Abstract
Rapid urbanization worldwide results in high demand for meat products, which in turn result in high numbers of animals being slaughtered for human consumption to meet food security demands, especially in low-income countries such as South Africa. The waste produced during slaughtering can serve as feedstock for biogas production. This study aims to determine the impacts of pasteurization and sterilization pre-treatments on high-throughput red meat abattoir solid slaughter waste’s physicochemical properties and biomethane yield when used as a feedstock for biogas production. Abattoir solid slaughter waste was collected from 45 high-throughput red meat abattoirs across South Africa and the various physicochemical properties were determined using standard methods, along with the impact of sterilization and pasteurization on red meat abattoir waste. Biomethane yield analysis was performed using AMPTS II with a hydraulic retention time of 40 days. Pasteurization and sterilization pretreatment was seen to increase physicochemical parameters such as pH, volatile solids, total solids, carbon, and nitrogen analyzed in all samples. Pasteurization and sterilization were also seen to increase biomethane yield, where methane production ranged from 610.67 Nml to 1756.30 Nml, 1592.20 Nml to 3319.30 Nml, and 949.57 Nml to 3297.87 Nml for untreated, sterilized, and pasteurized samples, respectively. There was no significant difference (p < 0.05) observed in the effect pasteurized and sterilized samples had on physicochemical properties and biomethane yield. It can be concluded that pasteurization and sterilization enhance the bioavailability of the physicochemical properties and biomethane yield of red meat solid slaughter waste when valorized as feedstock for biogas production.
1. Introduction
The growing demand for meat worldwide for human consumption has made the meat production industry become the fastest-growing agricultural-based industry in the world [1,2]. A steady growth in meat production and consumption has been estimated at a further 10 billion in developing countries and at 1.5 billion in industrialized countries by the year 2050 [2,3]. Most low-income countries have shown rapid urban growth, which results in extreme pressure for more animals to be slaughtered at abattoirs and in turn produces a large amount of slaughter waste [4,5,6]. The waste produced during slaughtering activities includes meat trimmings, fats, rumen contents, condemned organs, blood, green and red offal, and wastewater [7,8]. Approximately 20–50% of the weight of the slaughtered animal is not suitable for human consumption [1,2,3,4,5,6,7,8]. This waste, if not managed well, may be hazardous to the environment and animal and human health by spreading diseases and other toxins [7,8,9].
The European Commission has enforced regulation for abattoir slaughter waste (1069/2009/EC) [10] that requires stricter management control and higher hygienic treatment for this type of waste to prevent the outbreak and spread of disease and other environmental contaminants [11,12,13]. The implementation of this regulation has been reported in the literature to reduce the economic value of abattoir waste for rendering, thus resulting in this waste being disposed of using incineration [14]. Valuable nutrients which are contained in abattoir waste that can be beneficial for plants and soil are reported to be lost through incineration and can be harnessed using green technologies such as biogas production using anaerobic digestion (AD) [7,9]. AD is defined as the degradation of organic waste by microorganisms in the absence of oxygen to produce two by-products, i.e., biogas and bio-slurry (digestate). The 1069/2009/EC [10] regulation regards biogas production through AD as a suitable treatment and waste management option for solid abattoir slaughter waste, provided that thermal pre-treatment, either by pasteurization (70 °C for 1 h) or sterilization (133 °C at 3 bar for 15 min), is incorporated into the digestion process as a sanitary pre-caution [7,8,9]. In South Africa, category two and three abattoir slaughter waste is also mandated by the National Environmental Management (NEMA): Waste Act 59 of 2008 section 24(5) and Meat Safety Act (Act 40 of 2000) to be subjected to either pasteurization (70 °C for 1 h) or sterilization (133 °C at 3 bar for 15 min) prior to any biological treatment, including AD.
South Africa is a developing country with an agriculture-intensive industry, currently comprising about 430 abattoirs, with about 285 high-throughput red meat abattoirs registered, slaughtering over a million cattle, pigs, and sheep on an annual basis. South Africa is also the largest meat producer and exporter in Africa [9,15,16]. Currently, waste management at high-throughput red meat abattoir facilities in South Africa is difficult, and most local municipalities across the country have banned the disposal of this waste, especially solid slaughter waste in landfills, due to the high cost incurred during its treatment. This waste has become a hazard to the local communities living in the vicinity of the abattoir facilities, as most of this waste ends up being illegally dumped or buried in nearby fields, rivers, and streams. However, this waste can be converted into useful clean energy known as biogas (to assist in combating the current electricity crisis), and fertilizer in the form of a treated slurry known as digestate through the AD process, thus promoting a climate-smart agriculture through a circular economy (waste-energy).
Currently onsite treatment of red meat abattoir solid slaughter waste in South Africa using this waste as feedstock in AD for biogas production is gaining interest from local governments, the agricultural sector, the biogas industry, and the red meat abattoir industry. The AD of abattoir solid slaughter waste is not only explored as a treatment and management option but also to address the National Development Plan of reducing greenhouse gases by 50% by 2030 [7,9]. Treatment of abattoir solid slaughter waste using anaerobic digestion has been used in countries such as the USA, China, Canada, India, Tanzania, and European countries, incorporating thermal pre-treatment (pasteurization or sterilization) to enhance the biodegradability of the waste [2,16].
Research studies conducted on the effects of thermal pre-treatment of abattoir solid slaughter waste prior to AD have highlighted its ability to increase the breakdown of recalcitrant complex polymeric compounds present within the feedstock by increasing the solid reduction efficiency of the AD process [17,18,19,20,21,22]. A study by Harris and McCabe [23] observed an increase in biomethane production on pasteurized feedstock (co-digestion of food waste and cattle slaughter waste) under mesophilic conditions. A study by Liu et al. [24] also observed similar outcomes when using a digestion mixture of food waste, manure, and cattle slaughter waste. A study by Rodriguez-Abalde et al. [25] evaluated the effects of thermal pre-treatments on the methane yield of two solid slaughterhouse wastes, poultry, and pig slaughterhouse by-products, and observed that pasteurization and sterilization had no effect on biomethane production. Meanwhile, a study by Cuetos et al. [26] also reported no difference in biomethane production in the co-digestion of poultry slaughter waste and organic fraction of municipal solid waste (OFMSW). A study by Hejnfelt and Angelidaki [27] also observed similar results when evaluating the effects of pasteurization and sterilization on biomethane production of mixed pig and cattle slaughter waste and reported no significant increase (p < 0.05) in biomethane yield, observing instabilities in gas production in sterilized feedstock. In contrast to this, Baredar et al. [28] observed a four-fold increase in biomethane yield in pasteurized mixtures of cattle and pig slaughter waste by-products, food waste and liquid manure. Thermal pre-treatment of feedstock has been reported to also enhance the hydrolysis stage, promote digester stability, and accelerate the supply of nutrients required for microbial growth [12,19,20,21,29,30,31,32,33,34,35]. Even though sterilization and pasteurization of feedstock has been reported to enhance biomethane production, the results observed in the literature on its effect on biomethane yield vary with contradictory observations.
Study on the effects of mandatory pasteurization and sterilization pre-treatment of solid red meat slaughter waste used in mono-digestion on its physicochemical properties and biomethane production is limited worldwide, especially in South Africa, thus this study intends to close the gap by adding this knowledge to the literature. This study will also fill in research gaps by also evaluating the use of sheep solid slaughter waste as feedstock for AD, as research on this type of waste stream for biogas production has been minimally reported worldwide. As the biogas industry in South Africa is in its infancy stage and is currently initiating the use of solid slaughter waste from red meat abattoirs as potential feedstock for biogas production using AD, and to treat this waste, the existing knowledge on the effects of the mandatory thermal pre-treatment on physicochemical properties and biomethane yield is vital for policy making.
The highlights of AD during biogas production are grouped into three steps, i.e., (1) feedstock characterization (composition of the raw organic waste prior to AD); (2) biomethane production (during the AD process); (3) digestate characterization (final treated bio-slurry after AD process). The current study is related to the previous published study by Matjuda et al. [36], where the main focus was characterizing the final treated by-product, which is digestate, and its biofertilizer potential as well as its sanitary quality (step 3). The previous study by Matjuda et al. [36] did not present the physicochemical characteristics of red meat abattoir solid slaughter waste feedstock prior to AD (step 1) (using similar techniques) and its biomethane yield (step 2), hence this study intends to present the data and also fill the knowledge gaps stated in this current study. This study may serve as a model to develop processes to harvest value-added products such as green energy and biofertilizer from solid slaughter waste rather than disposing it in landfills, thus causing various forms of environmental pollution and promoting a circular economy through waste to energy. The data in this study may be beneficial for other researchers and organizations when implementing proper treatment processes and quality control standards and may also benefit the red meat industry in its waste treatment management processes.
Therefore, this project aims to determine the impacts of pasteurization and sterilization pre-treatments on red meat abattoir solid slaughter waste’s physicochemical properties and biomethane yield when used as a feedstock for biogas production.
2. Materials and Methods
2.1. Study Area
The study area is represented by 45 high-throughput red meat abattoirs selected from across South Africa. South Africa is a subtropical country located in the southernmost part of Africa, which covers an area of about 1,221,037 square kilometers, with a coastline of about 2798 km which stretches along the Atlantic and Indian Ocean. The country has a temperate climate due to it being surrounded by two oceans (Atlantic and Indian Ocean) on all three sides, as it is located in the climatically milder Southern Hemisphere. South Africa is a water-scarce country with an average rainfall of about 464 mm per annum. The list of the locations of sampling sites are presented in Table 1.

