Trace Metal Contamination in Community Garden Soils across the United States
Abstract
1. Introduction
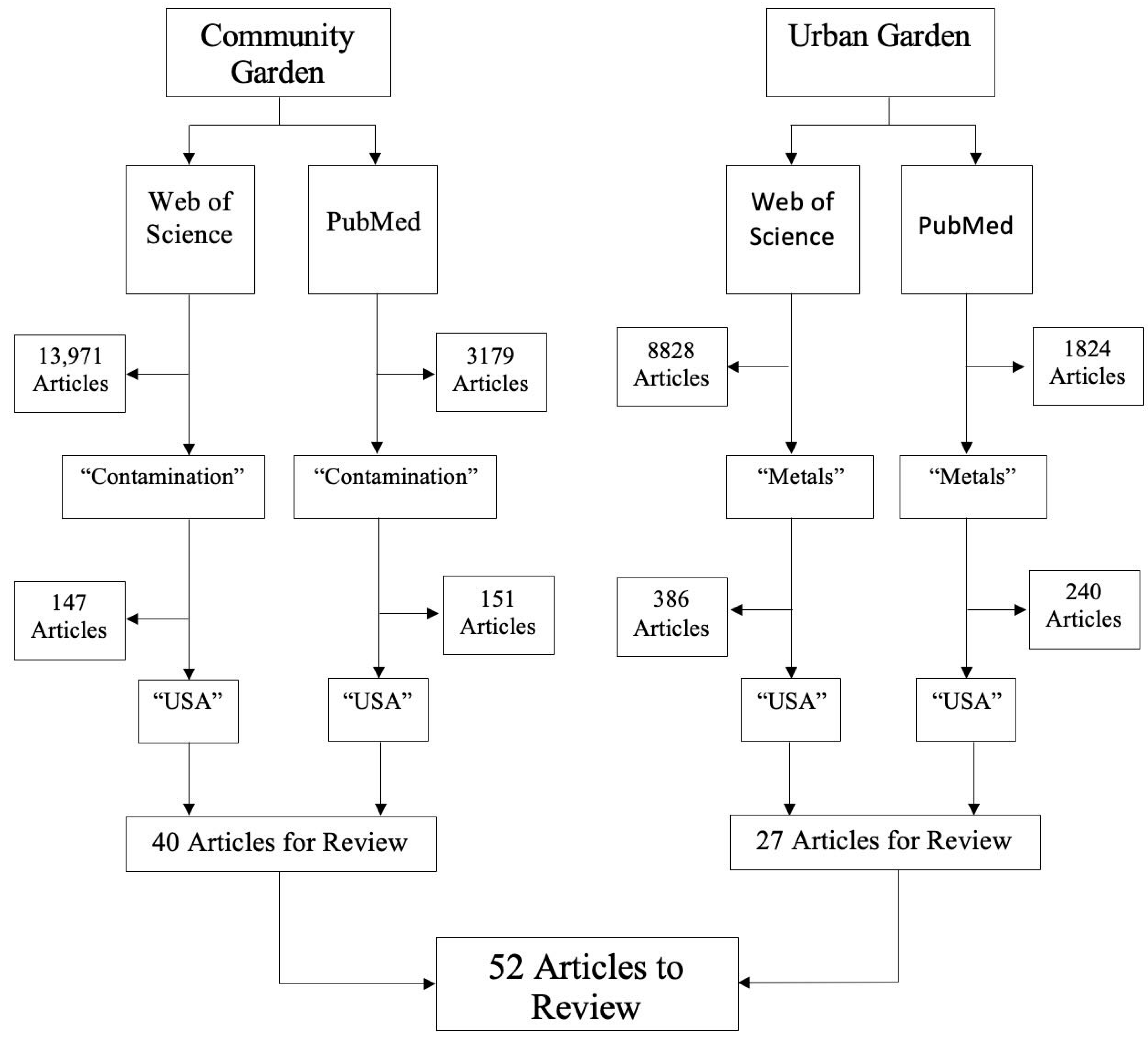
2. Method
3. Soil Contamination in Urban Community Gardens
3.1. Lead (Pb)
| Location | Residential Area Lead Standard (ppm) |
|---|---|
| Finland (Europe) * | 60 |
| California | 80 |
| World Health Organization | 85 |
| Canada | 140 |
| USEPA | 200 |
| Connecticut | 400 |
| New York | 400 |
| Location | Pb (ppm) | As (ppm) | Cd (ppm) | Zn (ppm) | Number of Gardens | Number of Samples | Type of Site |
|---|---|---|---|---|---|---|---|
| Aspen, CO, USA [33] | 172 (9.2–808) | _ | 2.5 (0.2–14.2) | 120 (8.4–484) | 65 | >195 | Former mine dump sites |
| Baltimore, MD, USA [23] | 104.5 (7.4–130.4) | 3.7 (0.2–13.5) | 1.4 | 139.7 (39.7–542) | 104 | 616 | Urban |
| Boston, MA, USA [34] | 130 (117–170) a | 30–39 b | _ | _ | 3 (88 plots) | Traffic, industrial | |
| Cleveland, OH, USA [35] | 224 (14–1241) | 15 (7–58) | 1.2 (0.5–2.5) | 197 (83–543) | _ | 65 | Vacant lots |
| Detroit, MI, USA [30] | 151 (17–882) | _ | _ | _ | 2 | 80 | Urban/residential |
| New Orleans, LA, USA [36] | 38.4 (1.4–9540) | 3 (0.7–61.7) | 0.318 (0.248–8.8) | 91.5 (17.8–7330) | 27 | appx. 600 | Urban/Suburban; backyards and community gardens |
| New York City, NY, USA [1] | 102 (11–2455) | 5.7 (<5.3–93.2) | <0.4 (<0.4–3.1) | 138 (21–2317) | 54 | 564 | Urban/residential |
