Copper-Plated Nanoporous Anodized Aluminum Oxide for Solar Desalination: An Experimental Study
Abstract
1. Introduction
2. Synthesis of Copper Electrodeposited Nanoporous Structure
2.1. Material
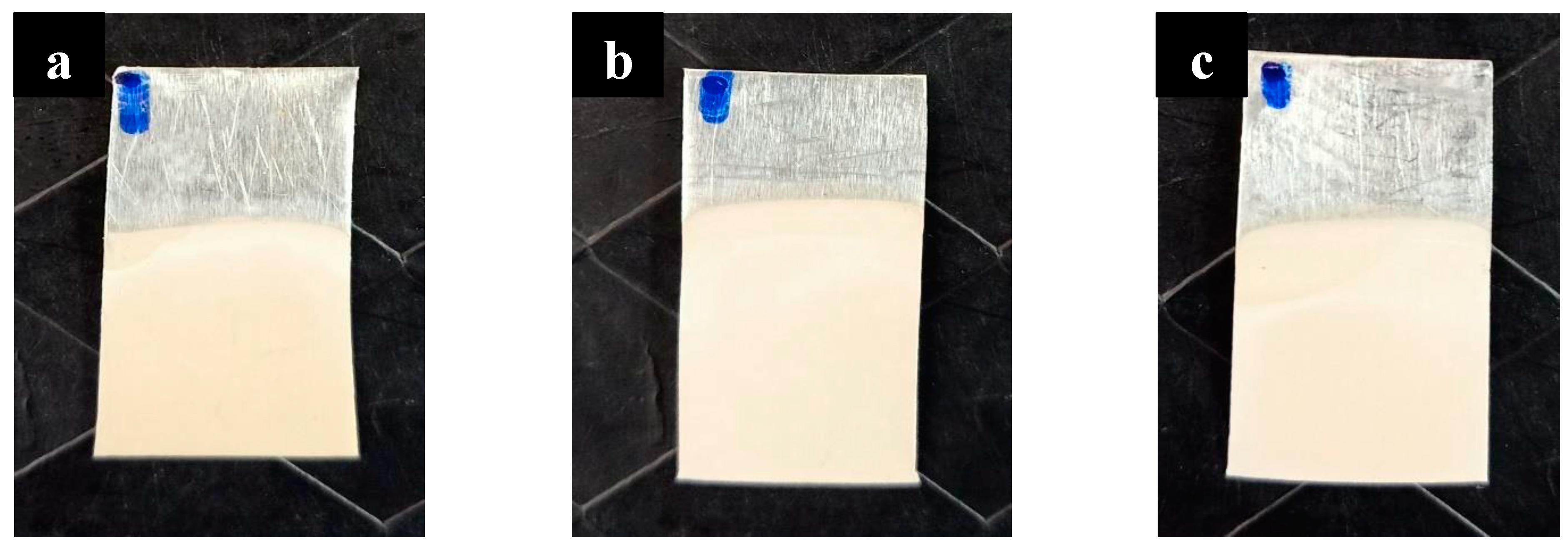
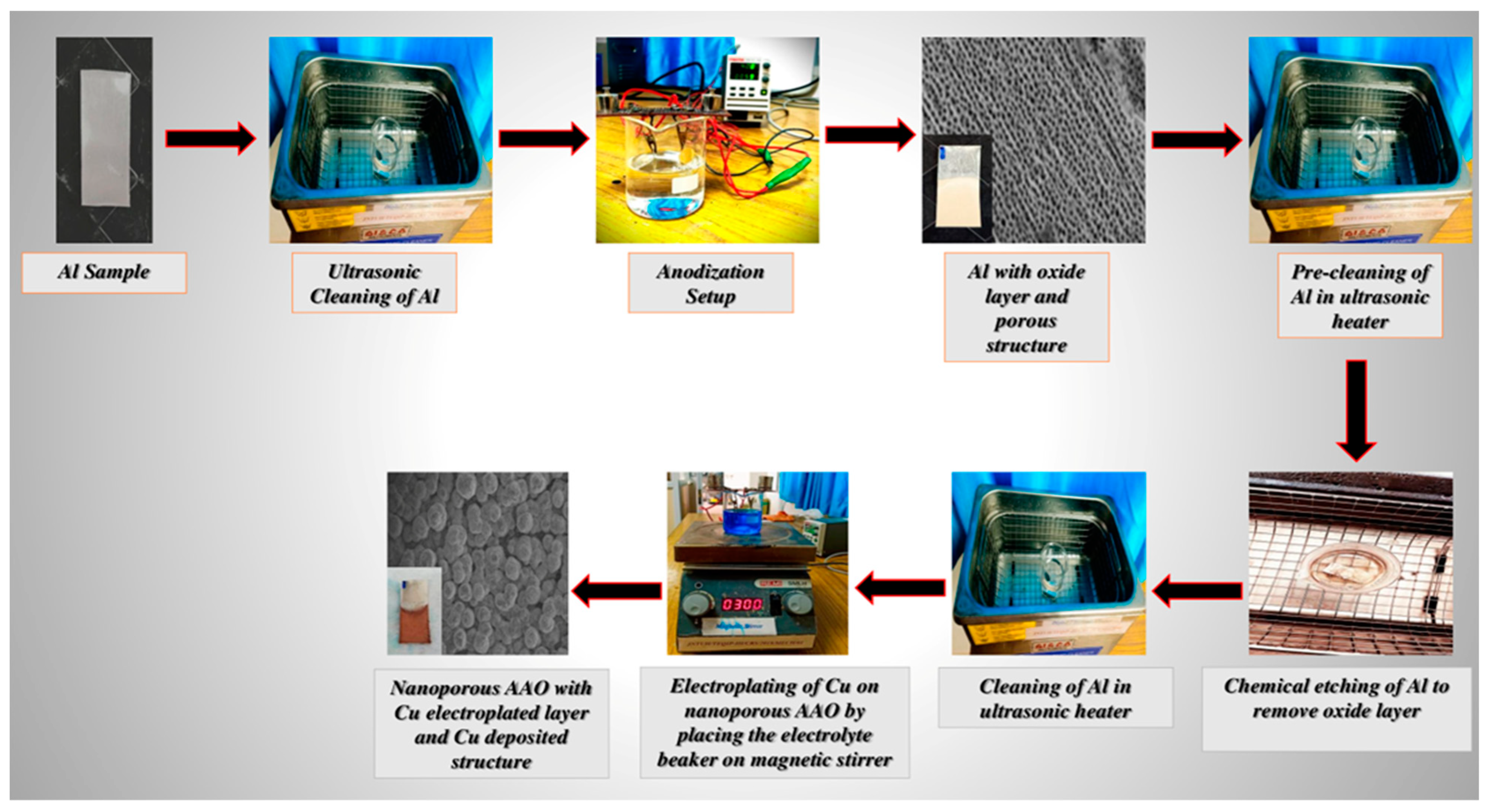
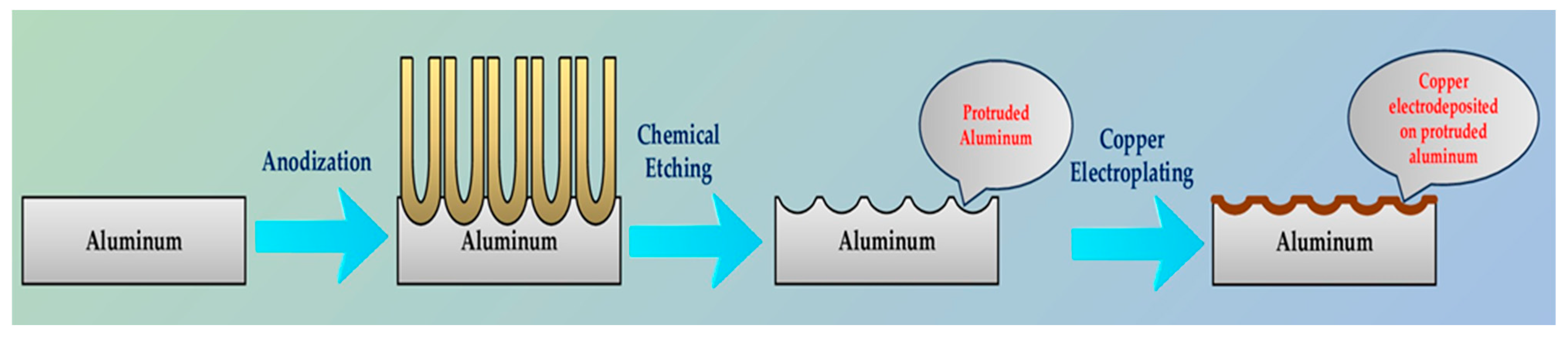