Table 1.
List of sampling sites of abattoirs for this study.
2.2. Sample Collection and Preparation
About 180 abattoir slaughter waste samples were collected from 45 high-throughput red meat abattoirs across South Africa. High-throughput abattoirs slaughtering more than 250 animals per week, obtained from feedlots and commercial farmers, were selected to partake in this study. Abattoirs were also selected according to the type (red meat only: cattle, sheep, and pig) of animals they slaughtered daily and the consent from abattoir management to partake in the study.
Abattoir slaughter waste such as meat trimmings, blood, offal (green and red), intestinal organs, and rumen contents were collected for a period of a year from March 2021 to February 2022. Samples including rumen contents, offal (green and red), intestinal organs, and meat trimmings were collected in polyester bags, while blood samples were collected in 1 L sterilized containers according to Matjuda et al. [36]. Samples were transported and prepared for physicochemical analysis in accordance with Matjuda et al. [35]. Samples were transported on ice from sampling points to the laboratory where they were cleaned by removing non-digestible materials such as plastics, tags, metals, stones, and bones. After the sampling period, similar waste from 45 high-throughput abattoir sampling sites was pooled together. From the pooled slaughter waste, a total of 20 representative samples for each treatment (i.e., untreated, sterilization, and pasteurization) were taken out for cattle, sheep, and pig slaughter waste (e.g., cattle slaughter waste had 20 for untreated, 20 for sterilization, and 20 for pasteurization). The rationale for pooling samples was that this study was not concentrated on determining the differences in characteristics of abattoir waste according to different sampling sites or sampling periods, or differences within similar or different animal breeds. This was because this study was concentrated on evaluating the biomethane potential of solid red meat abattoir slaughter waste as a single waste stream feedstock for biogas production and the effects thermal pretreatment has on its biogas yield and physicochemical properties. The pooling was also performed to assimilate commercial and medium-scale biogas plants’ operational condition in South Africa for treating red meat abattoir slaughter waste, where samples are pooled in accordance to waste types, i.e., cattle, sheep, and pig, and not according to sample location and breed. This is because the study was conducted to provide support in decision and policy making for both the biogas industry and red meat abattoirs in South Africa as to which thermal pre-treatment method to incorporate in their on-site biogas plant for enhancing the sanitary treatment of produced slaughter waste.
After sample preparation, samples were then kept in the freezer at −20 °C till further analysis. The samples were blended to a size of about 2 mm using a food processor and pre-treated using pasteurization (70 °C for 1 h) and sterilization (133 °C at 3 bar for 15 min) prior to AD, according to the Regulation (EC) No. 1069/2009 [10]. Abattoir waste that was not subjected to thermal pre-treatment is referred to as untreated abattoir samples in this study. After pre-treatment, samples were analyzed for their physicochemical properties and biomethane production. All analyses were performed in triplicate.
2.3. Determination of Feedstock’s Physicochemical Characteristics
The following physicochemical analyses were conducted on abattoir samples in accordance with methods described by Matjuda et al. [36]: pH, total solids (TS), volatile solids (VS), moisture content, chemical oxygen demand (COD), ammonia (NH4+), carbon, nitrogen (N). The VS (organics) were further broken down into primary constituents of fats, proteins and carbohydrates, where fats and proteins were determined in accordance with Commission Regulation 142/2011/EU, implementing the ABPR (European Commission, 2009, European Commission, 2011) as described by [37]. The difference between VS, fats, and protein content was designated as carbohydrates. The pH was read using a PHC101 probe and the pH meter was calibrated using buffers of pH 4.0, 7.0, and 10.0 (Hach (Pty) Ltd., Johannesburg, South Africa). NH4+ concentrations were read using a spectrophotometer (HACH DR 500) at absorbance of 495 nm and calculated against standard solutions of known concentrations. Determination of COD were read using a spectrophotometer (HACH DR 500) with the high COD range test program. The absorbance was measured spectrophotometrically at 495 nm using HACH DR 500 and all physicochemical properties analysis were conducted in triplicate.
2.4. Determination of Biomethane Production Potential of Abattoir Waste as AD Feedstock
The biochemical methane production potential tests were carried out using the Automatic Methane Potential Test System (AMPTS®) bioprocess control AMPTS II laboratory scale anaerobic digester. This instrument is made up of three components: (i) digester, (ii) CO2 fixing unit, and (iii) gas collecting unit (Figure 1). A batch system was set up and allowed to run for a period of 40 days hydraulic retention time (HRT). A 500 mL digester, with an effective volume of 400 mL, was used for biomethane production, which had a headspace of 150 mL. The experiment was conducted in triplicate. The temperature of the process was kept constant at a mesophilic temperature of 37 °C. A 3M NaOH solution (Sigma Aldrich (Pty) Ltd., Johannesburg South Africa) (120 g NaOH in ¾ of 1 L water) was prepared to be used as the scrubbing solution to absorb the impurities (automated by the AMPTS II system).
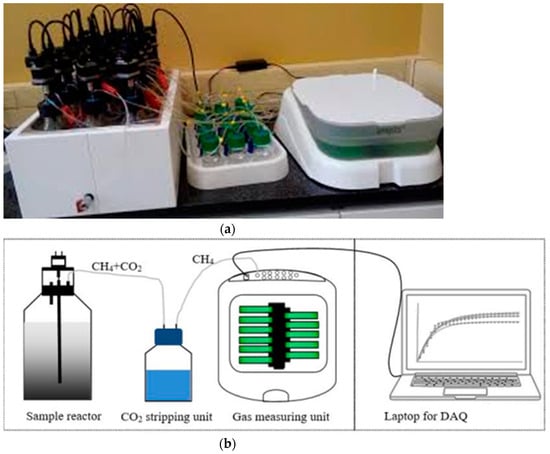
Figure 1.
(a) The bioprocess reactor used for BMP tests (AMPTS II); (b) schematic diagram of AMPTS II system. (Adopted from Xia et al. [38]).
A 0.4% Thymolphthalein solution was used as a pH-indicator to determine the saturation point for the cleaning solution to be replaced. Approximately 40 mg of Thymolphthalein was dissolved in 9 mL of ethanol (99.5%) followed by the addition of 1 mL distilled water. Thymolphthalein is insoluble in water, but it is freely soluble in ethanol. The NaOH solution containing the pH indicator was prepared by mixing 5 mL of the 0.4% Thymolphthalein solution per 1 L of 3M NaOH solution. Approximately 80 mL of the mixture containing the NaOH solution and Thymolphthalein pH indicator was added into each of the 15 glass bottles (100 mL) for the AMPTS II.
The biomethane production experiment was run using 500 mL digester bottles filled with 400 mL of sample, where inoculum was used as a negative control and cellulose as a positive control. The digesters were filled with a mixture of red meat abattoir samples and inoculum at a ratio of 2:1 (inoculum: substrate). The inoculum was a digestate taken from a commercial biogas plant treating abattoir slaughter waste located in Gauteng. The physicochemical characteristics of the inoculum are presented in Table 2. To limit background gas produced during the experiment, the inoculum was degassed by incubating it under a similar operational temperature range to the commercial and medium-scale biogas plant digesters and the experimental set-up (37 ± 2 °C).

Table 2.
Characteristics of inoculum used in AMPTSII experiment.
Prior to the digestion, the pH of the mixed liquor in the bioreactor was adjusted to 7.0 with the aid of NaOH (3M) and HCl (3M). To create an anaerobic environment in the bioreactors, nitrogen gas (N2) (100%) from Afrox Gas, South Africa was used to purge each reactor before starting the stirrers. The daily and cumulative biomethane production were collected using an in-built data-logger in the AMPTS II. The digester was connected to a 100 mL bottle (used as a scrubber) filled with 80 mL of the 3M NaOH solution. The gas exiting the CO2 fixing unit was sent to the flow cell (gas collection) where the volume of biomethane was determined as shown in Figure 1b.
2.5. Analysis of Biogas Composition of Abattoir Waste
A portable biogas analyzer, BIOGAS 5000 (Geotech, UK) was used to analyze the contents of the produced gas.
2.6. Determination of Solid Reduction Efficiency of AD of Abattoir Waste
The reduction efficiency of COD, VS, and TS during AD of abattoir waste were determined at the end of digestion, i.e., day 40. Solid reduction efficiency was calculated according to the equation below adopted from Latimer [39].
where:
- SR: solid reduction
- RW: raw waste
- ADW: anaerobic digested waste
It should also be noted that subsampling was not performed as the AMPTS II is a batch AD digester instrument (closed system).
2.7. Statistical Analysis
All analyses in this study were performed in triplicate. The statistical significance of the results was confirmed by two-way ANOVA (p < 0.05). The differences of each treatment were studied by t-test. For statistical analysis, the GENSTAT statistical program was used.
