Soil Salinity Estimation by 3D Spectral Space Optimization and Deep Soil Investigation in the Songnen Plain, Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling of the Soils and Waters and Laboratory Analysis
2.3. Satellite Imagery and Preprocessing
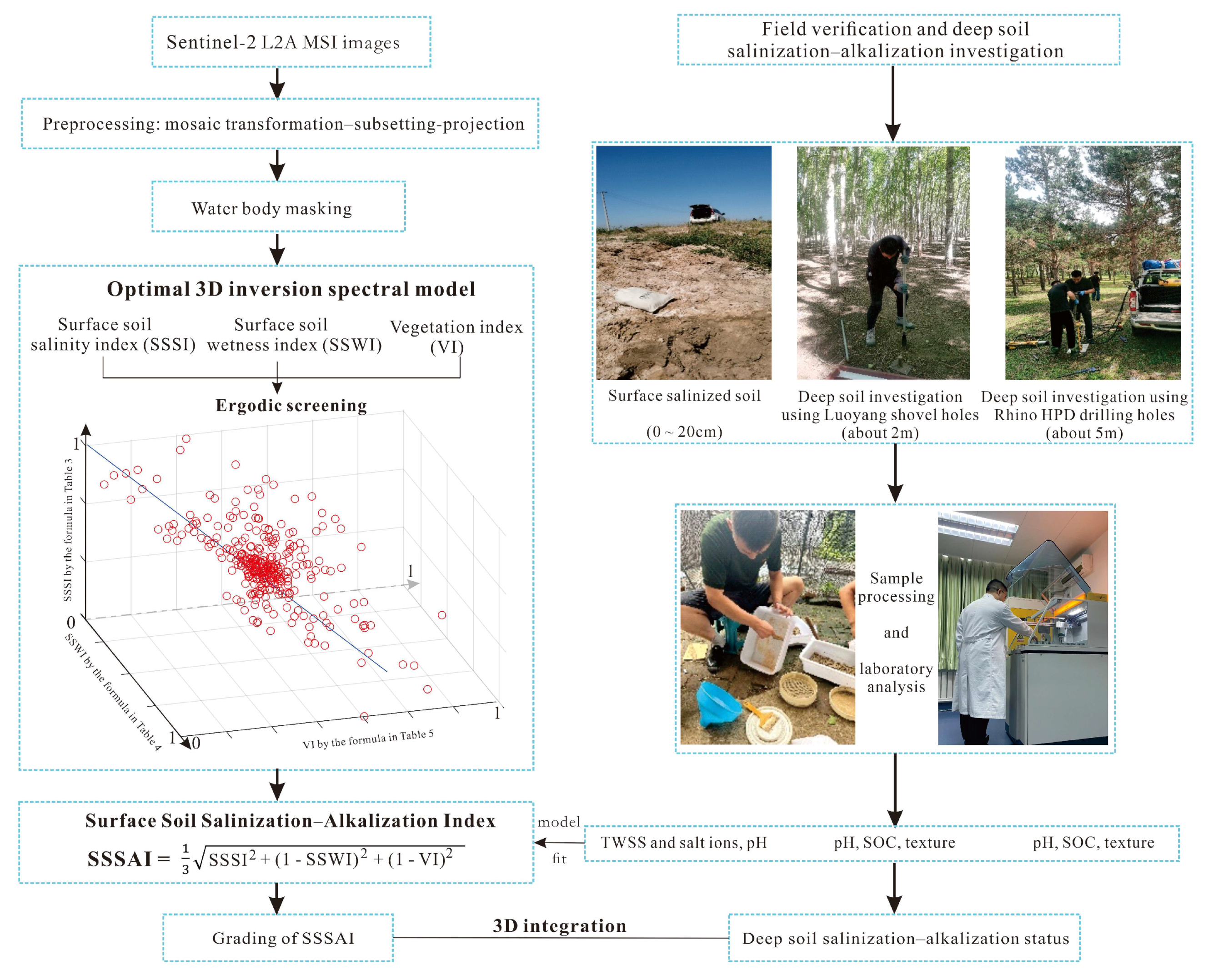
2.4. Methods
3. Results
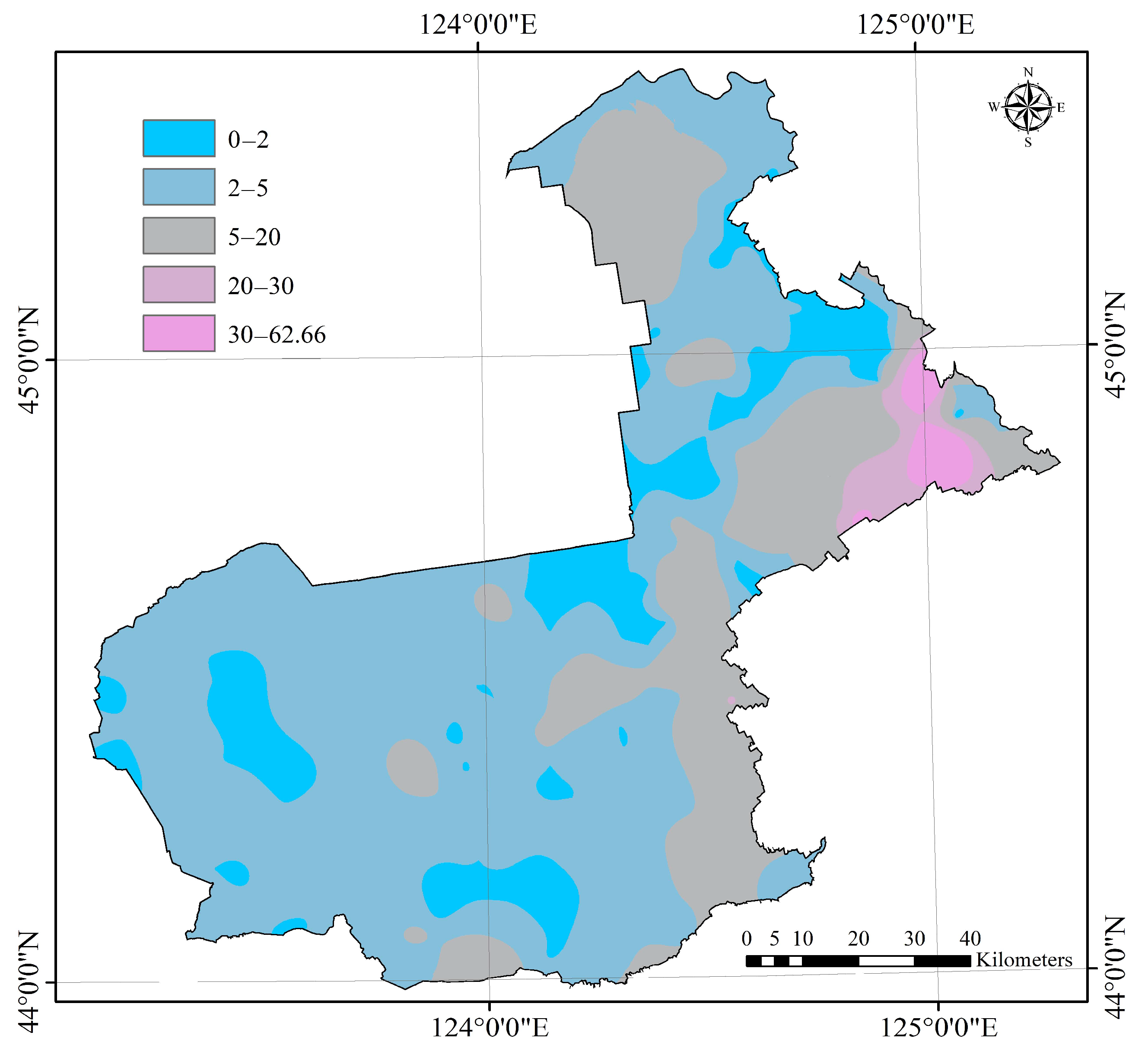
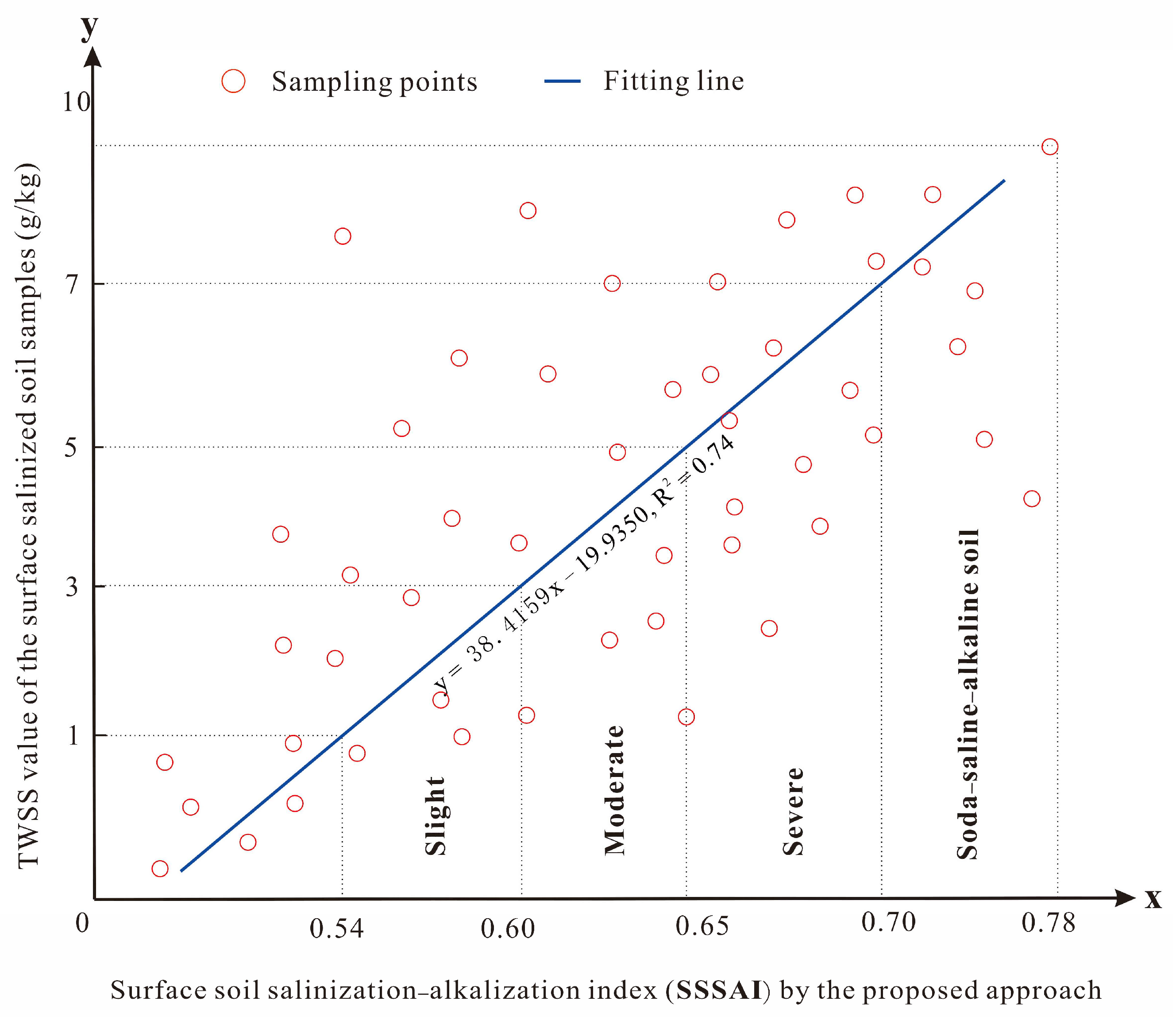
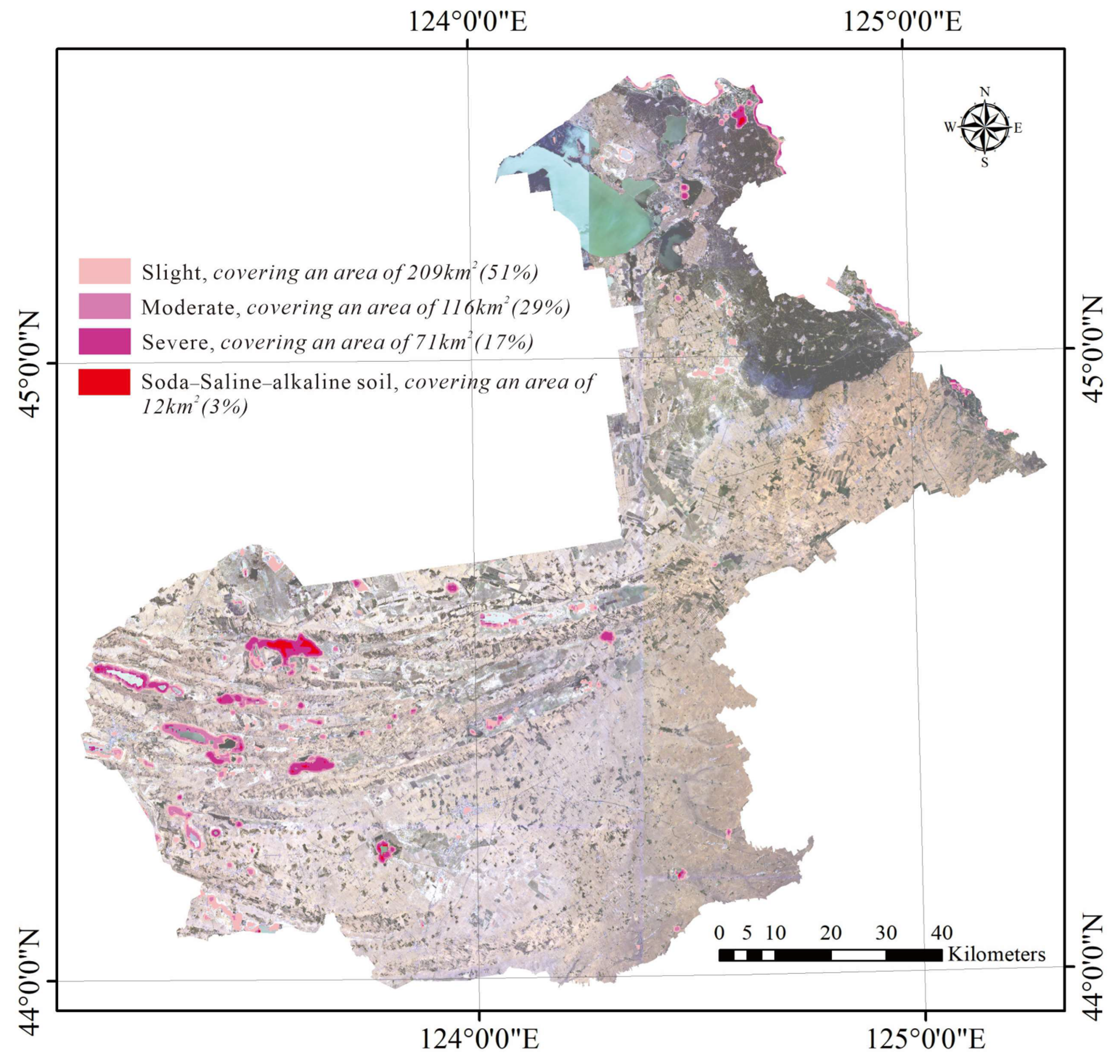
3.1. Prediction of SSS by the 3D Spectral Model and Its Accuracy
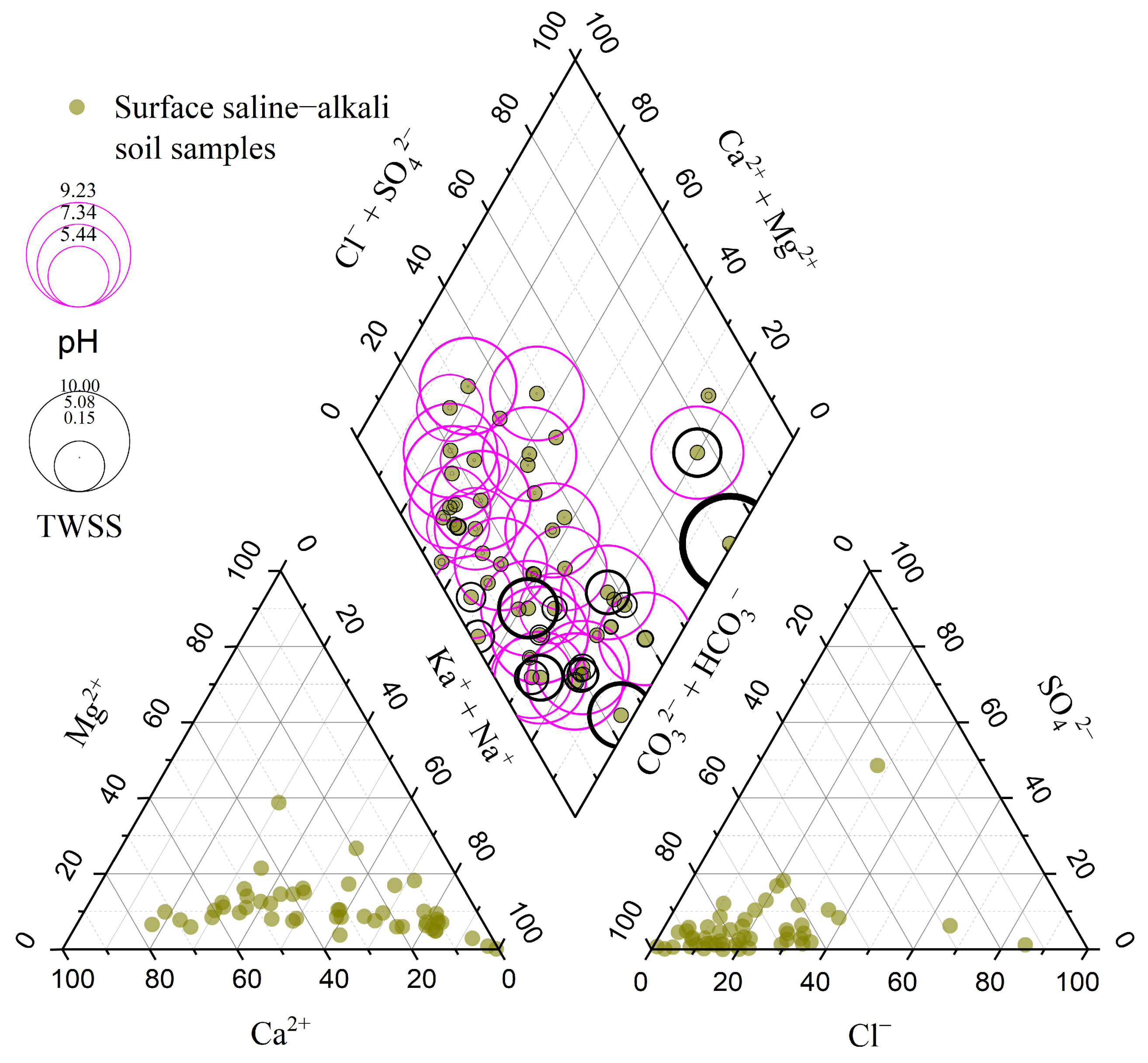
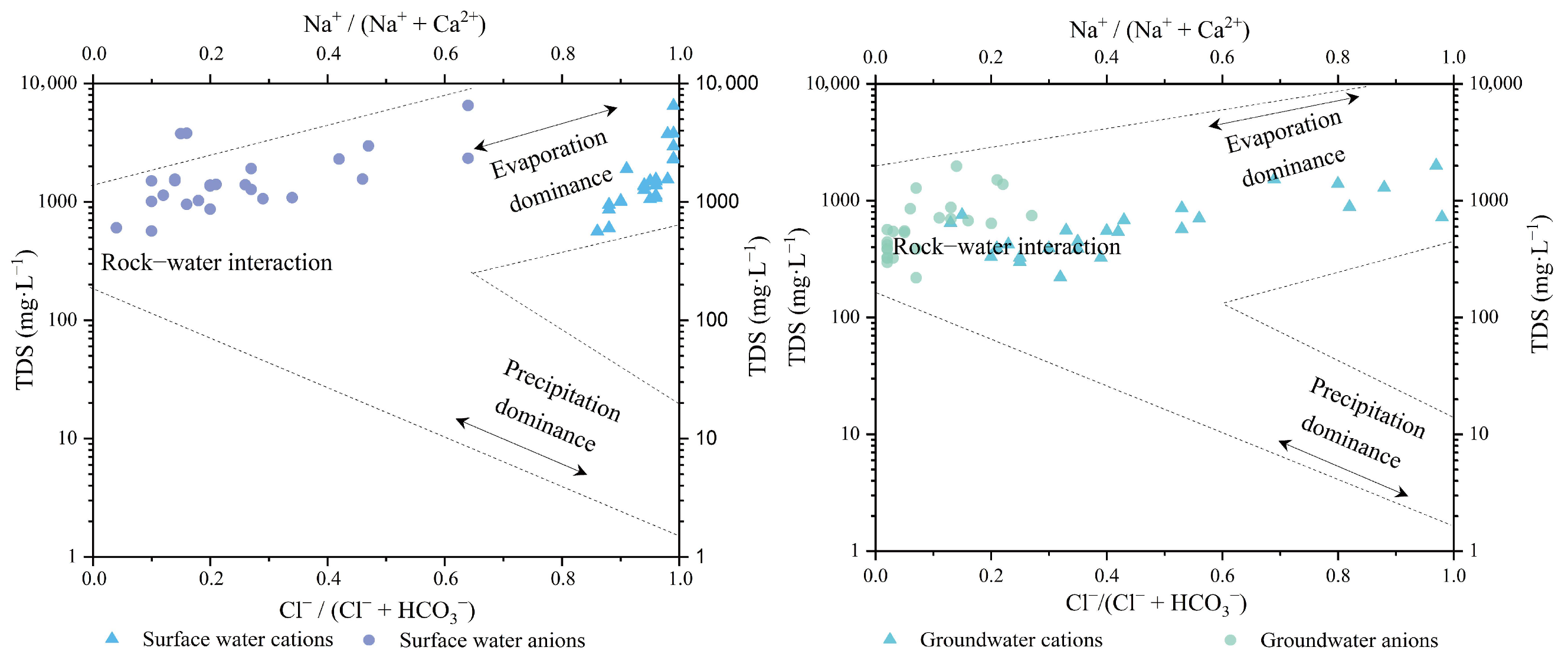
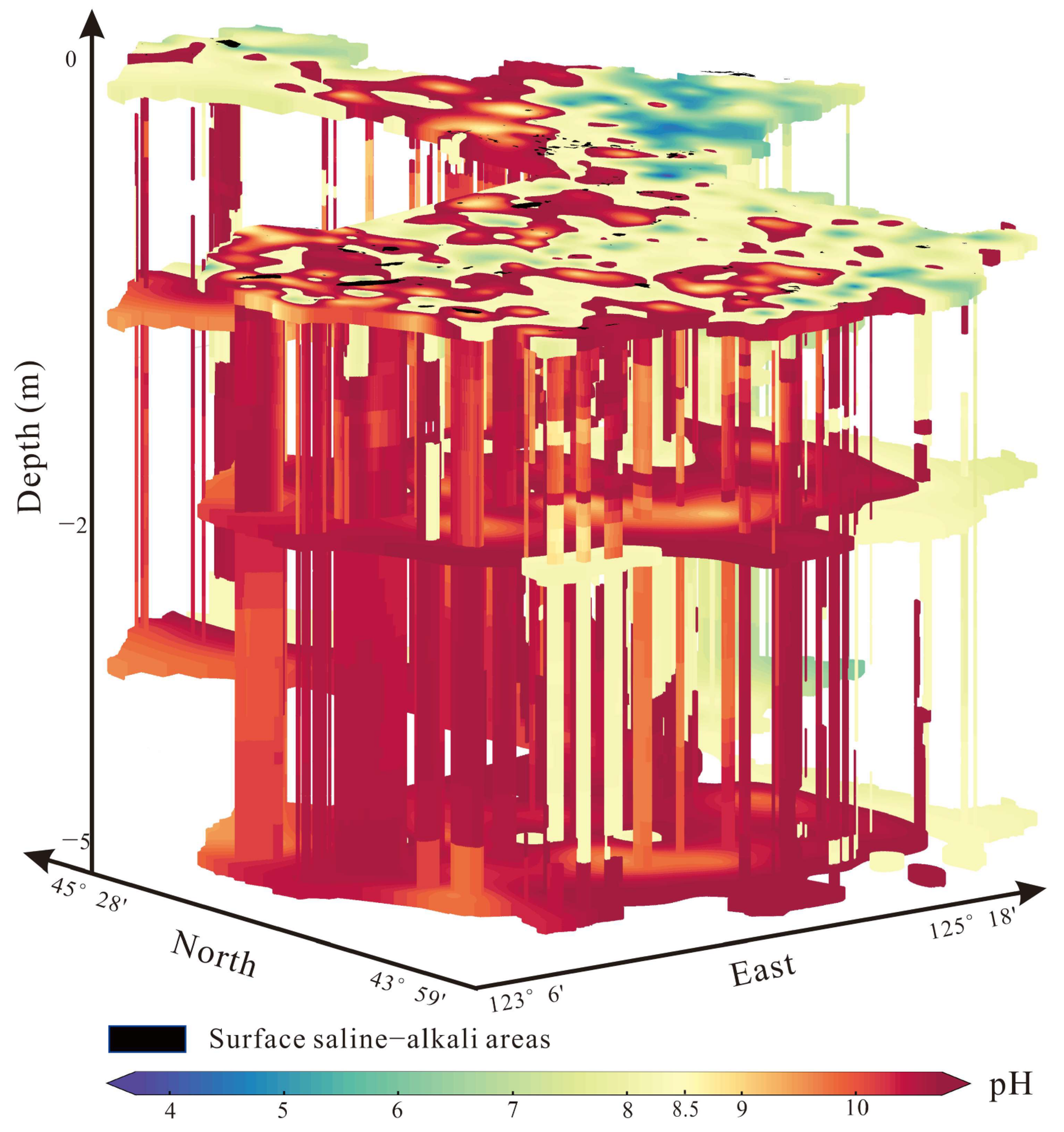
3.2. Soil Salinization–Alkalization Genesis of the Study Area
3.2.1. Origin of Salt Ions and the Deep Soil Saline–Alkaline Status
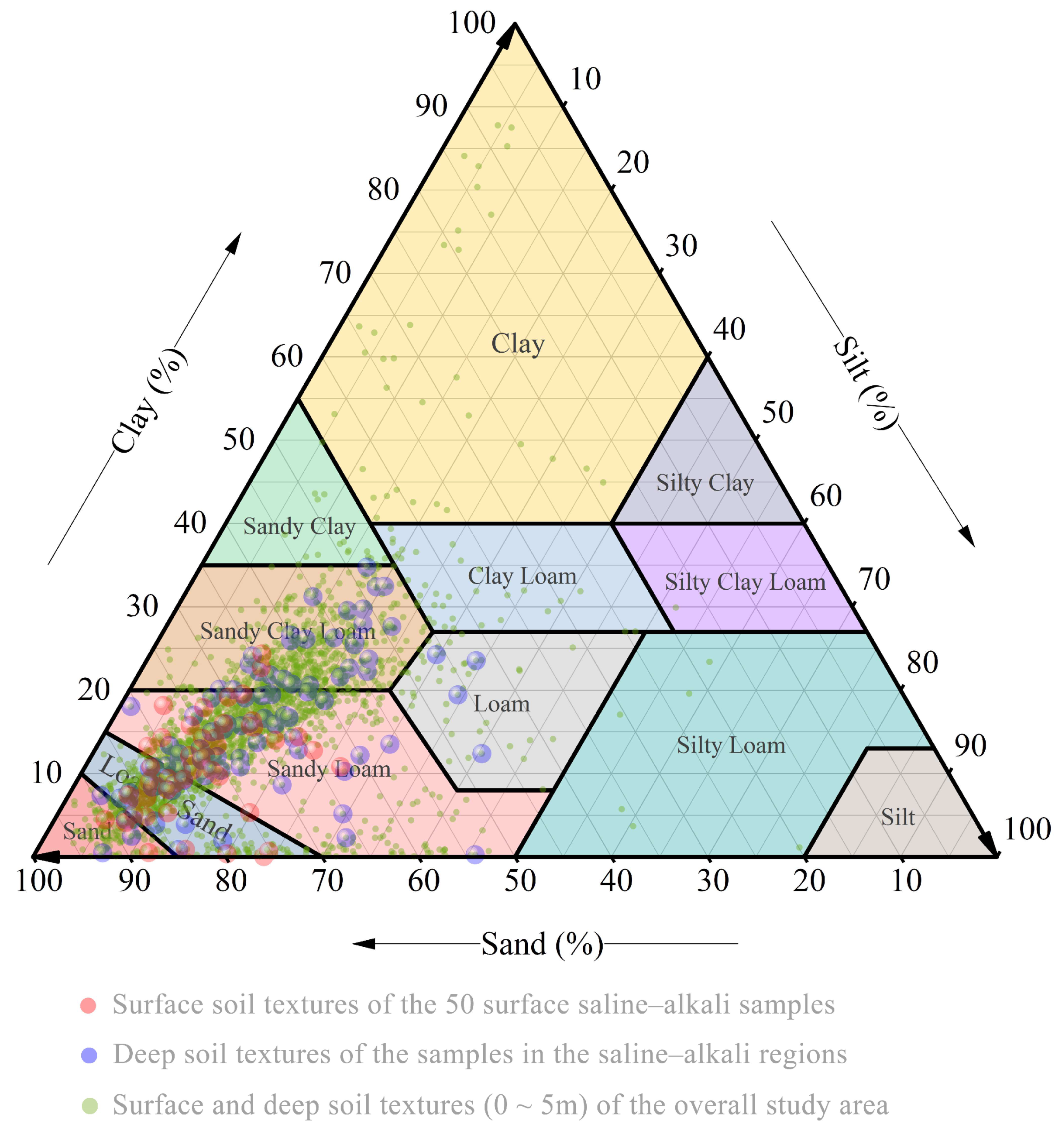
3.2.2. Surface and Deep Soil Texture Characteristics
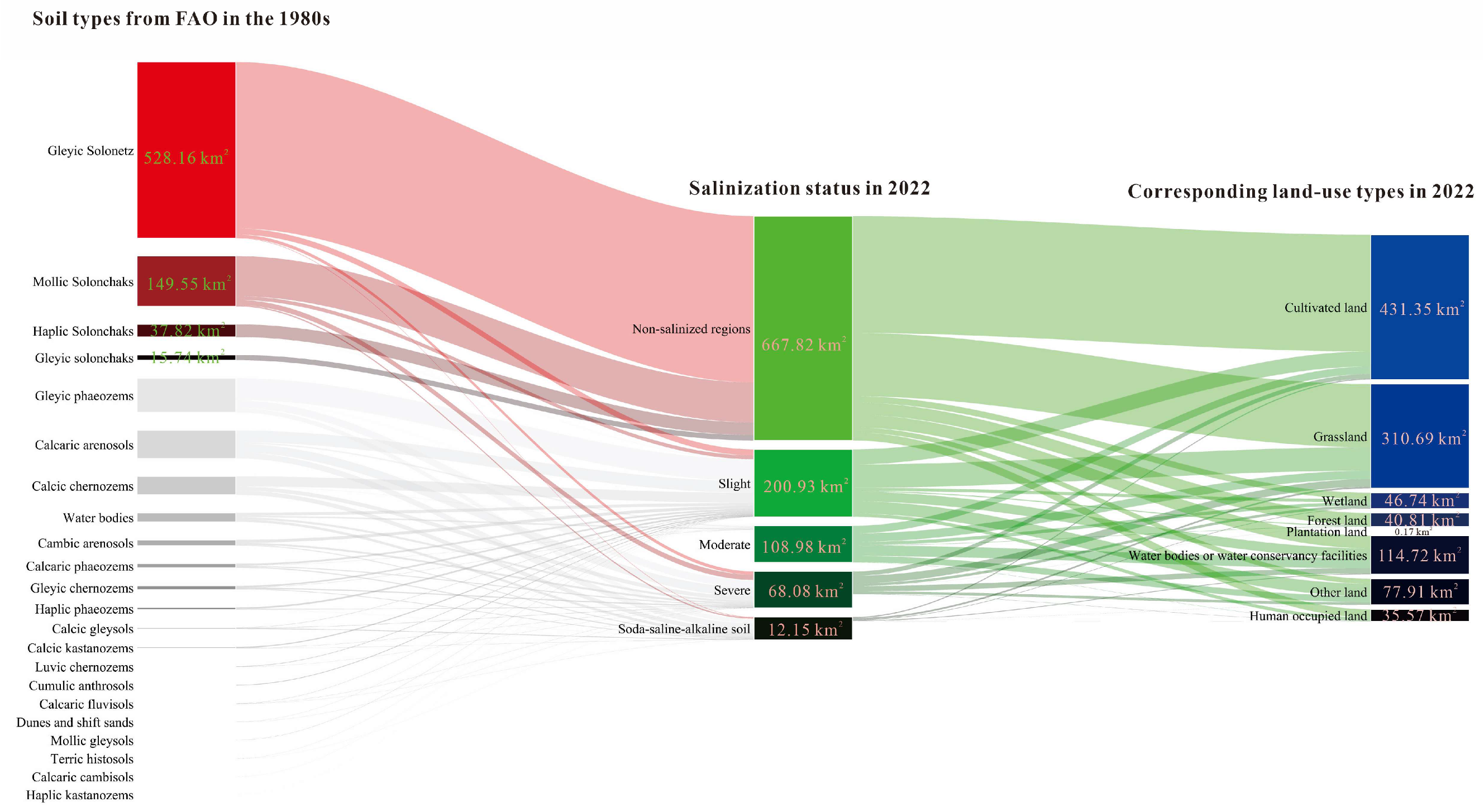
3.3. Changes in the Saline–Alkaline Status from the 1980s to 2022 and Land-Use Management Suggestions for the Alkaline Soils
4. Discussions
4.1. Uncertainty of Predicting SSS Using the Proposed Method and Grading of the Soil Salinity of the Study Area
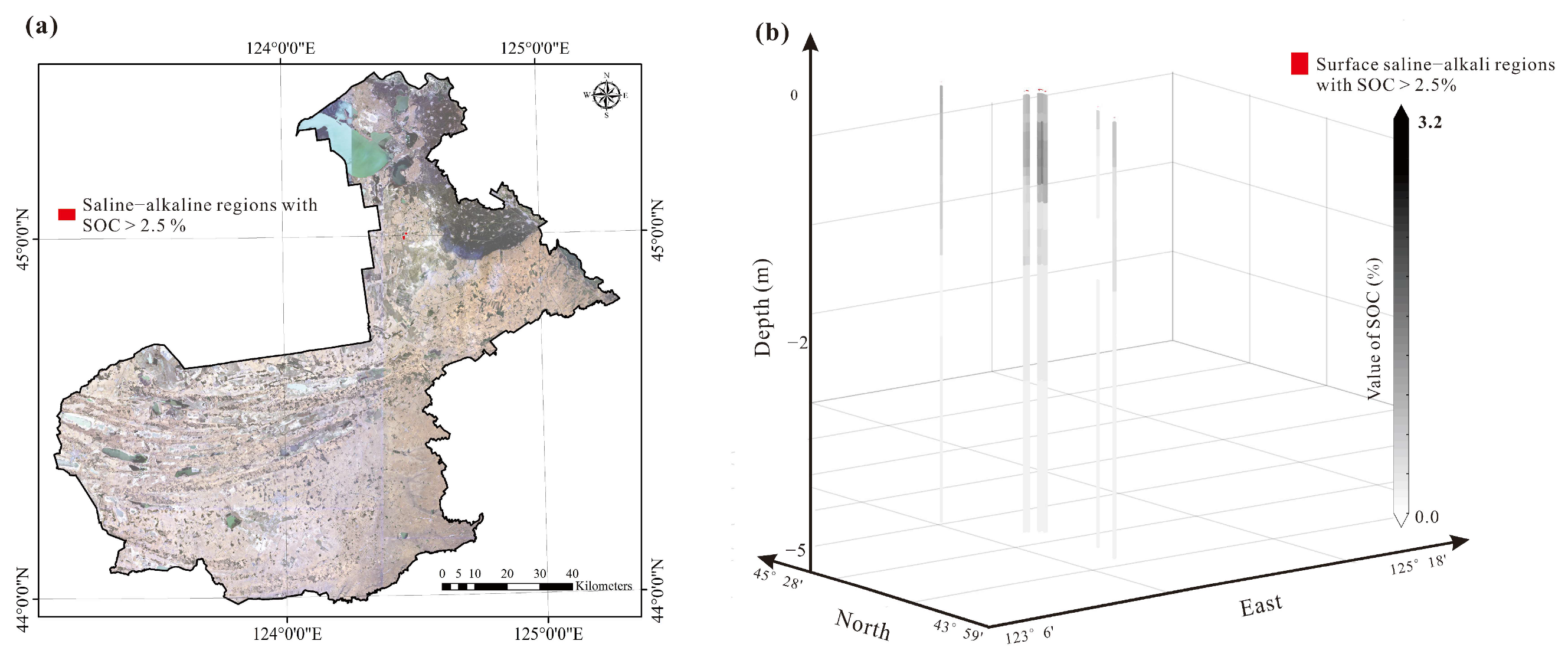
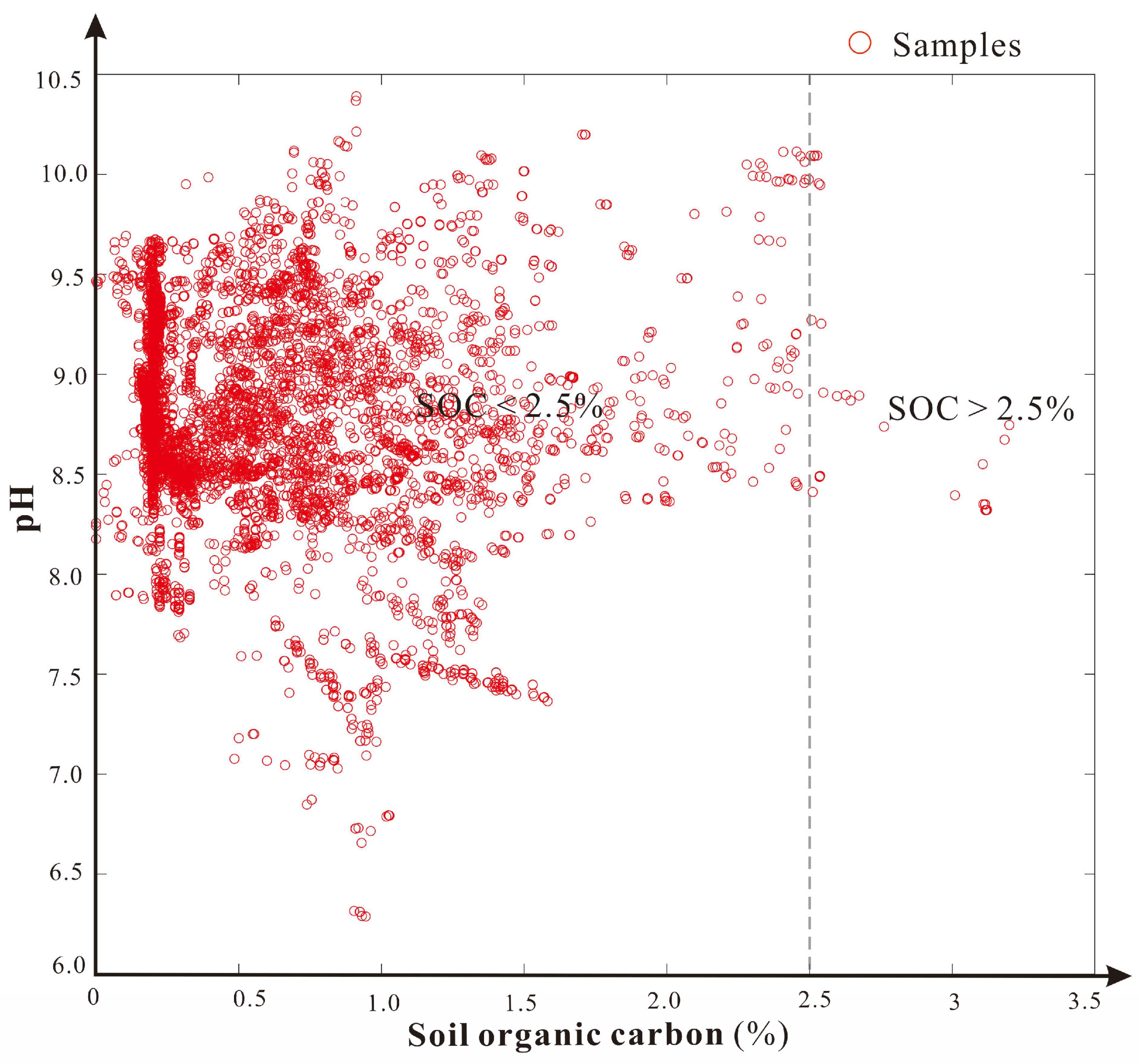
4.2. The Depth of Soil Salinization and Alkalization of the Study Area
4.3. Genesis of Soil Salinization and Alkalization of the Study Area
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; de Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point; Synthesis Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; pp. 10–14. [Google Scholar]
- FAO. Salt-Affected Soils. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/ (accessed on 12 January 2024).
- Al-Khaier, F. Soil Salinity Detection Using Satellite Remote Sensing. Master’s Thesis, International Institute for Geo-information Science and Earth Observation, Enschede, The Netherlands, 2003. [Google Scholar]
- Wallender, W.W.; Tanji, K.K. Agricultural Salinity Assessment and Management, 2nd ed.; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 1–1069. [Google Scholar]
- Zamann, M.; Shahidd, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer Open: Vienna, Austria, 2018; pp. 1–162. [Google Scholar]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Critical knowledge gaps and research priorities in global soil salinity. Adv. Agron. 2021, 169, 1–156. [Google Scholar]
- Li, X.; Li, Y.; Wang, B.; Sun, Y.; Cui, G.; Liang, Z. Analysis of spatial-temporal variation of the saline-sodic soil in the west of Jilin Province from 1989 to 2019 and influencing factors. Catena 2022, 217, 106492. [Google Scholar] [CrossRef]
