Enzyme-Assisted Circular Additive Manufacturing as an Enabling Technology for a Circular Bioeconomy—A Conceptual Review
Abstract
1. Introduction
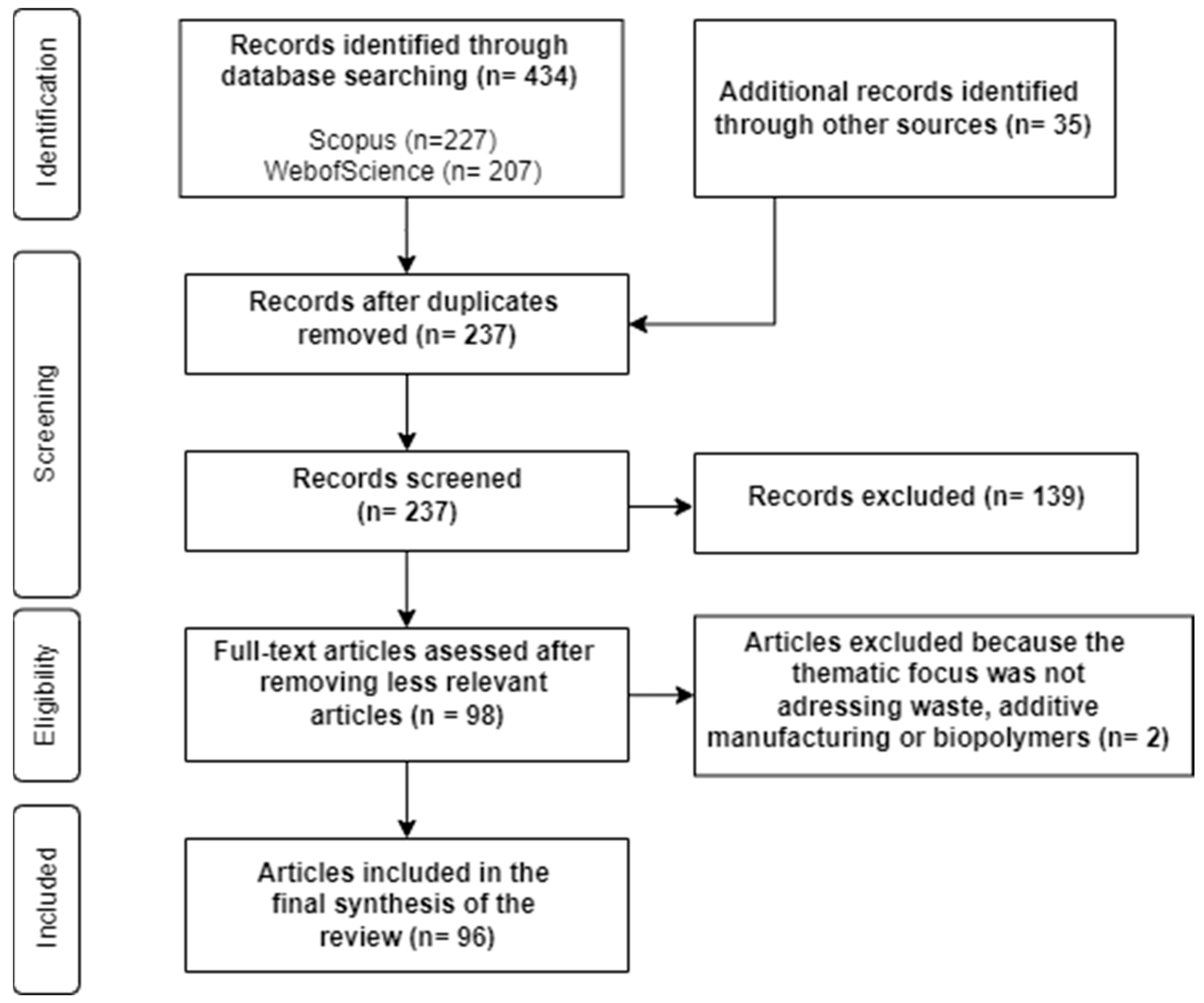
2. Methodology
3. Biopolymers in Additive Manufacturing
3.1. Areas of Application for Biopolymers in AM
- Bioprinting is the versatile deformation of cellular tissue. This is, for instance, an application of cells, growth factors and biomaterials that are combined to create products that mimic the properties of natural tissue [34,35]. A distinction is made between three- and four-dimensional technologies. In 3D bioprinting, fabric is created in three spatial dimensions and in 4D bioprinting, time is added, i.e., the ability to change over time by changing the product with the help of a programmable mat that reacts to environmental parameters (humidity, temperature, etc.) and thus changes its shape accordingly [36].
- Additive biomanufacturing describes any bio-based printing technology that uses non-traditional additive manufacturing technologies or materials and products that mimic the properties of natural tissues. EnCAM is an early example of these types of AM technologies.
3.2. Biopolymers as Raw Materials for Circular Additive Concepts
3.2.1. Chitin and Derived Biopolymers
3.2.2. Wood Powder and Derived Biopolymers
3.2.3. Alginate and Gelatin
| Material System | Printing Process | Applications | Reference |
| Chitin/Chitosan | |||
| DIW | Tissue engineering | [83,84,85,86,87,88] |
| DIW | Tissue engineering | [89] | |
| DIW | Tissue engineering | [90] | |
| DIW | Immobilization of microorganisms | [91] | |
| DIW | Drug release | [92] | |
| DIW | Tissue engineering | [93] | |
| PP | Tissue engineering | [94] | |
| PP | Wound treatment (bone) | [95] | |
| Cellulose | |||
| DIW | Soft tissue engineering | [96] |
| DIW | Cartilage tissue engineering | [97,98,99] | |
| DIW | Wound treatment | [100] | |
| DIW | General additive in bioink | [101] | |
| DLP | General additive in bioink | [102] | |
| DLP | Lightweight sustainable composite | [103] | |
| Wood powder | |||
| DIW | Wood products | [104] |
| FDM, FLM, | Cost reducing material | [105] | |
| DIW, SLS, BJ | Wood products | [106] | |
| FLM | Bio-based filler and coupling agent | [107] | |
| FDM | Bio-based filler and coupling agent | [108,109,110] | |
| FDM, BJ | By-product recycling | [111] | |
| BJ | Biodegradable ink | [112,113] | |
| SL | Higher mechanical strength | [114] | |
| PBF | Cement alternative | [115] | |
| Lignin | |||
| DIW | Biomedical engineering | [116] |
| FDM | Increased tensile strenght | [117] | |
| FDM | Biopolymer foaming | [118] | |
| FDM | Renewable material | [119] | |
| DLP | Packaging | [120] | |
| Alginate | |||
| DIW | Bone tissue engineering | [88,121] |
| DIW | Soft and hard tissue engineering | [122,123,124,125] | |
| DIW | Tissue engineering | [126,127] | |
| DIW | Tissue engineering | [128] | |
| DIW | Tissue engineering | [129] | |
| DIW | Tissue engineering | [123] | |
| Gelatin | |||
| DIW | Tissue engineering | [130] |
| DIW | Tissue engineering | [131] | |
| DIW | Biomedical devices | [132] | |
| DIW | Tissue engineering | [133] | |
| DIW | Tissue engineering | [134,135] | |
| DIW | Food design | [136] | |
4. Discussion
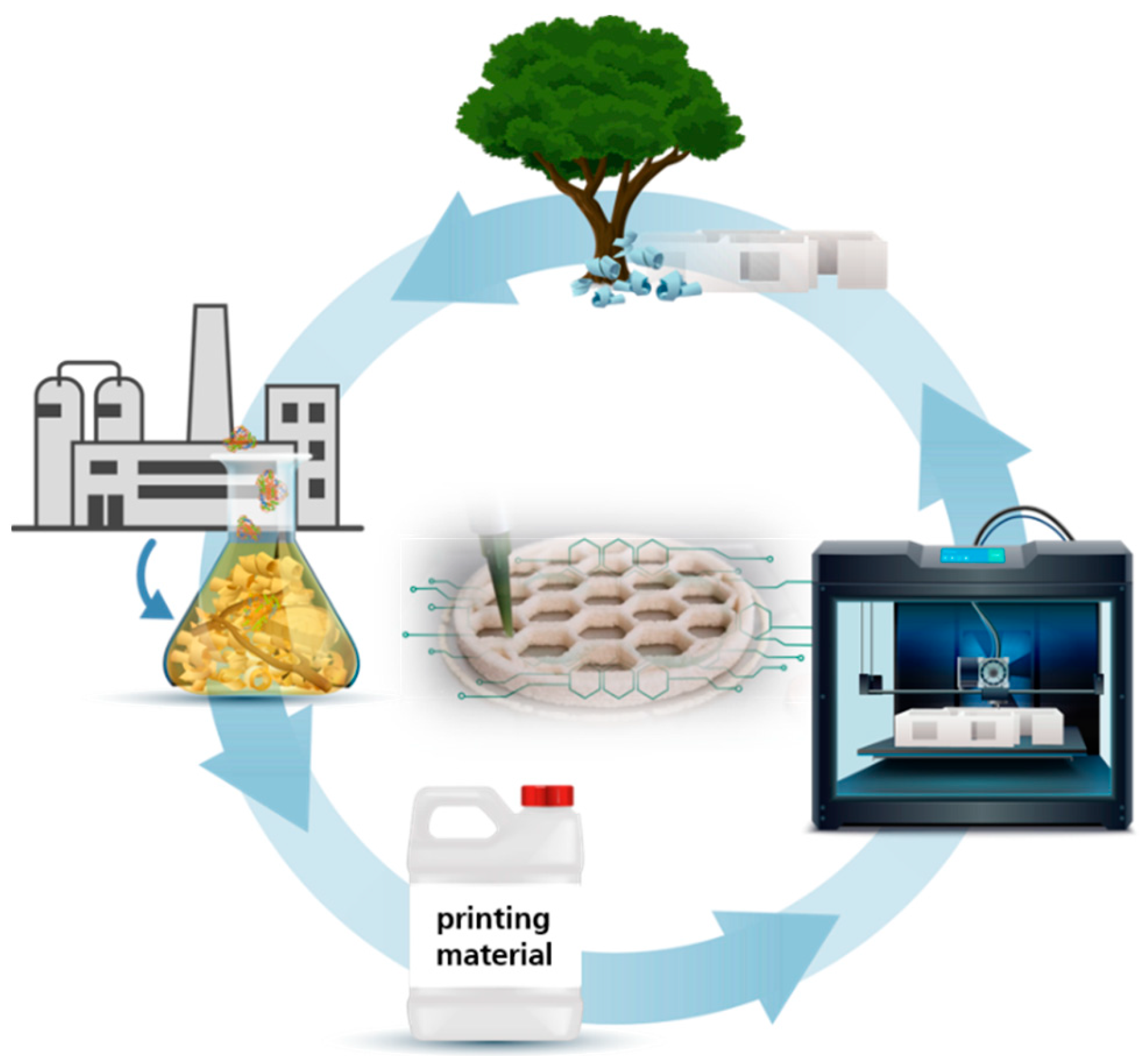
4.1. Process Concept for Enzyme-Assisted Circular Additive Manufacturing
4.2. Application Possibilities of Enzymes during Pre-Treatment
4.3. Application Possibilities of Enzymes during Functionalization