3. Results and Discussions
3.1. Impact of Thermal Pre-Treatment on the Physicochemical Properties of Red Meat Abattoir Solid Waste
The results for pH, TS, VS, and moisture characterization of abattoir feedstock are presented in Table 2. The pH of red meat abattoir slaughter waste feedstock was measured prior to AD at day 0. pH is a very important parameter in AD as it influences the stability of the digestion process [17,40,41,42]. The pH range for stability of anaerobic digestion has been recommended to be within the range of 6.5 to 8.5, with an optimal range of about 6.8 to 7.2 for stabilized anaerobic digestion and microbial activity [43,44,45]. The pH values for pre-treated samples were observed to be >6.5 in this study in all pre-treated samples (Table 3), thus indicating the ability of pasteurization and sterilization pre-treatments to enhance the stability of abattoir waste feedstock prior to AD. The pH values for pasteurized and sterilized pre-treated samples did not differ significantly (p ≤ 0.05) across all analyzed red meat abattoir slaughter samples, while pH values for untreated and pre-treated samples differed significantly (p ≤ 0.05). This may be attributed to the production of hydroxide ions (OH−) being higher than the production of hydrogen ions (H+) during pre-treatment. According to Budiyono et al. [46], Islam et al. [47], and Calicioglu et al. [46], methanogens thrive in slightly alkaline to neutral environments (6–8.5). The results observed in this study for both pasteurized and sterilized pre-treated samples fell within the optimal range of 6.5 to 8.5 for stable anaerobic digestion and microbial activity. This indicates that sterilization and pasteurization have the ability to create a more conducive environment for methanogen activity by offering sufficient buffering capacity against pH drop [41,47,48,49]. The results for pH reported in this study were lower as compared to those reported by Budiyono et al. [46], who observed a pH of 7.19 ± 0.06. The difference in results for pH observed in this study and those of Budiyono et al. [46] may be attributed to the difference in feedstock composition, as in their study, the abattoir waste included manure from holding pens and wastewater, which were not included in this study. Results observed in this study were similar to those reported by Bayr et al. [49], where a pH value range of 6.0 to 6.8 was reported.

Table 3.
Results for the pH, moisture, TS, and VS for untreated, sterilized, and pasteurized cattle, sheep, and pig abattoir slaughter waste feedstock. Results represent mean ± standard deviation (n = 20).
Results for VS and TS (Table 3) in this study were observed to be higher in pasteurized and sterilized samples as compared to untreated samples for cattle, sheep, and pig abattoir slaughter waste feedstock. The VS content of feedstock in AD is a very important parameter, as it determines the amount of organic matter contained in the feedstock that is biodegradable. The increase in TS contents in both sterilized and pasteurized pre-treated as compared to untreated samples may be attributed to the water contents of the red meat abattoir waste evaporating due to the high temperature, thus indicating that sterilization and pasteurization have the ability to enhance the solubilization of the pre-treated samples [50,51]. Results for TS and VS in this study differed significantly across untreated, pasteurized, and sterilized pre-treated samples (p ≤ 0.05). According to the literature, the VS of abattoir solid slaughter waste, comprising rumen content, intestinal organs, and meat trimmings, were reported to be around 80% of TS [50,51].
According to Jha et al. [51], a VS value ≥ 60% depicts the high proportion of feedstock fraction that is biodegradable and could be utilized as feedstock for mono-digestion for biogas production [16,40]. The VS values across all analyzed red meat abattoir samples in this study were observed to be >60%, with sterilized and pasteurized samples of VS being >72%. This indicated the effects of sterilization and pasteurization pre-treatment’s ability to enhance biodegradability in red meat abattoirs [51,52,53]. These increases in VS can be attributed to the capability of the high temperature of the pasteurization and sterilization imposed to release more volatile compounds within the samples, making them more readily available for digestion by anaerobes [51,52]. The results obtained in this study for TS and VS were similar to those observed by Alarcon et al. [52] and were lower compared to those reported by Ware and Power [14]. The difference in observed results for VS and TS in this study compared to Ware and Power [14] may be owing to the difference in feedstock composition, as in this study only solid red meat abattoir waste was used as a single waste stream (mono-digestion), while in their study the abattoir waste was co-digested with other organic waste such as food waste and activated municipal sludge. The TS and VS results obtained in this study for sterilized pig abattoir waste were similar to those reported by Budiyono et al. [46], who observed VS and TS increase due to thermal pre-treatment using similar pasteurization and sterilization treatment conditions. Budiyono et al. [46] reported a VS of 66.6% in untreated slaughter waste, 76.0% in pasteurized samples, and 74.6% in sterilized samples, and a TS value of 22.4%, 24.1%, and 26.6% for untreated, pasteurized, and sterilized slaughter waste, respectively.
Feedstock moisture content is one of the most important factors affecting the efficiency of AD, more especially the hydrolysis phase [54,55]. Although results in previous studies suggest that the efficiency of AD increases as feedstock moisture levels increase [46,54,56], there is no information on optimum moisture levels reported. In support of the studies by Zamri et al. [55], Cvetković et al. [54], and Kabeyi and Olanrewaju [57], a study by Khumalo [54] reported that activated sludge with 95% moisture content had a hydrolysis phase of six days, while sludge with 88% moisture content had a hydrolysis phase of 30 days. Similar observations were also observed in this study in sterilized cattle and pig abattoir slaughter samples (Table 3), as the moisture contents were observed to be 63.09 ± 2.07% and 69.95 ± 0.98%, respectively, and the hydrolysis phase was observed to be slower compared to pasteurized and untreated samples during AD. Sterilization pre-treatment was observed to reduce more moisture content in cattle, sheep, and pig abattoir slaughter waste in this study compared to untreated and pasteurized samples, and the results differed significantly (p < 0.05). This may be attributed to rapid water evaporation in the samples due to high temperatures during sterilization treatment [51,52,53]. There was no significant difference (p < 0.05) in the moisture content in cattle, sheep, and pig abattoir slaughter waste observed between untreated and pasteurized samples in this study (Table 3).
The results for COD concentration are presented in Table 4; in this study, COD varied from 12,530.35 ± 538.22 mg/L to 15,384.9 ± 333.17 mg/L across the studied abattoir waste samples (p ≤ 0.05). Pasteurization and sterilization pre-treatment in this study was observed to reduce COD concentration in abattoir samples; this may be due to the protein denaturing effect of high temperatures [52,58]. According to Otero et al. [53], Ortner et al. [59], and Obileke et al. [60], reduction in COD in feedstock occurs due to the degradation of complex organic compounds, which in turn increases the stability of the AD process by reducing organic acid production during digestion that will cause pH drop, thus enhancing microbial metabolism during AD. The results in this study were higher than those reported by Khumalo [54], Budiyono et al. [46], and Otero et al. [53], and were observed to be lower than those reported by Ortner et al. [59]; this is owing to the difference in feedstock composition.

Table 4.
Physicochemical composition of cattle, sheep, and pig slaughter waste. Results represent mean ± standard deviation (n = 20).
The organic carbon produced during AD of red meat abattoir waste is mostly produced during the degradation of carbohydrates by microorganisms [57,58]. Carbon as well as nitrogen are essential nutrients for regeneration and growth of methanogens during the AD process [54,59]. The results for carbon concentrations are presented in Table 4. In this study, carbon concentrations differed significantly (p ≤ 0.05) between untreated abattoir samples and treated abattoir samples. Carbon concentrations for pasteurized and sterilized samples were higher as compared to untreated samples; this may be due to the composition of carbohydrates being mostly carbon and water, and during heating the water evaporated, thus leaving C as the most available element within the feedstock [60]. The results obtained in this study were lower compared to those reported by Khumalo [54] and Ware and Power [14], who observed a C concertation of 65.60 ± 0.3% TS and 65.8% TS, respectively, in solid abattoir waste, which consisted of condemned meat, offal, and blood. In their study, Ware and Power [14] examined solid red meat abattoir feedstock that was co-digested with dissolved air flotation sludge and wastewater, while Khumalo [54] examined solid slaughter waste co-digested with winery sludge waste, whereas in this study, only solid red meat slaughter waste was used for mono-digestion.
Nitrogen (N) concentrations in this study were observed to range from 1.89 ± 0.74% to 2.68 ± 0.81% (p < 0.05) across the studied abattoir samples, with the highest nitrogen levels observed in sterilized cattle abattoir waste (Table 4). The C/N ratio is very important in AD of organic waste, as methanogens use carbon from the degradation of carbohydrates and N from the proteins as an energy source to produce biogas [60,61,62]. The optimum C/N ratio for AD was recommended in previous studies to range from 15 to 30 [62,63,64,65,66,67,68,69,70,71]. The results for the C/N ratios observed in this study (Table 4) ranged from 18.12 ± 0.41 to 23.91 ± 0.88 across the analyzed red meat abattoir waste and were within the recommended range, thus suggesting that abattoir waste can serve as a good substrate as a single waste stream for biogas production [66,72].
According to Wang et al. [17], Nnaemeka [43], and Kigozi et al. [45], it is important for the C/N ratio not to be higher than 30, as this may result in the quick depletion of nitrogen by bacteria, thus causing carbon to be in excess in the digester, which may result in low biogas yield. The low C/N ratio (<10) was also reported by Nnaemeka [43], Kigozi et al. [45], and Xia et al. [38], resulting in excess nitrogen in the digester, leading to the formation of ammonia, thus resulting in an increase in pH (>8.5) and a reduced biogas yield due to microbial inhibition. If the C/N ratio is higher or lower than the recommended range, co-digestion is recommended to increase the digestion process and increase biogas yield [17,38,42,45]. The C/N ratios observed in this study in untreated, pasteurized, and sterilized cattle, sheep, and pig slaughter waste were >10 and <30, indicating that red meat abattoir waste can be utilized as a single waste stream in AD. Pasteurization and sterilization pre-treatment was observed to increase the C/N ratio in this study, thus supporting previous observations reported by Kigozi et al. [45] and Xia et al. [38] that thermal pre-treatment has the ability to enhance the solubilization of organic matter, thereby enhancing feedstock biodegradability by microorganisms during AD.