- Yang, F.; An, F.; Ma, H.; Wang, Z.; Zhou, X.; Liu, Z. Variations on soil salinity and sodicity and its driving factors analysis under microtopography in different hydrological conditions. Water 2016, 8, 227. [Google Scholar] [CrossRef]
- Clark, R.N.; King, T.V.V.; Klejwa, M.; Swayze, G.A.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. 1990, 95, 12653–12680. [Google Scholar] [CrossRef]
- Metternicht, G.; Zinck, J.A. Spatial discrimination of salt- and sodium-affected soil surfaces. Int. J. Remote Sens. 1997, 18, 2571–2586. [Google Scholar] [CrossRef]
- Khan, N.M.; Rastoskuev, V.v.; Sato, Y.; Shiozawa, S. Assessment of hydrosaline land degradation by using a simple approach of remote sensing indicators. Agric. Water Manag. 2005, 77, 96–109. [Google Scholar] [CrossRef]
- Douaoui, A.E.K.; Nicolas, H.; Walter, C. Detecting salinity hazards within a semiarid context by means of combining soil and remote-sensing data. Geoderma 2006, 134, 217–230. [Google Scholar] [CrossRef]
- Bannari, A.; Guedon, A.M.; El-Harti, A.; Cherkaoui, F.Z.; El-Ghmari, A. Characterization of slightly and moderately saline and sodic soils in irrigated agricultural land using simulated data of advanced land imaging (EO-1) sensor. Commun. Soil Sci. Plan. 2008, 39, 2795–2811. [Google Scholar] [CrossRef]
- Bouaziz, M.; Matschullat, J.; Gloaguen, R. Improved remote sensing detection of soil salinity from a semi-arid climate in Northeast Brazil. Comptes Rendus Geosci. 2011, 343, 795–803. [Google Scholar] [CrossRef]
- Allbed, A.; Kumar, L.; Sinha, P. Soil salinity and vegetation cover change detection from multi-temporal remotely sensed imagery in Al Hassa Oasis in Saudi Arabia. Geocarto Int. 2018, 33, 830–846. [Google Scholar] [CrossRef]
- Abuelgasim, A.; Ammad, R. Mapping soil salinity in arid and semi-arid regions using Landsat 8 OLI satellite data. Remote Sens. Appl. 2019, 13, 415–425. [Google Scholar] [CrossRef]
- Tran, T.v.; Tran, D.X.; Myint, S.W.; Huang, C.; Pham, H.V.; Luu, T.H.; Vo, T.M.T. Examining spatiotemporal salinity dynamics in the Mekong River Delta using Landsat time series imagery and a spatial regression approach. Sci. Total Environ. 2019, 687, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Zewdu, S.; Suryabhagavan, K.V.; Balakrishnan, M. Geo-spatial approach for soil salinity mapping in Sego Irrigation Farm, South Ethiopia. J. Saudi Soc. Agric. Sci. 2017, 16, 16–24. [Google Scholar] [CrossRef]
- Suweis, S.; Rinaldo, A.; van der Zee, S.E.A.T.M.; Daly, E.; Maritan, A.; Porporato, A. Stochastic modeling of soil salinity. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Perri, S.; Molini, A.; Hedin, L.O.; Porporato, A. Contrasting effects of aridity and seasonality on global salinization. Nat. Geosci. 2022, 15, 375–381. [Google Scholar] [CrossRef]
