Impact of Cereal–Legume Intercropping on Changes in Soil Nutrients Contents under Semi–Arid Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Device
2.3. Plant and Soil Sampling
2.4. Measurements
2.5. Statistical Analyses
3. Results
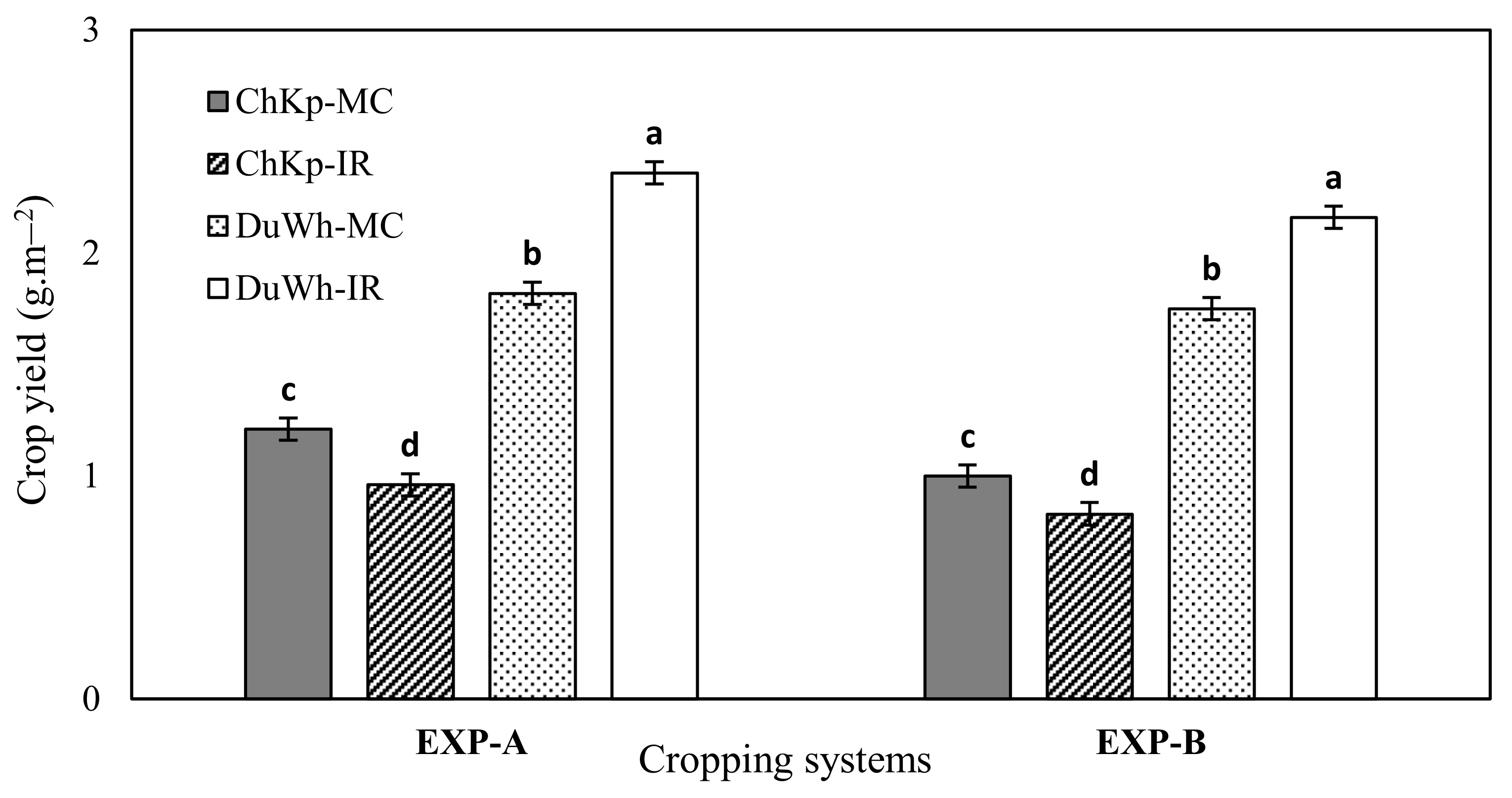
3.1. Crops Yield
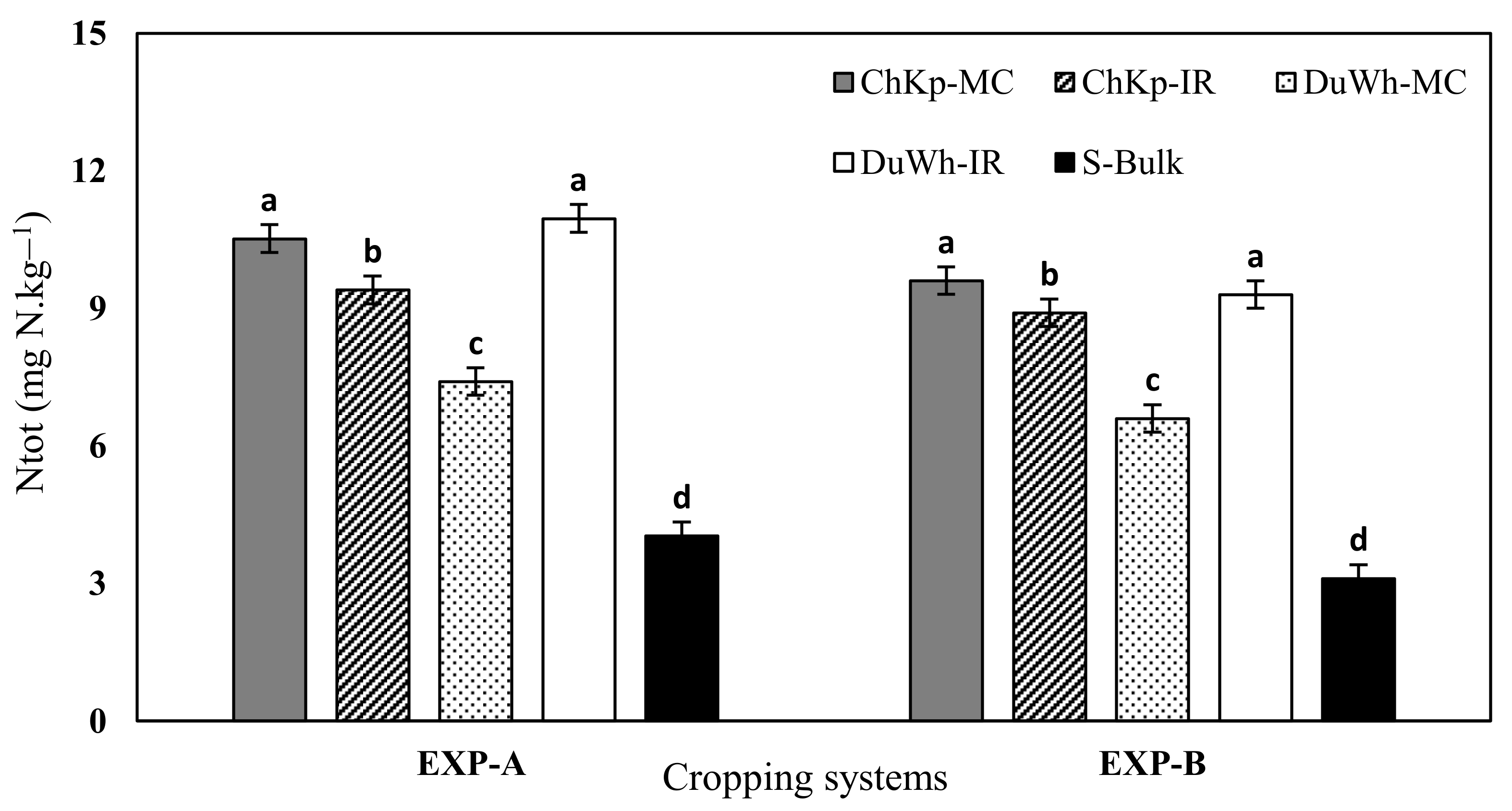
3.2. Nitrogen Content (Ntot) Soil Availability
3.3. Soil Microbial Biomass (SMB–N) Variation
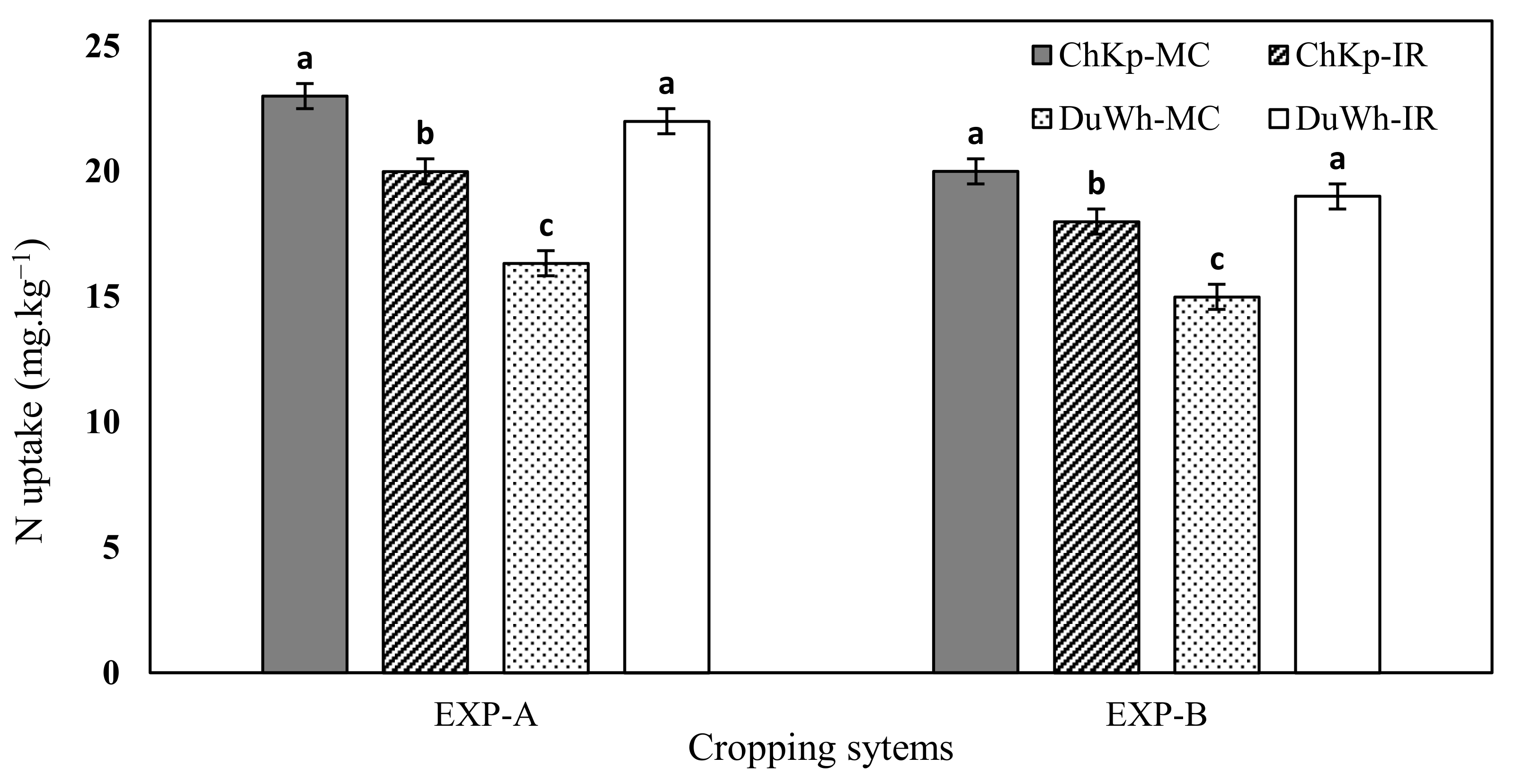
3.4. Nitrogen− Uptake
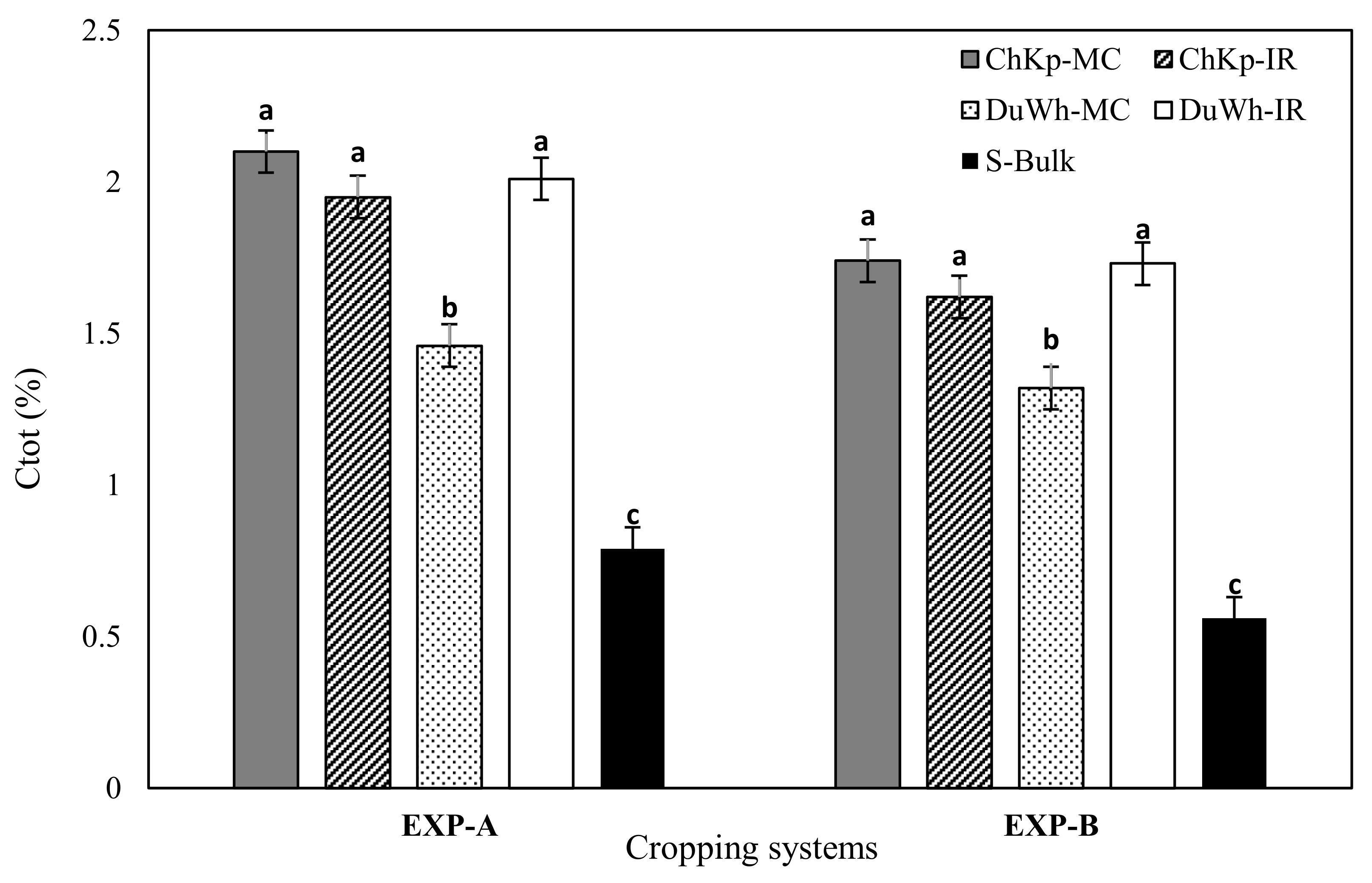
3.5. Total Soil Carbon Content (Ctot)
3.6. Microbial Biomass (SMB–C) Variation
4. Discussion
4.1. Impact of the Durum Wheat–Chickpea Intercropping on Crop Yield
4.2. Effect of Intercropping Cereal–Legume Systems on the Availability of Ntot, N− Uptake, and Variations in N within the SMB–N
4.3. Effect of Cereal–Legume Intercropping Systems on Ctot Content and SMB–C Variation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tribouillois, H.; Dürr, C.; Demilly, D.; Wagner, M.H.; Justes, E. Determination of Germination Response to Temperature and Water Potential for a Wide Range of Cover Crop Species and Related Functional Groups. PLoS ONE 2016, 11, e0161185. [Google Scholar] [CrossRef] [PubMed]
- Glaze-Corcoran, S.; Hashemi, M.; Saghpour, A.; Jahnazad, E.; Afshar, R.K.; Liu, X.; Herbert, S.J. Understanding intercropping to improve agricultural resiliency and environmental sustainability. Adv. Agron. 2020, 162, 199–256. [Google Scholar]