4.4. Application Possibilities of Enzymes during Printing Processes
5. Future Directions for Enzyme-Assisted Approaches in AM
- Protein engineering by recombinant production via the diversity generation strategy: The aim here is to improve the efficiency of the process by genetically altering the amino acid sequence of either an already-available enzyme or the formulation of an advanced enzyme activity. Therefore, the originally observed disadvantages of native enzymes can be overcome by using enzyme engineering tools. Some evolutionary strategies are applied to support this optimization procedure:
- Enzyme performance is critically influenced by the microenvironment. Therefore, the mechanistic study of immobilized enzymes is useful for developing improved biocatalysts. The study consists of the characterization of their effects on the properties of the enzymes associated with the particular microenvironment in the solid material. Key performance parameters are investigated. These include the enzyme activity, catalytic rate, and stability, which strongly depend on substrate and product concentrations, as well as the pH, ionic strength, reaction equilibrium and effective mass–action ratio in the solid particle, which can be influenced by changes in the critical concentrations [167,168].
- Cell-free systems consist of in vitro biochemical technologies extracting enzymes from outside the organism where they were originally located. Cell-free systems complement traditional cellular systems. Cell-free synthetic biology methods are useful in pathway prototyping for testing and optimizing biosynthetic pathways before implementation in live cells and scale-up, as well as for pathway-operation design and debugging in bio-circuitry [179,180]. The de novo biosynthesis of cellulose and chitin in cell-free systems has been shown to be possible. Generating covalent binding of material fragments of cellulose or chitin in the context of additive manufacturing would be a possible next step [181,182].
- A more advanced step than just improving proteins through random or targeted substitution within the 20 standard amino acids is to develop artificial amino acids and incorporate them into the gene sequence as artificial proteins with new properties. The development of completely new product classes, whose chemical synthesis was previously not possible through conventional protein engineering using the 20 standard amino acids, is expected [183,184].
- Screening strategies with high throughput screening using functional genomics, microbiome screening and looking for extremophiles:
- In the search for biocatalysts, especially for biomaterials, microbiome research enables the discovery of ever new enzymes. Insects, in particular, represent a very diverse group of organisms that can adapt to extremely different environmental conditions. Some of them, the herbivorous insects, have developed highly specialized systems that allow them to use a variety of plants as food sources. In the decomposition of leaves, stems and roots, the composition of the insect gut flora plays a decisive role for the development of food, but also for the decomposition of plastics and toxins [185,186,187].
- The search for specific metabolic activities in extremophilic organisms may reveal extremophilic enzyme functions that operate under extreme conditions such as high temperature and high ion concentrations, even in non-aqueous organic solvents. This would enable the design of a new generation of enzyme catalysts [188,189].
