Landscape Drivers Influence the Efficiency of Management of Aquatic Invasive Alien Rodents in Western France
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
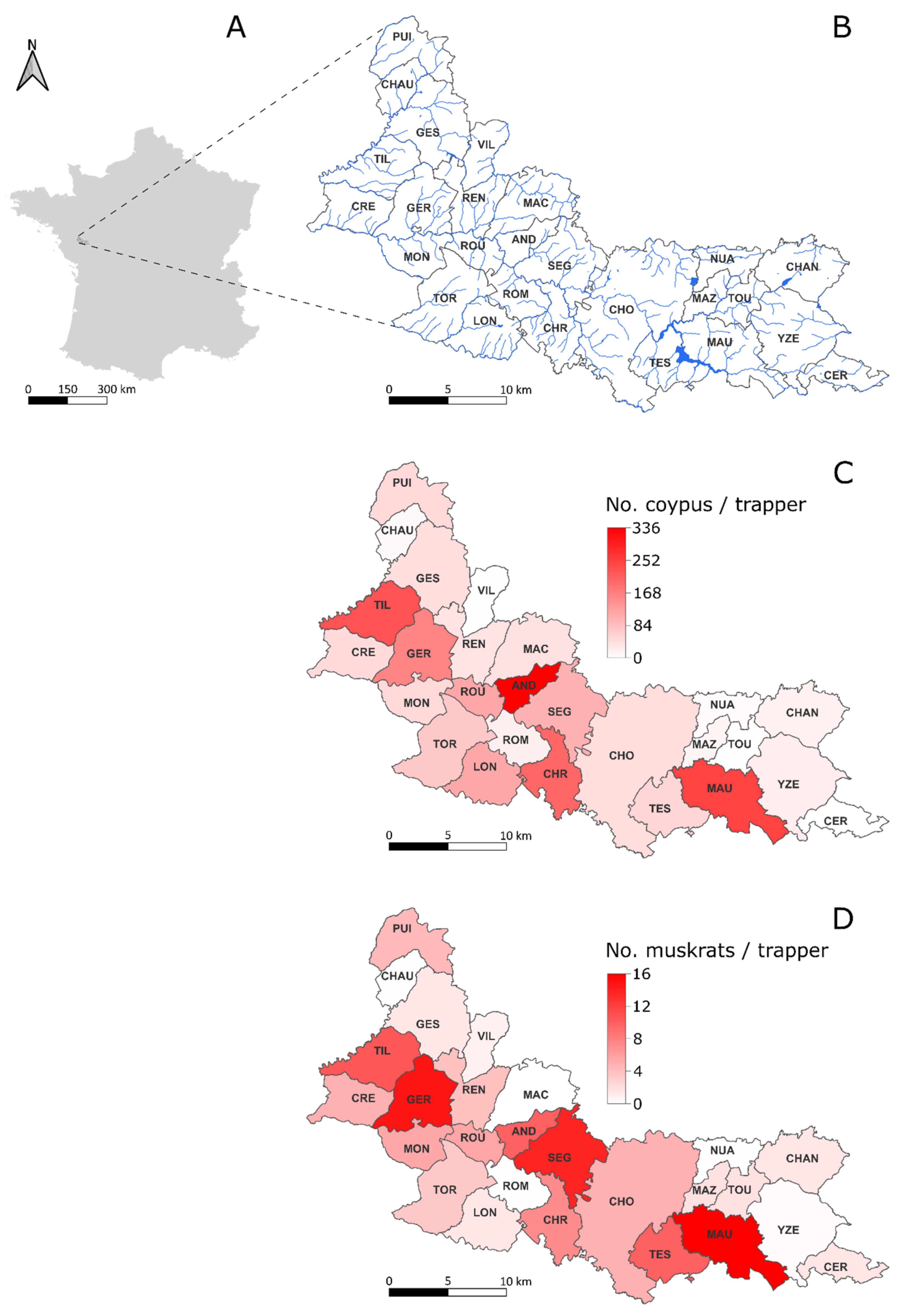
2.2. Study Area
2.3. Control Activities
2.4. Capture Data
2.5. Landscape Data
2.6. Data Analysis
3. Results
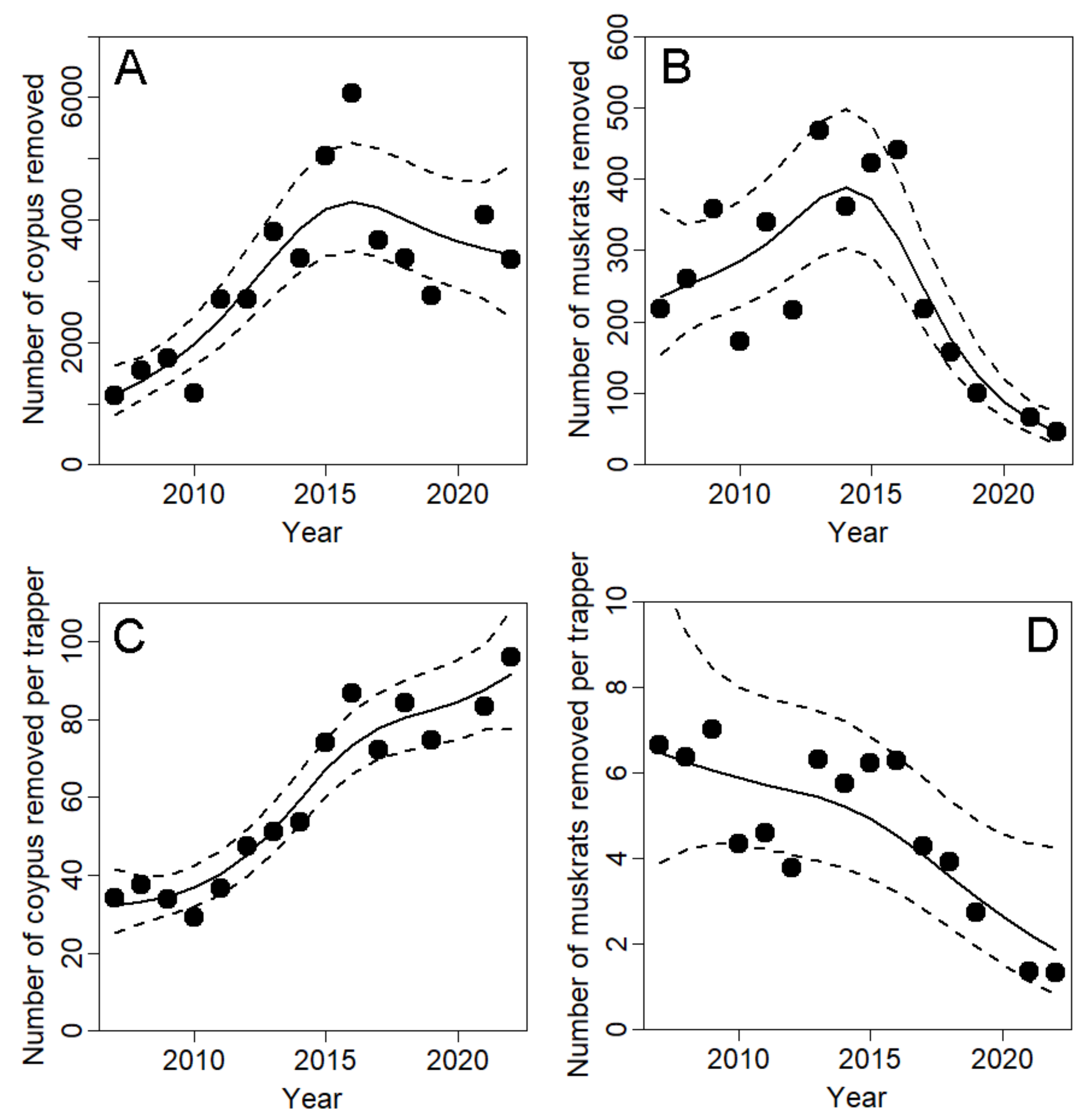
3.1. Temporal Trends of Captures and Volunteer Engagement
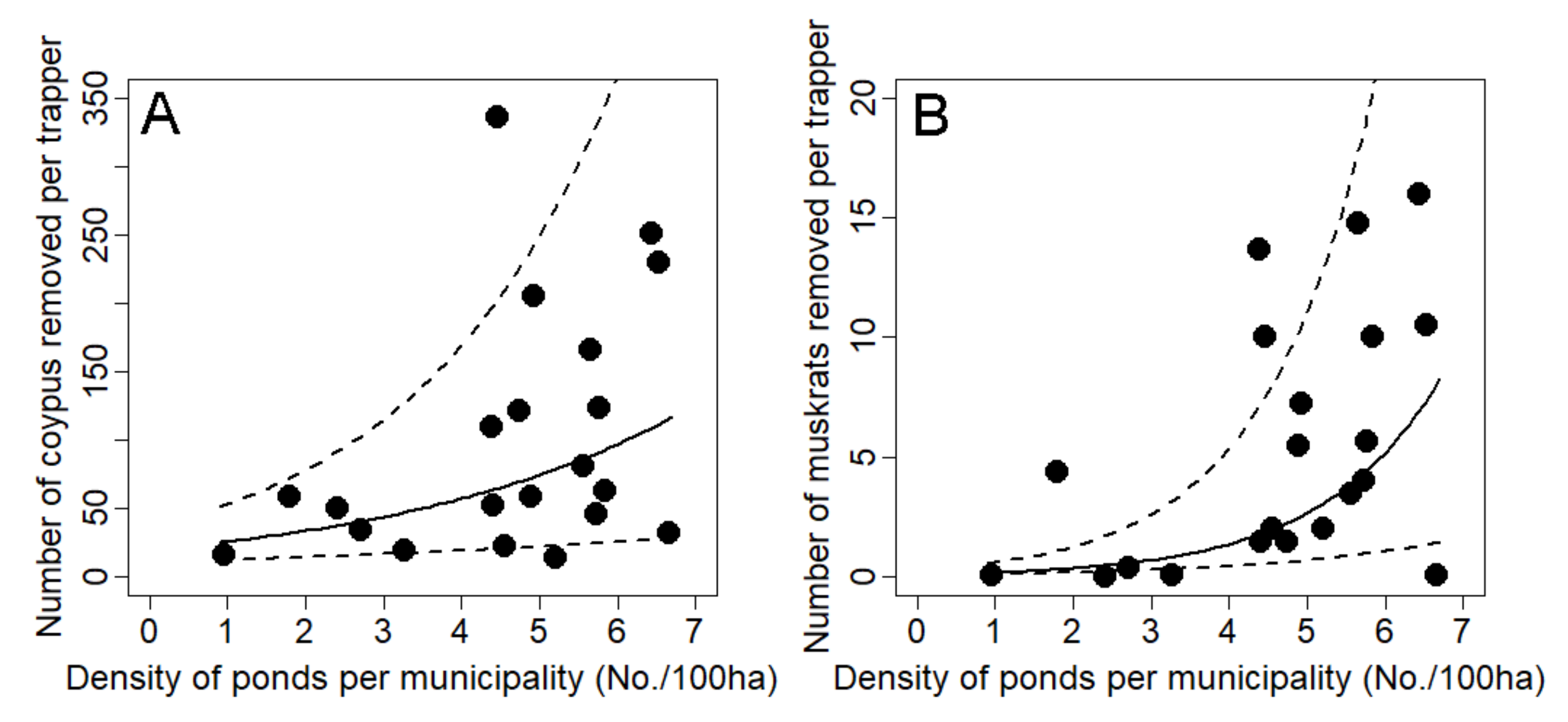
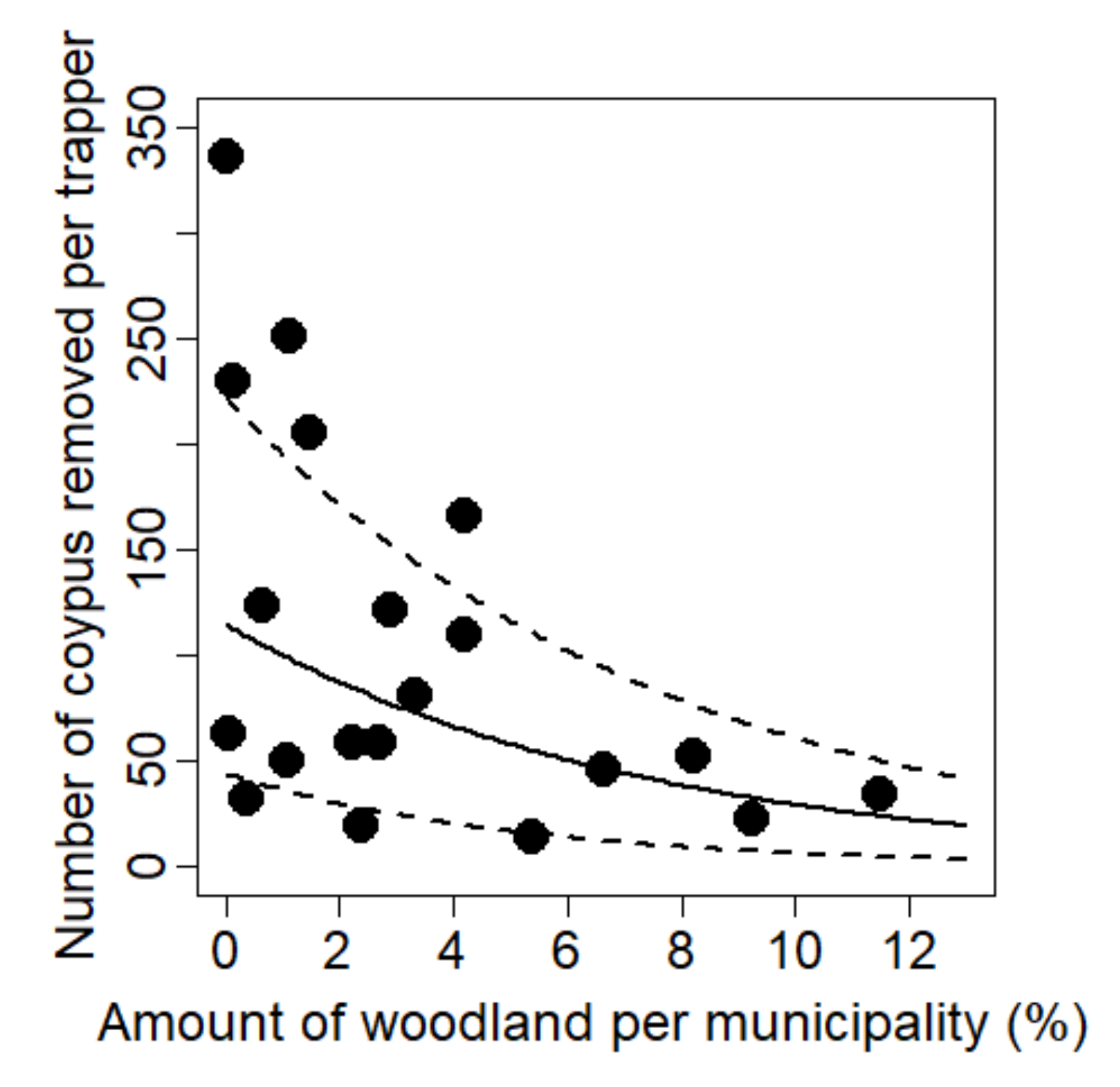
3.2. Effect of Landscape Drivers on Captures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doherty, T.S.; Glen, A.S.; Nimmo, D.G.; Ritchie, E.G.; Dickman, C.R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA 2016, 113, 11261–11265. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Roy, H.E., Pauchard, A., Stoett, P., Renard Truong, T., Bacher, S., Galil, B.S., Hulme, P.E., Ikeda, T., Sankaran, K.V., McGeoch, M.A., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2023; 56p. [Google Scholar] [CrossRef]
- Keller, R.P.; Geist, J.; Jeschke, J.; Kühn, I. Invasive species in Europe: Ecology, status, and policy. Environ. Sci. Eur. 2011, 23, 23. [Google Scholar] [CrossRef]
- Bradley, B.A.; Laginhas, B.B.; Whitlock, R.; Allen, J.M.; Bates, A.E.; Bernatchez, G.; Diez, J.M.; Early, R.; Lenoir, J.; Vilà, M.; et al. Disentangling the abundance–impact relationship for invasive species. Proc. Natl. Acad. Sci. USA 2019, 116, 9919–9924. [Google Scholar] [CrossRef]
- Kettenring, K.M.; Adams, C.R. Lessons learned from invasive plant control experiments: A systematic review and meta-analysis. J. Appl. Ecol. 2011, 48, 970–979. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Hulme, P.E. Non-native species, ecosystem services, and human well-being. In Impact of Biological Invasions on Ecosystem Services; Vilà, M., Hulme, P.E., Eds.; Springer International Publishing: Berlin, Germany; Cham, Switzerland, 2017; Volume 12, pp. 1–14. [Google Scholar] [CrossRef]
