Grapevine and Horseradish Leaves as Natural, Sustainable Additives for Improvement of the Microbial, Sensory, and Antioxidant Properties of Traditionally Fermented Low-Salt Cucumbers
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Plant Raw Material and Fermentation Process
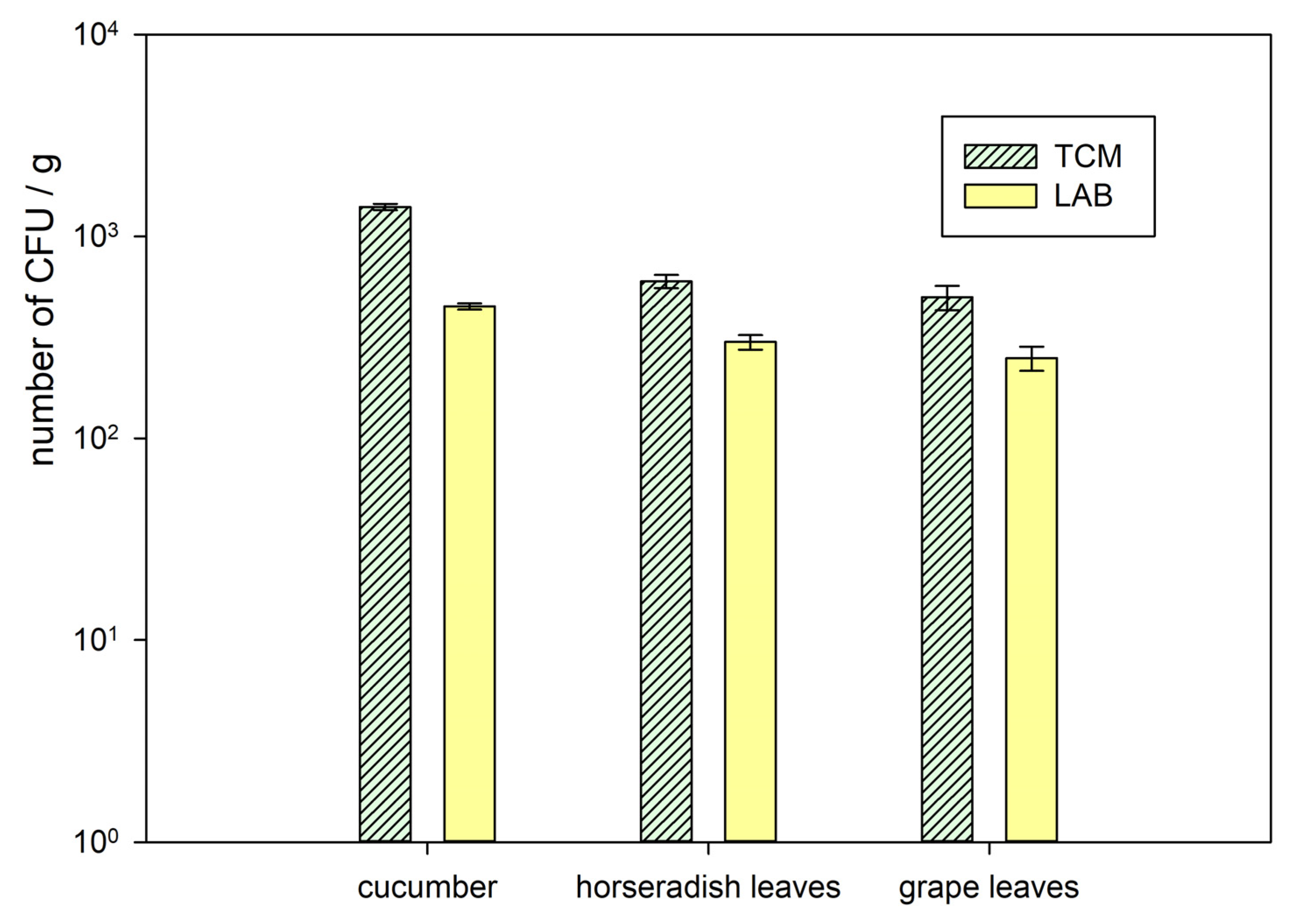
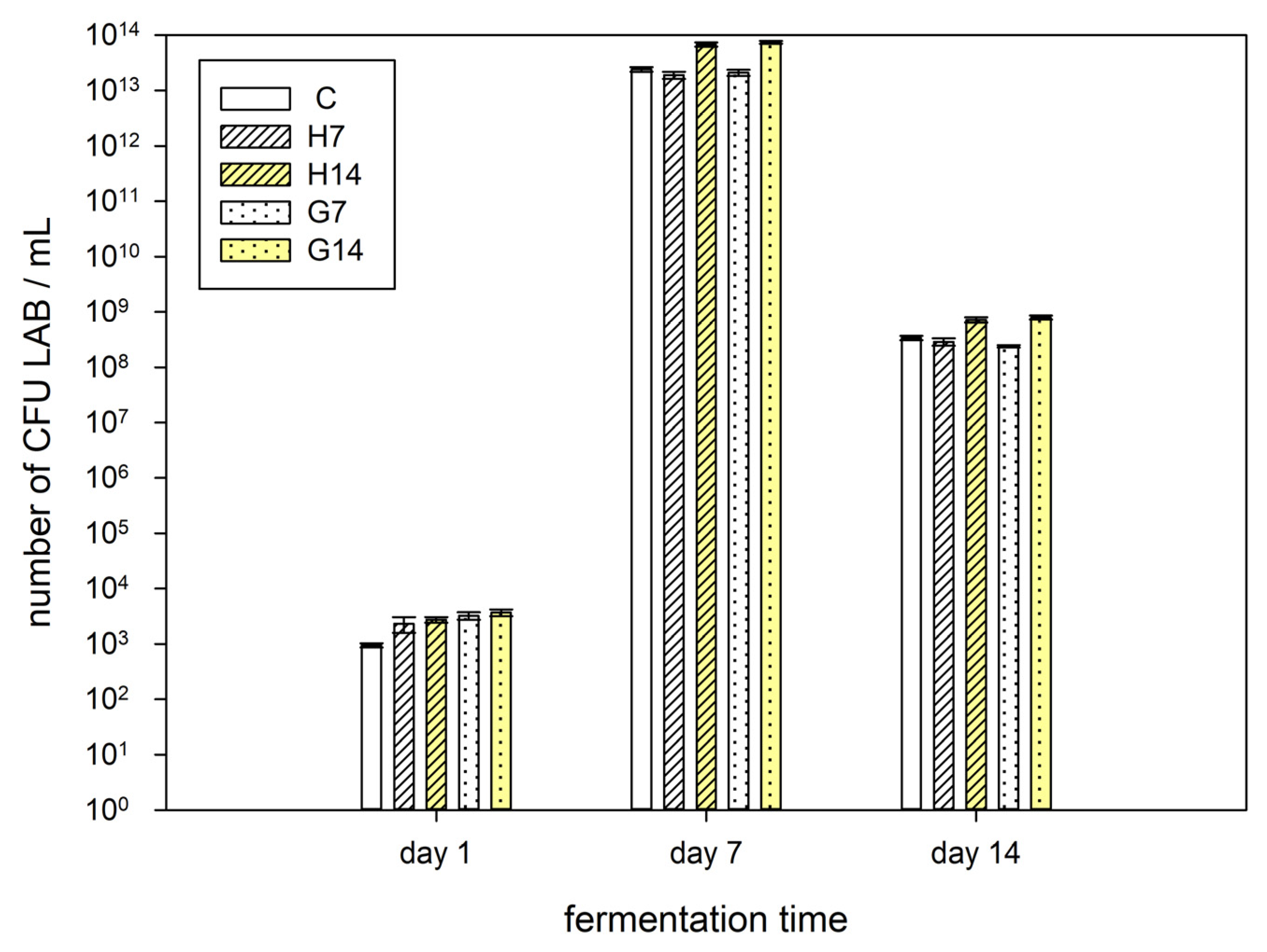
2.2. Microbiological Analysis
2.3. Bacteriocin Gene Expression Analysis
2.3.1. RNA Isolation
2.3.2. RT-qPCR Analysis
2.4. Gallic Acid and Elagic Acid Chromatography Analysis
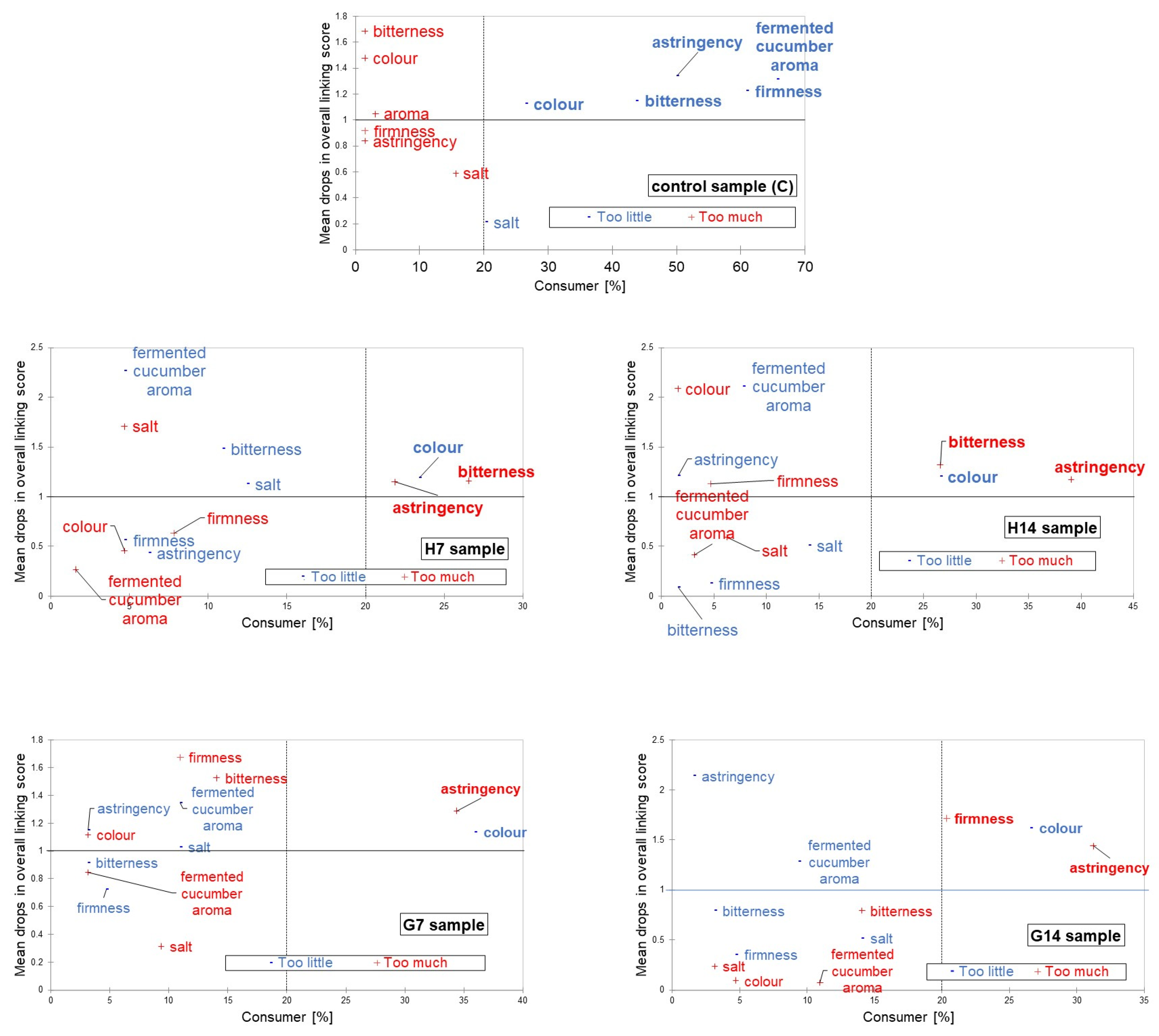
2.5. Consumer Test
2.6. Statistical Analysis
3. Results
3.1. Microbiology and pH Analysis
3.2. Bacteriocin Gene Expression Analysis
3.3. Gallic Acid and Ellagic Acid Analysis
3.4. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zabala, A. Ugly vegetables wasted. Nat. Sustain. 2018, 1, 457. [Google Scholar] [CrossRef]
- Heppner, S.; Livney, Y.D. Green leaves as a promising source for sustainable food protein: Seeking the productivity-functionality balance. Trends Food Sci. Technol. 2023, 142, 104207. [Google Scholar] [CrossRef]
- Danciu, C.-A.; Tulbure, A.; Stanciu, M.-A.; Antonie, I.; Capatana, C.; Zerbeș, M.V.; Giurea, R.; Rada, E.C. Overview of the Sustainable Valorization of Using Waste and By-Products in Grain Processing. Foods 2023, 12, 3770. [Google Scholar] [CrossRef]
- Hezarkhani, B.; Demirel, G.; Bouchery, Y.; Dora, M. Can “ugly veg” supply chains reduce food loss? Eur. J. Oper. Res. 2023, 309, 117–132. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Ora, A.; Häkkinen, S.T.; Ritala, A.; Räisänen, R.; Kallioinen-Mänttäri, M.; Melin, K. Innovative extraction technologies of bioactive compounds from plant by-products for textile colorants and antimicrobial agents. Biomass Conv. Bioref. 2023, 1. [Google Scholar] [CrossRef]