The results for NH4+ in this study are presented in Table 4 and ranged from 1.03 ± 0.09 g/L to 3.08 ± 0.31 g/L (p < 0.05) across the analyzed red meat abattoir slaughter waste. NH4+ was higher in sterilized cattle, sheep, and pig samples compared to untreated and pasteurized samples. This may be attributed to the higher temperatures of sterilization compared to pasteurization enhancing Maillard reactions in the feedstock, which may have produced sugar-amino acid compounds with low biodegradability. According to Wang et al. [17], NH4+ concentrations >3000 mg L−1 may cause inhibition of microbial growth during AD. The results obtained in this study were within the recommended limits reported by Wang et al. [17], except for the sterilized cattle abattoir waste, which was observed to have an NH4+ concentration of about 3.08 ± 0.31 g/L.
The studies by Wang et al. [17], Zhang et al. [18], and Renggaman et al. [73] reported that high pH (>8.5) and high temperature (>90 °C) were reported to increase NH4+, thus causing inhibition to microbial activity due to its high permeability to bacterial cell membranes and, as a result, reducing biomethane yield. That was evident in this study, as sterilization of cattle slaughter waste was conducted at 133 °C and resulted in NH4+ > 3000 mg/L. However, Edström et al. [28] indicated that when NH4+ concentration is >3000 mg/L, the AD process, particularly methanogen activity, is inhibited at any pH level, thus resulting in lower biomethane yield. That was also evident in this study, as sterilized cattle slaughter waste samples recorded a pH of 6.96, which was observed to be higher compared to other analyzed red meat slaughter samples, and biomethane yield was lower in sterilized cattle samples compared to untreated and pasteurized cattle samples from day 1 to day 23 HRT. In a similar study, Vavilin et al. [74] reported that an NH4+ concentration of 2500 mg/L inhibited methane production, while an NH4+-N concentration of 3300 mg/L inhibited methanogenesis, completely irrespective of pre-treatment temperature and pH. Similar observations reported by Vavilin et al. [74] were also noted in sterilized pig abattoir waste in this study, where NH4+ was 2.36 ± 0.36 g/L, though lower compared to the reported value of 2500 mg/L; methane production was lowest compared to untreated and pasteurized pig abattoir waste from day 1 to day 8 HRT.
In order to control NH4+ inhibition in AD, previous researchers reported that enhancing methanogens activities by acclimatizing and immobilizing microorganisms in the digester, along with pH (optimal range of 6.8–7.2) and temperature control, may contribute to the reduction of NH4+ concentration [14,17,71,75,76,77]. During this study, to minimize NH4+ toxicity in the digesters, the temperature was kept constant at 37 ± 2 °C (using a water bath) and acclimatization of the microorganism was not performed, which may have resulted in NH4+ toxicity in sterilized cattle and pig abattoir slaughter waste samples. As the AMPTS II is a closed system, it was not possible to monitor the pH value during the AD process, but the pH at day 40 of HRT was observed to range from 7.83 ± 0.06 to 8.11 ± 0.09 across the analyzed red meat abattoir samples in this study, which may indicate that the pH remained slightly alkaline throughout the AD process. The results obtained in this study for NH4+ were lower than those reported by Njoya [16], Tsegaye et al. [78], and Suanon et al. [79], who reported NH4+ concentrations were >4000 mg/L. The results of NH4+ concentrations observed in this study were also lower compared to those previously observed in AD of abattoir waste by Lauterböck et al. [80] and Musa et al. [81], where concentration of NH4+ exceeded 6000 mg/L.
The VS in the red meat slaughter waste was present in the form of protein, lipid, and carbohydrate contents (Table 4), with a concentration range of 33.45 ± 0.04% TS to 45.8 ± 1.25% TS, 13.21 ± 0.41% TS to 27.3 ± 0.77% TS, and 2.79 ± 0.19% TS to 14.24 ± 0.46% TS, respectively, for proteins, lipids, and carbohydrates across the analyzed samples in this study (p ≤ 0.05). As abattoir waste is characterized by high protein and lipid content, this was also observed in our study, as the concentration of proteins and lipids was higher compared to carbohydrate concentrations. Carbohydrate concentrations in untreated red meat abattoir slaughter samples in this study were observed to be higher than those of pasteurized and sterilized pre-treated samples, and results differed significantly across the analyzed samples (p ≤ 0.05).
The high carbohydrate concentration in untreated red meat slaughter waste was reported previously to be associated with partially digested lignocellulosic material, as well as the crude fiber concentration contained in ruminal content that was included as part of the feedstock in this study [82]. Pasteurization pre-treatments were also observed to increase carbohydrate concentration in cattle, sheep, and pig abattoir slaughter waste samples, attributed to the solubilization of complex molecules to simpler sugars which are easily biodegraded by methanogens during AD [73].
The high protein content in the abattoir samples in this study is consistent with the literature, where it has been reported that abattoir waste is rich in both proteins and lipids [73,74,75]. Pasteurization pre-treatment in this study was observed to increase the protein and lipid concentration as compared to untreated and sterilized samples due to the enhancement of organic matter solubilization. Sterilization had a negative impact on protein concentration, as the values were low compared to control and pasteurization treatments. This may be due to the denaturing effect of the high-temperature sterilization process on proteins [73,75].
3.2. Results: Impact of Thermal Pre-Treatment on Biomethane Potential Yield of Red Meat Abattoir Solid Slaughter Waste
Biomethane production for the studied abattoir waste samples in this study were performed with a 40-day HRT and the results are presented in Figure 2, Figure 3 and Figure 4. Biomethane accumulation in this study varied from 1756.33 Nml to 3297.87 Nml (1.77 m3CH4/kgVS to 3.30 m3CH4/kgVS) for cattle abattoir waste (Figure 2), 1125.4 Nml to 1821.73 Nml (1.23 m3CH4/kgVS to 1.82 m3CH4/kgVS) for sheep abattoir waste (Figure 3), and 610.67 Nml to 949.57 Nml (0.611 m3CH4/kgVS to 0.95 m3CH4/kgVS) for piggery waste (Figure 4). Biomethane production was observed from day 1 in all samples, and all pre-treated abattoir waste samples yielded more biomethane as compared to untreated abattoir waste samples at the end of the 40-day HRT. This can be attributed to the pre-treatment breaking down complex molecules into simpler substances, thus making them more bioavailable for degradation by methanogenic bacteria. The degradation of the complex molecules has been observed previously to enhance the hydrolysis stage of the anaerobic digestion process. This observation was also depicted in this study, where sterilized sheep abattoir slaughter waste samples and pasteurized samples yielded more biomethane in the early days of digestion as compared to untreated samples.
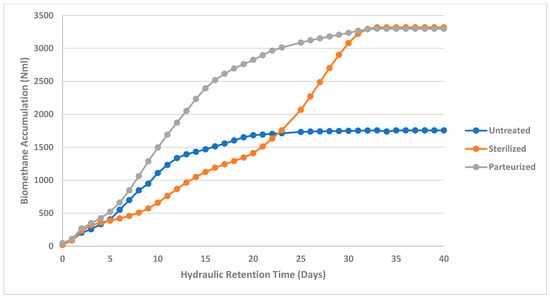
Figure 2.
Biomethane accumulation of cattle abattoir waste at a mesophilic temperature (37 °C) for untreated, sterilized, and pasteurized samples with an HRT of 40 days.
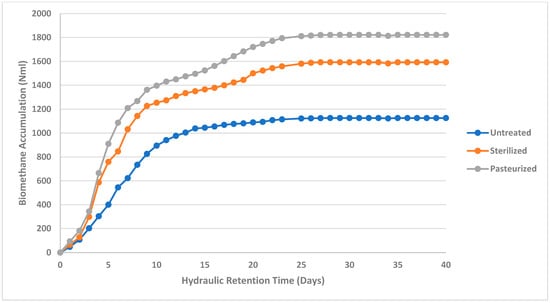
Figure 3.
Biomethane accumulation of sheep abattoir waste at a mesophilic temperature (37 °C) for untreated, sterilized, and pasteurized samples with an HRT of 40 days.
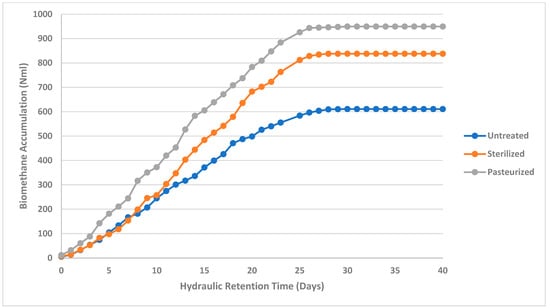
Figure 4.
Biomethane accumulation of pig slaughter waste at a mesophilic temperature (37 °C) for untreated, sterilized, and pasteurized samples with an HRT of 40 days.
The highest methane yields were obtained in sterilized pre-treated cattle waste despite its low biomethane production from day 1 to day 23 (Figure 2) as compared to the control and pasteurized cattle abattoir waste. The delay in methane production may be attributed to the production of long chain fatty acids (LCFAs) from the hydrolysis of fats within the samples, which were reported in previous studies to reduce methane activity [54,74]. Other authors have reported that sterilization causes the accumulation of microbial metabolic waste including the accumulation of metabolic acids which may impact the hydrolysis stage, thus affecting the microbial activity required for AD [14,54,59,60,74]. This also supports the findings reported by Kavuma et al. [15], who observed in their study that sterilization may alter the environmental conditions necessary for microbial growth due to the hypertonic environment caused by the high temperature and pressure during pre-treatment, which in turn reduced gas production during the early days of AD and led to the reduction in food nutrients necessary to support microbial growth.