2.2. Development of Nanoporous Structure
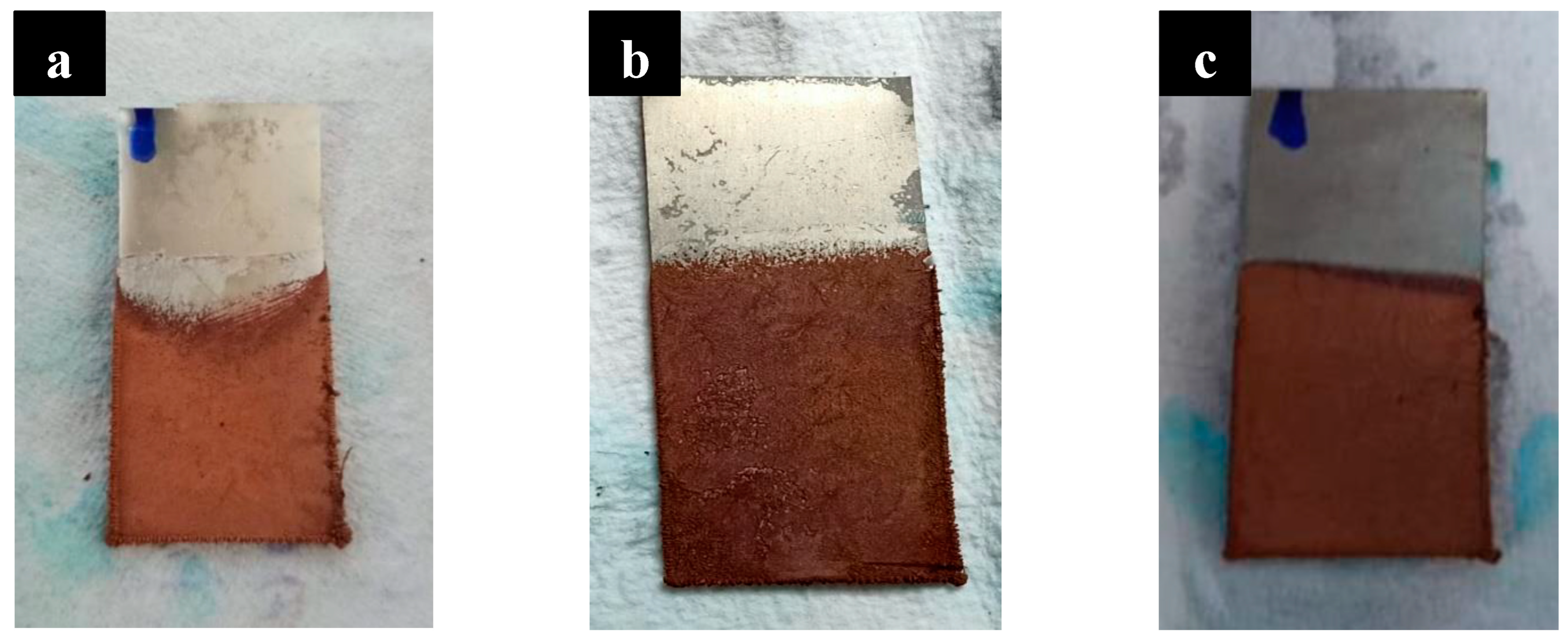
2.3. Electroplating of Nanoporous Structure
2.4. Characterization
3. Experimentation
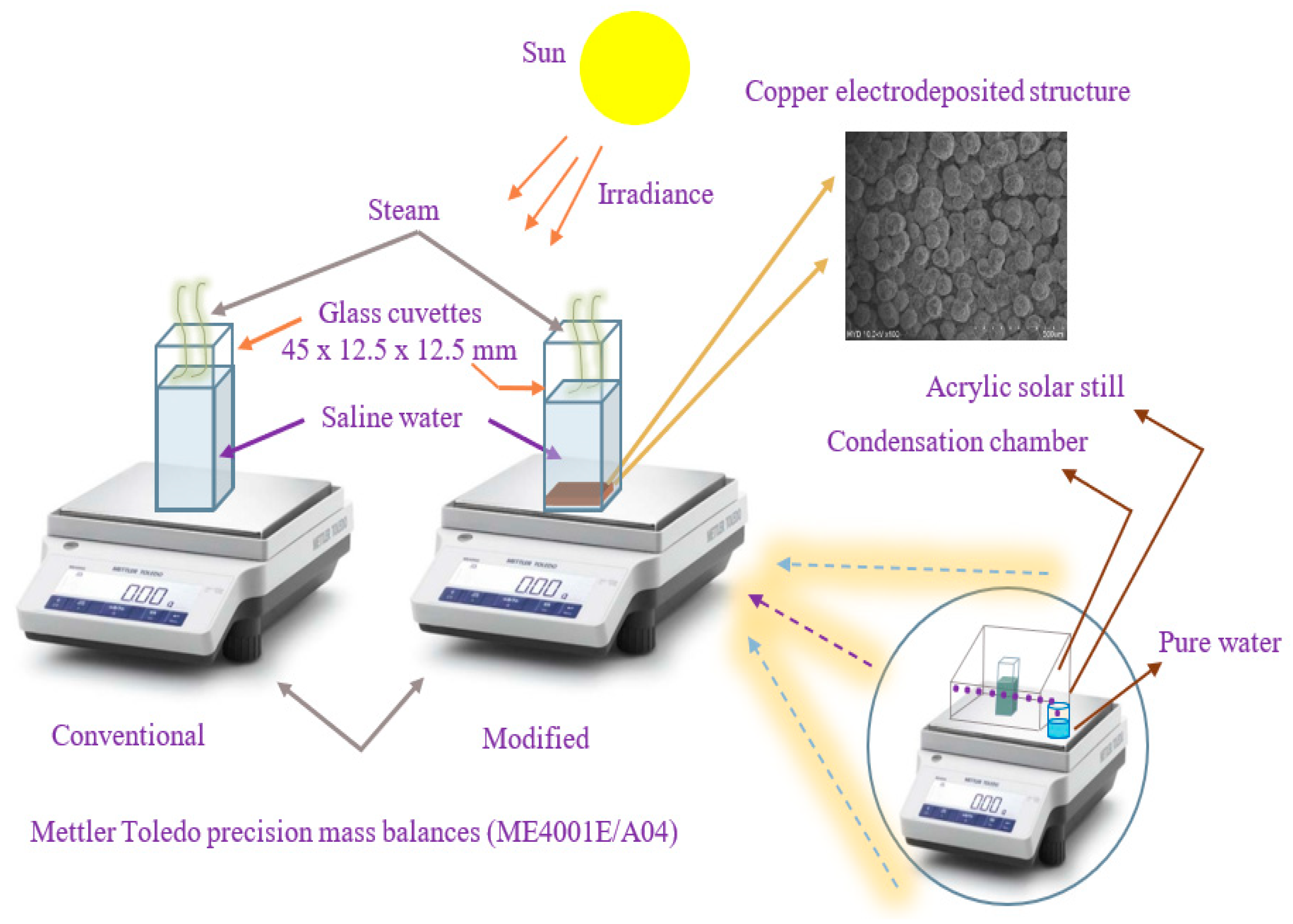
3.1. Experimental Apparatus
3.2. Experimental Procedure
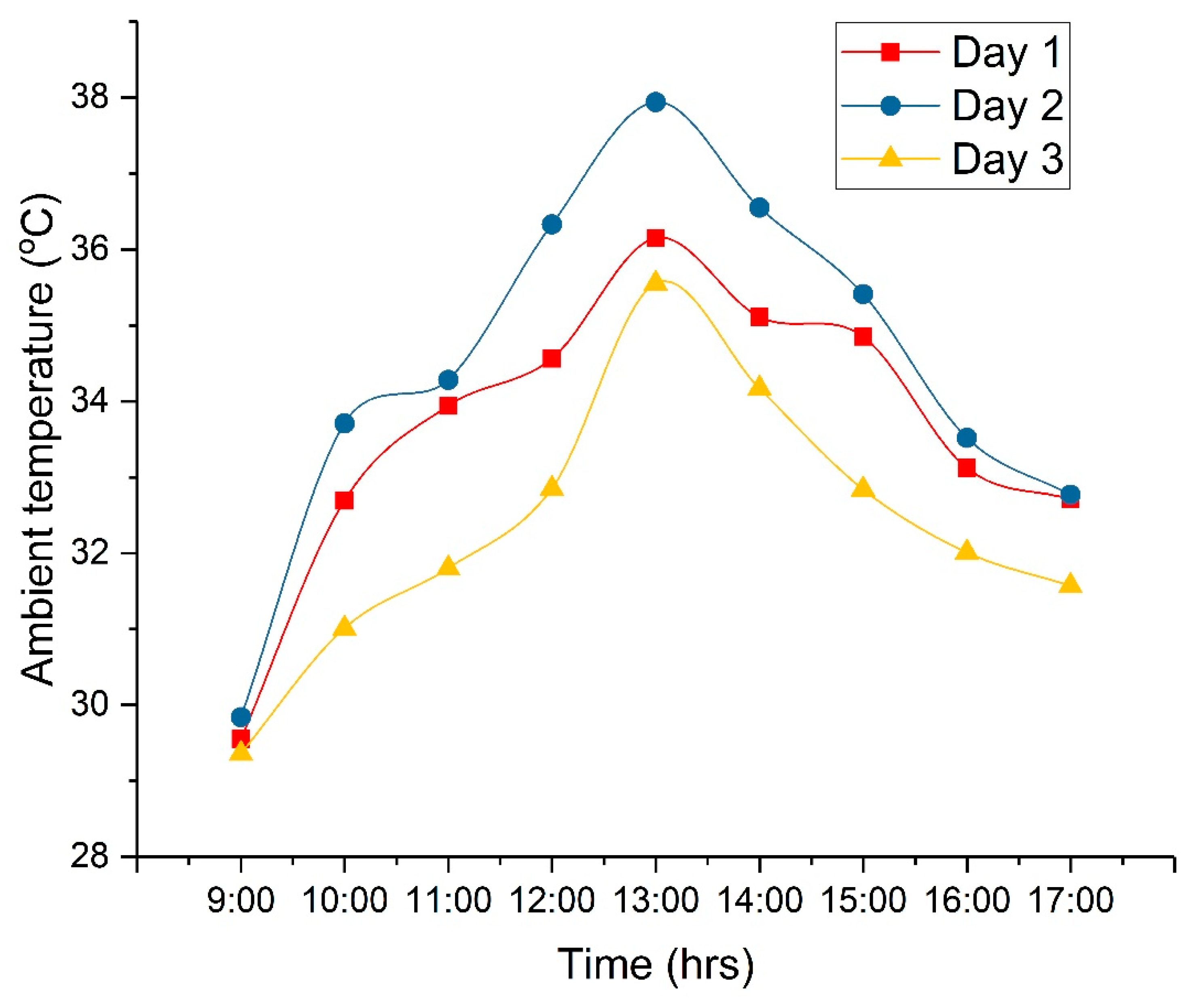