| New York City, NY, USA [24] | 600 (3–8912) | 12 (0.9–7.6) | 1.6 (0.1–11) | 327 (35–2352) | 905 | 1652 | Urban/residential |
| Oakland, CA, USA [14] | 47–326 | _ | _ | _ | 3 | 6 | Urban/residential |
| Philadelphia, PA, USA [21] | 47.6–351.4 | 0.9–9.6 | 0.1–1.4 | 177.4–936 | 11 | 78 | Urban |
| Philadelphia, PA, USA [21] | 10.3–185.5 | 0.77–3.22 | 0.2–0.5 | 39.2–158.5 | 5 | 24 | Suburban |
| Pittsburgh, PA, USA [21] | 83.1–232.9 | 1.9–11.4 | 0.2–1.3 | 110.9–237.9 | 5 | 20 | Urban |
| Roxbury and Dorchester, MA, USA [11] | 950 (80–3680) | _ | _ | _ | 141 | 692 | Backyard |
3.2. Arsenic (As)
3.3. Cadmium (Cd)
3.4. Zinc (Zn)
4. Plant Uptake of Trace Metals
5. Exposure
5.1. Exposure Pathways
5.2. Exposure to Children
6. Environmental Justice
7. Soil Remediation
7.1. Raised Bed
7.2. Replacement of Topsoil
7.3. pH, Compost, and Biochar
7.4. Limitations
8. Conclusions
8.1. Benefits of Community Gardens
8.2. Risks and Misconceptions
8.3. Useful Practices
8.4. Recommendations
- Minimizing the time spent for children at community gardens that are known to have trace metal contaminants.
- Using gloves and washing hands after gardening.
- Leaving gardening clothes and shoes outdoors.
- Education about potential trace metal contaminants.
- Washing produce.
- Selecting produce that will have less metal uptake.
