.01 | |
| season × spatial | 1.81 | 0.13 | |
| Functional richness | season | 0.75 | 0.5 |
| spatial | 0.8 | 0.4 | |
| season × spatial | 1.13 | 0.3 | |
| Functional divergence | season | 0.44 | 0.72 |
| spatial | 0.67 | 0.52 | |
| season × spatial | 0.68 | 0.66 | |
| Functional dispersion | season | 4.06 | <0.05 |
| spatial | 0.19 | 0.24 | |
| season × spatial | 0.42 | 0.86 | |
| Functional evenness | season | 0.35 | 0.78 |
| spatial | 1.28 | 0.29 | |
| season × spatial | 1.25 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, B.; Chang, S.; Ye, S.; Zhang, Y.; Wang, Y.; Wang, S.; Wu, L.; Zhang, T. Spatio-Temporal Dynamics of Fish Community and Influencing Factors in an Urban River (Haihe River), China. Sustainability 2025, 17, 231. https://doi.org/10.3390/su17010231
Tian B, Chang S, Ye S, Zhang Y, Wang Y, Wang S, Wu L, Zhang T. Spatio-Temporal Dynamics of Fish Community and Influencing Factors in an Urban River (Haihe River), China. Sustainability. 2025; 17(1):231. https://doi.org/10.3390/su17010231
Chicago/Turabian StyleTian, Biao, Suyun Chang, Shaowen Ye, Yantao Zhang, Yuncang Wang, Songqing Wang, Li Wu, and Tanglin Zhang. 2025. "Spatio-Temporal Dynamics of Fish Community and Influencing Factors in an Urban River (Haihe River), China" Sustainability 17, no. 1: 231. https://doi.org/10.3390/su17010231
APA StyleTian, B., Chang, S., Ye, S., Zhang, Y., Wang, Y., Wang, S., Wu, L., & Zhang, T. (2025). Spatio-Temporal Dynamics of Fish Community and Influencing Factors in an Urban River (Haihe River), China. Sustainability, 17(1), 231. https://doi.org/10.3390/su17010231