- Bian, J.; Tang, J.; Lin, N. Relationship between saline-alkali soil formation and neotectonic movement in Songnen Plain, China. Environ. Geol. 2008, 55, 1421–1429. [Google Scholar] [CrossRef]
- Salcedo, F.P.; Cutillas, P.P.; Cabañero, J.J.A.; Vivaldi, A.G. Use of remote sensing to evaluate the effects of environmental factors on soil salinity in a semi-arid area. Sci. Total Environ. 2022, 815, 152524. [Google Scholar] [CrossRef]
- Yan, Y.; Kayem, K.; Hao, Y.; Shi, Z.; Zhang, C.; Peng, J.; Liu, W.; Zuo, Q.; Ji, W.; Li, B. Mapping the Levels of Soil Salination and Alkalization by Integrating Machining Learning Methods and Soil-Forming Factors. Remote Sens. 2022, 14, 3020. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, K.; Qian, H.; Gao, Y.; Fang, Y.; Xiao, S.; Tang, S.; Zhang, Q.; Qu, W.; Ren, W. Characterization of soil salinization and its driving factors in a typical irrigation area of Northwest China. Sci. Total Environ. 2022, 837, 155808. [Google Scholar] [CrossRef]
- Everitt, J.H.; Escobar, D.E.; Gerbermann, A.H. Detecting saline soils with video imagery. Photogramm. Eng. Remote Sens. 1988, 54, 1283–1287. [Google Scholar]
- Peñuelas, J.; Isla, R.; Filella, I.; Araus, J.L. Visible and near-infrared reflectance assessment of salinity effects on barley. Crop Sci. 1997, 37, 198–202. [Google Scholar] [CrossRef]
- Fernandez-Buces, N.; Siebe, C.; Cram, S.; Palacio, J. Mapping soil salinity using a combined spectral response index for bare soil and vegetation: A case study in the former lake Texcoco, Mexico. J. Arid Environ. 2006, 65, 644–667. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zeng, S.L.; Gao, Y.; Ouyang, Z.T.; Li, B.; Fang, C.M.; Zhao, B. Using hyperspectral vegetation indices as a proxy to monitor soil salinity. Ecol. Indic. 2011, 11, 1552–1562. [Google Scholar] [CrossRef]
- Elmetwalli, A.M.H.; Tyler, A.N.; Hunter, P.D.; Salt, C.A. Detecting and distinguishing moisture-and salinity-induced stress in wheat and maize through in situ spectroradiometry measurements. Remote Sens. Lett. 2012, 3, 363–372. [Google Scholar] [CrossRef]
- Allbed, A.; Kumar, L.; Aldakheel, Y.Y. Assessing soil salinity using soil salinity and vegetation indices derived from IKONOS high-spatial resolution imageries: Applications in a date palm dominated region. Geoderma 2014, 230–231, 1–8. [Google Scholar] [CrossRef]
- Farifteh, J.; Farshad, A.; George, R.J. Assessing salt-affected soils using remote sensing, solute modelling, and geophysics. Geoderma 2006, 130, 191–206. [Google Scholar] [CrossRef]