- New types of nanozymes will be able to replace biocatalytic reactions of natural enzymes at low cost. “Nanozymes” are “nanomaterials with enzyme-like characteristics” [190]. Their unique characteristics over natural enzymes and even conventional artificial enzymes are as follows: suitability for mass production; robustness to harsh environments; high stability; possible long-term storage; recyclability; adjustable activity; size-, shape-, structure-, or composition-dependent properties; and responses to external stimuli (e.g., light) [191,192]. Many non-metallic materials, especially carbon-based nanomaterials, possess peroxidase activity, one of the non-specific catalytic options for lignin-based material fusion in AM processes.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.A.; Prasad, R. Basic principles of additive manufacturing: Different additive manufacturing technologies. In Additive Manufacturing. A Tool for Industrial Revolution 4.0; Manjaiah, M., Ed.; Woodhead Publishing Reviews: Sawston, UK; Elsevier Science & Technology: San Diego, CA, USA, 2021; Chapter 2; pp. 17–35. [Google Scholar]
- Singh, R.; Singh, S. Additive Manufacturing: An Overview. In Encyclopedia of Smart Materials; Olabi, A.G., Ed.; Elsevier: San Diego, CA, USA, 2022; pp. 258–269. [Google Scholar]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems. Pharm. Res. 2018, 36, 4. [Google Scholar] [CrossRef]
- Fina, F.; Gaisford, S.; Basit, A.W. Powder Bed Fusion: The Working Process, Current Applications and Opportunities. In 3D Printing of Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Kazmer, D. Three-Dimensional Printing of Plastics. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2017; pp. 617–634. [Google Scholar]
- Wangler, T.; Flatt, R.J.; Roussel, N.; Perrot, A.; Sonebi, M.; Wolfs, R.; Bos, F.; Lowke, D.; Freund, N.; Stephan, D.; et al. Printable Cement-Based Materials: Fresh Properties Measurements and Control. In Digital Fabrication with Cement-Based Materials; Roussel, N., Lowke, D., Eds.; RILEM State-of-the-Art Reports; Springer International Publishing: Cham, Switzerland, 2022; Volume 36, pp. 99–136. [Google Scholar]
- Miehe, R.; Finkbeiner, M.; Sauer, A.; Bauernhansl, T. A System Thinking Normative Approach towards Integrating the Environment into Value-Added Accounting—Paving the Way from Carbon to Environmental Neutrality. Sustainability 2022, 14, 13603. [Google Scholar] [CrossRef]
- Diegel, O. 10.02-Additive Manufacturing: An Overview. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; Volume 13, pp. 3–18. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Miehe, R.; Schneider, R.; Baaij, F.; Bauernhansl, T. Criticality of Material Resources in Industrial Enterprises—Structural Basics of an Operational Model. Procedia CIRP 2016, 48, 1–9. [Google Scholar] [CrossRef]
- Miehe, R.; Mueller, S.; Schneider, R.; Wahren, S.; Hornberger, M. Integrated hazardous materials management: Combining requirements from various environmental legislations to enable effective business compliance processes in industries. Int. J. Precis. Eng. Manuf. Green Tech. 2015, 2, 289–298. [Google Scholar] [CrossRef]
- Cruz Sanchez, F.A.; Boudaoud, H.; Camargo, M.; Pearce, J.M. Plastic recycling in additive manufacturing: A systematic literature review and opportunities for the circular economy. J. Clean. Prod. 2020, 264, 121602. [Google Scholar] [CrossRef]
- Colorado, H.A.; Velásquez, E.I.G.; Monteiro, S.N. Sustainability of additive manufacturing: The circular economy of materials and environmental perspectives. J. Mater. Res. Technol. 2020, 9, 8221–8234. [Google Scholar] [CrossRef]
- Ponis, S.; Aretoulaki, E.; Maroutas, T.N.; Plakas, G.; Dimogiorgi, K. A Systematic Literature Review on Additive Manufacturing in the Context of Circular Economy. Sustainability 2021, 13, 6007. [Google Scholar] [CrossRef]
- Mazur, K.E.; Borucka, A.; Kaczor, P.; Gądek, S.; Bogucki, R.; Mirzewiński, D.; Kuciel, S. Mechanical, Thermal and Microstructural Characteristic of 3D Printed Polylactide Composites with Natural Fibers: Wood, Bamboo and Cork. J. Polym. Environ. 2022, 30, 2341–2354. [Google Scholar] [CrossRef]
- Mandala, R.; Bannoth, A.P.; Akella, S.; Rangari, V.K.; Kodali, D. A short review on fused deposition modeling 3D printing of bio-based polymer nanocomposites. J. Appl. Polym. Sci. 2022, 139, 51904. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Guit, J.; Loos, K. Sustainable Photopolymers in 3D Printing: A Review on Biobased, Biodegradable, and Recyclable Alternatives. Macromol. Rapid Commun. 2021, 42, e2000475. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.; Styles, D.; Lens, P.N. Environmental performance comparison of bioplastics and petrochemical plastics: A review of life cycle assessment (LCA) methodological decisions. Resour. Conserv. Recycl. 2021, 168, 105451. [Google Scholar] [CrossRef]
- Bishop, G.; Styles, D.; Lens, P.N. Land-use change and valorisation of feedstock side-streams determine the climate mitigation potential of bioplastics. Resour. Conserv. Recycl. 2022, 180, 106185. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; McClements, D.J.; Weiss, J. Enzyme-Based Strategies for Structuring Foods for Improved Functionality. Annu. Rev. Food Sci. Technol. 2017, 8, 21–34. [Google Scholar] [CrossRef]
- Weiss, R.; Guebitz, G.M.; Pellis, A.; Nyanhongo, G.S. Harnessing the Power of Enzymes for Tailoring and Valorizing Lignin. Trends Biotechnol. 2020, 38, 1215–1231. [Google Scholar] [CrossRef]
- Gouseti, O.; Larsen, M.E.; Amin, A.; Bakalis, S.; Petersen, I.L.; Lametsch, R.; Jensen, P.E. Applications of Enzyme Technology to Enhance Transition to Plant Proteins: A Review. Foods 2023, 12, 2518. [Google Scholar] [CrossRef]
- Protte, K.; Schwarz, O. Additive manufacturing with chitin—Investigating the feasibility of an enzyme-assisted material approach for more sustainability. Procedia CIRP 2022, 107, 149–154. [Google Scholar] [CrossRef]
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312. [Google Scholar] [CrossRef]
- Liu, Z. A review on the emerging conversion technology of cellulose, starch, lignin, protein and other organics from vegetable-fruit-based waste. Int. J. Biol. Macromol. 2023, 242, 124804. [Google Scholar] [CrossRef] [PubMed]
- Kardung, M.; Cingiz, K.; Costenoble, O.; Delahaye, R.; Heijman, W.; Lovrić, M.; van Leeuwen, M.; M’Barek, R.; van Meijl, H.; Piotrowski, S.; et al. Development of the Circular Bioeconomy: Drivers and Indicators. Sustainability 2021, 13, 413. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- Lewandowski, I. Bioeconomy; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Ulrich, P.; Hill, W. Wissenschaftstheoretische Grundlagen der Betriebswirtschaftslehre—Teil I. Wirtsch. Stud. Z. Ausbild. Hochschulkontakt 1976, 5, 304–309. [Google Scholar]