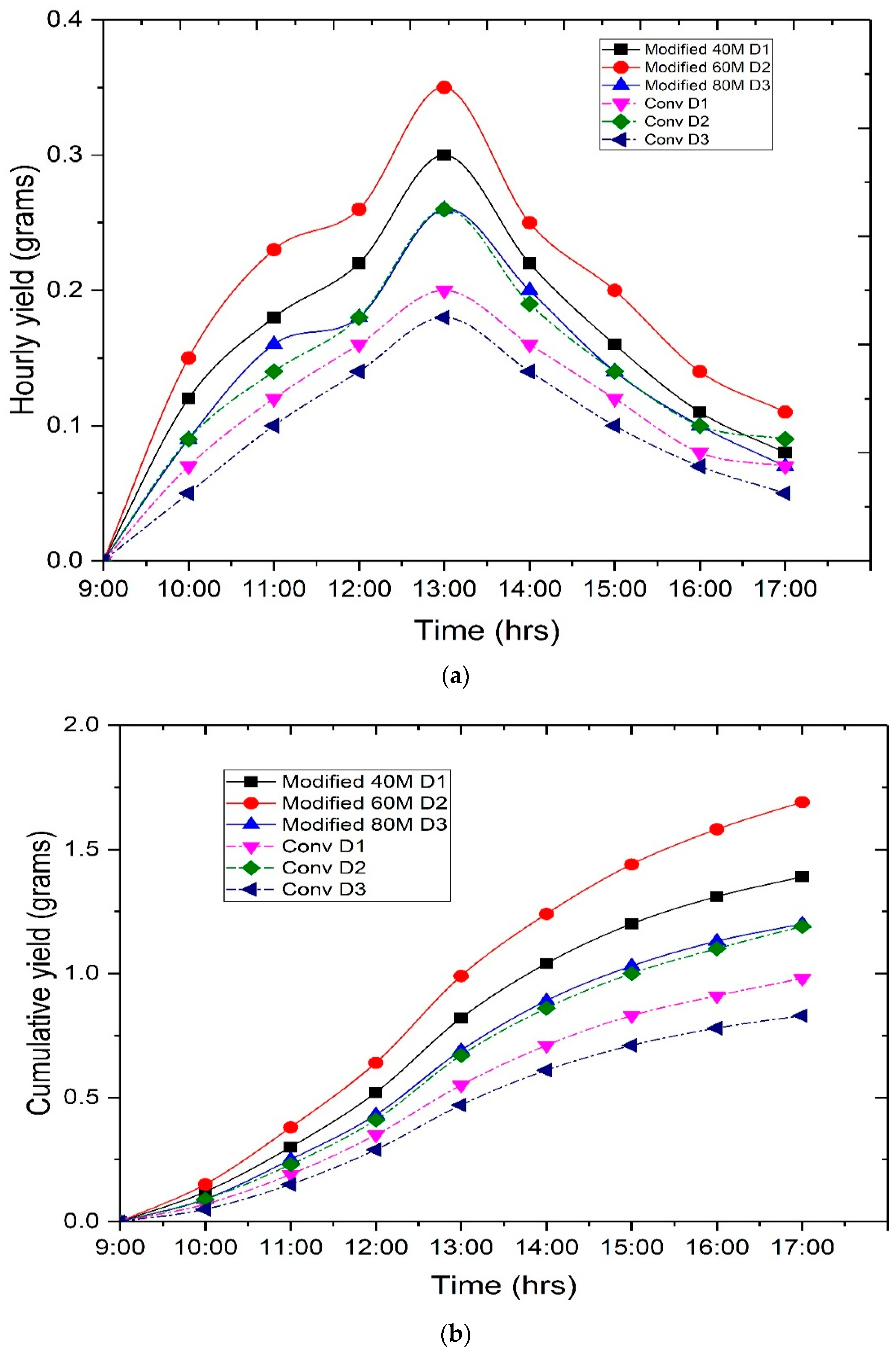
4. Results and Discussion
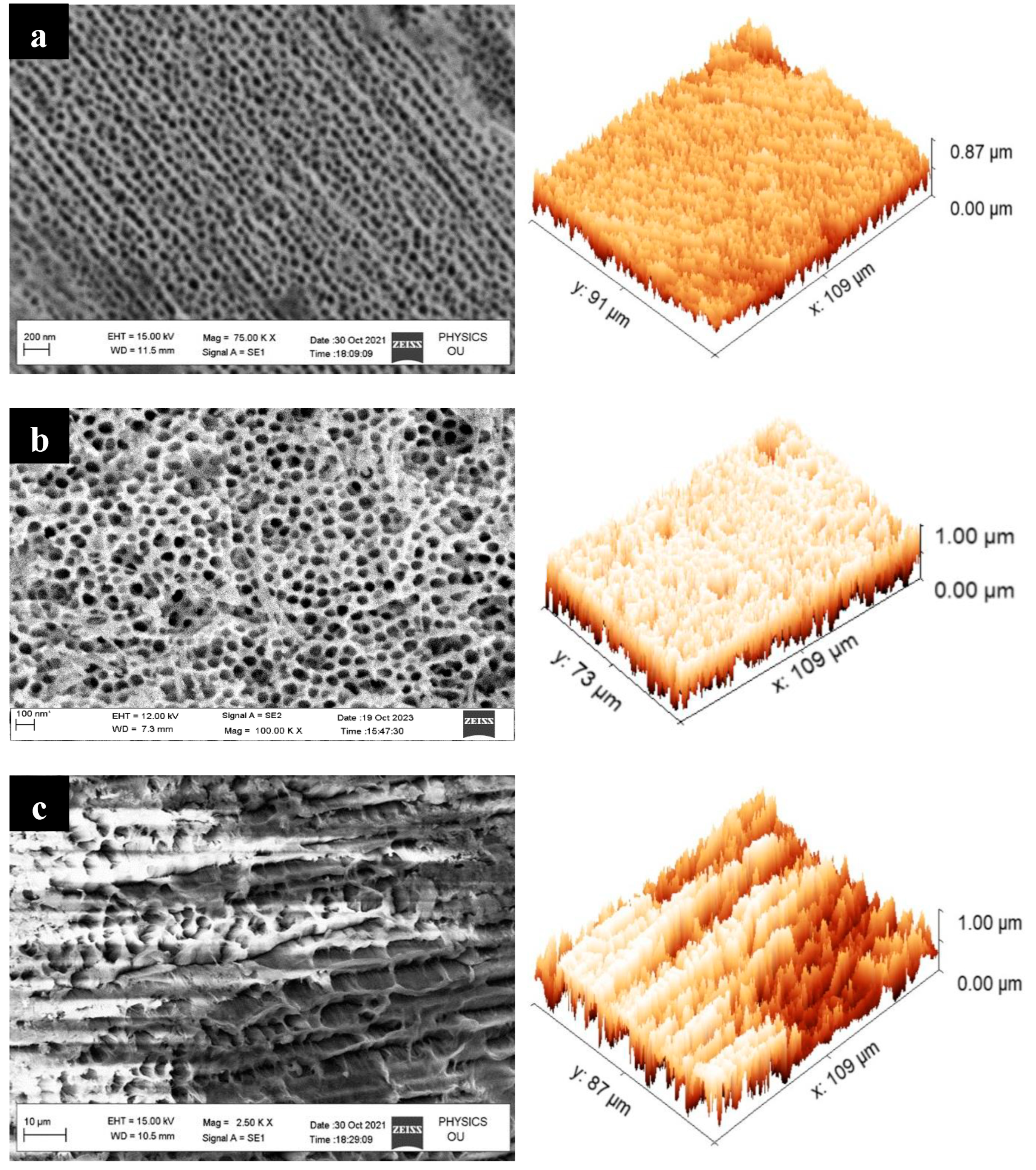
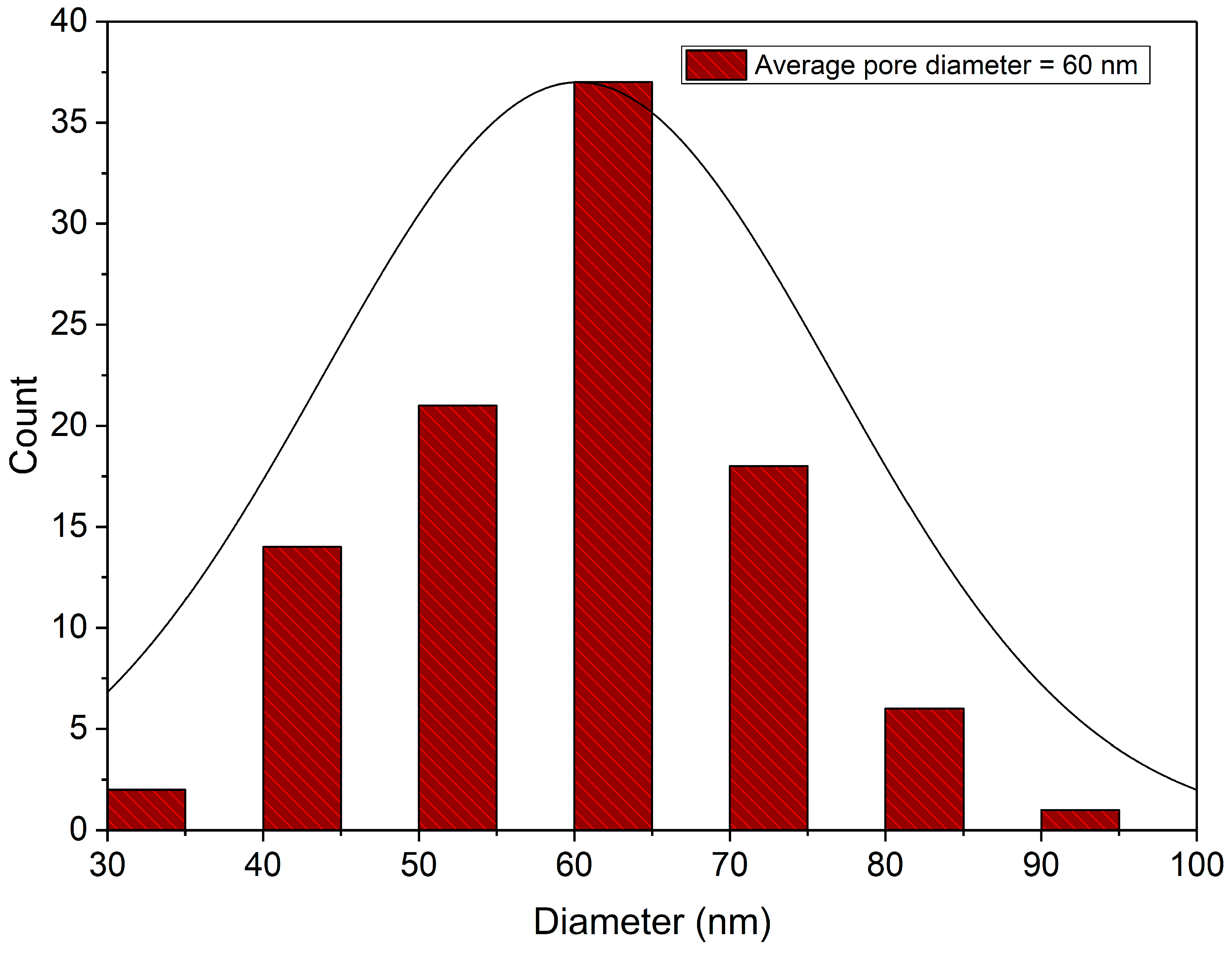
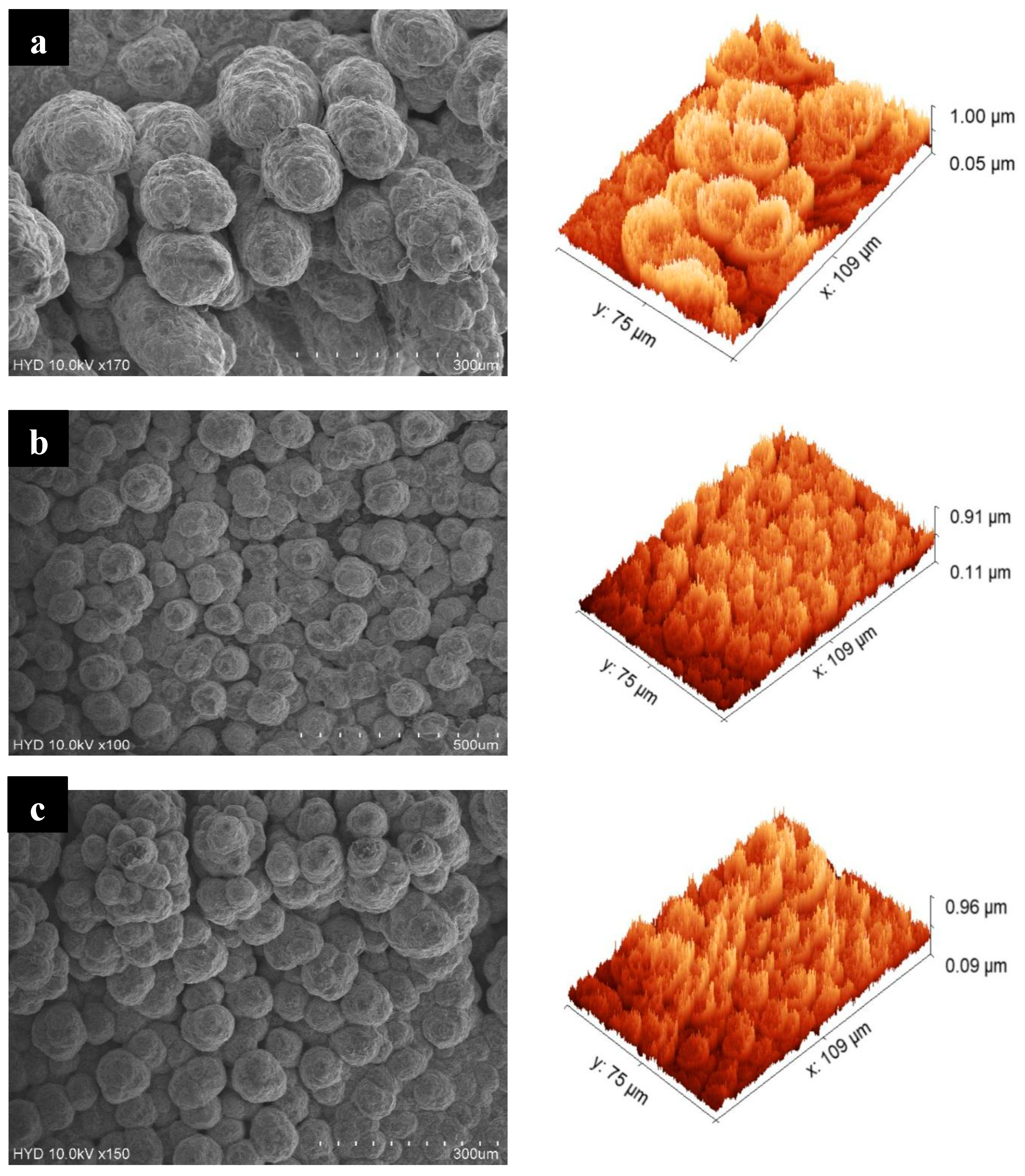
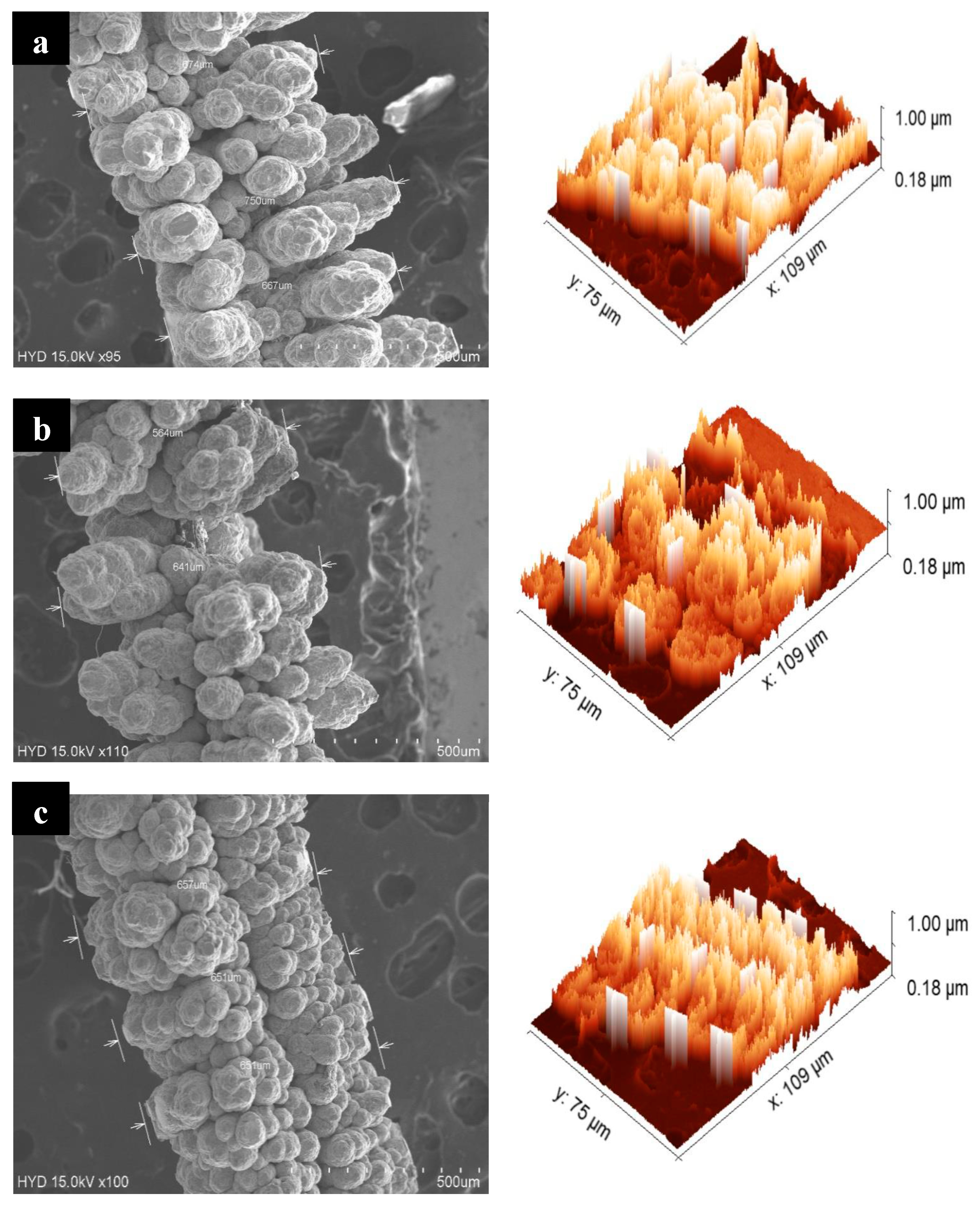
4.1. Morphology Examination
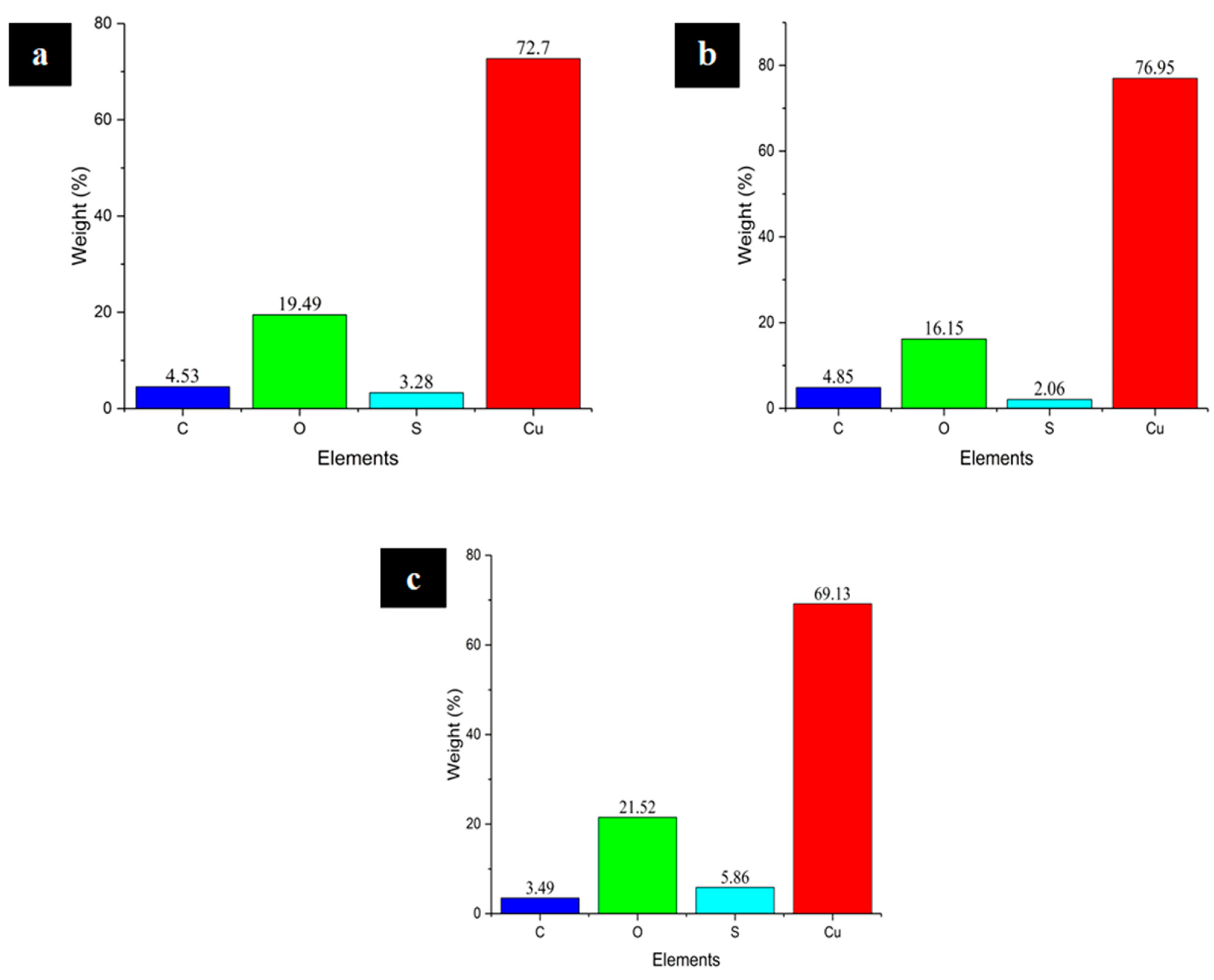