- Porter, S.D.; Reay, D.S.; Bomberg, E.; Higgins, P. Avoidable food losses and associated production-phase greenhouse gas emissions arising from application of cosmetic standards to fresh fruit and vegetables in Europe and the UK. J. Clean. Prod. 2018, 201, 869–878. [Google Scholar] [CrossRef]
- Mookerjee, S.; Cornil, Y.; Hoegg, J. From Waste to Taste: How “Ugly” Labels Can Increase Purchase of Unattractive Produce. J. Mark. 2021, 85, 62–77. [Google Scholar] [CrossRef]
- Walters, S.A. Horseradish: A Neglected and Underutilized Plant Species for Improving Human Health. Horticulturae 2021, 7, 167. [Google Scholar] [CrossRef]
- Priss, O.; Korchynskyy, I.; Kryvko, Y.; Korchynska, O. Leveraging horseradish’s bioactive substances for sustainable agricultural development. Int. J. Sustain. Dev. 2023, 18, 2563–2570. [Google Scholar] [CrossRef]
- Mihalcea, L.; Dinica, R.-M.; Vizireanu, C. Study on herbal actions of horseradish (Armoracia rusticana). J. Agroaliment. Proc. Technol. 2013, 19, 111–115. [Google Scholar]
- Păvăloiu, R.-D.; Sha’at, F.; Bubueanu, C.; Neagu, G.; Hlevca, C.; Sha’at, M.; Nechifor, G. Antioxidant Properties and Cytoprotective Effect Against H2O2-Induced Cytotoxicity in Mouse Fibroblasts Cells (L-929) of Horseradish Leaves. Proceedings 2019, 29, 30. [Google Scholar] [CrossRef]
- Drori, E.; Rahimi, O.; Marrano, A.; Henig, Y.; Brauner, H.; Salmon-Divon, M.; Netzer, Y.; Prazzoli, M.L.; Stanevsky, M.; Failla, O.; et al. Collection and characterization of grapevine genetic resources (Vitis vinifera) in the Holy Land, towards the renewal of ancient winemaking practices. Sci. Rep. 2017, 7, 44463. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.I.; Hong, G.; Albornoz, K.; Berlanga, M. Fresh grapevine (Vitis vinifera L.) leaves: Postharvest biology and handling recommendations. Sci. Hortic. 2022, 292, 110627. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. Vitr. 2010, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.R.W.; Carvalho, L.F.D.; Bertoli, S.L.; de Souza, C.K. Effect of refrigerated storage conditions on leafy vegetables. MOJ Food Process. Technol. 2019, 7, 75–77. [Google Scholar] [CrossRef]
- Pieńkos, M. Domowe Kiszonki z Owoców Warzyw i Ziół, 1st ed.; SBM Renata Gmitrzak: Warsaw, Poland, 2023. [Google Scholar]
- Yang, J.; Lee, J. Application of Sensory Descriptive Analysis and Consumer Studies to Investigate Traditional and Authentic Foods: A Review. Foods 2019, 8, 54. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Elhalis, H.; See, X.Y.; Osen, R.; Chin, X.H.; Chow, Y. Significance of Fermentation in Plant-Based Meat Analogs: A Critical Review of Nutrition, and Safety-Related Aspects. Foods 2023, 12, 3222. [Google Scholar] [CrossRef]
- Singh, J.; Rasane, P.; Kaur, R.; Kaur, H.; Garg, R.; Kaur, S.; Ercisli, S.; Choudhary, R.; Skrovankova, S.; Mlcek, J. Valorization of grape (Vitis vinifera) leaves for bioactive compounds: Novel green extraction technologies and food-pharma applications. Front. Chem. 2023, 11, 1290619. [Google Scholar] [CrossRef]