- Ohyama, T. The role of legume-rhizobium symbiosis in sustainable agriculture. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability; Sulieman, S., Phan Tran, L.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar]
- Andrés, P.; DoblaS–Miranda, E.; Silva-Sánchez, A.; Mattana, S.; Font, F. Physical, Chemical, and Biological Indicators of Soil Quality in Mediterranean Vineyards under Contrasting Farming Schemes. Agronomy 2022, 12, 2643. [Google Scholar] [CrossRef]
- Zuma, M.; Arthur, G.; Coopoosamy, R.; Naidoo, K. Incorporating cropping systems with eco-friendly strategies and solutions to mitigate the effects of climate change on crop production. J. Agric. Food Res. 2023, 14, 100722. [Google Scholar] [CrossRef]
- Lefebvre, M.; Midler, E.; Bontems, P. Adoption of Environment-Friendly Agricultural Practices with Background Risk: Experimental Evidence. Env. Resour. Econ. 2020, 76, 405–428. [Google Scholar] [CrossRef]
- Pansu, M.; Ibrahim, H.; Hatira, A.; Brahim, N.; Drevon, J.J.; Harmand, J.M.; Chotte, J.L.; Blavet, D. Modelling the continuous exchange of nitrogen between microbial decomposers, the organs and symbionts of plants, soil reserves, and the atmosphere. Soil Biol. Biochem. 2018, 125, 185–196. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Khugaev, C.V.; Utkina, A.O.; Isaev, K.V.; Mohamed, E.S.; Kucher, D.E. Contribution of Eco-Friendly Agricultural Practices in Improving and Stabilizing Wheat Crop Yield: A Review. Agronomy 2023, 13, 2400. [Google Scholar] [CrossRef]
- Vidal, D.F.; Trichet, P.; Puzos, L.; Bakker, M.R.; Delerue, F.; Augusto, L. Intercropping N–fixing shrubs in pine plantation forestry as an ecologically sustainable management option. For. Ecol. Manag. 2019, 437, 175–187. [Google Scholar] [CrossRef]
