Turopolje Pig: Between Conservation and Sustainability
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Pedigree Structure
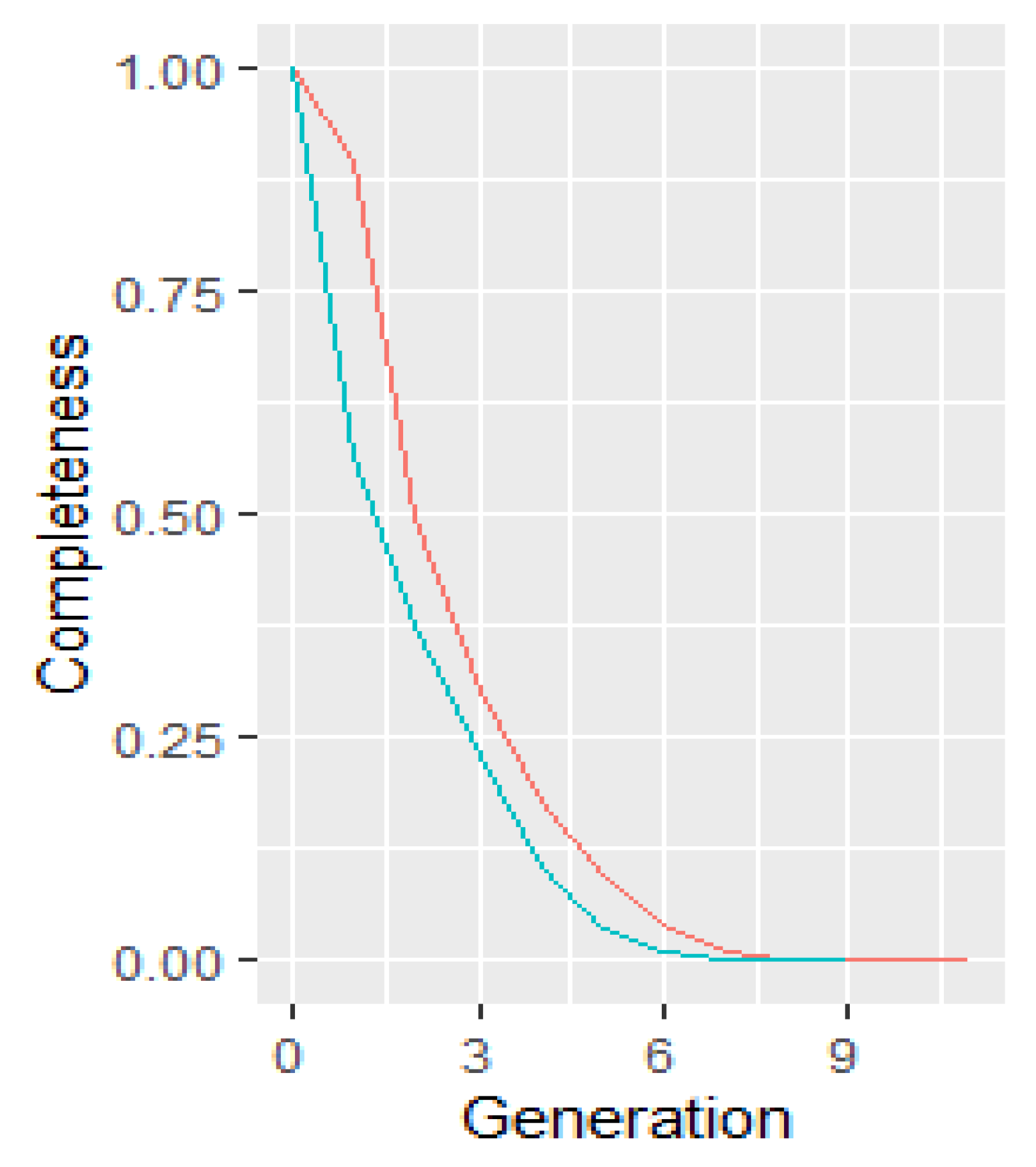
2.2. Pedigree Completeness
- –
- Number of fully traced generations (NTG)—this represents the number of generations separating an individual from its furthest ancestor;
- –
- Maximum number of complete generations (NCG)—this identifies the furthest generation with two known ancestors;
- –
- Number of equivalent complete generations (NECG)—this expresses the sum of all known ancestors, counting how many generations have been traced. It is calculated for those individuals having at least one known parent [13]. Also, pedigree completeness is visualized graphically by the mean completeness of the pedigrees of specified individuals within sexes.