4.2. Evaluation of Integrity of the Phases and the Elemental Mixture
4.3. Technical Analysis
5. Assessment of Water Quality
6. Conclusions
- ➢
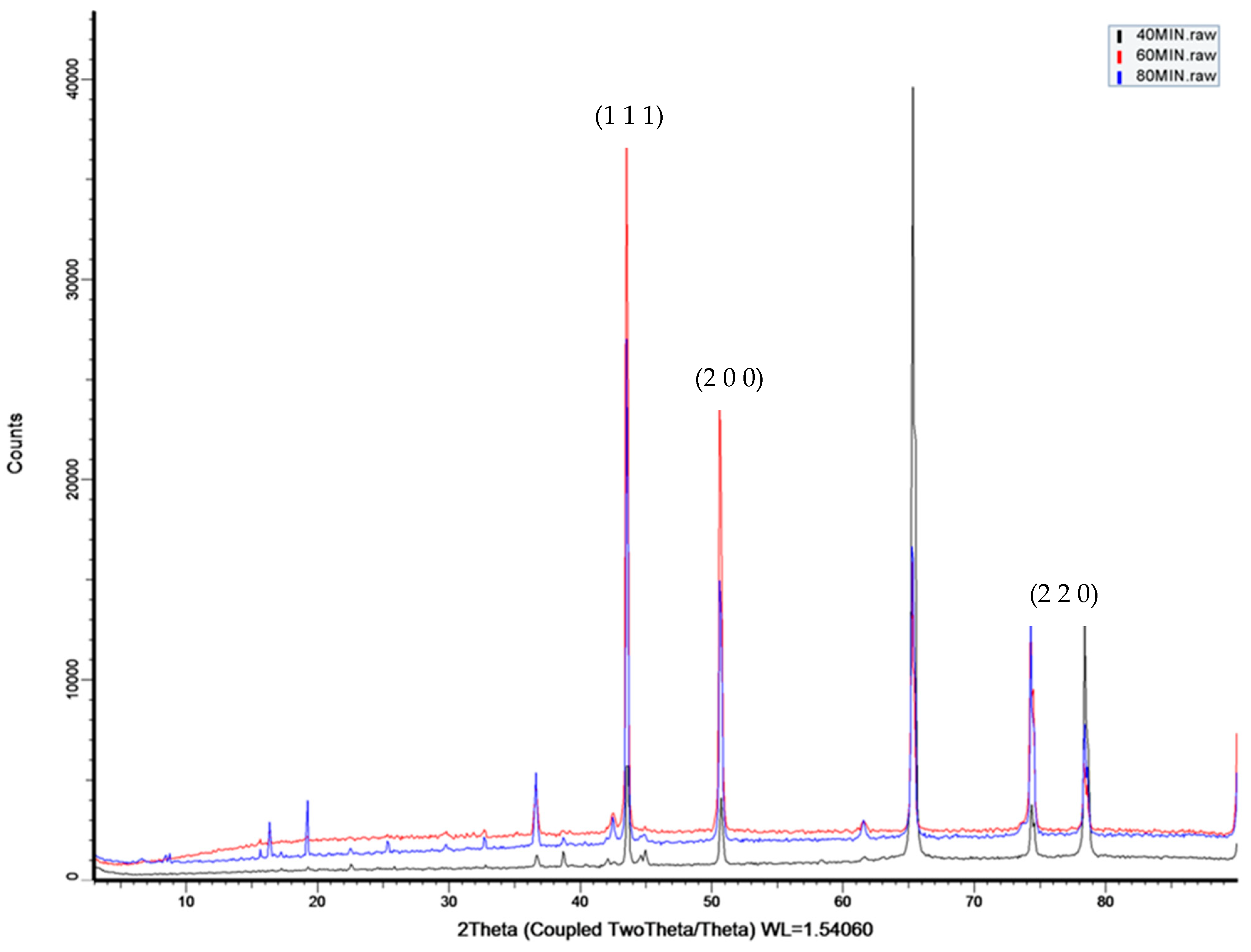
- For this study, we employed the anodization approach, a feasible and uncomplicated method for producing a nanoporous structure. The