- Cuthbert, R.; Pattison, Z.; Taylor, N.; Verbrugge, L.; Diagne, C.; Ahmed, D.A.; Leroy, B.; Angulo, E.; Briski, E.; Capinha, C.; et al. Global economic costs of aquatic invasive alien species. Sci. Total Environ. 2021, 775, 145238. [Google Scholar] [CrossRef] [PubMed]
- Chinchio, E.; Crotta, M.; Romeo, C.; Drewe, J.A.; Guitian, J.; Ferrari, N. Invasive Alien Species and Disease Risk: An Open Challenge in Public and Animal Health. PLoS Pathog. 2020, 16, e1008922. [Google Scholar] [CrossRef]
- Bertolino, S.; Perrone, A.; Gola, L. Effectiveness of Coypu Control in Small Italian Wetland Areas. Wildl. Soc. Bull. 2005, 33, 714–720. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Gozlan, R.; Vaissière, A.C.; Assailly, C.; Nuninger, L.; Roiz, D.; Jourdain, F.; Jarić, I.; Courchamp, F. InvaCost, a Public Database of the Economic Costs of Biological Invasions Worldwide. Sci. Data 2020, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Panzacchi, M.; Cocchi, R.; Genovesi, P.; Bertolino, S. Population control of coypu Myocastor coypus in Italy compared to eradication in UK: A cost-benefit analysis. Wildl. Biol. 2007, 13, 159–171. [Google Scholar] [CrossRef]
- Sofia., G.; Masin, R.; Tarolli, P. Prospects for Crowdsourced Information on the Geomorphic ‘Engineering’ by the Invasive Coypu (Myocastor Coypus). Earth Surf. Process. Landf. 2017, 42, 365–377. [Google Scholar] [CrossRef]
- Ayral, F.; Kodjo, A.; Guédon, G.; Boué, F.; Richomme, C. Muskrats Are Greater Carriers of Pathogenic Leptospira than Coypus in Ecosystems with Temperate Climates. PLoS ONE 2020, 15, e0228577. [Google Scholar] [CrossRef] [PubMed]
- Boorman, L.A.; Fuller, R.M. The changing status of reedswamp in the Norfolk Broads, England, UK. J. Appl. Ecol. 1981, 18, 241–269. [Google Scholar] [CrossRef]
- Bertolino, S.; Angelici, C.; Monaco, E.; Monaco, A.; Capizzi, D. Interactions between Coypu (Myocastor coypus) and Bird Nests in Three Mediterranean Wetlands of Central Italy. Hystrix Ital. J. Mamm. 2011, 22, 333–339. [Google Scholar] [CrossRef]
- Diggins, T.P.; Stewart, K.M. Evidence of large change in unionid mussel abundance from selective muskrat predation, as inferred by shell remains left on shore. Int. Rev. Hydrobiol. 2000, 85, 505–520. [Google Scholar] [CrossRef]