2.3. Population Parameters
- –
- Effective population size calculated as
2.4. Genetic Parameters
2.5. Optimization of Mating Plans
3. Results
3.1. Basic Pedigree Structure
3.2. Pedigree Completeness
3.3. Population Parameters
3.4. Genetic Parameters
3.5. Mating Optimization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karolyi, D.; Luković, Z.; Salajpal, K.; Škorput, D.; Vnučec, I.; Mahnet, Ž.; Klišanić, V.; Batorek-Lukač, V. Turopolje Pig (Turopoljska Svinja). In European Local Pig Breeds—Diversity and Performance. A Study of Project TREASURE; Candek-Potokar, M., Nieto Linan, R.M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Zorc, M.; Škorput, D.; Gvozdanović, K.; Margeta, P.; Karolyi, D.; Luković, Z.; Salajpal, K.; Savić, R.; Muñoz, M.; Bovo, S.; et al. Genetic Diversity and Population Structure of Six Autochthonous Pig Breeds from Croatia, Serbia, and Slovenia. Genet. Sel. Evol. 2022, 54, 30. [Google Scholar] [CrossRef]
- Barker, J.S.F. Conservation and Management of Genetic Diversity: A Domestic Animal Perspective. Can. J. For. Res. 2001, 31, 588–595. [Google Scholar] [CrossRef]
- Monteiro, N.T.R.A.; Wilfart, A.; Utzeri, V.J.; Batorek Lukac, N.; Tomažin, U.; Nanni Costa, L.; Čandek-Potokar, M.; Fontanesi, L.; Garcia-Launay, F. Environmental Impacts of Pig Production Systems Using European Local Breeds: The Contribution of Carbon Sequestration and Emissions from Grazing. J. Clean. Prod. 2019, 237, 117843. [Google Scholar] [CrossRef]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to Hot Climate and Strategies to Alleviate Heat Stress in Livestock Production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef] [PubMed]
- Fetting, C. The European Green Deal; European Sustainable Development Network (ESDN) Office: Vienna, Austria, 2020. [Google Scholar]
- Kasprzyk, A.; Walenia, A. Native Pig Breeds as a Source of Biodiversity—Breeding and Economic Aspects. Agriculutre 2023, 13, 1528. [Google Scholar] [CrossRef]
- Gourdine, J.L.; Sørensen, A.C.; Rydhmer, L. There Is Room for Selection in a Small Local Pig Breed When Using Optimum Contribution Selection: A Simulation Study. J. Anim. Sci. 2012, 90, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Toro, M.A. The Use of Mathematical Programming to Control Inbreeding in Selection Schemes. J. Anim. Breed. Genet. 1999, 116, 447–466. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E. Maximizing the Response of Selection with a Predefined Rate of Inbreeding. Maximizing the Response of Selection with a Predefined Rate of Inbreeding. J. Anim. Sci. 1997, 75, 934–940. [Google Scholar] [CrossRef]
- Sargolzaei, M.; Iwaisaki, H.; Colleau, J.J. CFC: A Tool for Monitoring Genetic Diversity. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Brazil, 13–18 August 2006; pp. 27–28. [Google Scholar]
- Maignel, L.; Boichard, D.; Verrier, E. Genetic Variability of French Dairy Breeds Estimated from Pedigree Infonnation. Interbull Bull. 1996, 14, 49. [Google Scholar]
- Meuwissen, T.H.E.; Luo, Z. Computing Inbreeding Coefficients in Large Populations. Genet. Sel. Evol. 1992, 24, 305–313. [Google Scholar] [CrossRef]
- Cervantes, I.; Goyache, F.; Molina, A.; Valera, M.; Gutierrez, J.P. Estimation of Effective Population Size from the Rate of Coancestry in Pedigreed Populations. J. Anim. Breed. Genet. 2011, 128, 56–63. [Google Scholar] [CrossRef]
- Wellmann, R. Optimum Contribution Selection for Animal Breeding and Conservation: The R Package. Bioinformatics 2019, 20, 25. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Groeneveld, E.; Kovač, M.; Mielenz, N. VCE User’s Guide and Reference Manual. Version 6.0; Institute of Farm Animal Genetics: Neustadt, Germany, 2010. [Google Scholar]
- Vandenberghe, L. The CVXOPT Linear and Quadratic Cone Program Solvers. 2010. Available online: https://www.seas.ucla.edu/~vandenbe/publications/coneprog.pdf (accessed on 1 December 2023).