- Bouras, F.-Z.; Hadjout, S.; Haddad, B.; Malek, A.; Aitmoumene, S.; Gueboub, F.; Metrah, L.; Zemmouri, B.; Kherif, O.; Rebouh, N.-Y.; et al. The Effect of Nitrogen Supply on Water and Nitrogen Use Efficiency by Wheat–Chickpea Intercropping System under Rain-Fed Mediterranean Conditions. Agriculture 2023, 13, 338. [Google Scholar] [CrossRef]
- Kherif, O.; Haddad, B.; Bouras, F.-Z.; Seghouani, M.; Zemmouri, B.; Gamouh, R.; Hamzaoui, N.; Larbi, A.; Rebouh, N.-Y.; Latati, M. Simultaneous Assessment of Water and Nitrogen Use Efficiency in Rain-Fed Chickpea-Durum Wheat Intercropping Systems. Agriculture 2023, 13, 947. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Crozat, Y. N2 fixation and N supply in organic pea (Pisum sativum L.) cropping systems as affected by weeds and pea weevil (Sitona lineatus L.). Eur. J. Agron. 2005, 22, 449–458. [Google Scholar] [CrossRef]
- Deveikyte, I.; Kadziuliene, Z.; Sarunaite, L. Weed suppression ability of spring cereal crops and peas in pure and mixed stands. Agron. Res. 2009, 7, 239–244. [Google Scholar]
- Amossé, C.; Jeuffroy, M.H.; Celette, F.; David, C. Relay-intercropped forage legumes help to control weeds in organic grain production. Eur. J. Agron. 2013, 49, 158–167. [Google Scholar] [CrossRef]