- Staninska-Pięta, J.; Czarny, J.; Wolko, Ł.; Cyplik, P.; Drożdżyńska, A.; Przybylak, M.; Ratajczak, K.; Piotrowska-Cyplik, A. Temperature, Salinity and Garlic Additive Shape the Microbial Community during Traditional Beetroot Fermentation Process. Foods 2023, 12, 3079. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Papadelli, M.; Pardali, E.; Mataragas, M.; Drosinos, E.H. Evaluation of plantaricin genes expression during fermentation of Raphanus sativus roots with a plantaricin-producing Lactobacillus plantarum starter. Curr. Microbiol. 2019, 76, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozes, N. Expression of Lactobacillus pentosus B96 bacteriocin genes under saline stress. Food Microbiol. 2011, 28, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Majhenič, A.; Venema, K.; Allison, G.; Matijašić, B.; Rogelj, I.; Klaenhammer, T. DNA analysis of the genes encoding acidocin LF221 A and acidocin LF221 B, two bacteriocins produced by Lactobacillus gasseri LF221. Appl. Microbiol. Biotechnol. 2004, 63, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Kamilari, E.; Tsaltas, D. Evolution of Bacterial Communities, Physicochemical Changes and Sensorial Attributes of Natural Whole and Cracked Picual Table Olives During Spontaneous and Inoculated Fermentation. Front. Microbiol. 2020, 11, 1128. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Chen, X.; Cui, B.; Bai, Z.; Zhuang, G. Variety features differentiate microbiota in the grape leaves. Can. J. Microbiol. 2020, 66, 653–663. [Google Scholar] [CrossRef]
- Mazzeo, M.F.; Lippolis, R.; Sorrentino, A.; Liberti, S.; Fragnito, F.; Siciliano, R.A. Lactobacillus acidophilus—Rutin Interplay Investigated by Proteomics. PLoS ONE 2015, 10, e0142376. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Fu, J.; Wang, L.; Sun, J.; Ju, N.; Jin, G. Malolactic Fermentation: New Approaches to Old Problems. Microorganisms 2022, 10, 2363. [Google Scholar] [CrossRef]