- Miehe, R.; Buckreus, L.; Kiemel, S.; Sauer, A.; Bauernhansl, T. A Conceptual Framework for Biointelligent Production—Calling for Systemic Life Cycle Thinking in Cellular Units. Clean Technol. 2021, 3, 844–857. [Google Scholar] [CrossRef]
- Full, J.; Miehe, R.; Kiemel, S.; Bauernhansl, T.; Sauer, A. The Biological Transformation of Energy Supply and Storage—Technologies and Scenarios for Biointelligent Value Creation. Procedia Manuf. 2019, 39, 1204–1214. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Campoli, G.; Borleffs, M.S.; Amin Yavari, S.; Wauthle, R.; Weinans, H.; Zadpoor, A.A. Mechanical properties of open-cell metallic biomaterials manufactured using additive manufacturing. Mater. Des. 2013, 49, 957–965. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Design for Additive Bio-Manufacturing: From Patient-Specific Medical Devices to Rationally Designed Meta-Biomaterials. Int. J. Mol. Sci. 2017, 18, 1607. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef]
- Rendón-Villalobos, R.; Ortíz-Sánchez, A.; Sánchez, E.T.; Flores-Huicochea, E. The Role of Biopolymers in Obtaining Environmentally Friendly Materials. In Composites from Renewable and Sustainable Materials; Poletto, M., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 8. [Google Scholar]
- Rao, M.G.; Bharathi, P.; Akila, R. A Comprehensive Review on Biopolymers. Sci. Rev. Chem. Commun. 2014, 4, 61–68. [Google Scholar]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Endres, H.-J.; Siebert-Raths, A. Technische Biopolymere. Rahmenbedingungen, Marktsituation, Herstellung, Aufbau und Eigenschaften; Hanser eLibrary: Munich, Germany, 2009. [Google Scholar]
- Theus, A.S.; Ning, L.; Hwang, B.; Gil, C.J.; Chen, S.; Wombwell, A.; Mehta, R.; Serpooshan, V. Bioprintability: Physiomechanical and Biological Requirements of Materials for 3D Bioprinting Processes. Polymers 2020, 12, 2262. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean Waste-Derived Chitosan: Antioxidant Properties and Future Perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Meticulous Research: Edible Insects Market Worth $9.60 Billion by 2030. Edible Insects Market by Product (Whole Insect, Insect Powder, Insect Meal, Insect Oil), Insect Type (Crickets, Black Soldier Fly, Mealworms), Application (Animal Feed, Protein Bar and Shakes, Bakery, Confectionery, Beverages), and Geography—Forecast to 2030. 2022. Available online: https://www.meticulousresearch.com/pressrelease/184/edible-insects-market-2030 (accessed on 8 December 2022).
- Kumaresapillai, N.; Ameer Basha, R.; Sathish, R. Production and Evaluation of Chitosan from Aspergillus Niger MTCC Strains. Iran. J. Pharm. Res. IJPR 2011, 10, 553–558. [Google Scholar] [PubMed]
- Muñoz, G.; Valencia, C.; Valderruten, N.; Ruiz-Durántez, E.; Zuluaga, F. Extraction of chitosan from Aspergillus niger mycelium and synthesis of hydrogels for controlled release of betahistine. React. Funct. Polym. 2015, 91, 1–10. [Google Scholar] [CrossRef]
- Xuemei, Z.; Hawkins, S.J. Interactions of aquaculture and waste disposal in the coastal zone. J. Ocean Univ. China 2002, 1, 8–12. [Google Scholar] [CrossRef]
- Gimeno, M.; Ramírez-Hernández, J.Y.; Mártinez-Ibarra, C.; Pacheco, N.; García-Arrazola, R.; Bárzana, E.; Shirai, K. One-solvent extraction of astaxanthin from lactic acid fermented shrimp wastes. J. Agric. Food Chem. 2007, 55, 10345–10350. [Google Scholar] [CrossRef]
- Xu, Y.; Gallert, C.; Winter, J. Chitin purification from shrimp wastes by microbial deproteination and decalcification. Appl. Microbiol. Biotechnol. 2008, 79, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; King, A.W.; Zaitchik, B.; Wullschleger, S.D.; Gregg, J.; Wang, S.; Kirk-Davidoff, D. Carbon sequestration via wood harvest and storage: An assessment of its harvest potential. Clim. Chang. 2013, 118, 245–257. [Google Scholar] [CrossRef]
- Pandey, S. Wood waste utilization and associated product development from under-utilized low-quality wood and its prospects in Nepal. SN Appl. Sci. 2022, 4, 168. [Google Scholar] [CrossRef]
- Manninen, K.; Judl, J.; Myllymaa, T. Life Cycle Environmental Impacts of Different Construction Wood Waste and Wood Packaging Waste Processing Methods; Valtioneuvosto Statsrådet: Helsinki, Finland, 2016. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The plant cell wall. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Annual Production of Pulp for Paper Worldwide from 1961 to 2020 (in Million Metric Tons); Statista: New York, NY, USA, 16 December 2021; Available online: https://www.statista.com/statistics/1333405/pulp-for-paper-production-worldwide/ (accessed on 28 January 2024).
- Fortune Business Insights. Market Size of Paper and Pulp Industry Worldwide from 2021 to 2029 (in Billion U.S. Dollars). Available online: https://www.statista.com/statistics/1073451/global-market-value-pulp-and-paper/ (accessed on 28 January 2024).
- Donaldson, L.; Nanayakkara, B.; Harrington, J. Wood Growth and Development. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Oxford, UK, 2017; pp. 203–210. [Google Scholar]
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81. [Google Scholar] [CrossRef]
- Gosselink, R.; de Jong, E.; Guran, B.; Abächerli, A. Co-ordination network for lignin—Standardisation, production and applications adapted to market requirements (EUROLIGNIN). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Malucelli, G.; Guan, J.-P. An overview on the use of lignin and its derivatives in fire retardant polymer systems. Lignin-Trends Appl. 2018, 9, 207–231. [Google Scholar]
- Gosselink, R.J.A. Lignin as a Renewable Aromatic Resource for the Chemical Industry; Wageningen University and Research: Wageningen, The Netherlands, 2011. [Google Scholar]
- Hodásová, L.; Jablonský, M.; Škulcová, A.; Ház, A. Lignin, potential products and their market value. Wood Res. 2015, 60, 973–986. [Google Scholar]
- Chiaoprakobkij, N.; Sanchavanakit, N.; Subbalekha, K.; Pavasant, P.; Phisalaphong, M. Characterization and biocompatibility of bacterial cellulose/alginate composite sponges with human keratinocytes and gingival fibroblasts. Carbohydr. Polym. 2011, 85, 548–553. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Grand View Research: Alginate Market Size, Share & Trends Analysis Report by Type (High M, High G), By Product (Sodium, Propylene Glycol), by Application (Pharmaceutical, Industrial), by Region, and Segment Forecasts, 2021–2028. Report ID: GVR-2-68038-244-0. Available online: https://www.grandviewresearch.com/industry-analysis/alginate-market (accessed on 23 January 2024).