- Lian, T.; Mu, Y.; Jin, J.; Ma, Q.; Cheng, Y.; Cai, Z. Impact of intercropping on the coupling between soil microbial community structure, activity, and nutrient–use efficiencies. Peer J. 2019, 7, 6412. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira, S.; FernandeS–Silva, A.; Reis, S.; Torcato, C.; Raimundo, F.; Ferreira, L.; Car-nide, V.; Marques, G. Inoculation of plant growth promoting bacteria and arbuS–cularmycorrhizal fungi improve chickpea performance under water deficit conditions. Appl. Soil. Ecol. 2021, 164, 103927. [Google Scholar] [CrossRef]
- Williams, A.; Birt, H.W.G.; Raghavendra, A.; Dennis, P.G. Cropping System Diversification Influences Soil Microbial Diversity in Subtropical Dryland Farming Systems. Microb. Ecol. 2023, 85, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Netthisinghe, A.M.; Galloway, H.O.; Agga, G.E.; Gunter, P.A.; Sistani, K.R. Effects of Cropping Systems on Soil Physicochemical Properties and Abundances and Spatial Distributions of Nitrogen–Cycling Bacteria. Agronomy 2023, 13, 1461. [Google Scholar] [CrossRef]
- Latati, M.; Rebouh, N.Y.; Aouiche, A.; Laouar, M. Modelling the functional role of the micro-organisms in the daily exchanges of carbon and nitrogen in intercropping system under Mediterranean conditions. Agron. Res. 2019, 17, 559–573. [Google Scholar]
- Homulle, Z.; George, T.S.; Karley, A.J. Root traits with team benefits: Understanding belowground interactions in intercropping systems. Plant Soil 2021, 471, 1–26. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, J.B.; Singh, R.; Kantwa, S.R.; Jha, P.K.; Ahamad, S.; Singh, A.; Ghosh, A.; Prasad, M.; Singh, S.; et al. Understanding Soil Carbon and Phosphorus Dynamics under GrasS–Legume Intercropping in a Semi-Arid Region. Agronomy 2023, 13, 1692. [Google Scholar] [CrossRef]