The hypertonic environment due to sterilization has been reported in previous studies to further result in the osmotic potential of the abattoir feedstock being higher than that of the microbial cell cytoplasm, thus affecting the growth of microorganisms in the digester, thereby reducing gas production [14,52,53,60,61]. This effect of reduced biogas production in the early days of AD was also evident in this study in sterilized cattle abattoir slaughter waste (Figure 2) and pig abattoir slaughter waste (Figure 4). Sterilization and pasteurization pre-treatment of red meat abattoir slaughter waste had a significant impact on the biomethane production yield (p < 0.05) in cattle, sheep, and piggery abattoir waste (Table 5).

Table 5.
Analysis of pre-treatment’s effect on the biomethane yield of abattoir waste digested.
The earlier start of biomethane production in all samples in this study may be attributed to the fact that the inoculum that was used already contained active microorganisms that were adapted to the reaction conditions and the type of feedstock in the digester, thereby reducing the lag phase [83,84]. The biomethane production of untreated and pre-treated samples in this study was observed to reach stable methane production by 28–30 days HRT and AD was terminated when daily biomethane yield was less than 1% of the total biomethane volume produced throughout the AD process for four consecutive days, in accordance with Ware and Power [14]. Termination of AD was performed at day 40 of HRT.
Biomethane yield in pasteurized samples in this study was observed to be higher than the untreated and sterilized samples (Figure 2, Figure 3 and Figure 4); this may suggest that pasteurization produced significant thermal particle disintegration and hydrolysis, realizing more readily biodegradable compounds [20,51,52,53,54,59,60]. Biomethane accumulation of sterilized cattle and pig abattoir slaughter waste started slow, and only picked up at day 20 and day 9, respectively. This may be due to the particle disintegration and the hydrolysis process being affected by high sterilization temperature, which may have denatured proteins in the feedstock, thereby affecting the chemical structure and composition of the nutrients needed for microbial growth [22,29,54]. The impact of sterilization pre-treatment on protein denaturation is also evident in this study, where protein concentrations for sterilized samples were lower as compared to control and pasteurized samples (Table 4). Thus, sterilization leads to lower bioavailability of biodegradable compounds.
Even though biomethane accumulation in sterilized samples started slow as compared to untreated and pasteurized samples, both sterilized and pasteurized samples were observed to have produced higher biomethane when the experiment was terminated at day 40 HRT. This increase due to thermal pre-treatment, with positive effects on anaerobic digestion performance, has been reported for many different types of organic waste, i.e., for sewage sludge by Oludare et al. [75] and pig manure by Edström et al. [28].
The results obtained in this study for biomethane accumulation in pasteurized and sterilized cattle abattoir waste were higher compared to those reported by Li et al. [20] (0.46 m3CH4/kgVS to 0.58 m3CH4/kgVS) [26] (0.23 m3CH4/kgVS to 0.62 m3CH4/kgVS) and Palatsi et al. [84] (0.63 m3CH4/kgVS to 0.78 m3CH4/kgVS). The difference in biomethane yield in this study as compared to that reported by Rodriguez-Abalde et al. [25], Palatsi et al. [84], and Hejnfelt and Angelidaki [27] may be due to the difference in HRT, operational temperature, inoculum used and the physicochemical properties of the feedstock. The results of biomethane accumulation for cattle abattoir waste in this study were also higher than those reported by Yenigün and Demirel [77], who observed a methane yield of 0.681 m3CH4/kgVS during AD of slaughter waste (cattle rumen contents mixed with blood). This may be due to the composition of the feedstock used as well as the AD operational temperature, as in their study rumen content, fat, and blood were used as feedstock with an AD operation temperature of 55 °C. The results for cattle abattoir waste observed in this study were also higher compared to those reported by Ware and Power [14], where the authors reported a biomethane yield of about 0.64 m3CH4/kgVS. The AD of cattle abattoir slaughter waste feedstock in a study by Ware and Power [14] was operated under a mesophilic temperature of 39 ± 2 °C, which was slightly higher than the 37 ± 2 °C that was used in this study, which could be the reason for the difference in biomethane yield observed. It was also noted that the C/N ratio is a critical parameter for anaerobes in AD; the C/N ratio in this study was higher compared to those reported by Ware and Power [14], which may be the other contributor to higher biomethane yield observed in this study. The results for piggery biomethane accumulation in this study were observed to be in a range similar to those reported by Díaz et al. [78], where biomethane accumulation for pasteurized and sterilized pre-treated piggery abattoir waste ranged from 0.58 m3CH4/kgVS to 0.96 m3CH4/kgVS. The biomethane accumulation observed in this study was also higher than that observed by Bayr et al. [49], Rodriguez-Abalde et al. [25], and Hejnfelt and Angelidaki [27], who reported a biomethane accumulation of 0.43 m3CH4/kgVS, 0.58 m3CH4/kgVS, and 0.23–0.62 m3CH4/kgVS, respectively, for pasteurized pig abattoir waste.
During this study, it was observed that pre-treatment had a significant impact on biomethane accumulation (p ≤ 0.05) (Table 5), where a twofold increase was observed in both pasteurized and sterilized cattle, sheep, and piggery abattoir samples at day 40 HRT. Similar observations were also reported by Edström et al. [28], but they observed a fourfold increase in biomethane yield after pasteurization pre-treatment of abattoir waste at 70 °C for 1 h. The high methane yield of pre-treated samples as compared to untreated samples was attributed to the fact that pre-treatment decreased particle sizes, thus leading to an increase in the accessible active sites to which exocellular enzymes can attach and cleave complex macromolecules into simpler and more biodegradable constituents [29].
The high methane yield in pre-treated samples may also be due to the increased accessibility of lipids to the microorganisms resulting from the heat treatments, i.e., pasteurization and sterilization [29,75]. In contrast to the observed results, Hejnfelt and Angelidaki [27] reported that pre-treatment (pasteurization at 70 °C for 1 h and sterilization at 133 °C, 3 bar for 20 min) had no effects on the biomethane accumulation of abattoir waste. Until now, there has been limited or no research conducted on solid sheep abattoir waste and this study may be the first to report on the AD of such feedstock.
3.3. Results for Biogas Composition
The results for biogas composition are presented in Table 6. The biogas composition in this study ranged from 54.69 ± 1.66% to 65.17 ± 3.99% CH4, 129 ± 10.87 to 177 ± 36.98 ppm NH3, 24.83 ± 1.17% to 39.4 ± 2.18% CO2, 0.4 ± 0.00% to 1.6 ± 0.03% O2, and 113 ± 33.74 ppm to 318 ± 31.56 ppm H2S. The H2S has been reported to cause corrosion in digesters and may lead to a further impact on methanogen activity due to sulfide inhibition [76,79]. The results in this study were higher compared to those observed by Kavuma et al. [15], who observed a methane volume range of 40.6% to 50.4%. The methane composition in this study was also higher than that reported by Budiyono et al. [46], where they observed a biogas CH4 volume of about 48.89%. The methane volumes observed in this study were comparable to those reported by Tsegaye et al. [79], where the CH4 volume in biogas from AD of abattoir waste ranged from 53.4% to 66%.

Table 6.
Biogas composition of cattle, sheep, and pig abattoir waste in relationship to sample pre-treatment. Results represent mean ± standard deviation (n = 20).
The methane volume in this study was lower compared to that reported by Njoya [16], where the CH4 volume in biogas was observed to be 72.33%. The biogas composition observed in this study was comparable to that reported by Ware and Power [14], where they reported a CH4 concentration of about 63%.
Methane production in the study here was higher in pasteurized samples as compared to untreated and sterilized abattoir samples, and the biogas composition differed significantly amongst pre-treatments (p < 0.05). Pasteurized samples gave the highest methane production; this may be attributed to the high C/N ratio and VS and lower NH4+ concentrations as compared to untreated and sterilized samples. The concentration of gaseous NH3 in this study ranged from 29 ± 10.87 to 77 ± 36.98 across the samples analyzed in this study. NH3, CO2, and H2S are very corrosive gases that can affect the biogas quality downstream and damage generators and steel pipes. NH3 affects the overall quality of biogas, impacting its calorific value and energy density. High NH3 levels can lower the efficiency of biogas utilization, leading to decreased energy production and reduced economic viability.
The volume of CO2 reported in this study was lower than that reported by Nnaemeka [43], where the reported volume was 47.87%. A study by Njoya [16] reported a CO2 volume of about 23.33% in biogas produced during AD of abattoir waste, which is lower than the CO2 volume reported in this study. The CO2 volume in this study was lower than that reported by Ware and Power [14], where a CO2 concentration of about 37% was observed during the AD of abattoir waste. The presence of CO2 and H2S in biogas in high concentrations results in a low heat value of the produced biogas and reducing CO2 and H2S content significantly improves the quality of biogas produced. Pasteurization was observed to result in a lower concentration of CO2 and H2S as compared to untreated and sterilization pre-treatment.