- Dey, P.; Ramanujam, R.; Venkatesan, G.; Nagarathnam, R. Sodium alginate potentiates antioxidant defense and PR proteins against early blight disease caused by Alternaria solani in Solanum lycopersicum Linn. PLoS ONE 2019, 14, e0223216. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Wang, Y.; Moradali, M.F.; Rehman, Z.U.; Rehm, B.H.A. Genetics and regulation of bacterial alginate production. Environ. Microbiol. 2014, 16, 2997–3011. [Google Scholar] [CrossRef]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef]
- Ahmady, A.; Abu Samah, N.H. A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.; Lee, T.C.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Hassan, M.; Hussain, D.; Kanwal, T.; Xiao, H.-M.; Ghulam Musharraf, S. Methods for detection and quantification of gelatin from different sources. Food Chem. 2024, 438, 137970. [Google Scholar] [CrossRef]
- Zarubin, N.Y.; Kharenko, E.N.; Bredikhina, O.V.; Arkhipov, L.O.; Zolotarev, K.V.; Mikhailov, A.N.; Nakhod, V.I.; Mikhailova, M.V. Application of the Gadidae Fish Processing Waste for Food Grade Gelatin Production. Mar. Drugs 2021, 19, 455. [Google Scholar] [CrossRef]
- Toniciolli Rigueto, C.V.; Rosseto, M.; Alessandretti, I.; Oliveira R de Wohlmuth, D.A.R.; Ferreira Menezes, J.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Gelatin films from wastes: A review of production, characterization, and application trends in food preservation and agriculture. Food Res. Int. 2022, 162, 112114. [Google Scholar] [CrossRef]
- Grand View Research. Hydrocolloids Market Size, Share & Trends Analysis Report by Product (Gelatin, Xanthan Gum, Carrageenan, Alginates, Pectin, Guar Gum, Carboxy Methyl Cellulose), by Function, by Application, by Region, and Segment Forecasts, 2023–2030. Report ID: GVR-3-68038-145-0. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/hydrocolloids-market (accessed on 23 January 2024).
- Polaris Market Research. Gelatin Market Share, Size, Trends, Industry Analysis Report, by Raw Material (Pig Skin, Cattle Bones, Bovine Hides, Fish & Poultry, and Others); By Function; By Application; By Region; Segment Forecast, 2022–2030. Report ID: PM1271. 2021. Available online: https://www.polarismarketresearch.com/industry-analysis/global-gelatin-market (accessed on 23 January 2024).
- Grand View Research. Gelatin Market Size, Share & Trends Analysis Report by Source (Bovine, Porcine), by Function (Stabilizer, Thickener), by Application (Food & Beverages, Healthcare), by Region, and Segment Forecasts, 2024–2030. Report ID: 978-1-68038-110-8. 2024. Available online: https://www.grandviewresearch.com/industry-analysis/gelatin-market-analysis (accessed on 28 January 2024).
- Wu, Q.; Therriault, D.; Heuzey, M. Processing and Properties of Chitosan Inks for 3D Printing of Hydrogel Microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sirous, F.; Hussain, C.M. Current achievements in 3D bioprinting technology of chitosan and its hybrids. New J. Chem. 2021, 45, 10565–10576. [Google Scholar] [CrossRef]
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.-C. 3D Printing of Microstructured and Stretchable Chitosan Hydrogel for Guided Cell Growth. Adv. Biosys. 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Ramirez Caballero, S.S.; Saiz, E.; Montembault, A.; Tadier, S.; Maire, E.; David, L.; Delair, T.; Grémillard, L. 3-D printing of chitosan-calcium phosphate inks: Rheology, interactions and characterization. J. Mater. Sci. Mater. Med. 2018, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Chavanne, P.; Stevanovic, S.; Wüthrich, A.; Braissant, O.; Pieles, U.; Gruner, P.; Schumacher, R. 3D printed chitosan/hydroxyapatite scaffolds for potential use in regenerative medicine. Biomed. Eng. Biomed. Tech. 2013, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Zhou, L.; Ramezani, H.; Sun, M.; Xie, M.; Nie, J.; Lv, S.; Cai, J.; Fu, J.; He, Y. 3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents. Biomater. Sci. 2020, 8, 5020–5028. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Neufurth, M.; Wang, S.; Link, T.; Al-Nawas, B.; Wang, X. A new printable and durable N,O-carboxymethyl chitosan-Ca2+-polyphosphate complex with morphogenetic activity. J. Mater. Chem. B 2015, 3, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Condi Mainardi, J.; Rezwan, K.; Maas, M. Genipin-crosslinked chitosan/alginate/alumina nanocomposite gels for 3D bioprinting. Bioprocess Biosyst. Eng. 2022, 45, 171–185. [Google Scholar] [CrossRef]
- Xu, Y.; Han, J.; Lin, H. Fabrication and characterization of a self-crosslinking chitosan hydrogel under mild conditions without the use of strong bases. Carbohydr. Polym. 2017, 156, 372–379. [Google Scholar] [CrossRef]
- Carillo, G.; Sullivan, M.C.; Islam, M.; Martinez-Duarte, R. 3D Printing of Carbides Using Renewable Resources. Meet. Abstr. 2018, 85, 37. [Google Scholar] [CrossRef]
- He, Y.; Wang, F.; Wang, X.; Zhang, J.; Wang, D.; Huang, X. A photocurable hybrid chitosan/acrylamide bioink for DLP based 3D bioprinting. Mater. Des. 2021, 202, 109588. [Google Scholar] [CrossRef]
- Parkatzidis, K.; Chatzinikolaidou, M.; Kaliva, M.; Bakopoulou, A.; Farsari, M.; Vamvakaki, M. Multiphoton 3D Printing of Biopolymer-Based Hydrogels. ACS Biomater. Sci. Eng. 2019, 5, 6161–6170. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Polez, R.T.; Kimiaei, E.; Madani, Z.; Rojas, O.J.; Österberg, M.; Seppälä, J. 3D printing and properties of cellulose nanofibrils-reinforced quince seed mucilage bio-inks. Int. J. Biol. Macromol. 2021, 192, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate-Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Martínez Ávila, H.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1, 22–35. [Google Scholar] [CrossRef]
- Rees, A.; Powell, L.C.; Chinga-Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.E.; Thomas, D.W. 3D Bioprinting of Carboxymethylated-Periodate Oxidized Nanocellulose Constructs for Wound Dressing Applications. BioMed Res. Int. 2015, 2015, 925757. [Google Scholar] [CrossRef]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N. Characterization of κ-carrageenan/methylcellulose/cellulose nanocrystal hydrogels for 3D bioprinting. Polym. Int. 2022, 71, 181–191. [Google Scholar] [CrossRef]