- Collaborating with the local universities and organizations for regular soil testing.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, R.G.; Spliethoff, H.M.; Ribaudo, L.N.; Lopp, D.M.; Shayler, H.A.; Marquez-Bravo, L.G.; Lambert, V.T.; Ferenz, G.S.; Russell-Anelli, J.M.; Stone, E.B.; et al. Lead (Pb) and other metals in New York City community garden soils: Factors influencing contaminant distributions. Environ. Pollut. 2014, 187, 162–169. [Google Scholar] [CrossRef]
- Siewell, N.; Aguirre, S.; Thomas, M. Building Sustainable Neighborhoods through Community Gardens: Enhancing Residents’ Well-being through University—Community Engagement Initiative. Metrop. Univ. 2015, 18, 173–190. [Google Scholar]
- Kim, B.F.; Poulsen, M.N.; Margulies, J.D.; Dix, K.L.; Palmer, A.M.; Nachman, K.E. Urban Community Gardeners’ Knowledge and Perceptions of Soil Contaminant Risks. PLoS ONE 2014, 9, e87913. [Google Scholar] [CrossRef]
- Hunter, C.M.; Williamson, D.H.Z.; Gribble, M.O.; Bradshaw, H.; Pearson, M.; Saikawa, E.; Ryan, P.B.; Kegler, M. Perspectives on Heavy Metal Soil Testing Among Community Gardeners in the United States: A Mixed Methods Approach. Int. J. Environ. Res. Public Health 2019, 16, 2350. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; Webb, M. Community Gardens as Environmental Health Interventions: Benefits Versus Potential Risks. Curr. Environ. Health Rep. 2017, 4, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M.; Felix, D.; Alcantara, F.; Zaslavsky, I.; Work, A.; Watson, P.L.; Pezzoli, K.; Yu, Q.; Zhu, D.; Scavo, A.J.; et al. Monitoring and mitigation of toxic heavy metals and arsenic accumulation in food crops: A case study of an urban community garden. Plant Direct 2020, 4, e00198. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Ali, S.H.; O’Connor, J.; Tozan, Y.; Jones, A.M.; Capasso, A.; Foreman, J.; DiClemente, R.J. Food insecurity among households with children during the COVID-19 pandemic: Results from a study among social media users across the United States. Nutr. J. 2021, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.L.; Williams, M.L.; Basta, N.; Hand, M.; Huber, S. When Vacant Lots Become Urban Gardens: Characterizing the Perceived and Actual Food Safety Concerns of Urban Agriculture in Ohio. J. Food Prot. 2015, 78, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Glass, K.; Morris, S.; Zhang, H.; McRae, I.; Anderson, N.; Alfieri, A.; Egendorf, S.P.; Holberton, S.; Owrang, S.; et al. Sediment exchange to mitigate pollutant exposure in urban soil. J. Environ. Manag. 2018, 214, 354–361. [Google Scholar] [CrossRef]
- Malone, M. Seeking justice, eating toxics: Overlooked contaminants in urban community gardens. Agric. Hum. Values 2022, 39, 165–184. [Google Scholar] [CrossRef]
- Clark, H.F.; Hausladen, D.M.; Brabander, D.J. Urban gardens: Lead exposure; recontamination mechanisms, and implications for remediation design. Environ. Res. 2008, 107, 312–319. [Google Scholar] [CrossRef]
- Geiger, T.; Norton, U. Effects of Garden Amendments on Soil Available Lead and Plant Uptake in a Contaminated Calcareous Soil. Appl. Sci. 2021, 11, 5777. [Google Scholar] [CrossRef]
- Goswami, O.; Rouff, A.A. Soil Lead Concentration and Speciation in Community Farms of Newark. New Jersey, USA. Soil Syst. 2020, 5, 2. [Google Scholar] [CrossRef]
- McClintock, N. Assessing soil lead contamination at multiple scales in Oakland, California: Implications for urban agriculture and environmental justice. Appl. Geogr. 2012, 35, 460–473. [Google Scholar] [CrossRef]
- Schwarz, K.; Pouyat, R.; Yesilonis, I. Legacies of Lead in Charm City’s Soil: Lessons from the Baltimore Ecosystem Study. Int. J. Environ. Res. Public Health 2016, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Manjón, I.; Ramírez-Andreotta, M.D.; Sáez, A.E.; Root, R.A.; Hild, J.; Janes, M.K.; Alexander-Ozinskas, A. Ingestion and inhalation of metal(loid)s through preschool gardening: An exposure and risk assessment in legacy mining communities. Sci. Total Environ. 2020, 718, 134639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lee, L.; Dayan, S.; Grinshtein, M.; Shaw, R. Speciation of heavy metals in garden soils: Evidences from selective and sequential chemical leaching. J. Soils Sediments 2011, 11, 628–638. [Google Scholar] [CrossRef]