anodization process was carried out using oxalic acid at room temperature and a fixed voltage of 40 V. To generate three distinct nanoporous structures, we utilized three anodization periods of 40 min, 60 min, and 80 min.
- ➢
- To deposit copper onto the nanoporous structure, the samples underwent chemical etching to remove the oxide layer. Following this, copper electroplating was carried out on the three samples for a duration of 30 min.
- ➢
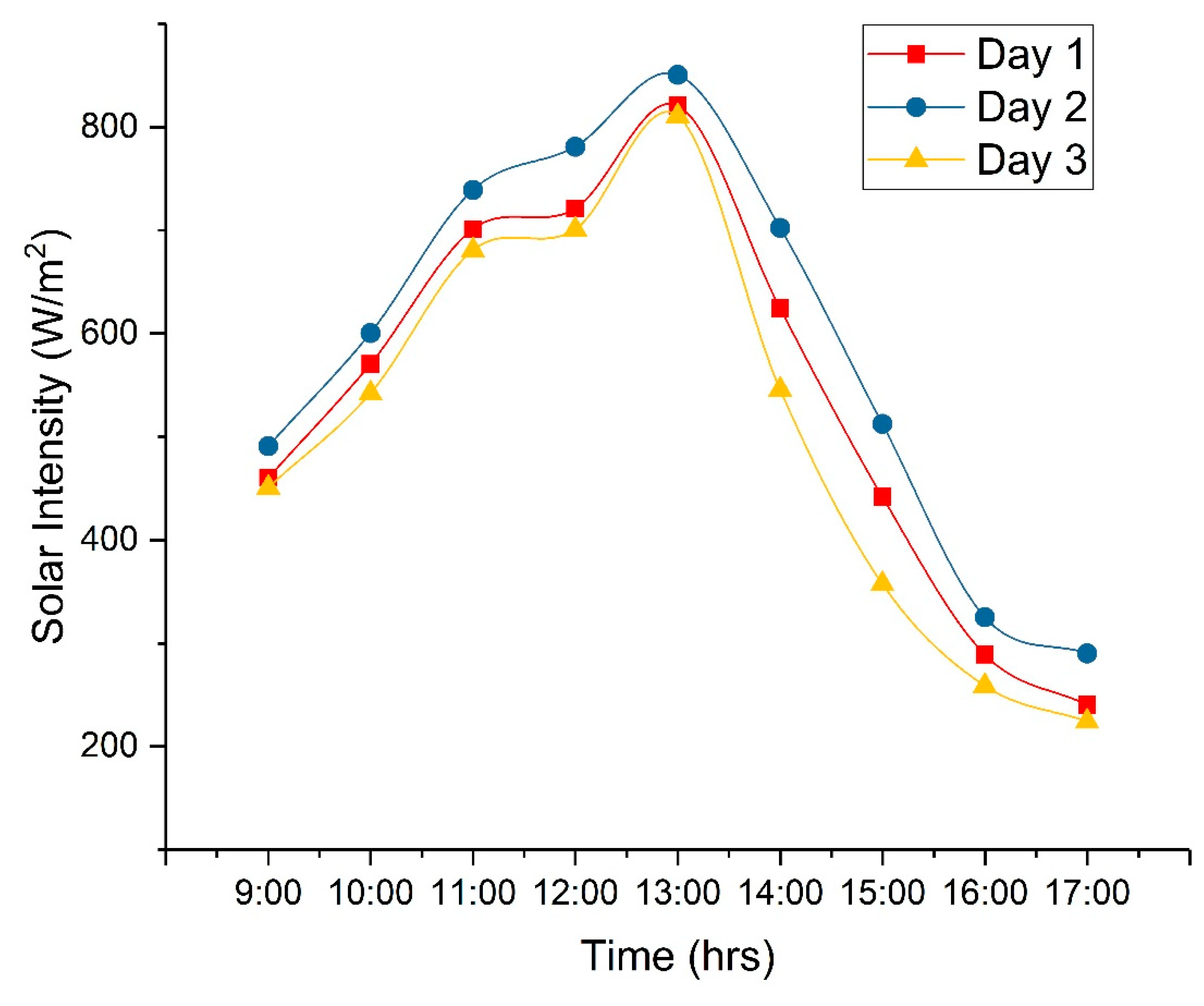
- On three separate days, three distinct copper-electroplated samples were utilized in the process of solar desalination.