- Wang, J.; Chiappone, A.; Roppolo, I.; Shao, F.; Fantino, E.; Lorusso, M.; Rentsch, D.; Dietliker, K.; Pirri, C.F.; Grützmacher, H. All-in-One Cellulose Nanocrystals for 3D Printing of Nanocomposite Hydrogels. Angew. Chem. 2018, 57, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose Nanocrystal Inks for 3D Printing of Textured Cellular Architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Rosenthal, M.; Henneberger, C.; Gutkes, A.; Bues, C.-T. Liquid Deposition Modeling: A promising approach for 3D printing of wood. Eur. J. Wood Prod. 2018, 76, 797–799. [Google Scholar] [CrossRef]
- Das, A.K.; Agar, D.A.; Rudolfsson, M.; Larsson, S.H. A review on wood powders in 3D printing: Processes, properties and potential applications. J. Mater. Res. Technol. 2021, 15, 241–255. [Google Scholar] [CrossRef]
- Kariz, M.; Sernek, M.; Kuzman, M.K. Use of wood powder and adhesive as a mixture for 3D printing. Eur. J. Wood Prod. 2016, 74, 123–126. [Google Scholar] [CrossRef]
- Liu, L.; Lin, M.; Xu, Z.; Lin, M. Polylactic acid-based wood-plastic 3D printing composite and its properties. BioRes 2019, 14, 8484–8498. [Google Scholar] [CrossRef]
- Obielodan, J.; Vergenz, K.; Aqil, D.; McEllistrem, L. Characterization of PLA/Lignin Biocomposites for 3D Printing. In Proceedings of the 2019 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 11–14 August 2019; University of Texas at Austin: Austin, TX, USA, 2019. [Google Scholar]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A Biopolymer from Forestry Biomass for Biocomposites and 3D Printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, H.; Li, Z.; Li, P.; Shi, S.Q. Development and Application of Wood Flour-Filled Polylactic Acid Composite Filament for 3D Printing. Materials 2017, 10, 339. [Google Scholar] [CrossRef]
- Plarre, R.; Zocca, A.; Spitzer, A.; Benemann, S.; Gorbushina, A.; Li, Y.; Waske, A.; Funk, A.; Wilbig, J.; Günster, J. Searching for biological feedstock material: 3D printing of wood particles from house borer and drywood termite frass. PLoS ONE 2021, 16, e0246511. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Hao, N.; Ragauskas, A.J. Stereolithography 3D Printing of Lignin-Reinforced Composites with Enhanced Mechanical Properties. ACS Omega 2019, 4, 20197–20204. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Lignin-Containing Photoactive Resins for 3D Printing by Stereolithography. ACS Appl. Mater. Interfaces 2018, 10, 36456–36463. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Guo, Y.; Jiang, K.; Yu, Z.; Liu, Y.; Shen, Y.; Deng, J.; Wang, P. Laser intensity effect on mechanical properties of wood-plastic composite parts fabricated by selective laser sintering. J. Thermoplast. Compos. Mater. 2013, 26, 125–136. [Google Scholar] [CrossRef]
- Henke, K.; Treml, S. Wood based bulk material in 3D printing processes for applications in construction. Eur. J. Wood Prod. 2013, 71, 139–141. [Google Scholar] [CrossRef]
- Jiang, B.; Yao, Y.; Liang, Z.; Gao, J.; Chen, G.; Xia, Q.; Mi, R.; Jiao, M.; Wang, X.; Hu, L. Lignin-Based Direct Ink Printed Structural Scaffolds. Small 2020, 6, e1907212. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H.; Park, J.H.; Kim, O.Y.; Hwang, S.-H. Preparation of Chemically Modified Lignin-Reinforced PLA Biocomposites and Their 3D Printing Performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef]
- Mimini, V.; Sykacek, E.; Syed Hashim, S.N.A.; Holzweber, J.; Hettegger, H.; Fackler, K.; Potthast, A.; Mundigler, N.; Rosenau, T. Compatibility of Kraft Lignin, Organosolv Lignin and Lignosulfonate with PLA in 3D Printing. J. Wood Chem. Technol. 2019, 39, 14–30. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Bowland, C.C.; Naskar, A.K. A general method to improve 3D-printability and inter-layer adhesion in lignin-based composites. Appl. Mater. Today 2018, 12, 138–152. [Google Scholar] [CrossRef]
- Zhang, X.; Keck, S.; Qi, Y.; Baudis, S.; Zhao, Y. Study on Modified Dealkaline Lignin as Visible Light Macromolecular Photoinitiator for 3D Printing. ACS Sustain. Chem. Eng. 2020, 8, 10959–10970. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112525. [Google Scholar] [CrossRef] [PubMed]
- Curti, F.; Drăgușin, D.-M.; Serafim, A.; Iovu, H.; Stancu, I.-C. Development of thick paste-like inks based on superconcentrated gelatin/alginate for 3D printing of scaffolds with shape fidelity and stability. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111866. [Google Scholar] [CrossRef] [PubMed]
- Hazur, J.; Detsch, R.; Karakaya, E.; Kaschta, J.; Teßmar, J.; Schneidereit, D.; Friedrich, O.; Schubert, D.W.; Boccaccini, A.R. Improving alginate printability for biofabrication: Establishment of a universal and homogeneous pre-crosslinking technique. Biofabrication 2020, 12, 045004. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zheng, X.; Zhang, C.; Wang, H.; Li, H. Gelatin-Sodium Alginate Hydrogel Processing by Low-Temperature 3D Printing. In Intelligent Robotics and Application; Liu, H., Kubota, N., Zhu, X., Dillmann, R., Zhou, D., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2015; Volume 9245, pp. 523–532. [Google Scholar]
- You, F.; Wu, X.; Chen, X. 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 299–306. [Google Scholar] [CrossRef]
- Bertuola, M.; Aráoz, B.; Gilabert, U.; Gonzalez-Wusener, A.; Pérez-Recalde, M.; Arregui, C.O.; Hermida, É.B. Gelatin–alginate–hyaluronic acid inks for 3D printing: Effects of bioglass addition on printability, rheology and scaffold tensile modulus. J. Mater. Sci. 2021, 56, 15327–15343. [Google Scholar] [CrossRef]
- Monavari, M.; Homaeigohar, S.; Fuentes-Chandía, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A.R. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO2-CaO nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Zhang, X.; Rahman, S.E.; Su, S.; Wei, J.; Ning, F.; Hu, Z.; Martínez-Zaguilán, R.; Sennoune, S.R.; et al. 3D printed agar/calcium alginate hydrogels with high shape fidelity and tailorable mechanical properties. Polymer 2021, 214, 123238. [Google Scholar] [CrossRef]
- Dávila, J.L.; d’Ávila, M.A. Rheological evaluation of Laponite/alginate inks for 3D extrusion-based printing. Int. J. Adv. Manuf. Technol. 2019, 101, 675–686. [Google Scholar] [CrossRef]
- Das, S.; Pati, F.; Choi, Y.-J.; Rijal, G.; Shim, J.-H.; Kim, S.W.; Ray, A.R.; Cho, D.-W.; Ghosh, S. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233–246. [Google Scholar] [CrossRef]
- Eltaher, H.M.; Abukunna, F.E.; Ruiz-Cantu, L.; Stone, Z.; Yang, J.; Dixon, J.E. Human-scale tissues with patterned vascular networks by additive manufacturing of sacrificial sugar-protein composites. Acta Biomater. 2020, 113, 339–349. [Google Scholar] [CrossRef]