- Nagayama, S.; Kume, M.; Oota, M.; Mizushima, K.; Mori, S. Common Coypu Predation on Unionid Mussels and Terrestrial Plants in an Invaded Japanese River. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 37. [Google Scholar] [CrossRef]
- Mori, E.; Andreoni, A.; Cecere, F.; Magi, M.; Lazzeri, L. Patterns of activity rhythms of invasive coypus Myocastor coypus inferred through camera-trapping. Mamm. Biol. 2020, 100, 591–599. [Google Scholar] [CrossRef]
- Gosling, L.M. Climatic determinants of spring littering by feral coypus, Myocastor coypus. J. Zool. 1981, 195, 281–288. [Google Scholar] [CrossRef]
- Simberloff, D.; Keitt, B.; Will, D.; Holmes, N.; Pickett, E.; Genovesi, P. Yes we can! Exciting progress and prospects for controlling invasives on islands and beyond. West. N. Am. Nat. 2018, 78, 942–958. [Google Scholar] [CrossRef]
- Gosling, L.M.; Baker, S.J. The eradication of muskrats and coypus from Britain. Biol. J. Linn. Soc. 1989, 38, 39–51. [Google Scholar] [CrossRef]
- Anderson, D.P.; Pepper, M.A.; Travers, S.; Michaels, T.A.; Sullivan, K.; Ramsey, D.S.L. Confirming the broadscale eradication success of nutria (Myocastor coypus) from the Delmarva Peninsula, USA. Biol. Invasions 2022, 24, 3509–3521. [Google Scholar] [CrossRef]
- Braysher, M. Managing Vertebrate Pests: Principles and Strategies; Bureau of Resource Sciences, Australian Government Publishing Service: Canberra, Australia, 1993; 50p.
- Bonnet, M.; Guédon, G.; Pondaven, M.; Bertolino, S.; Padiolleau, D.; Pénisson, V.; Gastinel, F.; Angot, F.; Renaud, P.C.; Frémy, A.; et al. Aquatic invasive alien rodents in Western France: Where do we stand today after decades of control? PLoS ONE 2021, 16, e0249904. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Guédon, G.; Bertolino, S.; Harmange, C.; Pagano, A.; Picard, D.; Pays, O. Improving the management of aquatic invasive alien rodents in France: Appraisal and recommended actions. Manag. Biol. Invasions 2023, 14, 625–640. [Google Scholar] [CrossRef]