- Dos Santos, A.S.; de Albuquerque, T.M.R.; de Brito Alves, J.L.; de Souza, E.L. Effects of Quercetin and Resveratrol on in vitro Properties Related to the Functionality of Potentially Probiotic Lactobacillus Strains. Front. Microbiol. 2019, 10, 2229. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Al Azzaz, J.; Al Tarraf, A.; Heumann, A.; Da Silva Barreira, D.; Laurent, J.; Assifaoui, A.; Rieu, A.; Guzzo, J.; Lapaquette, P. Resveratrol Favors Adhesion and Biofilm Formation of Lacticaseibacillus paracasei subsp. paracasei Strain ATCC334. Int. J. Mol. Sci. 2020, 21, 5423. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arietta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Casciano, F.; Mayr, H.; Nissen, L.; Putti, A.; Zoli, F.; Gianotti, A.; Conterno, L. Red Beetroot Fermentation with Different Microbial Consortia to Develop Foods with Improved Aromatic Features. Foods 2022, 11, 3055. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Inês, A.; Vilela, A. Consumer’s acceptability and health consciousness of probiotic and prebiotic of non-dairy products. Food Res. Int. 2022, 151, 110842. [Google Scholar] [CrossRef]
- Syaputri, Y.; Lei, J.; Ratningsing, N.; Rossiana, N.; Safitri, R.; Wulandari, A.P. Heterologous Expression and Partial Purification of Plantaricin Produced by Lactiplantibacillus plantarum COY2906. Appl. Food Biotechnol. 2023, 10, 271–278. [Google Scholar] [CrossRef]
- Sand, S.L.; Nissen-Meyer, J.; Sand, O.; Haug, T.M. Plantaricin A, a cationic peptide produced by Lactobacillus plantarum, permeabilizes eukaryotic cell membranes by a mechanism dependent on negative surface charge linked to glycosylated membrane proteins. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 249–259. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, L.; Wei, Y.; Shang, N.; Zhang, X.; Li, P. Two-peptide bacteriocin PlnEF causes cell membrane damage to Lactobacillus plantarum. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 274–280. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; Li, P.; Gu, Q. Heterologous expression of class IIb bacteriocin plantaricin JK in Lactococcus lactis. Protein Expr. Purif. 2019, 159, 10–16. [Google Scholar] [CrossRef]
- Maldonado, A.; Jiménez-Díaz, R.; Ruiz-Barba, J.L. Induction of Plantaricin Production in Lactobacillus plantarum NC8 after Coculture with Specific Gram-Positive Bacteria Is Mediated by an Autoinduction Mechanism. J. Bacteriol. 2004, 186, 5. [Google Scholar] [CrossRef]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic. Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Bartoshuk, L.M.; Fillingim, R.B.; Dotson, C.D. Exploring Ethnic Differences in Taste Perception. Chem. Senses 2016, 41, 449–456. [Google Scholar] [CrossRef] [PubMed]

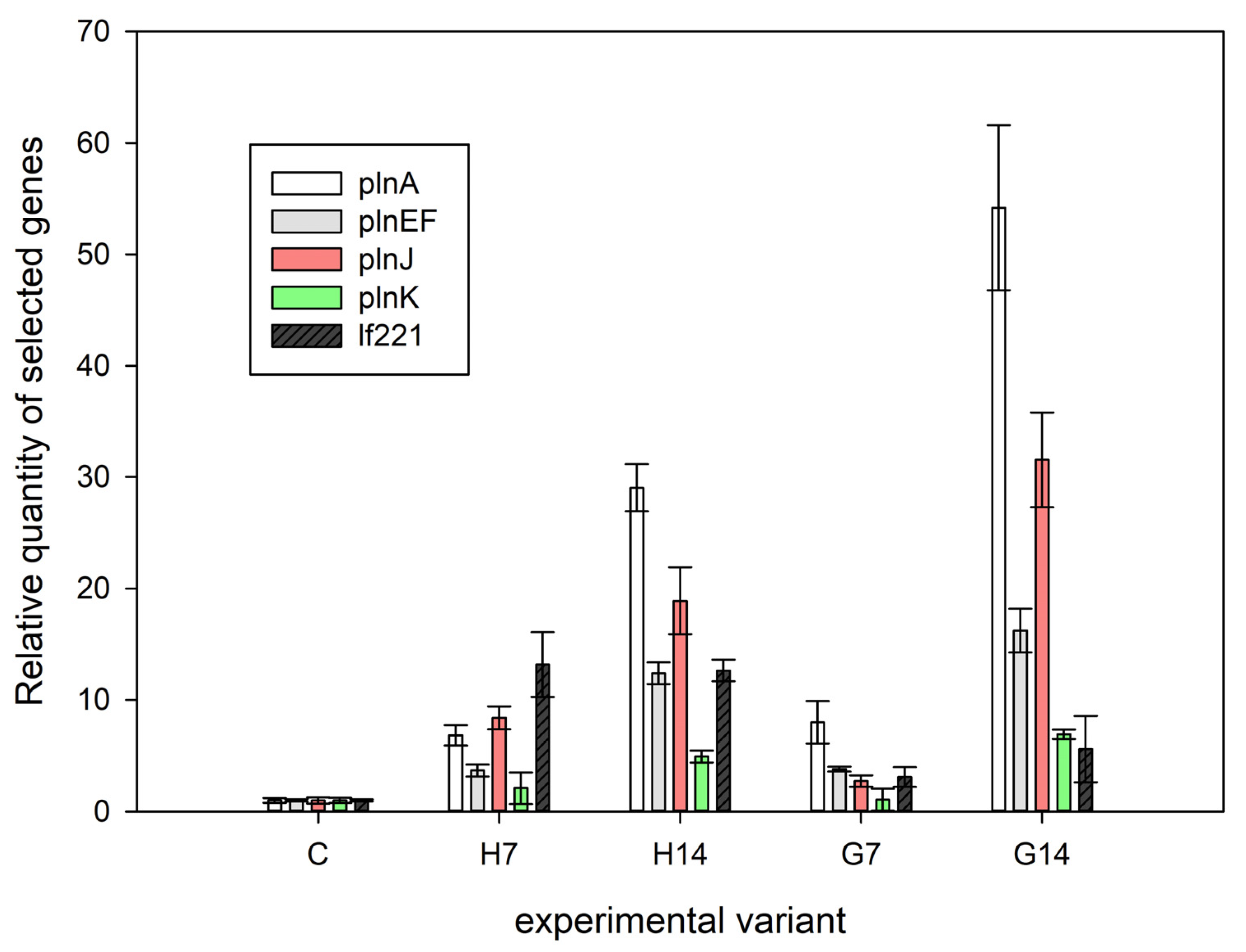

| Gene Name | Primer Name | Abbreviation | Sequence (5′-3′) | Reference |
|---|---|---|---|---|
| Plantaricin A | PlnAF | plnA | AAAATTCAAATTAAAGGTATGAAGCAA | [22] |
| PlnAR | CCCCATCTGCAAAGAATACG | |||
| Plantaricin EF | PlnEFF | plnEF | GTTTTAATCGGGGCGGTTAT | [22] |
| PlnEFR | ATACCACGAATGCCTGCAAC | |||
| Plantaricin J | PlnJF | plnJ | TAAGTTGAACGGGGTTGTTG | [23] |
| PlnJR | TAACGACGGATTGCTCTGC | |||
| Plantaricin K | PlnKF | plnK | TTCTGGTAACCGTCGGAGTC | [23] |
| PlnKR | ATCCCTTGAACCACCAAGC | |||
| 16srRNA | 16srRNAF | 16srRNA | GATGCATAGCCGACCTGAGA | [22] |
| 16srRNAR | CTCCGTCAGACTTTCGTCCA | |||