- Erkoc, P.; Uvak, I.; Nazeer, M.A.; Batool, S.R.; Odeh, Y.N.; Akdogan, O.; Kizilel, S. 3D Printing of Cytocompatible Gelatin-Cellulose-Alginate Blend Hydrogels. Macromol. Biosci. 2020, 20, e2000106. [Google Scholar] [CrossRef]
- Landers, R.; Hübner, U.; Schmelzeisen, R.; Mülhaupt, R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 2002, 23, 4437–4447. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Pataky, K.; Braschler, T.; Negro, A.; Renaud, P.; Lutolf, M.P.; Brugger, J. Microdrop printing of hydrogel bioinks into 3D tissue-like geometries. Adv. Mater. 2012, 24, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Riantiningtyas, R.R.; Sager, V.F.; Chow, C.Y.; Thybo, C.D.; Bredie, W.L.P.; Ahrné, L. 3D printing of a high protein yoghurt-based gel: Effect of protein enrichment and gelatine on physical and sensory properties. Food Res. Int. 2021, 147, 110517. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.G.; Boyce, S.; Tipton, K.F. ExplorEnz: The primary source of the IUBMB enzyme list. Nucleic Acids Res. 2009, 37, D593–D597. [Google Scholar] [CrossRef] [PubMed]
- Quartinello, F.; Guebitz, G.M.; Ribitsch, D. Surface functionalization of polyester. In Methods in Enzymology: Enzymatic Polymerizations; Bruns, N., Loos, K., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 13; Volume 627, pp. 339–360. [Google Scholar]
- Bhardwaj, H.; Gupta, R.; Tiwari, A. Communities of Microbial Enzymes Associated with Biodegradation of Plastics. J. Polym. Environ. 2013, 21, 575–579. [Google Scholar] [CrossRef]
- Yang, S.-T. Bioprocessing—From Biotechnology to Biorefinery. In Bioprocesses for Value-Added Products from Renewable Resources. New Technologies and Applications; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2006; Chapter 1; pp. 1–24. [Google Scholar]
- Yang, S.-T. (Ed.) Bioprocesses for Value-Added Products from Renewable Resources. New Technologies and Applications; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2006. [Google Scholar]
- Wang, S.-J.; Zhong, J.-J. Chapter 6-Bioreactor Engineering. In Bioprocesses for Value-Added Products from Renewable Resources. New Technologies and Applications; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2006; Chapter 6; pp. 131–161. [Google Scholar]
- Kumar, N.; Panghal, A.; Garg, M.K. Thermal Food Engineering Operations; Bioprocessing in Food Science; Wiley: Hoboken, NJ, USA; Scrivener Publishing: Beverly, MA, USA, 2022. [Google Scholar]
- Joshi, S.; Mohapatra, A.; Singh, L.; Sahu, J.K. Spray Drying: Principles and Applications. In Thermal Food Engineering Operations; Kumar, N., Panghal, A., Garg, M.K., Eds.; Wiley: Hoboken, NJ, USA, 2022; Volume 125, pp. 141–177. [Google Scholar]
- Stadler, R.H.; Studer, A. Acrylamide Formation Mechanisms. In Acrylamide in Food: Analysis, Content and Potential Health Effects; Gökmen, V., Ed.; Academic Press: Amsterdam, The Netherlands, 2016; Chapter 1; pp. 1–17. [Google Scholar]
- Rao, J.K.; Ramesh, D.V.; Rao, K.P. Implantable controlled delivery systems for proteins based on collagen—pHEMA hydrogels. Biomaterials 1994, 15, 383–389. [Google Scholar] [CrossRef]
- Silber, N.; Hessel, J.; Eigner, S.; Gamero, E.; Lambart, A.-L.; Protte, K.; Full, J.; Bauernhansl, T.; Miehe, R.; Schwarz, O. Towards an Enzymatic Approach to Valorize Wood Residues for Industrial Production in a Circular Bioeconomy. Procedia CIRP 2023, 116, 450–455. [Google Scholar] [CrossRef]
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Hammami, A.; Hajji, S.; Jridi, M.; Nasri, M.; Nasri, R. Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. Int. J. Biol. Macromol. 2017, 101, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; BeMiller, J.N. Enzymatic Approaches for Structuring Starch to Improve Functionality. Annu. Rev. Food Sci. Technol. 2023, 14, 271–295. [Google Scholar] [CrossRef]
- Dun, Y.; Li, Y.; Xu, J.; Hu, Y.; Zhang, C.; Liang, Y.; Zhao, S. Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. Int. J. Biol. Macromol. 2019, 123, 420–426. [Google Scholar] [CrossRef]
- Corchero, J.L.; Vázquez, E.; García-Fruitós, E.; Ferrer-Miralles, N.; Villaverde, A. Recombinant protein materials for bioengineering and nanomedicine. Nanomedicine 2014, 9, 2817–2828. [Google Scholar] [CrossRef]
- Upadrasta, L.; Garlapati, V.K.; Lakdawala, N.; Banerjee, R. Enzyme-Triggered Hydrogels for Pharmaceutical and Food Applications. In Research Advancements in Pharmaceutical, Nutritional, and Industrial Enzymology; Bharati, S.L., Chaurasia, P.K., Eds.; Advances in Medical Technologies and Clinical Practice (AMTCP) Book Series; Medical Information Science Reference: Hershey, PA, USA, 2018; pp. 159–177. [Google Scholar]
- Jaffur, B.N.; Kumar, G.; Jeetah, P.; Ramakrishna, S.; Bhatia, S.K. Current advances and emerging trends in sustainable polyhydroxyalkanoate modification from organic waste streams for material applications. Int. J. Biol. Macromol. 2023, 253, 126781. [Google Scholar] [CrossRef]
- Zolqadri, R.; Heidari Damani, M.; Malekjani, N.; Saeed Kharazmi, M.; Mahdi Jafari, S. Rice bran protein-based delivery systems as green carriers for bioactive compounds. Food Chem. 2023, 420, 136121. [Google Scholar] [CrossRef]
- Javed, S.; Azeem, F.; Hussain, S.; Rasul, I.; Siddique, M.H.; Riaz, M.; Afzal, M.; Kouser, A.; Nadeem, H. Bacterial lipases: A review on purification and characterization. Prog. Biophys. Mol. Biol. 2018, 132, 23–34. [Google Scholar] [CrossRef]
- Lim, S.Y.; Steiner, J.M.; Cridge, H. Lipases: It’s not just pancreatic lipase! Am. J. Vet. Res. 2022, 83. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Bhunia, B. Industrial Application of Lipase: A Review. Biopharm. J. 2015, 1, 41–47. [Google Scholar]
- Anobom, C.D.; Pinheiro, A.S.; De-Andrade, R.A.; Aguieiras, E.C.G.; Andrade, G.C.; Moura, M.V.; Almeida, R.V.; Freire, D.M. From structure to catalysis: Recent developments in the biotechnological applications of lipases. BioMed Res. Int. 2014, 2014, 684506. [Google Scholar] [CrossRef]
- Kudanga, T.; Prasetyo, E.N.; Sipilä, J.; Nousiainen, P.; Widsten, P.; Kandelbauer, A.; Nyanhongo, G.S.; Guebitz, G. Laccase-Mediated Wood Surface Functionalization. Eng. Life Sci. 2008, 8, 297–302. [Google Scholar] [CrossRef]
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, H.; Wu, X.; Yu, H.; Lou, S.; Sun, F.; Han, S.; Zhang, Y.; Xu, H. A new strategy for the hydrophobization of bamboo via laccase catalyzed dodecyl gallate coupling with hydrothermal pretreatment. Ind. Crops Prod. 2023, 192, 115992. [Google Scholar] [CrossRef]