- Wang, G.; Sheng, L.; Zhao, D.; Sheng, J.; Wang, X.; Liao, H. Allocation of Nitrogen and Carbon Is Regulated by Nodulation and Mycorrhizal Networks in Soybean/Maize Intercropping System. Front. Plant Sci. 2016, 7, 1901. [Google Scholar] [CrossRef] [PubMed]
- Willey, R. Intercropping: Its importance and research needs. 1. Competition and yield advantages. Field Crops Abstr. 1979, 32, 1–10. [Google Scholar]
- Lal, B.; Rana, K.S.; Rana, D.S.; Shivay, Y.S.; Sharma, D.K.; Meena, B.P.; Gautam, P. Biomass, yield, quality and moisture use of Brassica carinata as influenced by intercropping with chickpea under semiarid tropics. J. Saudi Soc. Agric. Sci. 2017, 18, 61–71. [Google Scholar] [CrossRef]
- Raza, M.A.; Yasin, H.S.; Gul, H.; Qin, R.; Mohi Ud Din, A.; Khalid, M.H.B.; Hussain, S.; Gitari, H.; Saeed, A.; Wang, J.; et al. Maize/soybean strip intercropping produces higher crop yields and saves water under semi-arid conditions. Front. Plant Sci. 2022, 13, 1006720. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, J.; van Noordwijk, M.; Anderson, J.; Ong, C.; Perfecto, I. Global change and multispecies agroecosystems: Concepts and issues. Agric. Ecosyst. Environ. 1998, 67, 1–22. [Google Scholar] [CrossRef]
- Hamdi, W.; Noura, Z.; Ernest, K.; Blavet, D.; Seffen, M. Effect of the soils properties on the sorption capacity of phosphorus and ammonium by alkaline soils of the semi-arid areas. IOSR-JA C 2015, 5, 34–42. [Google Scholar]