- Li, H.Y.; Shi, Z.; Webster, R.; Triantafilis, J. Mapping the three-dimensional variation of soil salinity in a rice-paddy soil. Geoderma 2013, 195–196, 31–41. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Zhang, X.; Wang, X.; Lv, Z.; Yang, J.; Shao, H.; Yu, S. GIS-mapping spatial distribution of soil salinity for Eco-restoring the Yellow River Delta in combination with Electromagnetic Induction. Ecol. Eng. 2016, 94, 306–314. [Google Scholar] [CrossRef]
- Jiang, Q.; Peng, J.; Biswas, A.; Hu, J.; Zhao, R.; He, K.; Shi, Z. Characterising dryland salinity in three dimensions. Sci. Total Environ. 2019, 682, 190–199. [Google Scholar] [CrossRef]
- Dehni, A.; Kheloufi, N.; Bouakkaz, K. Implicit modeling of salinity reconstruction by using 3D combined models. Environ. Earth Sci. 2020, 79, 440. [Google Scholar] [CrossRef]
- FAO. Harmonized World Soil Database v12. Available online: https://www.fao.org/soils-portal/soil-survey/soil-maps-and-databases/harmonized-world-soil-database-v12/en/ (accessed on 12 January 2024).
- Qin, Y.; Bai, Y.; Chen, G.; Liang, Y.; Li, X.; Wen, B.; Lu, X.; Li, X. The effects of soil freeze–thaw processes on water and salt migrations in the western Songnen Plain, China. Sci. Rep. 2021, 11, 3888. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ge, H.; Xu, Y.; Zhuang, L.; Wang, F.; Gu, Q.; Li, X. Estimating Soil Salinity Using Multiple Spectral Indexes and Machine Learning Algorithm in Songnen Plain, China. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2023, 16, 7041–7050. [Google Scholar]
- Yu, H.; Wang, Z.; Mao, D.; Jia, M.; Chang, S.; Li, X. Spatiotemporal variations of soil salinization in China’s West Songnen Plain. Land Degrad. Dev. 2023, 34, 2366–2378. [Google Scholar] [CrossRef]
- Li, P. Soil Analysis in Xinjiang; People’s Publishing House in Xinjiang: Urumqi, China, 1983; pp. 1–207. (In Chinese) [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; United States Salinity Laboratory Staff: Washington, DC, USA, 1954; pp. 8–17. [Google Scholar]
- Page, A.L.; Miller, R.H.; Jeeney, D.R. Methods of soil analysis, part 2. In Chemical and Mineralogical Properties; Soil Science Society of American Publication: Madison, WI, USA, 1992; p. 1159. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000; pp. 1–495. (In Chinese) [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA Publication: Washington, DC, USA, 2005. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part III, 3rd ed.; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Baver, L.; David, L. Soil Physics, 3rd ed.; Wiley: New York, NY, USA, 1956. [Google Scholar]