- Bryce, R.; Oliver, M.K.; Davies, L.; Gray, H.; Urquhart, J.; Lambin, X. Turning back the tide of American mink invasion at an unprecedented scale through community participation and adaptive management. Biol. Conserv. 2011, 144, 575–583. [Google Scholar] [CrossRef]
- de Sá Dechoum, M.; Giehl, E.L.H.; Sühs, B.G.R.; Silveira, T.C.L.; Ziller, S.R. Citizen engagement in the management of non-native invasive pines: Does it make a difference? Biol. Invasions 2019, 21, 175–188. [Google Scholar] [CrossRef]
- Sheail, J. The grey squirrel (Sciurus carolinensis)—A UK historical perspective on a vertebrate pest species. J. Environ. Manag. 1999, 55, 145–156. [Google Scholar] [CrossRef]
- Dedah, C.O.; Kazmierczak, R.F.; Walter, R.K. The role of bounties and human behavior on Louisiana nutria harvests. J. Agric. Appl. Econ. 2010, 42, 133–142. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.C.; Kim, A.; Lim, J.; Kim, T.S.; Park, S.G.; Kim, M.; Lee, J.H.; Lee, J.R.; Lee, D.H. Distribution and Management of Nutria (Myocastor coypus) Populations in South Korea. Sustainability 2019, 11, 4169. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Van Loon, E.E.; Bos, D.; van Hellenberg Hubar, C.J.; Ydenberg, R.C. A historical perspective on the effects of trapping and controlling the muskrat (Ondatra zibethicus) in the Netherlands. Pest Manag. Sci. 2017, 73, 305–312. [Google Scholar] [CrossRef]
- Bos, D.; Kentie, R.; La Haye, M.; Ydenberg, R.C. Evidence for the effectiveness of controlling muskrat (Ondatra zibethicus L.) populations by trapping. Eur. J. Wildl. 2019, 65, 45. [Google Scholar] [CrossRef]
- Bertolino, S.; Viterbi, R. Long-Term Cost-Effectiveness of Coypu (Myocastor coypus) Control in Piedmont (Italy). Biol. Invasions 2010, 12, 2549–2558. [Google Scholar] [CrossRef]
- Higgins, S.; Richardson, D.; Cowling, R. Modeling invasive plant spread: The role of plant-environment interactions and model structure. Ecology 1996, 77, 2043–2054. [Google Scholar] [CrossRef]
- With, K.A. The Landscape Ecology of Invasive Spread. Conserv. Biol. 2002, 16, 1192–1203. [Google Scholar] [CrossRef]