- ➢
- On the first day, the copper sample, specifically the copper deposited on the 40 min anodized sample, exhibited a 14.28% faster rate of evaporation compared to the conventional still. On the second day, the copper sample, specifically the copper deposited on the 60 min anodized sample, had an evaporation rate that is 22.22% higher than the conventional still. On the third day, the copper sample, specifically the copper deposited on the 80 min anodized sample, had an evaporation rate that is 11.11% higher than the evaporation rate of the conventional still.
- ➢
- Upon analyzing the three samples, it was seen that the sample which underwent anodization for 60 min followed by copper deposition displayed a higher percentage of evaporation compared to the other two samples anodized for 40 and 80 min, respectively.
- ➢
- The enhanced evaporation was a consequence of the porous structure attained during anodization, along with the efficient and extensive deposition of copper within these pores. The initial pH of the groundwater was 8.2, which was subsequently reduced to 7.4 for the CSS and 7.2 for the MSS. The initial concentration of total dissolved solids (TDS) in the groundwater was 450 parts per million (ppm). However, after undergoing the desalination process, the TDS levels were significantly decreased and dropped within the permitted limits set by the World Health Organization (WHO) and the Bureau of Indian Standards (BIS).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Kuravi, S.; Kota, K.; Park, Y.H.; Xu, P. Low-Cost and Reusable Carbon Black Based Solar Evaporator for Effective Water Desalination. Desalination 2020, 483, 114412. [Google Scholar] [CrossRef]
- Wang, X.; Ou, G.; Wang, N.; Wu, H. Graphene-Based Recyclable Photo-Absorbers for High-Efficiency Seawater Desalination. ACS Appl. Mater. Interfaces 2016, 8, 9194–9199. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, J.; Liu, S.; Wang, Y.; Li, B.; Jiao, T. Facile Synthesis of Ag/Pd Nanoparticle—Loaded Poly(Ethylene Imine) Composite Hydrogels with Highly Efficient Catalytic Reduction of 4-Nitrophenol. ACS Omega 2020, 5, 3725–3733. [Google Scholar] [CrossRef]
- Chen, C.; Kuang, Y.; Hu, L. Challenges and Opportunities for Solar Evaporation. Joule 2019, 3, 683–718. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Recent Progress in Solar-Driven Interfacial Water Evaporation: Advanced Designs and Applications. Nano Energy 2019, 57, 507–518. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-Driven Interfacial Evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, H.; Wang, Y.; Ouyang, Z.; Wang, W.; Yang, B.; Liu, H.; Hu, X. Enhanced Water Yield of Solar Desalination by Thermal Concentrated Multistage Distiller. Desalination 2020, 477, 114260. [Google Scholar] [CrossRef]
- Ni, G.; Miljkovic, N.; Ghasemi, H.; Huang, X.; Boriskina, S.V.; Lin, C.T.; Wang, J.; Xu, Y.; Rahman, M.M.; Zhang, T.J.; et al. Volumetric Solar Heating of Nanofluids for Direct Vapor Generation. Nano Energy 2015, 17, 290–301. [Google Scholar] [CrossRef]
- He, S.; Chen, C.; Kuang, Y.; Mi, R.; Liu, Y.; Pei, Y.; Kong, W.; Gan, W.; Xie, H.; Hitz, E.; et al. Nature-Inspired Salt Resistant Bimodal Porous Solar Evaporator for Efficient and Stable Water Desalination. Energy Environ. Sci. 2019, 12, 1558–1567. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Chen, F.; Zhu, X.; Dai, J.; Li, Y.; Yang, Z.; Yan, X.; Song, J.; Wang, Y.; et al. Plasmonic Wood for High-Efficiency Solar Steam Generation. Adv. Energy Mater. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Geng, L.; Li, L.; Zhang, H.; Zhong, M.; Mu, P.; Li, J. Interfacial Solar Evaporator Synergistic Phase Change Energy Storage for All-Day Steam Generation. J. Mater. Chem. A 2022, 10, 15485–15496. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, F.; Zhou, X.; Chen, Z.; Yu, G. Tailoring Nanoscale Surface Topography of Hydrogel for Efficient Solar Vapor Generation. Nano Lett. 2019, 19, 2530–2536. [Google Scholar] [CrossRef]
- Li, Y.; Gao, T.; Yang, Z.; Chen, C.; Kuang, Y.; Song, J.; Jia, C.; Hitz, E.M.; Yang, B.; Hu, L. Graphene Oxide-Based Evaporator with One-Dimensional Water Transport Enabling High-Efficiency Solar Desalination. Nano Energy 2017, 41, 201–209. [Google Scholar] [CrossRef]
- Huang, L.; Pei, J.; Jiang, H.; Hu, X. Water Desalination under One Sun Using Graphene-Based Material Modified PTFE Membrane. Desalination 2018, 442, 1–7. [Google Scholar] [CrossRef]
- Hong, Z.; Pei, J.; Wang, Y.; Cao, B.; Mao, M.; Liu, H.; Jiang, H.; An, Q.; Liu, X.; Hu, X. Characteristics of the Direct Absorption Solar Collectors Based on Reduced Graphene Oxide Nanofluids in Solar Steam Evaporation. Energy Convers. Manag. 2019, 199, 112019. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, F.; Guo, Y.; Zhang, Y.; Yu, G. A Hydrogel-Based Antifouling Solar Evaporator for Highly Efficient Water Desalination. Energy Environ. Sci. 2018, 11, 1985–1992. [Google Scholar] [CrossRef]
- Kashyap, V.; Al-Bayati, A.; Sajadi, S.M.; Irajizad, P.; Wang, S.H.; Ghasemi, H. A Flexible Anti-Clogging Graphite Film for Scalable Solar Desalination by Heat Localization. J. Mater. Chem. A 2017, 5, 15227–15234. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Farokhnia, N.; Irajizad, P.; Hasnain, M.; Ghasemi, H. Flexible Artificially-Networked Structure for Ambient/High Pressure Solar Steam Generation. J. Mater. Chem. A 2016, 4, 4700–4705. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, L.; Yu, J.; Wang, Y.; Nie, S.; Zhu, S.; Zhu, J. Dual Functional Asymmetric Plasmonic Structures for Solar Water Purification and Pollution Detection. Nano Energy 2018, 51, 451–456. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S.; Zhu, J. 3D Self-Assembly of Aluminium Nanoparticles for Plasmon-Enhanced Solar Desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Liu, P.F.; Miao, L.; Deng, Z.; Zhou, J.; Su, H.; Sun, L.; Tanemura, S.; Cao, W.; Jiang, F.; Zhao, L.D. A Mimetic Transpiration System for Record High Conversion Efficiency in Solar Steam Generator under One-Sun. Mater. Today Energy 2018, 8, 166–173. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Hong, Z.; Pu, Z.; Yao, Q.; Shi, J.; Yang, G.; Mi, B.; Yang, B.; Liu, X.; et al. A Bioinspired Capillary-Driven Pump for Solar Vapor Generation. Nano Energy 2017, 42, 115–121. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Tang, M.; Zhou, L.; Zhu, B.; Zhu, S.; Zhu, J. Graphene Oxide-Based Efficient and Scalable Solar Desalination under One Sun with a Confined 2D Water Path. Proc. Natl. Acad. Sci. USA 2016, 113, 13953–13958. [Google Scholar] [CrossRef]
- Murphy, C.J. Materials Science: Nanocubes and Nanoboxes. Science 2002, 298, 2139–2141. [Google Scholar] [CrossRef]