3.4. Results for Solid Reduction Efficiency Anaerobic Digestion of Abattoir Waste
Reduction of TS, VS, and COD during anaerobic digestion is referred to as solids/organic load reduction, which occurs when microbes degrade organic substrates [43,72,80]. The reduction efficiency of AD on TS, VS, and COD is represented in Table 7. The results for COD reduction in this study ranged from 73.19 ± 1.23% to 86.87 ± 1.09%, 71.33 ± 2.49% to 81.66 ± 1.99%, and 79.59 ± 0.99% to 87.61 ± 1.19% for cattle, sheep, and pig abattoir waste, respectively. The substrate COD is released in the medium due to microbial activities and their subsequent consumption as carbon sources [80]. Usually, COD removal is one key value reflecting the efficiency of anaerobic digestion of the liquid substrate, and for a typical stable anaerobic digestion system, the value of COD removal is generally above 80% [17]. COD is used as a carbon source by microorganisms during the anaerobic digestion of organic waste [72].

Table 7.
Solid reduction efficacy of TS, COD, and TS. Results mean ± SD (n = 20).
According to Lauterböck et al. [81], COD reduction of >80% shows that AD has excellent and stable performance for COD removal and thus has great potential for the biological treatment of red meat abattoir slaughter waste. The results obtained in this study for COD reduction during AD of red meat slaughter waste were >80% in all pre-treated samples, indicating that pasteurization and sterilization pre-treatment of red meat slaughter has the ability to promote higher COD reduction efficiency. There was a significant difference (p < 0.05) in COD reduction efficiency observed amongst both sterilized and pasteurized pre-treatments during AD of abattoir waste compared to untreated samples.
The results observed in this study for COD removal were lower compared to Musa et al. [82], where the authors observed a >95% COD reduction in AD-treated abattoir waste with HRT ranging from 50 days to 90 days. This can be attributed to the longer HRT of 50 to 90 days as compared to the 40 days HRT in this study, as well as the feeding method, as they used continuous feeding, whereas in this study batch feeding was utilized. The COD removal at day 40 HRT observed by Musa et al. [82] was >83%, which is comparable to the observation of COD removal in this study. The results in this study for COD removal in abattoir waste were also lower as compared to those reported by Hernández et al. [85], where the COD removal efficiency of AD ranged from 82.58% to 91.41% with a 40-day HRT. The results for COD in this study were higher than those observed by Maria et al. [86], where they reported a removal efficacy of about 79% during AD of abattoir waste with 20 days HRT. It should also be noted that comparison for day 20 HRT in this study with that observed by Maria et al. [86] was not possible, as in our study the experiment was a batch AD in a closed system. The results reported in this study for COD were comparable to those reported by Sunada et al. [87], where the researchers observed a COD reduction efficacy of 85.9% with similar HRT and operational AD conditions with pasteurized and sterilized red meat slaughter waste.
The difference in COD removal efficacy observed in this study for pre-treated abattoir samples may be attributed to the impact of the different high temperatures imposed on simple organic compounds, making them more readily available for microorganisms in the digester [87]. High COD removal may also be attributed to longer HRT, which has been observed in previous studies by Musa et al. [82], Hernández et al. [85], Sunada et al. [87] to decrease VFA, thus causing a decrease in the accumulation of these acids in the digester and in turn an increase in COD removal efficacy. With the significant reduction in COD of >70% during the AD of red meat slaughter waste observed in this study, as well as the reduction reported in literature, it is evident the AD can be utilized for both waste management and treatment to minimize the environmental impact COD has in causing the eutrophication of receiving aerosols.
The reduction efficiency at 40 days HRT for VS was observed to range from 78.58 ± 2.01%–80.45 ± 2.55%, 78.53 ± 1.66%–88.65 ± 1.97%, and 81.13 ± 1.36%–84.35 ± 2.79% for cattle, sheep, and pig abattoir waste, respectively (Table 7). The VS reduction efficacy differed significantly between treatments (p < 0.05). The results observed in this study were higher than those observed by Sunada et al. [87], where VS reduction efficacy was reported to be 70.44% with 28 days HRT. The results in this study were also higher as compared to those reported by Alfa et al. [88], where the VS reduction was observed to be 47.12%. The high VS reduction during AD indicates that most of the organic matter contained in the cattle, sheep, and pig solid slaughter waste was converted to biogas [89,90].
At 40 days HRT, TS reduction ranged from 68.37 ± 2.01% to 76.98 ± 2.03%, 67.62 ± 1.83% to 78.96 ± 2.44%, 73.42 ± 2.02% to 79.86 ± 2.89% for cattle, sheep, and pig abattoir waste, respectively (Table 7). The TS reduction efficacy differed significantly (p < 0.05) amongst pre-treatments. Reduction efficacy in this study was observed to be higher in pasteurized samples as compared to control and sterilized samples. The results in this study were higher than those observed by Alfa et al. [88], where the TS reduction efficacy in AD of abattoir waste was 53% for anaerobically digested pasteurized cattle abattoir waste. The lower TS reduction observed by Alfa et al. [88] as compared to this study may be due to their HRT of 30 days, whereas in this study HRT was 40 days.
4. Conclusions
The organic waste streams from the sampled abattoir facilities proved to have high potential as feedstock for anaerobic digestion when treated as a single waste stream. The physicochemical characteristics of abattoir waste such as pH, VS, C/N ratio, etc., fell within the recommended values indicative of a good feedstock for anaerobic digestion treatment. The results of this study showed that pasteurization and sterilization of abattoir waste prior to anaerobic digestion had a positive impact on the physicochemical properties of red meat abattoir solid waste, as it increased its nutrient bioavailability and solubilization, which are essential for the performance and stability of AD, as well as the growth of the AD microbial community. It was observed in this study that high temperatures (sterilization) denatured proteins while pasteurization increased their concentration.
Pasteurization and sterilization pre-treatment of abattoir waste prior to anaerobic digestion was observed to enhance biomethane production. Higher biomethane production was observed in pasteurized abattoir samples as compared to sterilized abattoir samples. It was observed in this study that high temperatures (sterilization) denature proteins, thus impacting on biomethane production. The HRT had an influence on increasing the reduction efficacy of TS, VS, and COD during AD of abattoir waste (>70%), and a longer retention time will be recommended to yield even higher organic reduction. The high solid reduction efficacy in this study revealed that abattoir waste is biodegradable and most of the organic solid can be converted into biogas. Pasteurized abattoir samples yielded more biomethane and high solid reduction efficiency compared to the control and sterilized samples. Thus, this study recommends that pasteurization be adopted as a means of pretreatment for anaerobic digestion during AD of red meat abattoir solid waste in South Africa.
Author Contributions
Methodology, D.S.M., M.T. and M.-J.T.-C.; validation: D.S.M., M.T. and M.-J.T.-C.; formal analysis: D.S.M., M.T. and M.-J.T.-C.; investigation D.S.M.; resources: D.S.M. and M.-J.T.-C.; data curation: D.S.M., M.T. and M.-J.T.-C.; writing—original draft preparation: D.S.M.; writing—review and editing: D.S.M., M.T. and M.-J.T.-C.; supervision, M.T. and M.-J.T.-C.; project administration, D.S.M. and M.-J.T.-C.; funding acquisition: M.-J.T.-C. All authors have read and agreed to the published version of the manuscript.
Funding
National Research Foundation (NRF); United Nations Industrial Development Organization (UNIDO).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented in this manuscript.
Acknowledgments
We would like to acknowledge Bekezela Dube, a Senior Researcher at the Agricultural Research Council-Animal Production (ARC-AP) in the Animal Breeding and Genetics department, for the assistance with statistical analysis. The authors would also like to show appreciation and gratitude to the National Research Foundation and United Nations Industrial Development Organization (UNIDO) for funding this study. The authors would also like to show appreciation to the Agricultural Research Council Animal Production and Agricultural Engineering for the facilities used during this study. The authors would also like to acknowledge the 45 abattoirs which granted the authors permission to carry out sampling.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Kefalew, T.; Lami, M. Biogas and bio-fertilizer production potential of abattoir waste: Implication in sustainable waste management in Shashemene City, Ethiopia. Heliyon 2021, 7, e08293. [Google Scholar] [CrossRef] [PubMed]
- Yasir, S.; Siddiki, A.; Uddin, M.N.; Mofijur, M.; Fattah, I.M.R.; Chyuan, H. Theoretical calculation of biogas production and greenhouse gas emission reduction potential of livestock, poultry, and slaughterhouse waste in Bangladesh. Environ. Chem. Eng. 2021, 9, 105204. [Google Scholar]
- Food Northwest Water and Wastewater Use in the Food Processing Industry-Meat and Poultry Processing. 2018. Available online: http://www.foodnorthwest.org (accessed on 21 June 2018).
- Tolera, S.T.; Alemu, F.K. Potential of Abattoir Waste for Bioenergy as Sustainable Management, Eastern Ethiopia, 2019. J. Energy 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Ezeoha, S.L.; Ugwuishiwu, B.O. Status of slaughterhouse wastes research in Nigeria. Niger. J. Technol. 2011, 30, 143–148. [Google Scholar]
- Heinz, G.; Food and Agricultural Organization (FAO). Abattoir Development, Options and Designs for Hygienic Basic and Medium-Sized, Corporate Document Repository Animal Production and Health Commission for Asia and the Pacific: Bangkok, Thailand. 2010. Available online: https://www.farm-d.org/app/uploads/2013/08/Abbatoir-design.pdf (accessed on 12 December 2023).