- Rodewald, A.D.; Arcese, P. Direct and Indirect Interactions between Landscape Structure and Invasive or Overabundant Species. Curr. Landsc. Ecol. Rep. 2016, 1, 30–39. [Google Scholar] [CrossRef]
- Della Rocca, F.; Milanesi, P. The Spread of the Japanese Beetle in a European Human-Dominated Landscape: High Anthropization Favors Colonization of Popillia japonica. Diversity 2022, 14, 658. [Google Scholar] [CrossRef]
- Bertolino, S.; Ingegno, B. Modelling the distribution of an introduced species: The coypu Myocastor coypus (Mammalia, Rodentia) in Piedmont region. Ital. J. Zool. 2009, 76, 340–346. [Google Scholar] [CrossRef]
- Ruys, T.; Lorvelec, O.; Marre, A.; Bernez, I. River management and habitat characteristics of three sympatric aquatic rodents: Common muskrat, coypu and European beaver. Eur. J. Wildl. 2011, 57, 851–864. [Google Scholar] [CrossRef]
- Guichón, M.L.; Cassini, M.H. Local determinants of coypu distribution along the Luján River, eastcentral Argentina. J. Wildl. Manag. 1999, 63, 895–900. [Google Scholar] [CrossRef]
- Leggieri, L.R.; Guichón, M.L.; Cassini, M.H. Landscape correlates of the distribution of coypu Myocastor coypus (Rodentia, Mammalia) in Argentinean Pampas. Ital. J. Zool. 2011, 78, 124–129. [Google Scholar] [CrossRef]
- Turner, C.K.; Lantz, T.C.; Fisher, J.T. Muskrat distributions in a changing Arctic delta are explained by patch composition and configuration. Arct. Sci. 2020, 2, 77–94. [Google Scholar] [CrossRef]
- Kua, Z.X.; Stella, J.C.; Farrell, J.M. Local disturbance by muskrat, an ecosystem engineer, enhances plant diversity in regionally-altered wetlands. Ecosphere 2020, 11, e03256. [Google Scholar] [CrossRef]
- Ecke, F.; Henry, A.; Danell, K. Landscape-based prediction of the occurrence of the invasive muskrat (Ondatra zibethicus). Ann. Zool. Fenn. 2014, 51, 325–334. [Google Scholar] [CrossRef]
- Plateforme Ouverte des Données Publiques Françaises. Available online: https://data.gouv.fr/ (accessed on 1 May 2023).
- Sysma. Available online: https://sysma.io/ (accessed on 1 May 2023).