- Škorput, D.; Ceranac, D.; Luković, Z. Control of Inbreeding in Banija Spotted Pig Population Using Optimisation Methods. J. Cent. Eur. Agric. 2023, 24, 53–60. [Google Scholar] [CrossRef]
- Casellas, J.; Ibáñez-Escriche, N.; Varona, L.; Rosas, J.P.; Noguera, J.L. Inbreeding Depression Load for Litter Size in Entrepelado and Retinto Iberian Pig Varieties. J. Anim. Sci. 2019, 97, 1979–1986. [Google Scholar] [CrossRef]
- Posta, J.; Szabó, P.; Komlósi, I. Pedigree Analysis of Mangalica Pig Breeds. Ann. Anim. Sci. 2016, 16, 701–709. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Secondary Guidelines for Development of National Farm Animal Genetic Resources Management Plans: Management of Small Populations at Risk; Food and Agriculture Organization (FAO): Rome, Italy, 2000. [Google Scholar]
- Crovetti, A.; Sirtori, F.; Pugliese, C.; Franci, O.; Bozzi, R. Pedigree Analysis of Cinta Senese and Mora Romagnola Breeds. Acta Agric. Slov. 2013, 2013 (Suppl. 4), 41–44. [Google Scholar]
- Druml, T.; Salajpal, K.; Dikic, M.; Urosevic, M.; Grilz-seger, G.; Baumung, R. Genetic Diversity, Population Structure and Subdivision of Local Balkan Pig Breeds in Austria, Croatia, Serbia and Bosnia-Herzegovina and Its Practical Value in Conservation Programs Genetic Diversity, Population Structure and Subdivision of Local Bal. Genet. Sel. Evol. 2012, 44, 5. [Google Scholar] [CrossRef]
- Schiavo, G.; Bovo, S.; Muñoz, M.; Ribani, A.; Alves, E.; Araújo, J.P.; Bozzi, R.; Čandek-Potokar, M.; Charneca, R.; Fernandez, A.I.; et al. Runs of Homozygosity Provide a Genome Landscape Picture of Inbreeding and Genetic History of European Autochthonous and Commercial Pig Breeds. Anim. Genet. 2021, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Paixão, G.; Martins, Â.; Esteves, A.; Payan-Carreira, R.; Carolino, N. Genetic Parameters for Reproductive, Longevity and Lifetime Production Traits in Bísaro Pigs. Livest. Sci. 2019, 225, 129–134. [Google Scholar] [CrossRef]
- Saura, M.; Fernández, A.; Varona, L.; Fernández, A.I.; de Cara, M.Á.R.; Barragán, C.; Villanueva, B. Detecting Inbreeding Depression for Reproductive Traits in Iberian Pigs Using Genome-Wide Data. Genet. Sel. Evol. 2015, 47, 1. [Google Scholar] [CrossRef]
- Noguera, J.L.; Casellas, J.; Rosas, J.P.; Varona, L. Genetic Parameters and Direct, Maternal and Heterosis Effects on Litter Size in a Diallel Cross among Three Commercial Varieties of Iberian Pig. Animal 2019, 13, 2765–2772. [Google Scholar] [CrossRef]
- Sell-Kubiak, E. Selection for Litter Size and Litter Birthweight in Large White Pigs: Maximum, Mean and Variability of Reproduction Traits. Animal 2021, 15, 100352. [Google Scholar] [CrossRef]
- Zaalberg, R.M.; Chu, T.T.; Bovbjerg, H.; Jensen, J.; Villumsen, T.M. Genetic Parameters for Early Piglet Weight, Litter Traits and Number of Functional Teats in Organic Pigs. Animal 2023, 17, 100717. [Google Scholar] [CrossRef]
- Leeb, C.; Rudolph, G.; Bochicchio, D.; Edwards, S.; Früh, B.; Holinger, M.; Holmes, D.; Illmann, G.; Knop, D.; Prunier, A.; et al. Effects of Three Husbandry Systems on Health, Welfare and Productivity of Organic Pigs. Animal 2019, 13, 2025–2033. [Google Scholar] [CrossRef]
- Woolliams, J.A.; Berg, P.; Dagnachew, B.S.; Meuwissen, T.H.E. Genetic Contributions and Their Optimization. J. Anim. Breed. Genet. 2015, 132, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Obšteter, J.; Jenko, J.; Hickey, J.M.; Gorjanc, G. Efficient Use of Genomic Information for Sustainable Genetic Improvement in Small Cattle Populations. J. Dairy Sci. 2019, 102, 9971–9982. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Regulation (EU) 2023/2177 of 9 October 2023 on Entering a Name in the Register of Protected Designations of Origin and Protected Geographical Indications (Meso Turopoljske Svinje (PDO)). Available online: http://data.europa.eu/eli/reg_impl/2023/2177/oj (accessed on 8 December 2023).