- Ali, M.M.; Ndongo, M.; Bilal, B.; Yetilmezsoy, K.; Youm, I.; Bahramian, M. Mapping of biogas production potential from livestock manures and slaughterhouse waste: A case study for African countries. Clean. Prod. 2020, 256, 120499. [Google Scholar] [CrossRef]
- Arshad, M.; Bano, I.; Khan, N.; Shahzad, M.I.; Younus, M.; Abbas, M. Electricity generation from biogas of poultry waste: An assessment of potential and feasibility in Pakistan. Renew. Sustain. Energy Rev. 2018, 81, 1241–1246. [Google Scholar] [CrossRef]
- Agricultural Research Council (ARC). Estimating the Biogas Potential for Electricity Generation from the Agro-Waste Industry: A Resource Assessment for South Africa. South African-German Energy Programme (SAGEN). South Africa. 2016, pp. 1–32. Available online: https://www.sagen.org.za/publications/energy-efficiency-investment/15-assessment-of-biogas-potential-from-agro-waste-in-south-africa/file (accessed on 19 May 2019).
- Regulation (EC) No. 1069/2009 of the European Parliament and of the Council of 2009 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption. Off. J. Eur. Union 2009, L 300, 1–33.
- Regulation (EC) No. 1774/2002 of the European Parliament and of the Council laying down health rules concerning animal by-products not intended for human consumption. Off. J. 2002, L 273, 1–95.
- Klintenberg, P.; Jamieson, M.; Kinyaga, V.; Odlare, M. Assessing biogas potential of slaughter waste: Can biogas production solve a serious waste problem at abattoirs? Energ. Procedia 2014, 61, 2600–2603. [Google Scholar] [CrossRef][Green Version]
- Alvarez, R.; Lidén, G.P. The effect of temperature variation on biomethanation at high altitude. Bioresour. Technol. 2008, 99, 7278–7284. [Google Scholar] [CrossRef] [PubMed]
- Ware, A.; Power, N. Biogas from cattle slaughterhouse waste: Energy recovery towards an energy self-sufficient industry in Ireland. Renew. Energy 2016, 97, 541–549. [Google Scholar] [CrossRef]
- Kavuma, C.; Ekwar, I.; Nabaterega, R.; Lwanyaga, J.D.; Sserumaga, P. Biogas potential from slaughterhouse wastes at ambient temperatures in lira municipality of Northern Uganda. Res. Sq. 2020, 13, 1–11. [Google Scholar]
- Njoya, M. Anaerobic Digestion of High Strength Wastewater in High-Rate Anaerobic Bioreactor Systems: Case of Poultry Slaughterhouse Wastewater (psw). Ph.D. Thesis, Faculty of Engineering & the Built Environment, Cape Peninsula University of Technology, Cape Town, South Africa, 2019. [Google Scholar]
- Wang, Y.; Zhu, Y.; Zhang, S.; Yongqiang, W. What could promote farmers to replace chemical fertilizers with organic fertilizers? Clean. Prod. 2018, 199, 882–890. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]-EDDS via improving metals bioavailability. J. Water Res. 2015, 84, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Akunna, J.C. Anaerobic Waste-Wastewater Treatment and Biogas Plants: A Practical Handbook; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Li, Y.; Jin, Y.; Li, H.; Borrion, A.; Yu, Z.; Li, J. Kinetic studies on organic degradation and its impacts on improving methane production during anaerobic digestion of food waste. Appl. Energy 2018, 213, 136–147. [Google Scholar] [CrossRef]
- Kim, M.; Li, D.; Choi, O.; Sang, B.-I.; Chiang, P.C.; Kim, H. Effects of supplement additives on anaerobic biogas production. Korean J. Chem. Eng. 2017, 34, 2678–2685. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Harris, P.W.; McCabe, B.K. Review of pre-treatments used in anaerobic digestion and their potential application in high-fat cattle slaughterhouse wastewater. Appl. Energy 2015, 155, 560–575. [Google Scholar] [CrossRef]
- Liu, Y.; Wachemo, A.C.; Yuan, H.; Li, X. Anaerobic digestion performance and microbial community structure of corn stover in three-stage continuously stirred tank reactors. Bioresour. Technol. 2019, 287, 121339. [Google Scholar] [CrossRef]
- Rodríguez-Abalde, A.; Fernández, B.; Silvestre, G.; Flotats, X. Effects of thermal pre-treatment on solid slaughterhouse waste methane potential. Waste Manag. 2011, 31, 1488–1493. [Google Scholar] [CrossRef]
- Cuetos, M.; Gómez, X.; Otero, M.; Morán, A. Anaerobic digestion and co-digestion of slaughterhouse waste (SHW): Influence of heat and pressure pre-treatment in biogas yield. Waste Manag. 2010, 30, 1780–1789. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic digestion of slaughterhouse by-products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Edström, M.; Nordberg, Å.; Thyselius, S. Anaerobic Treatment of Animal by-products from Slaughterhouses at Laboratory and Pilot Scale. Appl. Biochem. Biotechnol. 2003, 19, 127–138. [Google Scholar] [CrossRef]
- Baredar, P.; Suresh, S.; Kumar, A.; Krishnakumar, P. A Review on Enhancement of Biogas Yield by Pre-treatment and addition of Additives. MATEC Web Conf. 2016, 62, 06002. [Google Scholar] [CrossRef]
- Song, Z.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of seven chemical pre-treatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 2014, 9, e93801. [Google Scholar]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2001, 11, 364–373. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Desseaux, V.; Tazi, S.; Puigserver, A. Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem. 2008, 107, 1244–1252. [Google Scholar] [CrossRef]
- Dwyer, J.; Starrenburg, D.; Tait, S.; Barr, K.; Batstone, D.J.; Lant, P. Decreasing activated sludge thermal hydrolysis temperature reduces product colour, without decreasing degradability. Water Res. 2008, 42, 4699–4709. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Lacalle, F.; Blanco, M.; Pinto, I.; Díaz, A.D. Semi-continuous anaerobic digestion of solid slaughterhouse waste. J. Environ. Chem. Eng. 2014, 2, 819–825. [Google Scholar] [CrossRef]
- Valta, K.; Kosanovic, T.; Malamis, D.; Moustakas, K.; Loizidou, M. Overview of water usage and wastewater management in the food and beverage industry. Desalination Water Treat. 2014, 53, 3335–3347. [Google Scholar] [CrossRef]
- Matjuda, D.S.; Tekere, M.; Thaela-Chimuka, M.-J. Characterization of the physicochemical composition of anaerobically digested (digestate) high throughput red meat abattoir waste in South Africa and the determination of its quality as a potential biofertilizer. Heliyon 2023, 9, e21647. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein Determination—Method Matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, D.K.; Kong, Y.; Ungerfeld, E.M.; Seviour, R.; Massé, D.I. Anaerobic digestibility of beef hooves with swine manure or slaughterhouse sludge. Waste Manag. 2015, 38, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Li, B.; Dinkler, K.; Zhao, N.; Sobhi, M.; Merkle, W.; Liu, S.; Dong, R.; Oechsner, H.; Guo, J. Influence of anaerobic digestion on the labile phosphorus in pig, chicken, and dairy manure. Sci. Total Environ. 2020, 737, 140–234. [Google Scholar] [CrossRef]
- Leng, L.; Bogush, A.A.; Roy, A.; Stegemann, J.A. Characterization of ashes from waste biomass power plants and phosphorus recovery. Sci. Total. Environ. 2019, 690, 573–583. [Google Scholar] [CrossRef]
- Díaz, J.P.; Reyes, I.P.; Lundin, M.; Horváth, I.S. Co-digestion of different waste mixtures from agro-industrial activities: Kinetic evaluation and synergetic effects. Bioresour. Technol. 2011, 102, 10834–10840. [Google Scholar] [CrossRef]
- Nnaemeka, U.S. The Use of Enhanced Anaerobic Digestion Process for Energy Recovery and Phosphorus Release from Agro-industrial Wastes. Ph.D. thesis, College of Science and Engineering, University of South Africa, Tshwane, South Africa, 2020. [Google Scholar]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities—A review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Kigozi, R.; Muzenda, E.; Aboyade, A.O. Biogas technology: Current trends, opportunities, and challenges. In Proceedings of the 6th International Conference GREEN Technology, Renewable Energy & Environmental Engineering, Cape Town, South Africa, 27–28 November 2014. [Google Scholar]
- Budiyono, I.N.; Widiasa, S.; Johari, M.; Sunarso, S.H. Study on Slaughterhouse Wastes Potency and Characteristic for Biogas Production. Waste Resour. 2011, 1, 4–7. [Google Scholar]
- Islam, A.; Biswas, P.; Sabuj, A.A.; Haque, Z.F.; Saha, C.K.; Alam, M.M.; Rahman, M.T.; Saha, S. Microbial load in bio-slurry from different biogas plants in Bangladesh. J. Adv. Veter Anim. Res. 2019, 6, 376–383. [Google Scholar] [CrossRef]
- Calicioglu, O.; Shreve, M.J.; Richard, T.L.; Brennan, R.A. Effect of pH and temperature on microbial community structure and carboxylic acid yield during the acidogenic digestion of duckweed. Biotechnol. Biofuels 2018, 11, 1–19. [Google Scholar] [CrossRef]