- Hamdi, W.; Hamdi, N.; Jellali, S.; Seffen, M. Effect of background electrolytes on the adsorption of phosphorus (P) onto southern Tunisia natural clays. Phys. Chem. Earth 2022, 127, 103160. [Google Scholar] [CrossRef]
- Leoni, F.; Lazzaro, M.; Carlesi, S.; Moonen, A.C. Screening suitable legumes for living mulches to support nitrogen dynamics and weed control in a durum wheat-forage sorghumm crop sequence. Field Crops Res. 2024, 307, 109246. [Google Scholar] [CrossRef]
- Huss, C.P.; Holmes, K.D.; Blubaugh, C.K. Benefits and Risks of Intercropping for Crop Resilience and Pest Management. J. Econ. Entomol. 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- Boudreau, M.A. Diseases in intercropping systems. Annu. Rev. Phytopathol. 2013, 51, 499–519. [Google Scholar] [CrossRef]
- Brenas, S.V.; Celette, F.; Pissaloux, A.P.; David, C. Biotic and abiotic factors impacting establishment and growth of relay intercropped forage legumes. Eur. J. Agron. 2016, 81, 169–177. [Google Scholar] [CrossRef]
- Kchaou, R.; Benyoussef, S.; Jebari, S.; Harbaoui, K.; Berndtsson, R. Forage Potential of Cereal–Legume Mixtures as an Adaptive Climate Change Strategy under Low Input Systems. Sustainability 2023, 15, 338. [Google Scholar] [CrossRef]
- Delhoumi, M.; Zaabar, W.; Ben Rhouma, A.; Achouri, M.S. Effects of agricultural practices and abiotic factors on woo-dlice diversity across two agroecosystems in Tunisia. Vie et milieu-life and enVironment 2018, 68, 253–261. [Google Scholar]
- Mtimet, A.; Lasram, M. Atlas Des Sols Tunisiens; Ministère de l’agriculture: Tunis, France, 1999.