- Teng, S.C.; Hsai, C.C.; Hseung, Y. hydrometer method of mechanical analysis of soils. ACTA Pedol. Sin. 1958, 6, 70–83. [Google Scholar]
- Gao, J.J.; Liu, J.H.; Zou, J.J.; Song, X.H.; Zhu, A.M. Determination of Dissolved Organic Carbon in Seawater by Combustion Oxidation-non-dispersive Infrared Absorption Method. Adv. Mar. Sci. 2009, 27, 477–482. (In Chinese) [Google Scholar]
- Gilmore, K.R.; Luong, H.V. Improved method for measuring total dissolved solids. Anal. Lett. 2016, 49, 1772–1782. [Google Scholar] [CrossRef]
- Maiorova, A.V.; Belozerova, A.A.; Mel’chakov, S.Y.; Mashkovtsev, M.A.; Suvorkina, A.S.; Shunyaev, K.Y. Determination of Arsenic and Antimony in Ferrotungsten by Inductively Coupled Plasma Atomic Emission Spectrometry. J. Anal. Chem. 2019, 74, 18–26. [Google Scholar] [CrossRef]
- Pohl, P.; Stecka, H.; Jamroz, P. Solid phase extraction with flame atomic absorption spectrometry for determination of traces of Ca, K, Mg and Na in quality control of white sugar. Food Chem. 2012, 130, 441–446. [Google Scholar] [CrossRef]
- Hodge, E.M.; Martinez, P.; Sweetin, D. Determination of inorganic cations in brine solutions by ion chromatography. J. Chromatogr. A 2000, 884, 223–227. [Google Scholar] [CrossRef]
- Abd El-Hamid, H.T.; Alshehri, F.; El-Zeiny, A.M.; Nour-Eldin, H. Remote sensing and statistical analyses for exploration and prediction of soil salinity in a vulnerable area to seawater intrusion. Mar. Pollut. Bull. 2023, 187, 114555. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.K.; Brijesh, K.R.; Dwivedi, P. Spatial modelling of soil alkalinity in GIS environment using IRS data. In Proceedings of the 18th Asian Conference in Remote Sensing, Kuala Lumpur, Malaysia, 20–24 October 1997. [Google Scholar]
- Amalo, L.F.; Hidayat, R.; Sulma, S. Analysis of Agricultural Drought in East Java Using Vegetation Health Index. AGRIVITA J. Agric. Sci. 2018, 40, 63–73. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S. Using remote sensing techniques for appraisal of irrigated soil salinity. In Proceedings of the International Congress on Modelling and Simulation, Land, Water and Environmental Management: Integrated Systems for Sustainability, Christchurch, New Zealand, 10–13 December 2007. [Google Scholar]
- Abbas, A.; Khan, S.; Hussain, N.; Hanjra, M.A.; Akbar, S. Characterizing soil salinity in irrigated agriculture using a remote sensing approach. Phys. Chem. Earth 2013, 55–57, 43–52. [Google Scholar] [CrossRef]
- Scudiero, E.; Skaggs, T.H.; Corwin, D.L. Regional scale soil salinity evaluation using Landsat 7, Western San Joaquin Valley, CA, USA. Geoderma Reg. 2014, 2–3, 82–90. [Google Scholar] [CrossRef]