- Mielke, H.W. Nature and extent of metal-contaminated soils in urban environments (keynote talk). Environ. Geochem. Health 2016, 38, 987–999. [Google Scholar] [CrossRef]
- Glaeser, E.L.; Kahn, M.E.; Rappaport, J. Why do the poor live in cities? The role of public transportation. J. Urban Econ. 2008, 63, 1–24. [Google Scholar] [CrossRef]
- Spliethoff, H.M.; Mitchell, R.G.; Shayler, H.; Marquez-Bravo, L.G.; Russell-Anelli, J.; Ferenz, G.; McBride, M. Estimated lead (Pb) exposures for a population of urban community gardeners. Environ. Geochem. Health 2016, 38, 955–971. [Google Scholar] [CrossRef]
- Bassetti, O.G.; McDonough, R.A.; Shakya, K.M. Soil contamination in community gardens of Philadelphia and Pittsburgh, Pennsylvania. Environ. Monit. Assess. 2023, 195, 782. [Google Scholar] [CrossRef]
- Latimer, J.C.; Van Halen, D.; Speer, J.; Krull, S.; Weaver, P.; Pettit, J.; Foxx, H. Soil Lead Testing at a High Spatial Resolution in an Urban Community Garden: A Case Study in Relic Lead in Terre Haute. Indiana. J. Environ. Health 2016, 79, 28–35. [Google Scholar]
- Lupolt, S.N.; Santo, R.E.; Kim, B.F.; Green, C.; Codling, E.; Rule, A.M.; Chen, R.; Scheckel, K.G.; Strauss, M.; Cocke, A.; et al. The Safe Urban Harvests Study: A Community-Driven Cross-Sectional Assessment of Metals in Soil, Irrigation Water, and Produce from Urban Farms and Gardens in Baltimore, Maryland. Environ. Health Perspect. 2021, 129, 117004. [Google Scholar] [CrossRef]
- Cheng, Z.; Paltseva, A.; Li, I.; Morin, T.; Huot, H.; Egendorf, S.; Su, Z.; Yolanda, R.; Singh, K.; Lee, L.; et al. Trace Metal Contamination in New York City Garden Soils. Soil Sci. 2015, 180, 167–174. [Google Scholar] [CrossRef]
- Clarke, L.W.; Jenerette, G.D.; Bain, D.J. Urban legacies and soil management affect the concentration and speciation of trace metals in Los Angeles community garden soils. Environ. Pollut. 2015, 197, 1–12. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Silva, M.R.D.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Chaney, R.L.; Hettiarachchi, G.M. Lead in Urban Soils: A Real or Perceived Concern for Urban Agriculture? J. Environ. Qual. 2016, 45, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Tuccillo, M.E.; Blue, J.; Koplos, J.; Kelly, J.; Wilkin, R.T. Complexities in attributing lead contamination to specific sources in an industrial area of Philadelphia, PA. Heliyon 2023, 9, e15666. [Google Scholar] [CrossRef] [PubMed]
- Bugdalski, L.; Lemke, L.D.; McElmurry, S.P. Spatial Variation of Soil Lead in an Urban Community Garden: Implications for Risk-Based Sampling: Spatial Variation of Soil Lead in an Urban Community Garden. Risk Anal. 2014, 34, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Shargo, I.; Khanjar, N.; Howard, J.; Schmidt, L.; Deng, A.; Edwards, C.; Berman, I.; Galarraga, J.; Wilson, S. Proximity of Urban Farms to Hazards With and Without Heavy Metal Contamination in Baltimore, Maryland. Environ. Justice 2021, 14, 56–69. [Google Scholar] [CrossRef]
- Paltseva, A.A.; Cheng, Z.; Egendorf, S.P.; Groffman, P.M. Remediation of an urban garden with elevated levels of soil contamination. Sci. Total Environ. 2020, 722, 137965. [Google Scholar] [CrossRef]
- Boon, D.Y.; Soltanpour, P.N. Lead, Cadmium, and Zinc Contamination of Aspen Garden Soils and Vegetation. J. Environ. Qual. 1992, 21, 82–86. [Google Scholar] [CrossRef]
- Heiger-Bernays, W.; Fraser, A.; Burns, V.; Diskin, K.; Pierotti, D.; Merchant-Borna, K.; McClean, M.; Brabander, D.; Hynes, H.P. Characterization and Low-Cost Remediation of Soils Contaminated by Timbers in Community Gardens. Int. J. Soil Sediment Water 2009, 2, 5. [Google Scholar]
- Minca, K.K.; Basta, N.T. Comparison of plant nutrient and environmental soil tests to predict Pb in urban soils. Sci. Total Environ. 2013, 445–446, 57–63. [Google Scholar] [CrossRef]