| Acidocin LF221 | LFAF | lf221 | GTTGCAGGATCATGTG | [24] |
| LFAR | TGTTGCAGCTCCGTTA |
| Control Sample | H7 Sample | H14 Sample | G7 Sample | G14 Sample | |
|---|---|---|---|---|---|
| Overall linking score | 5.16 a | 8.16 b | 7.73 b | 6.17 c | 6.66 c |
| Standard deviation (SD) | 1.08 | 1.05 | 0.99 | 1.23 | 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staninska-Pięta, J.; Cyplik, P.; Drożdżyńska, A.; Piotrowska-Cyplik, A. Grapevine and Horseradish Leaves as Natural, Sustainable Additives for Improvement of the Microbial, Sensory, and Antioxidant Properties of Traditionally Fermented Low-Salt Cucumbers. Sustainability 2024, 16, 2431. https://doi.org/10.3390/su16062431
Staninska-Pięta J, Cyplik P, Drożdżyńska A, Piotrowska-Cyplik A. Grapevine and Horseradish Leaves as Natural, Sustainable Additives for Improvement of the Microbial, Sensory, and Antioxidant Properties of Traditionally Fermented Low-Salt Cucumbers. Sustainability. 2024; 16(6):2431. https://doi.org/10.3390/su16062431
Chicago/Turabian StyleStaninska-Pięta, Justyna, Paweł Cyplik, Agnieszka Drożdżyńska, and Agnieszka Piotrowska-Cyplik. 2024. "Grapevine and Horseradish Leaves as Natural, Sustainable Additives for Improvement of the Microbial, Sensory, and Antioxidant Properties of Traditionally Fermented Low-Salt Cucumbers" Sustainability 16, no. 6: 2431. https://doi.org/10.3390/su16062431
APA StyleStaninska-Pięta, J., Cyplik, P., Drożdżyńska, A., & Piotrowska-Cyplik, A. (2024). Grapevine and Horseradish Leaves as Natural, Sustainable Additives for Improvement of the Microbial, Sensory, and Antioxidant Properties of Traditionally Fermented Low-Salt Cucumbers. Sustainability, 16(6), 2431. https://doi.org/10.3390/su16062431