- Fayaz, G.; Soleimanian, Y.; Mhamadi, M.; Turgeon, S.L.; Khalloufi, S. The applications of conventional and innovative mechanical technologies to tailor structural and functional features of dietary fibers from plant wastes: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2149–2199. [Google Scholar] [CrossRef]
- Badali, E.; Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic Crosslinked Hydrogels for Biomedical Application. Polym. Sci. Ser. A 2021, 63, S1–S22. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Liu, Y. A Review of the Mechanism, Properties, and Applications of Hydrogels Prepared by Enzymatic Cross-linking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef]
- Araujo, T.R.; Bresolin, D.; Oliveira D de Sayer, C.; Araújo, P.H.H.; de Oliveira, J.V. Conventional lignin functionalization for polyurethane applications and a future vision in the use of enzymes as an alternative method. Eur. Polym. J. 2023, 188, 111934. [Google Scholar] [CrossRef]
- Mican, J.; Jaradat, D.M.; Liu, W.; Weber, G.; Mazurenko, S.; Bornscheuer, U.T.; Damborsky, J.; Wei, R.; Bednar, D. Exploring new galaxies: Perspectives on the discovery of novel PET-degrading enzymes. Appl. Catal. B Environ. 2024, 342, 123404. [Google Scholar] [CrossRef]
- Emisha, L.; Wilfred, N.; Kavitha, S.; Halder, G.; Haldar, D.; Patel, A.K.; Singhania, R.R.; Pandey, A. Biodegradation of microplastics: Advancement in the strategic approaches towards prevention of its accumulation and harmful effects. Chemosphere 2024, 346, 140661. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Zhang, L.; Wu, J.; Zeng, X.; Shi, X.; Liu, L.; Chen, J. A comprehensive review on enzymatic biodegradation of polyethylene terephthalate. Environ. Res. 2024, 240, 117427. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Fixe, F.; Dufva, M.; Telleman, P.; Christensen, C.B.V. One-step immobilization of aminated and thiolated DNA onto poly(methylmethacrylate) (PMMA) substrates. Lab Chip 2004, 4, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Fixe, F. Functionalization of poly(methyl methacrylate) (PMMA) as a substrate for DNA microarrays. Nucleic Acids Res. 2004, 32, e9. [Google Scholar] [CrossRef] [PubMed]
- Sebra, R.P.; Masters, K.S.; Bowman, C.N.; Anseth, K.S. Surface Grafted Antibodies: Controlled Architecture Permits Enhanced Antigen Detection. Langmuir 2005, 21, 10907–10911. [Google Scholar] [CrossRef]
- Cen, L.; Neoh, K.G.; Ying, L.; Kang, E.T. Surface modification of polymeric films and membranes to achieve antibacterial properties. Surf. Interface Anal. 2004, 36, 716–719. [Google Scholar] [CrossRef]
- Chevallier, P.; Janvier, R.; Mantovani, D.; Laroche, G. In vitro Biological Performances of Phosphorylcholine-Grafted ePTFE Prostheses through RFGD Plasma Techniques. Macromol. Biosci. 2005, 5, 829–839. [Google Scholar] [CrossRef]
- Xu, Z.-K.; Dai, Q.-W.; Wu, J.; Huang, X.-J.; Yang, Q. Covalent Attachment of Phospholipid Analogous Polymers to Modify a Polymeric Membrane Surface: A Novel Approach. Langmuir 2004, 20, 1481–1488. [Google Scholar] [CrossRef]
- Hodgman, C.E.; Jewett, M.C. Cell-free synthetic biology: Thinking outside the cell. Metab. Eng. 2012, 14, 261–269. [Google Scholar] [CrossRef]
- Kelwick, R.J.R.; Webb, A.J.; Freemont, P.S. Biological Materials: The Next Frontier for Cell-Free Synthetic Biology. Front. Bioeng. Biotechnol. 2020, 8, 399. [Google Scholar] [CrossRef]
- Endoh, T.; Kanai, T.; Sato, Y.T.; Liu, D.V.; Yoshikawa, K.; Atomi, H.; Imanaka, T. Cell-free protein synthesis at high temperatures using the lysate of a hyperthermophile. J. Biotechnol. 2006, 126, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Lepthien, S.; Merkel, L.; Budisa, N. In vivo double and triple labeling of proteins using synthetic amino acids. Angew. Chem. 2010, 49, 5446–5450. [Google Scholar] [CrossRef]
- Schipp, C.J.; Ma, Y.; Al-Shameri, A.; D’Alessio, F.; Neubauer, P.; Contestabile, R.; Budisa, N.; Di Salvo, M.L. An Engineered Escherichia coli Strain with Synthetic Metabolism for in-Cell Production of Translationally Active Methionine Derivatives. ChemBiochem 2020, 21, 3525–3538. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Ciesielski, S.; Kosewska, O. Changes in the gut microbiome and enzymatic profile of Tenebrio molitor larvae biodegrading cellulose, polyethylene and polystyrene waste. Environ. Pollut. 2020, 256, 113265. [Google Scholar] [CrossRef]
- Brandon, A.M.; Garcia, A.M.; Khlystov, N.A.; Wu, W.-M.; Criddle, C.S. Enhanced Bioavailability and Microbial Biodegradation of Polystyrene in an Enrichment Derived from the Gut Microbiome of Tenebrio molitor (Mealworm Larvae). Environ. Sci. Technol. 2021, 55, 2027–2036. [Google Scholar] [CrossRef]
- Galloway-Peña, J.; Hanson, B. Tools for Analysis of the Microbiome. Dig. Dis. Sci. 2020, 65, 674–685. [Google Scholar] [CrossRef]
- Karan, R.; Capes, M.D.; Dassarma, S. Function and biotechnology of extremophilic enzymes in low water activity. Aquat. Biosyst. 2012, 8, 4. [Google Scholar] [CrossRef]
- Laye, V.J.; Solieva, S.; Voelz, V.A.; Dassarma, S. Effects of Salinity and Temperature on the Flexibility and Function of a Polyextremophilic Enzyme. Int. J. Mol. Sci. 2022, 23, 15620. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Meunier, B. How to Define a Nanozyme. ACS Nano 2022, 16, 6956–6959. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protte-Freitag, K.; Gotzig, S.; Rothe, H.; Schwarz, O.; Silber, N.; Miehe, R. Enzyme-Assisted Circular Additive Manufacturing as an Enabling Technology for a Circular Bioeconomy—A Conceptual Review. Sustainability 2024, 16, 2167. https://doi.org/10.3390/su16052167
Protte-Freitag K, Gotzig S, Rothe H, Schwarz O, Silber N, Miehe R. Enzyme-Assisted Circular Additive Manufacturing as an Enabling Technology for a Circular Bioeconomy—A Conceptual Review. Sustainability. 2024; 16(5):2167. https://doi.org/10.3390/su16052167
Chicago/Turabian StyleProtte-Freitag, Kristin, Sophia Gotzig, Hannah Rothe, Oliver Schwarz, Nadine Silber, and Robert Miehe. 2024. "Enzyme-Assisted Circular Additive Manufacturing as an Enabling Technology for a Circular Bioeconomy—A Conceptual Review" Sustainability 16, no. 5: 2167. https://doi.org/10.3390/su16052167
APA StyleProtte-Freitag, K., Gotzig, S., Rothe, H., Schwarz, O., Silber, N., & Miehe, R. (2024). Enzyme-Assisted Circular Additive Manufacturing as an Enabling Technology for a Circular Bioeconomy—A Conceptual Review. Sustainability, 16(5), 2167. https://doi.org/10.3390/su16052167