- Bayr, S.; Rantanen, M.; Kaparaju, P.; Rintala, J. Mesophilic and thermophilic anaerobic co-digestion of rendering plant and slaughterhouse wastes. Bioresour. Technol. 2012, 104, 28–36. [Google Scholar] [CrossRef]
- Gebrekidan, T.; Egigu, M.C.; Muthuswamy, M. Efficiency of biogas production from cactus fruit peel co-digestion with cow dung. Int. J. Adv. Res. 2014, 2, 916–923. [Google Scholar]
- Jha, A.K.; Li, J.; Zhang, L.; Ban, Q.; Jin, Y. Comparison between Wet and Dry Anaerobic Digestions of Cow Dung under Mesophilic and Thermophilic Conditions. Adv. Water Resour. Protect. 2013, 1, 28–38. [Google Scholar]
- Alarcon, P.; Fèvre, E.M.; Murungi, M.K.; Muinde, P.; Akoko, J.; Dominguez-Salas, P.; Kiambi, S.; Ahmed, S.; Häsler, B.; Rushton, J. Mapping of beef, sheep, and goat food systems in Nairobi—A framework for policy making and the identification of structural vulnerabilities and deficiencies. Agric. Syst. 2017, 152, 1–17. [Google Scholar] [CrossRef]
- Otero, A.; Mendoza, M.; Carreras, R.; Fernández, B. Biogas production from slaughterhouse waste: Effect of blood content and fat saponification. Waste Manag. 2021, 133, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, S.C. Anaerobic Co-Digestion of Abattoir and Winery Solid Waste for Enhanced Biogas Production. Master’s Thesis, Chemical Engineering, Faculty of Engineering and the Built Environment, Cape Peninsula University of Technology, Cape Town, South Africa, 2020. [Google Scholar]
- Zamri, M.F.M.A.; Hasmady, S.; Akhiar, A.; Ideris, F.; Shamsuddin, A.H.; Mofijur, M.; Fattah, I.M.R.; Mahlia, T.M.I. A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste. Solid Waste. Renew. Sustain. Energy Rev. 2020, 137, 110637. [Google Scholar] [CrossRef]
- Cvetković, S.; Radoičić, T.K.; Vukadinović, B.; Kijevčanin, M. Potentials and status of biogas as energy source in the Republic of Serbia. Renew. Sustain. Energy Rev. 2014, 31, 407–416. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Optimization of Biogas Production for Optimal Abattoir Waste Treatment with Bio-Methanation as Solution to Nairobi Slaughterhouses Waste Disposal. In Proceedings of the 2nd African International Conference on Industrial Engineering and Operations Management Harare, Harare, Zimbabwe, 7–10 December 2020. [Google Scholar]
- Dawana, D.; Kassa, K. Characterization and evaluation of biogas generation of Arba Minch Town slaughterhouse wastewater, Ethiopia. Water Pract. Technol. 2020, 15, 899–909. [Google Scholar] [CrossRef]
- Ortner, M.; Wöss, D.; Schumergruber, A.; Pröll, T.; Fuchs, W. Energy self-supply of large abattoir by sustainable waste utilization based on anaerobic mono-digestion. Appl. Energy 2015, 143, 460–471. [Google Scholar] [CrossRef]
- Obileke, K.; Nwokolo, N.; Makaka, G.; Mukumba, P.; Onyeaka, H. Anaerobic digestion: Technology for biogas production as a source of renewable energy—A review. Energy Environ. 2020, 32, 191–225. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Borja, R.; Rincón, B. Biogas production. Ref. Modul. Life Sci. 2017, 2, 1–24. [Google Scholar] [CrossRef]
- Riya, S.; Suzuki, K.; Terada, A.; Hosomi, M.; Zhou, S. Influence of C/N Ratio on Performance and Microbial Community Structure of Dry-Thermophilic Anaerobic Co-Digestion of Swine Manure and Rice Straw. J. Med Bioeng. 2016, 5. [Google Scholar] [CrossRef][Green Version]
- Shi, H.; Mahinpey, N.; Aqsha, A.; Silbermann, R. Characterization, thermochemical conversion studies, and heating value modelling of municipal solid waste. Waste Manag. 2016, 48, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Braz, C.E.M.; Crnkovic, P.C.G.M. Physical-chemical characterization of biomass samples for application in pyrolysis process. Chem. Eng. Trans. 2014, 37, 523–528. [Google Scholar]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef]
- Lin, J.; Zou, J.N.; Ga, L.L.; Li, P.; Liu, F.L.; Wang, K.; Chen, L.; Gan, H. Effect of mixture ratio on anaerobic co-digestion with fruit and vegetable waste (FVW) and food waste (FW) of China. Environ. Sci. 2011, 23, 60572–60574. [Google Scholar]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Habiba, L.; Hassib, B.; Moktar, H. Improvement of activated sludge stabilization and filterability during anaerobic digestion by fruit and vegetable waste addition. Bioresour. Technol. 2009, 100, 1555–1560. [Google Scholar] [CrossRef]
- Schattauer, A.; Weiland, P. Handreichung Biogassgewinnung und-nutzung (Guidelines for Biogas Production and Use); Fachagentur Nachwachsende Rohstoffe e.V.: Gülzow, Germany, 2004. [Google Scholar]
- Kayhanian, M. Ammonia inhibition in high-solids biosification: An overview and practical solutions. Environ. Technol. 1999, 20, 355–365. [Google Scholar] [CrossRef]
- Ye, J.; Hu, A.; Ren, G.; Chen, M.; Tang, J.; Zhang, P.; Zhou, S.; He, Z. Enhancing sludge methanogenesis with improved redox activity of extracellular polymeric substances by hematite in red mud. Water Res. 2018, 134, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Renggaman, A.; Choi, H.L.; Sudiarto, S.I.A.; Febrisiantosa, A.; Ahn, D.H.; Choung, Y.W.; Suresh, A. Biochemical Methane Potential of Swine Slaughter Waste, Swine Slurry, and Its Codigestion Effect. Energies 2021, 14, 7103. [Google Scholar] [CrossRef]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Oludare, J.O.; Ebenezer, L.O.; Ayorinde, B.; Olayomi, A.F.; Funso, A. Anaerobic digestion of abattoir wastes for biogas production: Optimization via performance evaluation comparison. Cogent Eng. 2022, 9, 2122150. [Google Scholar] [CrossRef]
- Odejobi, O.J.; Odekanle, E.L.; Bamimore, A.; Falowo, O.A.; Akeredolu, F. Anaerobic digestion of abattoir wastes for biogas production: Optimization via performance evaluation comparison. Cogent Eng. 2022, 9. [Google Scholar] [CrossRef]
- Yenigün, R.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Tsegaye, G.; Asefa, B.; Kiros, S. Optimization of biogas production from slaughterhouse wastes. Afr. J. Environ. Sci. Technol. 2018, 12, 283–295. [Google Scholar]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.-P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef]
- Lauterböck, B.; Ortner, M.; Haider, R.; Fuchs, W. Counteracting ammonia inhibition in anaerobic digestion by removal with a hollow fiber membrane contactor. Water Res. 2012, 46, 4861–4869. [Google Scholar] [CrossRef]
- Musa, M.A.; Idrus, S.; Harun, M.R.; Marzuki, T.F.T.M.; Wahab, A.M.A. A Comparative Study of Biogas Production from Cattle Slaughterhouse Wastewater Using Conventional and Modified Upflow Anaerobic Sludge Blanket (UASB) Reactors. Int. J. Environ. Res. Public Heal. 2020, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Gómez, X.; Otero, M.; Morán, A. Anaerobic digestion of solid slaughterhouse waste (SHW) at laboratory scale: Influence of co-digestion with the organic fraction of municipal solid waste (OFMSW). Biochem. Eng. 2008, 4, 99–106. [Google Scholar] [CrossRef]
- Palatsi JVinas, M.; Guivernau, M.; Fernandez, B.; Flotats, X. Anaerobic digestion of slaughterhouse wastes: Main process limitations and microbial community interactions. Bioresour. Technol. 2011, 102, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.C.; Jiménez, L.D.; Bueno Garcia, J.A. Potential of energy production from slaughterhouse wastewater. Interciencia 2018, 43, 558–565. [Google Scholar]
- Maria, O.D.; Gita, P.P.; Budiyono, S.S.; Tutuk, D.K. The effect of pH and operation mode for COD removal of slaughterhouse wastewater with Anaerobic Batch Reactor. J. Waste Technol. 2015, 3, 7–13. [Google Scholar]
- Sunada, N.d.S.; Orrico, A.C.A.; Júnior, M.A.P.O.; Junior, F.M.d.V.; Garcia, R.G.; Fernandes, A.R.M. Potential of biogas and methane production from anaerobic digestion of poultry slaughterhouse effluent. Rev. Bras. Zootec. 2012, 41, 2379–2383. [Google Scholar] [CrossRef]
- Alfa, M.I.; Ojeleye, O.A.; Wamyil, F.B.; Makolo, D. Anaerobic Digestion of Abattoir Waste: A Combined Strategy for Biogas and Biofertilizer Production, and Waste Management. Asian J. Biotechnol. Bioresour. Technol. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Azadbakht, M.; Safieddin Ardebili, S.M.; Rahmani, M. Potential for the production of biofuels from agricultural waste, livestock, and slaughterhouse waste in Golestan province, Iran. Biomass Convers. Biorefinery 2021, 13, 3123–3133. [Google Scholar] [CrossRef]
- Hu, C.; Yan, B.; Wang, K.-J.; Xiao, X.-M. Modelling the performance of anaerobic digestion reactor by the anaerobic digestion system model (ADSM). Environ. Chem. Eng. 2018, 6, 2095–2104. [Google Scholar] [CrossRef]
- Khalil, M.; Berawi, M.A.; Heryanto, R.; Rizalie, A. Waste to energy technology: The potential of sustainable biogas production from animal waste in Indonesia. Renew. Sustain. Energy Rev. 2019, 105, 323–331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).