- Masoud, A.A.; Koike, K. Arid land salinization detected by remotely-sensed landcover changes: A case study in the Siwa region, NW Egypt. J. Arid Environ. 2006, 66, 151–167. [Google Scholar] [CrossRef]
- Wang, J.; Ding, J.; Yu, D.; Teng, D.; He, B.; Chen, X.; Ge, X.; Zhang, Z.; Wang, Y.; Yang, X.; et al. Machine learning-based detection of soil salinity in an arid desert region, Northwest China: A comparison between Landsat-8 OLI and Sentinel-2 MSI. Sci. Total Environ. 2020, 707, 136092. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Jones, S.B.; Philpot, W.D. A linear physically-based model for remote sensing of soil moisture using short wave infrared bands. Remote Sens. Environ. 2015, 164, 66–76. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J. NMDI: A normalized multi-band drought index for monitoring soil and vegetation moisture with satellite remote sensing. Geophys. Res. Lett. 2007, 34, 1–5. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Ustin, S.L. Modeling Canopy Water Content for Carbon Estimates from MODIS data at Land EOS Validation Sites. In Proceedings of the International Geoscience and Remote Sensing Symposium, Sydney, Australia, 9–13 July 2001. [Google Scholar]
- Xiao, X.; Boles, S.; Liu, J.; Zhuang, D.; Frolking, S.; Li, C.; William, S.; Berrien, M. Mapping paddy rice agriculture in southern China using multi-temporal MODIS images. Remote Sens. Environ. 2005, 95, 480–492. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Pearson, R.L.; Miller, L.D. Remote mapping of standing crop biomass for estimation of the productivity of the short-grass Prairie, Pawnee National Grasslands, Colorado. In Proceedings of the 8th International Symposium on Remote Sensing of Environment, Ann Arbor, MI, USA, 2–6 October 1972. [Google Scholar]
- Richardson, A.J.; Wiegand, C.L. Distinguishing vegetation from soil background information. Photogramm. Eng. Remote Sens. 1977, 43, 1541–1552. [Google Scholar]
- Baret, F.; Guyot, G. Potentials and limits of vegetation indices for LAI and APAR assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Crist, E.P.A. TM Tasseled Cap equivalent transformation for reflectance factor data. Remote Sens. Environ. 1985, 17, 301–306. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Available online: http://www.moa.gov.cn/ztzl/dscqgtrpc/zywj/202307/t20230720_6432535.htm (accessed on 12 January 2024). (In Chinese)
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- USDA. Soil Taxonomy, a Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 1999; pp. 9–18.