- Álvarez, I.; Royo, L.J.; Gutiérrez, J.P.; Fernández, I.; Arranz, J.J.; Goyache, F. Relationship between Genealogical and Microsatellite Information Characterizing Losses of Genetic Variability: Empirical Evidence from the Rare Xalda Sheep Breed. Livest. Sci. 2008, 115, 80–88. [Google Scholar] [CrossRef]
- Kennedy, B.W.; Trus, D. Considerations on Genetic Connectedness between Management Units under an Animal Model. J. Anim. Sci. 1993, 71, 2341–2352. [Google Scholar] [CrossRef]
- Škorput, D.; Gorjanc, G.; Luković, Z. Evaluation of Connectedness between the Management Units of Landrace Breed of Pigs in Croatia. Acta Agric. Slov. 2012, 100, 181–185. [Google Scholar]


| Individuals in total | 1155 |
| Inbreds in total | 260 |
| Sires | 160 |
| Dams | 271 |
| Founders | 135 |
| Non-founders | 1020 |
| Parameter | Value |
|---|---|
| Number of maximum generations | 1.96 |
| Number of full generations | 1.35 |
| Number of equivalent generations | 3.45 |
| Parameter | Value |
|---|---|
| Inbreeding coefficient | 0.03 |
| Average F * in inbred animals | 0.10 |
| Average relatedness | 0.06 |
| Effective population size | 29.14 |
| Δcij | 0.17 |
| σ2ph | σ2p | σ2a | σ2e | |
|---|---|---|---|---|
| Variances | 3.43 | 0.00 | 0.05 | 3.39 |
| p2 | h2 | e2 | ||
| Ratio of phenotypic variances | 0.00 | 0.01 | 0.98 |
| Upper Bound on Kinship | Selected Number of Candidates | F | Average Kinship among Candidates | Maximal Male Contribution | Maximal Female Contribution |
|---|---|---|---|---|---|
| <0.7 | No solution | ||||
| 0.07 * | 5/31 | 0.01 | 0.74 | 0.12 | 0.07 |
| 0.10 | 5/120 | 0.02 | 0.09 | 0.12 | 0.01 |
| 0.25 | 5/120 | 0.06 | 0.11 | 0.11 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škorput, D.; Kaić, A.; Špehar, M.; Karolyi, D.; Luković, Z. Turopolje Pig: Between Conservation and Sustainability. Sustainability 2024, 16, 1786. https://doi.org/10.3390/su16051786
Škorput D, Kaić A, Špehar M, Karolyi D, Luković Z. Turopolje Pig: Between Conservation and Sustainability. Sustainability. 2024; 16(5):1786. https://doi.org/10.3390/su16051786
Chicago/Turabian StyleŠkorput, Dubravko, Ana Kaić, Marija Špehar, Danijel Karolyi, and Zoran Luković. 2024. "Turopolje Pig: Between Conservation and Sustainability" Sustainability 16, no. 5: 1786. https://doi.org/10.3390/su16051786
APA StyleŠkorput, D., Kaić, A., Špehar, M., Karolyi, D., & Luković, Z. (2024). Turopolje Pig: Between Conservation and Sustainability. Sustainability, 16(5), 1786. https://doi.org/10.3390/su16051786