- IGN BD TOPO. Available online: https://geoservices.ign.fr/ (accessed on 1 May 2023).
- Copernicus Land Monitoring Service information. Available online: https://land.copernicus.eu/ (accessed on 1 May 2023).
- QGIS Development Team. QGIS Geographic Information System. Open-Source Geospatial Foundation Project. 2022. Available online: http://qgis.osgeo.org/en/site/ (accessed on 1 May 2023).
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; 574p. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Brian, D.M. Flexible smoothing with B-splines and penalties. Stat. Sci. 1996, 11, 89–121. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: New York, NY, USA, 2006; 410p. [Google Scholar]
- Wood, S.N. On p-values for smooth components of an extended generalized additive model. Biometrika 2013, 100, 221–228. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Monographs on Statistics and Applied Probability 43; Chapman and Hall/CRC: New York, NY, USA, 1990; 352p. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. Available online: http://www.R-project.org (accessed on 1 July 2023).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3. 1. 2019. Available online: https://svn.r-project.org/R-packages/trunk/nlme/ (accessed on 1 July 2023).
- Reggiani, G.; Boitani, L.; de Stefano, R. Population Dynamics and Regulation in the Coypu Myocastor coypus in Central Italy. Ecography 2006, 18, 138–146. [Google Scholar] [CrossRef]
- Baker, S. The eradication of coypus (Myocastor coypus) from Britain: The elements required for a successful campaign. In Assessment and Control of Biological Invasion Risks; Koike, F., Clout, M.N., Kawamichi, M., De Poorter, M., Iwatsuki, K., Eds.; Shoukadoh Book Sellers: Kyoto, Japan; IUCN: Gland, Switzerland, 2006; pp. 142–147. [Google Scholar]
- Gosling, L.M.; Baker, S.J. Planning and monitoring an attempt to eradicate coypus from Britain. Symp. Zool. Soc. Lond. 1987, 58, 99–113. [Google Scholar]
- Evans, J. About Nutria and Their Control; Resource Publication; United States Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1970; Volume 86, 65p.