- WRB. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, Update 2015; FAO: Rome, Italy, 2015; pp. 40–41. [Google Scholar]
- Mandal, A.K. Necessity for quantified measurement of soil sodicity and selection of suitable gypsum amendment for proper reclamation of sodic soils. Pedosphere 2023, 33, 231–235. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.; Fu, Q.; Guo, B.; Lin, Y.; Liu, X. Long-term rice cultivation improved coastal saline soil properties and multifunctionality of subsoil layers. Soil Use Manag. 2023, 40, e12918. [Google Scholar] [CrossRef]
- Karavanova, E.; Shrestha, D.P.; Orlov, D.S. Application of remote sensing techniques for the study of soil salinity in semi-arid Uzbekistan. In Response to Land Degradation; CRC Press: Boca Raton, FL, USA, 1999; pp. 261–273. [Google Scholar]
- Abrol, I.; Yadav, J.S.P.; Massoud, F. Salt-Affected Soils and Their Management; Food & Agriculture Organization: Rome, Italy, 1988; Volume 39. [Google Scholar]
- Schoeneberger, P.; Wysocki, D.; Benham, E.; Broderson, W. Field Book for Describing and Sampling Soils, Version 2.0; Natural Resources Conservation Service, National Soil Survey Center: Lincoln, OR, USA, 2002; pp. 2–72. [Google Scholar]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Ibrakhimov, M.; Khamzina, A.; Forkutsa, I.; Paluasheva, G.; Lamers, J.P.A.; Tischbein, B.; Vlek, P.L.G.; Martius, C. Groundwater table and salinity: Spatial and temporal distribution and influence on soil salinization in Khorezm region (Uzbekistan, Aral Sea Basin). Irrig. Drain. Syst. 2007, 21, 219–236. [Google Scholar] [CrossRef]
- Luo, J.M.; Yang, F.; Wang, Y.J.; Ya, Y.J.; Deng, W.; Zhang, X.P.; Liu, Z.J. Mechanism of soil sodification at the local scale in Songnen Plain, northeast China, as affected by shallow groundwater table. Arid Land Res. Manag. 2011, 25, 234–256. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, G.X.; Yin, X.R.; Liu, Z.J.; Huang, Z.G. Study on capillary rise from shallow groundwater and critical water table depth of a saline-sodic soil in western Songnen Plain of China. Environ. Earth Sci. 2011, 64, 2119–2126. [Google Scholar] [CrossRef]
- FAO. Global Map of Salt-Affected Soils. Available online: https://www.fao.org/global-soil-partnership/gsasmap/en (accessed on 12 January 2024).
- Ren, J.; Xie, R.; Zhao, Y.; Zhang, Z. Fractal Approach to Measuring Electrical Conductivity Values of Soda Saline-Alkali Soils with Desiccation Cracks in the Songnen Plain, China. J. Soil Sci. Plant Nut. 2023, 23, 1953–1966. [Google Scholar] [CrossRef]

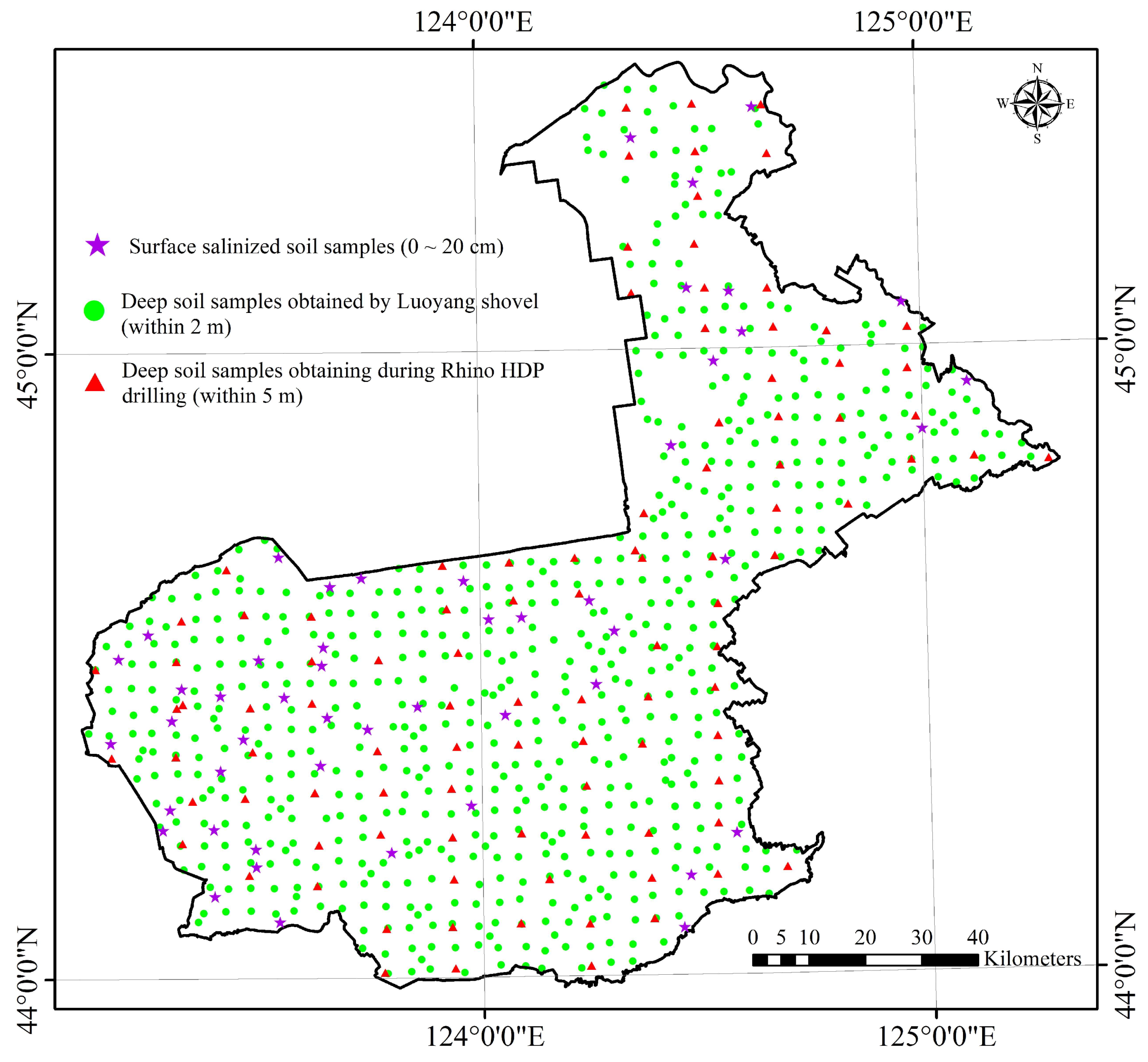




| Data Source Type | Quantity | Depth |
|---|---|---|
| TWSS and salt ions of surface salinized soil samples | 50 | 20 cm |
| pH, SOC, and texture of deep soil samples | 1955 | 2 m |
| 407 | 5 m | |
| TDS and salt ions of surface water samples | 25 | surface |
| TDS and salt ions of groundwater samples | 25 | underground |
| Band Number | Central Wavelength (μm) | Spatial Resolution(m) |
|---|---|---|
| Band1 Coastal Aerosol | 0.443 | 60 |
| Band2 Blue | 0.490 | 10 |
| Band3 Green | 0.560 | 10 |
| Band4 Red | 0.665 | 10 |
| Band5 Vegetation Red Edge1 | 0.705 | 20 |
| Band6 Vegetation Red Edge2 | 0.740 | 20 |
| Band7 Vegetation Red Edge3 | 0.783 | 20 |
| Band8 NIR | 0.842 | 10 |
| Band8A Vegetation Red Edge | 0.865 | 20 |
| Band9 Water Vapor | 0.945 | 60 |
| Band10 SWIR Cirrus | 1.375 | 60 |
| Band11 SWIR1 | 1.610 | 20 |
| Band12 SWIR2 | 2.190 | 20 |
| Number | Name | Formula | References |
|---|---|---|---|
| 1 | NDSI | (BRed − BNIR)/(BRed + BNIR) | [12,16,19,54] |
| 2 | NSI | (BSWIR1 − BSWIR2)/(BSWIR1 + BNIR) | [17] |
| 3 | ASTER_SI | (BSWIR1 − BSWIR2)/(BSWIR1 + BSWIR2) | [4,14,15,16] |
| 4 | ESI | 2.5 × (BRed − BNIR)/(BNIR + 6 × BRed − 7.5 × BBlue + 1) | [18] |
| 5 | Intensity Indices | (BGreen + BRed + BNIR)/3 | [13,15] |
| 6 | Intensity Indices | sqrt(BGreen2 + BRed2 + BNIR2) | [13,15,16,21] |
| 7 | Intensity Indices | sqrt(BRed2 + BGreen2) | [13,15,16,21] |
| 8 | Brightness Index | sqrt(BRed2 + BNIR2) | [12,13,16] |
| 9 | Brightness Index | sqrt(BGreen2 + BNIR2) | [15,16] |
| 10 | Brightness Index | sqrt(BRed2 + BRed_edge32) | [14] |
| 11 | Ratio salt index | (BBlue − BSWIR2)/(BBlue + BSWIR2) | [8] |
| 12 | Band Ratio SI | BBlue/BGreen | [16] |