- Kjeldahl, J. A new method for the determination of nitrogen in organic matter. Zeitschrift für Analytische Chemie 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with NaHCO3; USDA Cir.939; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Valizadeh, G.R.; Rengel, Z.; Rate, A.W. Response of wheat genotypes efficient in P utilization and genotypes responsive to P fertilization to different P banding depths and watering regimes. Aust. J. Agric. Res. 2003, 54, 59–65. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Leo, M.W.M. Determination of soil carbonates by a rapidegasometric method. J. Agric. Food Chem. 1963, 11, 452–455. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Boulelouah, N.; Berbache, M.R.; Bedjaoui, H.; Selama, N.; Rebouh, N.Y. Influence of Nitrogen Fertilizer Rate on Yield, Grain Quality and Nitrogen Use Efficiency of Durum Wheat (Triticum durum Desf.) under Algerian Semiarid Conditions. Agriculture 2022, 12, 1937. [Google Scholar] [CrossRef]
- Dahmardeh, M.; Ghanbari, A.; Syahsar, B.A.; Ramrodi, M. The role of intercropping maize (Zea mays L.) and cowpea (Vigna unguiculata L.) on yield and soil chemical properties. Afr. J. Agric. Res. 2010, 5, 631–636. [Google Scholar]
- Tang, X.; Yu, Y.; Shen, J.; Van der Werf, W.; Zhang, F. Intercropping legumes and cereals increases phosphorus use efficiency: A meta-analysis. Plant Soil 2021, 460, 89–104. [Google Scholar] [CrossRef]
- Huňady, I.; Hochman, M. Potential of legume-cereal intercropping for increasing yields and yield stability for self-sufficiency with animal fodder in organic farming. Czech J. Gen. Plant Breed. 2014, 50, 185–194. [Google Scholar] [CrossRef]
- Chhetri, B.; Sinha, A.C. Advantage of maize (Zea mays)-based intercropping system to different nutrient management practices. Indian J. Agron. 2020, 65, 25–32. [Google Scholar] [CrossRef]
- Lazali, M.; Brahimi, S.; Drevon, J.J. High yields in a low–P tolerant recombinant inbred line of common bean under field conditions. Rhizosphere 2018, 8, 27–33. [Google Scholar] [CrossRef]
- Takim, F.O. Advantages of maize-cowpea intercropping over sole cropping through competition indices. J. Agric. Biodivers. Res. 2012, 1, 53–59. [Google Scholar]
- Latati, M.; Pansu, M.; Drevon, J.J.; Ounane, S.M. Advantage of intercropping maize (Zea mays L.) and common bean (Phaseolus vulgaris L.) on yield and nitrogen uptake in Northeast Algeria. IJRAS 2013, 1, 1–7. [Google Scholar]
- Wehmeye, H.; De Guia, A.H.; Connor, M. Reduction of Fertilizer Use in South China Impacts and Implications on Smallholder Rice Farmers. Sustainability 2020, 12, 2240. [Google Scholar] [CrossRef]
- Yong, T.W.; Ping, C.; Qian, D.; Qing, D.U.; Feng, Y.; Wang, X.; Liu, W.; Yang, W.Y. Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize-soybean relay intercropping system. J. Integr. Agric. 2018, 17, 664–676. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Gooding, M.; Ambus, P.; Corre-Hellou, G.; Crozat, Y.; Dahlmann, C.; Dibet, A.; von Fragstein, P.; Pristeri, A.; Monti, M.; et al. Pea-barley intercropping for efficient symbiotic N2-fixation, soil N acquisition and use of other nutrients in European organic cropping systems. Field Crops Res. 2009, 113, 64–71. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Fustec, J.; Crozat, Y. Interspecific competition for soil N and its interaction with N2 fixation, leaf expansion and crop growth in pea-Barley intercrops. Plant Soil. 2006, 282, 195–208. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bernard, L.; Brauman, A.; Daufresne, T.; Deleporte, P.; Desclaux, D.; Souche, G.; Placella, S.A.; Hinsinger, P. Increase in microbial biomass and phosphorus availability in the rhizosphere of intercropped cereal and legumes under field conditions. Soil Biol. Biochem. 2014, 75, 86–93. [Google Scholar] [CrossRef]
- Chenene, Y.; Blavet, D.; Belalmi, M.; Kaci, G.; Teffahi, M.; Ounane, S.M. Variation of chickpea nodulation in a Mediterranean agroecosystem: Relationship with soil characteristics and thresholds for significant contribution to plant growth. Agron. Res. 2021, 19, 42–56. [Google Scholar]
- Szumigalski, A.R.; Van Acker, R.C. Nitrogen yield and land use efficiency in annual sole crops and intercrops. Agron. J. 2006, 98, 1030–1040. [Google Scholar] [CrossRef]
- Arshad, M. Fortnightly dynamics and relationship of growth, dry matter partition and productivity of maize based sole and intercropping systems at different elevations. Eur. J. Agron. 2021, 130, 126377. [Google Scholar] [CrossRef]
- Chapagain, T.; Riseman, A. Barley–pea intercropping: Effects on land productivity, carbon and nitrogen transformations. Field Crops Res. 2014, 166, 18–25. [Google Scholar] [CrossRef]
- Scalise, A.; Tortorella, D.; Aurelio, P.; Petrov, B.; Gelsomino, A.; Lindstrom, K.; Monti, M. Legume-barley intercropping stimulates soil N supply and crop yield in the succeeding durum wheat in a rotation under rained conditions. Soil Biol. Biochem. 2015, 89, 150–161. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attallah, A.; Hamdi, W.; Souid, A.; Farissi, M.; L’taief, B.; Messiga, A.J.; Rebouh, N.Y.; Jellali, S.; Zagrarni, M.F. Impact of Cereal–Legume Intercropping on Changes in Soil Nutrients Contents under Semi–Arid Conditions. Sustainability 2024, 16, 2725. https://doi.org/10.3390/su16072725
Attallah A, Hamdi W, Souid A, Farissi M, L’taief B, Messiga AJ, Rebouh NY, Jellali S, Zagrarni MF. Impact of Cereal–Legume Intercropping on Changes in Soil Nutrients Contents under Semi–Arid Conditions. Sustainability. 2024; 16(7):2725. https://doi.org/10.3390/su16072725
Chicago/Turabian StyleAttallah, Amal, Wissem Hamdi, Amira Souid, Mohamed Farissi, Boulbaba L’taief, Aimé J. Messiga, Nazih Yacer Rebouh, Salah Jellali, and Mohamed Faouzi Zagrarni. 2024. "Impact of Cereal–Legume Intercropping on Changes in Soil Nutrients Contents under Semi–Arid Conditions" Sustainability 16, no. 7: 2725. https://doi.org/10.3390/su16072725
APA StyleAttallah, A., Hamdi, W., Souid, A., Farissi, M., L’taief, B., Messiga, A. J., Rebouh, N. Y., Jellali, S., & Zagrarni, M. F. (2024). Impact of Cereal–Legume Intercropping on Changes in Soil Nutrients Contents under Semi–Arid Conditions. Sustainability, 16(7), 2725. https://doi.org/10.3390/su16072725