- Ahlers, A.A.; Heske, E.J. Empirical Evidence for Declines in Muskrat Populations Across the United States. J. Wildl. Manag. 2017, 81, 1408–1416. [Google Scholar] [CrossRef]
- Jubase, N.; Shackleton, R.T.; Measey, J. Motivations and contributions of volunteer groups in the management of invasive alien plants in South Africa’s Western Cape province. Bothalia 2021, 51, a3. [Google Scholar] [CrossRef]
- Anđelković, A.A.; Handley, L.L.; Marchante, E.; Adriaens, T.; Brown, P.M.; Tricarico, E.; Verbrugge, L.N. A review of volunteers’ motivations to monitor and control invasive alien species. NeoBiota 2022, 73, 153–175. [Google Scholar] [CrossRef]
- Schertler, A.; Rabitsch, W.; Moser, D.; Wessely, J.; Essl, F. The potential current distribution of the coypu (Myocastor coypus) in Europe and climate change included shifts in the near future. Neobiota 2020, 58, 129–160. [Google Scholar] [CrossRef]
- Fahrig, L.; Rytwinski, T. Effects of roads on animal abundance: An empirical review and synthesis. Ecol. Soc. 2009, 14, 21. [Google Scholar] [CrossRef]
- Ruiz-Capillas, P.; Estacio, C.M.; Malo, J.E. Road verges are refuges for small mammal populations in extensively managed Mediterranean landscapes. Biol. Conserv. 2013, 158, 223–229. [Google Scholar] [CrossRef]
- Galantinho, A.; Santos, S.; Eufrázio, S.; Silva, C.; Carvalho, F.; Alpizar-Jara, R.; Mira, A. Effects of roads on small-mammal movements: Opportunities and risks of vegetation management on roadsides. J. Environ. Manag. 2022, 316, 115272. [Google Scholar] [CrossRef]

| Variables | χ2 | est.df | p | Deviance Explained |
|---|---|---|---|---|
| Number of coypus removed | 48.88 | 2.952 | <0.001 | 80.3 |
| Number of muskrats removed | 71.49 | 3.488 | <0.001 | 85.6 |
| Number of trappers | 22.91 | 3.144 | <0.001 | 70.7 |
| Number of coypus removed per trapper | 107.3 | 1.741 | <0.001 | 93.6 |
| Number of muskrats removed per trapper | 6.107 | 1.891 | 0.050 | 72.5 |
| Variables in the Municipality (x) | Number of Coypus Removed per Trapper (y1) | Number of Muskrats Removed per Trapper (y2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | θ1 ± SE (x) | F | p | R2 | Model | θ1 ± SE (x) | F | p | R2 | |
| Surface of municipality (ha) | Ln(y1) = θ0 + θ1.x | 1.397 | 0.251 | 0.019 | y2 = θ0 + θ1.x | 0.938 | 0.344 | 0.047 | ||
| Density of watercourses (m/ha) | Ln(y1) = θ0 + θ1.x | 2.284 | 0.147 | 0.060 | y2 = θ0 + θ1.x | 0.570 | 0.459 | 0.029 | ||
| Amount of pond surface (%) | Ln(y1) = θ0 + θ1.x | 0.956 | 0.340 | 0.050 | y2 = θ0 + θ1.x | 2.539 ± 1.212 | 4.386 | 0.049 | 0.145 | |