- Mirkin, C.A. The Beginning of a Small Revolution. Small 2005, 1, 14–16. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Pavan Kumar, Y.; Swetha, S.; Kumar Kaviti, A. Development of Nano Structures for Solar Driven Desalination. Mater. Today Proc. 2023, 82, 270–278. [Google Scholar] [CrossRef]
- Manzoor, S.; Ashraf, M.W.; Tayyaba, S.; Tariq, M.I.; Hossain, M.K. Recent Progress of Fabrication, Characterization, and Applications of Anodic Aluminum Oxide (AAO) Membrane: A Review. C.-Comput. Model. Eng. Sci. 2023, 135, 1007–1052. [Google Scholar] [CrossRef]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural Features of Oxide Coatings on Aluminum. J. Electrochem. Soc. 1953, 100, 411. [Google Scholar] [CrossRef]
- Li, J.M.; Cai, C.; Song, L.X.; Li, J.F.; Zhang, Z.; Xue, M.Z.; Liu, Y.G. Electrodeposition and Characterization of Nano-Structured Black Nickel Thin Films. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2013, 23, 2300–2306. [Google Scholar] [CrossRef]
- Ma, P.; Geng, Q.; Gao, X.; Yang, S.; Liu, G. Cu1.5Mn1.5O4-Based Ceramic Spectrally Selective Coatings for Efficient Solar Absorber Applications. J. Alloys Compd. 2016, 675, 423–432. [Google Scholar] [CrossRef]
- Randich, E.; Allred, D.D. General film behaviour 393 chemically vapor-deposited ZrB2 as a selective solar absorber. Thin Solid Films 1981, 83, 393–398. [Google Scholar] [CrossRef]
- Wackelgard, E.; Bartali, R.; Gerosa, R.; Laidani, N.; Mattsson, A.; Micheli, V.; Rivolta, B. New Cermet Coatings for Mid-Temperature Applications for Solar Concentrated Combine Heat and Power System. Energy Procedia 2014, 48, 242–249. [Google Scholar] [CrossRef]
- Lizama-Tzec, F.I.; Macías, J.D.; Estrella-Gutiérrez, M.A.; Cahue-López, A.C.; Arés, O.; de Coss, R.; Alvarado-Gil, J.J.; Oskam, G. Electrodeposition and Characterization of Nanostructured Black Nickel Selective Absorber Coatings for Solar–Thermal Energy Conversion. J. Mater. Sci. Mater. Electron. 2015, 26, 5553–5561. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Mu, A.; Cheetham, A.K. Nanomaterials Chemistry; Rao, C.N.R., Müller, A., Cheetham, A.K., Eds.; Wiley: Hoboken, NJ, USA, 2007; ISBN 9783527316649. [Google Scholar]
- Tang, X.J.; Zhang, G.; Zhao, Y.P. Electrochemical Characterization of Silver Nanorod Electrodes Prepared by Oblique Angle Deposition. Nanotechnology 2006, 17, 4439–4444. [Google Scholar] [CrossRef]
- Krishna, S.A.M.; Shridhar, T.N.; Krishnamurthy, L. Computational Investigation on Thermal Conductivity Behavior of Al 6061-SiC-Gr Hybrid Metal Matrix Composites. Int. J. Comput. Mater. Sci. Eng. 2015, 4, 1–24. [Google Scholar] [CrossRef]
- Mendes, L.F.; Moraes, A.S.; Santos, J.S.; Leite, F.L.; Trivinho-Strixino, F. Investigation of Roughness and Specular Quality of Commercial Aluminum (6061 Alloy) for Fabrication of Nanoporous Anodic Alumina Films. Surf. Coatings Technol. 2017, 310, 199–206. [Google Scholar] [CrossRef]
- For, S.M.; Copper, P.; Aluminum, O.N. 20020411 126 Suggested Method for Plating Copper on Aluminum; Technology utilization report, NASA SP-5025; NASA: Washington, DC, USA.
- Milusheva, V.; Georgieva, M.; Tzaneva, B.; Petrova, M. Electroless Copper Deposition into Anodic Aluminium Oxide on Aluminum Substrate. In Proceedings of the 2018 IEEE XXVII International Scientific Conference Electronics—ET, Sozopol, Bulgaria, 13–15 September 2018. [Google Scholar] [CrossRef]
- Poznyak, A.; Knörnschild, G.; Karoza, A.; Norek, M.; Pligovka, A. Peculiar Porous Aluminum Oxide Films Produced via Electrochemical Anodizing in Malonic Acid Solution with Arsenazo-I Additive. Materials 2021, 14, 5118. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.A.; Knörnschild, G.H.; Pligovka, A.N.; Larin, T.D. Anodic Alumina Prepared in Aqueous Solutions of Chelating Complex Zinc and Cobalt Compounds. Tech. Phys. 2022, 67, 411–422. [Google Scholar] [CrossRef]
- Poznyak, A.; Pligovka, A.; Turavets, U.; Norek, M. On-Aluminum and Barrier Anodic Oxide: Meeting the Challenges of Chemical Dissolution Rate in Various Acids and Solutions. Coatings 2020, 10, 875. [Google Scholar] [CrossRef]
- Kanakarn, D.; Venkatachalam, L.; Narasimhan, R.V.; Subramanian, R. Plating on Anodised Aluminium. Bull. Electrochem. 1986, 2, 273–275. [Google Scholar]
- Kumar, A.; Ram, S. Results in Engineering Influence of Anodization Time on Al 2 O 3 Nanoporous Morphology and Optical Properties Using Energy Band Gap at Room Temperature. Results Eng. 2023, 17, 100816. [Google Scholar] [CrossRef]
- Kolassa, N.; Punzengruber, C.; Suko, J.; Makinose, M. Mechanism of Calcium-Independent Phosphorylation of Sarcoplasmic Reticulum ATPase by Orthophosphate. Evidence of Magnesium-Phosphoprotein Formation. FEBS Lett. 1979, 108, 495–500. [Google Scholar] [CrossRef]
- Liu, A.; Shi, Z.; Reddy, R.G. Mechanism Study of Cu-Zn Alloys Electrodeposition in Deep Eutectic Solvents. Ionics 2020, 26, 3161–3172. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, A.; Baredar, P.V. Experimental Investigation on Modified Solar Still Using Nanoparticles and Water Sprinkler Attachment. Front. Mater. 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Mohiuddin, S.A.; Kaviti, A.K.; Rao, T.S.; Atchuta, S.R. Experimental Assessment of Productivity and Sustainability of Nanoporous Cr-Mn-Fe Oxide Nanocoating in Solar-Powered Desalination. Process Saf. Environ. Prot. 2022, 162, 61–71. [Google Scholar] [CrossRef]
- Kaviti, A.K.; Akkala, S.R.; Sikarwar, V.S.; Sai Snehith, P.; Mahesh, M. Camphor-Soothed Banana Stem Biowaste in the Productivity and Sustainability of Solar-Powered Desalination. Appl. Sci. 2023, 13, 1652. [Google Scholar] [CrossRef]


| Parameters of Water Quality | Before Desalination | After Desalination (CSS) | After Desalination (MSS) | Maximum Permissible Limits of Drinking Water (WHO and BIS Standards) [50] |
|---|---|---|---|---|
| Hardness (mg/L) | 315 | 165 | 130 | 200 |
| pH | 8.2 | 7.4 | 7.2 | 8.5 |
| Chloride (mg/L) | 58.7 | 8.5 | 6.6 | 250 |
| Fluoride (mg/L) | 0.6 | 0.40 | 0.34 | 1.5 |
| TDS (ppm) | 450 | 25 | 17 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaviti, A.K.; Kumar, Y.P.; Sikarwar, V.S. Copper-Plated Nanoporous Anodized Aluminum Oxide for Solar Desalination: An Experimental Study. Sustainability 2024, 16, 2220. https://doi.org/10.3390/su16052220
Kaviti AK, Kumar YP, Sikarwar VS. Copper-Plated Nanoporous Anodized Aluminum Oxide for Solar Desalination: An Experimental Study. Sustainability. 2024; 16(5):2220. https://doi.org/10.3390/su16052220
Chicago/Turabian StyleKaviti, Ajay Kumar, Yerolla Pavan Kumar, and Vineet Singh Sikarwar. 2024. "Copper-Plated Nanoporous Anodized Aluminum Oxide for Solar Desalination: An Experimental Study" Sustainability 16, no. 5: 2220. https://doi.org/10.3390/su16052220
APA StyleKaviti, A. K., Kumar, Y. P., & Sikarwar, V. S. (2024). Copper-Plated Nanoporous Anodized Aluminum Oxide for Solar Desalination: An Experimental Study. Sustainability, 16(5), 2220. https://doi.org/10.3390/su16052220