- Moller, K.; Hartwell, J.; Simon-Friedt, B.; Wilson, M.; Wickliffe, J. Soil Contaminant Concentrations at Urban Agricultural Sites in New Orleans, Louisiana: A Comparison of Two Analytical Methods. J. Agric. Food Syst. Community Dev. 2018, 8, 139–149. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Parsania, S.; Mohammadi, P.; Soudi, M.R. Biotransformation and removal of arsenic oxyanions by Alishewanella agri PMS5 in biofilm and planktonic states. Chemosphere 2021, 284, 131336. [Google Scholar] [CrossRef] [PubMed]
- Walter, W.W. Arsenic. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Smolders, E.; Mertens, J. Cadmium. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Preer, J.; Abdi, A.; Sekhon, H.; Murchison, G. Metals in Urban Gardens—Effect of Lime and Sludge. J. Environ. Sci. Health Part-Environ. Sci. Eng. Toxic Hazard. Subst. Control 1995, 30, 2041–2056. [Google Scholar] [CrossRef]
- McIvor, K.; Cogger, C.; Brown, S. Effects of Biosolids Based Soil Products on Soil Physical and Chemical Properties in Urban Gardens. Compost Sci. Util. 2012, 20, 199–206. [Google Scholar] [CrossRef]
- Mertens, J.; Smolders, E. Zinc. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Zhou, H.; Yang, W.-T.; Zhou, X.; Liu, L.; Gu, J.-F.; Wang, W.-L.; Zou, J.-L.; Tian, T.; Peng, P.-Q.; Liao, B.-H. Accumulation of Heavy Metals in Vegetable Species Planted in Contaminated Soils and the Health Risk Assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Assessing Dermal Exposure from Soil; U.S. Environmental Protection Agency: Washington, DC, USA, 2015.
- U.S. Environmental Protection Agency. Why Urban Waters? U.S. Environmental Protection Agency: Washington, DC, USA, 2013.
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef]
- Smollin, L.; Lubitow, A. Environmental Justice and Interventions to Prevent Environmental Injustice in the United States. Encycl. Environ. Health Second Ed. Ref. Module Earth Syst. Environ. Sci. 2019, 561–568. [Google Scholar] [CrossRef]
- Li, L.; Mao, K.; Ippolito, J.A.; Xing, W.; Chen, X.; Zhu, W.; Cheng, Y. Calcium amendments affect heavy metal bioavailability in acidic and calcareous soils. Int. J. Environ. Sci. Technol. 2022, 19, 10067–10076. [Google Scholar] [CrossRef]
- Xu, Y.; Seshadri, B.; Sarkar, B.; Wang, H.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; Yang, X.; Bolan, N. Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci. Total Environ. 2018, 621, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Paltseva, A.A.; Deeb, M.; Di Iorio, E.; Circelli, L.; Cheng, Z.; Colombo, C. Prediction of bioaccessible lead in urban and suburban soils with Vis-NIR diffuse reflectance spectroscopy. Sci. Total Environ. 2022, 809, 151107. [Google Scholar] [CrossRef] [PubMed]
- Young, S.D. Chemistry of Heavy Metals and Metalloids in soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the influence of compost and Biochar Amendments on the Mobility and Uptake of Heavy Metals by Green Leafy vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Gable, L.; Rivera-Nunez, Z. Perceived Benefits of Participation and Risks of Soil Contamination in St Louis Urban Community Gardens. J. Community Health 2018, 43, 604–610. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malone, M.; Shakya, K.M. Trace Metal Contamination in Community Garden Soils across the United States. Sustainability 2024, 16, 1831. https://doi.org/10.3390/su16051831
Malone M, Shakya KM. Trace Metal Contamination in Community Garden Soils across the United States. Sustainability. 2024; 16(5):1831. https://doi.org/10.3390/su16051831
Chicago/Turabian StyleMalone, Maeve, and Kabindra M. Shakya. 2024. "Trace Metal Contamination in Community Garden Soils across the United States" Sustainability 16, no. 5: 1831. https://doi.org/10.3390/su16051831
APA StyleMalone, M., & Shakya, K. M. (2024). Trace Metal Contamination in Community Garden Soils across the United States. Sustainability, 16(5), 1831. https://doi.org/10.3390/su16051831