| 13 | Band Ratio SI | BBlue/BRed | [13,16,31,57,58] |
| 14 | Band Ratio SI | BBlue/BNIR | [16] |
| 15 | Band Ratio SI | BGreen/BRed | [16] |
| 16 | Band Ratio SI | BGreen/BNIR | [16] |
| 17 | Band Ratio SI | BRed/BNIR | [16] |
| 18 | Band Ratio SI | (BGreen × BRed)/BBlue | [31,57,58] |
| 19 | Band Ratio SI | (BBlue × BRed)/BGreen | [16,31,57] |
| 20 | Band Ratio SI | (BRed × BNIR)/BGreen | [15,27] |
| 21 | SI-T | (BRed/BNIR) × 100 | [55] |
| 22 | SI-2 | (BBlue − BRed)/(BBlue + BRed) | [31,57] |
| 23 | SI-4 | (BSWIR1 × BRed)/BGreen | [8] |
| 24 | SI | (BGreen + BRed)/2 | [13,15] |
| 25 | SI | BSWIR1/BSWIR2 | [14,15,16] |
| 26 | SI | (BRed − BSWIR1)/(BRed + BSWIR1) | [14] |
| 27 | SI | sqrt(BBlue × BRed) | [12,14,16,31,57,58] |
| 28 | SI | sqrt(BGreen × BRed) | [12,13,15,16,31,40,59] |
| 29 | SI | sqrt(BRed × BNIR) | [16] |
| 30 | SSSI-1 | BSWIR1 − BSWIR2 | [14] |
| 31 | SSSI-2 | (BSWIR1 × BSWIR2 − BSWIR2 × BSWIR2)/BSWIR1 | [14] |
| Number | Name | Formula | References |
|---|---|---|---|
| 1 | Tasseled cap wetness | 0.1509 × BBlue + 0.1973 × BGreen + 0.3273 × BRed + 0.3406 × BNIR − 0.7112 × BSWIR1 − 0.4573 × BSWIR2 | [60,61] |
| 2 | NDWI | (BNIR − BSWIR1)/(BNIR + BSWIR1) | [18] |
| 3 | STR | 0.5 × (1 − BSWIR22)/BSWIR2 | [62] |
| 4 | NMDI | BNIR − (BSWIR1 − BSWIR2)/BNIR + BSWIR1 − BSWIR2 | [63] |
| 5 | SRWI | BVegetation RedEdge/BSWIR1 | [64] |
| 6 | LSWI | (BVegetation RedEdge − BSWIR1)/(BVegetation RedEdge + BSWIR1) | [65] |
| Number | Name | Formula | References |
|---|---|---|---|
| 1 | NDVI | (BNIR − BRed)/(BRed + BNIR) | [13,15,40,58,59] |
| 2 | EVI | 2.5 × (BNIR − BRed)/(BNIR + 6 × BRed − 7.5 × BBlue + 1) | [15,40,59] |
| 3 | SAVI | 1.5 × (BNIR − BRed)/(BRed + BNIR + 0.5) | [13,15,31,40,60,66] |
| 4 | RVI | BNIR/BRed | [67] |
| 5 | DVI | BNIR − BRed | [13,40] |
| 6 | PVI | (BNIR − (a × BRed + b))/sqrt(1 + a2) | [13,68] |
| 7 | TSAVI | a × (BNIR − (a × BRed + b))/(BRed + a × (BNIR − b) + 0.08 × (1 + a2)) | [13,69] |
| 8 | Tasseled cap greenness | −0.0635 × BCoastal − 0.1128 × BBlue − 0.1680 × BGreen − 0.3480 × BRed − 0.3303 × BRedEdge1 + 0.0852 × BRedEdge2 + 0.3302 × BRedEdge3 + 0.3165 × BNIR + 0.3625 × BVegetation RedEdge + 0.0467 × BWaterVapor − 0.4578 × BSWIR1 − 0.4064 × BSWIR2 | [70] |
| 9 | CRSI | sqrt((BNIR × BRed − BRed × BBlue)/(BNIR × BRed + BRed × BBlue)) | [16,59] |
| Taxonomies | Indicators | Saline–Alkaline Classification/Grading | |
|---|---|---|---|
| USSL Staff 1954 | EC*, ESP, pH | EC* ≥ 4, ESP < 15%, pH < 8.5 | saline |
| EC* ≥ 4, ESP ≥ 15%, pH ≥ 8.5 | saline–sodic | ||
| EC* < 4, ESP ≥ 15%, pH > 8.5 | sodic | ||
| FAO 1988 | EC* | 0 ≤ EC* < 2 | non-saline |
| 2 ≤ EC* < 4 | slightly saline | ||
| 4 ≤ EC* < 8 | moderately saline | ||
| 8 ≤ EC* < 16 | strongly saline | ||
| 16 ≤ EC* | very strongly saline | ||
| USDA 2002 | EC* | 0 ≤ EC* < 2 | non-saline |
| 2 ≤ EC* < 4 | very slightly saline | ||
| 4 ≤ EC* < 8 | slightly saline | ||
| 8 ≤ EC* < 16 | moderately saline | ||
| 16 ≤ EC* | strongly saline | ||
| CTNSS 2022 | TWSS | 1 k·kg−1 ≤ TWSS < 3 k·kg−1 | slightly saline |
| 3 k·kg−1 ≤ TWSS < 5 k·kg−1 | moderately saline | ||
| 5 k·kg−1 ≤ TWSS < 7 k·kg−1 | strongly saline | ||
| 7 k·kg−1 ≤ TWSS | sodic saline | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Hao, Y.; Huang, Q.; Liu, Y.; Xiu, L.; Gao, Q. Soil Salinity Estimation by 3D Spectral Space Optimization and Deep Soil Investigation in the Songnen Plain, Northeast China. Sustainability 2024, 16, 2069. https://doi.org/10.3390/su16052069
Ma M, Hao Y, Huang Q, Liu Y, Xiu L, Gao Q. Soil Salinity Estimation by 3D Spectral Space Optimization and Deep Soil Investigation in the Songnen Plain, Northeast China. Sustainability. 2024; 16(5):2069. https://doi.org/10.3390/su16052069
Chicago/Turabian StyleMa, Min, Yi Hao, Qingchun Huang, Yongxin Liu, Liancun Xiu, and Qi Gao. 2024. "Soil Salinity Estimation by 3D Spectral Space Optimization and Deep Soil Investigation in the Songnen Plain, Northeast China" Sustainability 16, no. 5: 2069. https://doi.org/10.3390/su16052069
APA StyleMa, M., Hao, Y., Huang, Q., Liu, Y., Xiu, L., & Gao, Q. (2024). Soil Salinity Estimation by 3D Spectral Space Optimization and Deep Soil Investigation in the Songnen Plain, Northeast China. Sustainability, 16(5), 2069. https://doi.org/10.3390/su16052069