| Density of ponds (nb/100 ha) | Ln(y1) = θ0 + θ1.x | 0.264 ± 0.122 | 4.642 | 0.044 | 0.154 | Ln(y2) = θ0 + θ1.x | 0.665 ± 0.235 | 7.972 | 0.011 | 0.259 |
| Density of water infrastructures (nb/ha) | y1 = θ0 + θ1.x | 0.211 | 0.650 | 0.008 | y2 = θ0 + θ1.x | 1.279 | 0.272 | 0.013 | ||
| Amount of human-settled areas (%) | y1 = θ0 + θ1.x | 3.655 | 0.071 | 0.117 | y2 = θ0 + θ1.x | 0.768 | 0.391 | 0.038 | ||
| Amount of cereal (%) | y1 = θ0 + θ1.x | 0.001 | 0.984 | 0.001 | Ln(y2) = θ0 + θ1.x | 0.143 | 0.709 | 0.007 | ||
| Amount of woodland a | Ln(y1) = θ0 + θ1.x | −0.037 ± 0.016 | 5.293 | 0.032 | 0.217 | Ln(y2) = θ0 + θ1.x | 3.203 | 0.089 | 0.104 | |
| Amount of woodland b | Ln(y1) = θ0 + θ1.x | −0.136 ± 0.056 | 5.989 | 0.025 | 0.208 | Ln(y2) = θ0 + θ1.x | 2.641 | 0.122 | 0.122 | |
| Amount of pasture (%) | Ln(y1) = θ0 + θ1.x | 0.944 | 0.343 | 0.047 | y2 = θ0 + θ1.x | 0.034 | 0.854 | 0.002 | ||
| Density of riparian vegetation (m/ha) | y1 = θ0 + θ1.x | 0.311 | 0.583 | 0.016 | Ln(y2) = θ0 + θ1.x | 0.898 | 0.355 | 0.045 | ||
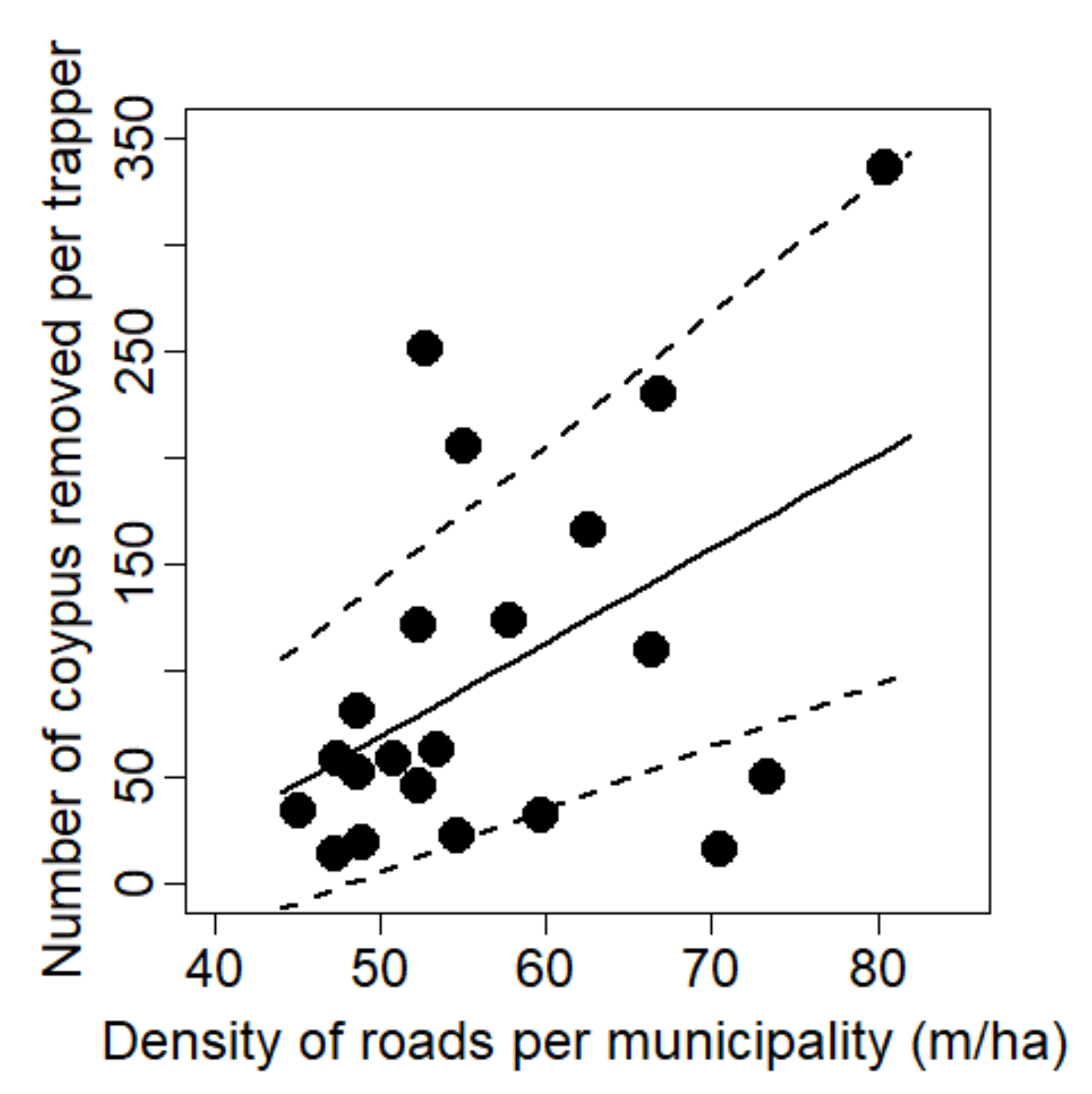
| Density of roads (m/ha) | y1 = θ0 + θ1.x | 4.402 ± 1.867 | 5.561 | 0.029 | 0.226 | y2 = θ0 + θ1.x | 1.530 | 0.231 | 0.074 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pays, O.; Bonnet, M.; Marchand, E.; Harmange, C.; Bertolino, S.; Pagano, A.; Picard, D.; Grillo, X.; Grimault-Frémy, A. Landscape Drivers Influence the Efficiency of Management of Aquatic Invasive Alien Rodents in Western France. Sustainability 2024, 16, 1970. https://doi.org/10.3390/su16051970
Pays O, Bonnet M, Marchand E, Harmange C, Bertolino S, Pagano A, Picard D, Grillo X, Grimault-Frémy A. Landscape Drivers Influence the Efficiency of Management of Aquatic Invasive Alien Rodents in Western France. Sustainability. 2024; 16(5):1970. https://doi.org/10.3390/su16051970
Chicago/Turabian StylePays, Olivier, Manon Bonnet, Ewen Marchand, Clément Harmange, Sandro Bertolino, Alain Pagano, Damien Picard, Xavier Grillo, and Antonin Grimault-Frémy. 2024. "Landscape Drivers Influence the Efficiency of Management of Aquatic Invasive Alien Rodents in Western France" Sustainability 16, no. 5: 1970. https://doi.org/10.3390/su16051970
APA StylePays, O., Bonnet, M., Marchand, E., Harmange, C., Bertolino, S., Pagano, A., Picard, D., Grillo, X., & Grimault-Frémy, A. (2024). Landscape Drivers Influence the Efficiency of Management of Aquatic Invasive Alien Rodents in Western France. Sustainability, 16(5), 1970. https://doi.org/10.3390/su16051970








