1. Introduction
In most Southern African countries, including Mozambique, low soil fertility has consistently been identified as a major constraint to agricultural productivity. Soils with low inherent fertility often exhibit low levels of organic matter, high acidity, and consequently, limited nutrient availability and are responsible for low yields [
1,
2]. The loss of soil fertility in Sub-Saharan Africa is primarily attributed to continuous cultivation and is widely recognized as the greatest threat to maintaining crop productivity [
3,
4]. Farming practices that contribute to a decrease in soil pH, such as the long-term use of nitrogen (N) fertilizers, must also be avoided [
5,
6]. To counter the trend of soil fertility loss, which can jeopardize the food security of small-scale farmers, it is usually recommended to use organic amendments and adopt practices that increase the amount of crop residues left on the soil [
7,
8]. If crop residues are not removed from the field, they can enhance soil organic matter and nutrient cycling, particularly important in acidic tropical soils with a sandy texture [
9,
10,
11]. Intercropping practices can also benefit soil fertility, being seen as very important for regions of the world where farmers have limited access to industrial fertilizers, and, not requiring external inputs, they are easy to implement [
1,
2].
Farmers commonly practice intercropping, and the plant combinations most studied by scholars typically involve legumes and grasses. This is primarily based on the legumes’ ability to access atmospheric N and the high avidity of the grasses in absorbing N from the soil [
12]. Nodulated legumes have access to atmospheric N by establishing symbiotic relationships with N-fixing microorganisms, known as diazotrophs. This allows them to obtain N for their normal growth without the need for external addition of the nutrient [
13]. The benefits of cultivating legumes extend beyond the use of the nutrient fixed by the legume itself, as part of the N entering the system can be used by species grown in intercropping or that follow in rotation [
12,
13].
The intercropping of cereals and legumes in several spatial arrangements usually leads to improved land productivity, N availability, and economic returns, making it an essential component in the development of more sustainable farming systems [
14]. In Algeria, Benider et al. [
15] studied the effect of three cereals, namely triticale (×
Triticosecale Wittmack), oats (
Avena sativa L.), and barley (
Hordeum vulgare L.), in association with forage pea (
Pisum sativum L.) and common vetch (
Vicia sativa L.), on grain yield. The results showed that the triticale-pea and barley-pea intercrops provided the highest grain yields and average weights of 1000 grains. Maize (
Zea mays L.)-legume intercrops can also provide protein-rich food in low-input agricultural systems, bringing overall benefits to soil fertility conservation, pest management, and animal feed [
16,
17]. In Malawi, a three-year study was carried out to compare the performance of maize–legume intercropping under conservation agriculture and conventional tillage on twelve farms [
18]. Legume production varied with species and farm, but maize production was higher in the second (by 1.4 t ha
−1) and third (by 3.2 t ha
−1) growing seasons in conservation agriculture compared to conventional cultivation. In North China, Guo et al. [
19] observed that maize–peanut (
Arachis hypogaea L.) intercropping led to an increase in beneficial soil microorganisms, enhancing phosphonate and phosphinate metabolism, providing more available phosphorus (P) for crops.
Intercropping, however, does not always lead to increased productivity. The most used intercropping strategy, planting legumes in between or within the same maize rows, can create excessive competition between the two crops [
20]. The relative contribution of each of the ‘4C effects’ (complementarity, cooperation, compensation, and competition) determines the result and can emphasize the yield advantages of intercropping [
21]. The challenge to improve the intercropping consists of optimizing agronomic traits that reduce the negative effect of competition and, at the same time, improve the aspects of complementarity, cooperation, and compensation [
17,
21]. Estimating the land equivalent ratio (LER) in intercrops also leads to an understanding of the interactions affecting yields. LER is defined as the relative land area required as sole crops to produce the same yields as intercropping [
20] and has been successively used in many studies, providing valuable information for the management of intercrops [
14,
18,
22,
23,
24].
However, the results published so far show that the benefits of intercropping can vary with the species involved and the local agroecological conditions. In Sub-Saharan Africa, numerous studies on intercropping have already been conducted [
18,
25,
26]. However, the subject continues to draw the attention of many researchers due to the inherent opportunity for achieving sustainable soil fertility management in small-scale farming systems. Mozambique is a Southern African country, where the studies already conducted have not yet produced sufficient data to enable small-scale farmers to adopt intercropping techniques for profit optimization. Some of the most important crops in the country are maize and cowpea (
Vigna unguiculata L., Walp.). The productions of these crops in 2021 were 2,100,000 and 81,819 t, respectively, but yields were low (1123, and 238 kg ha
−1, respectively) and have practically not increased in the last thirty years [
27]. This indicates that greater effort is needed to optimize cropping practices on a local scale.
This study reports a field experiment carried out in sandy soil in the Vilankulo district, Southern Mozambique, where a maize–cowpea intercrop was compared with sole cropping of maize and cowpea. The effects of treatments were evaluated using data on grain yield, elemental composition of plant tissues, nutrient removal by plants, soil fertility variables, and competition indices. The hypothesis put forward is that intercropping brings measurable advantages to the system compared to sole cropping of each species.
2. Materials and Methods
2.1. Site Description
This study was carried out in Vilankulo district, Southern Mozambique, at the experimental farm of the Escola Superior de Desenvolvimento Rural, Eduardo Mondlane University (ESUDER–UEM), in the geographic coordinates 21°59′31″ S, 35°16′18″ E.
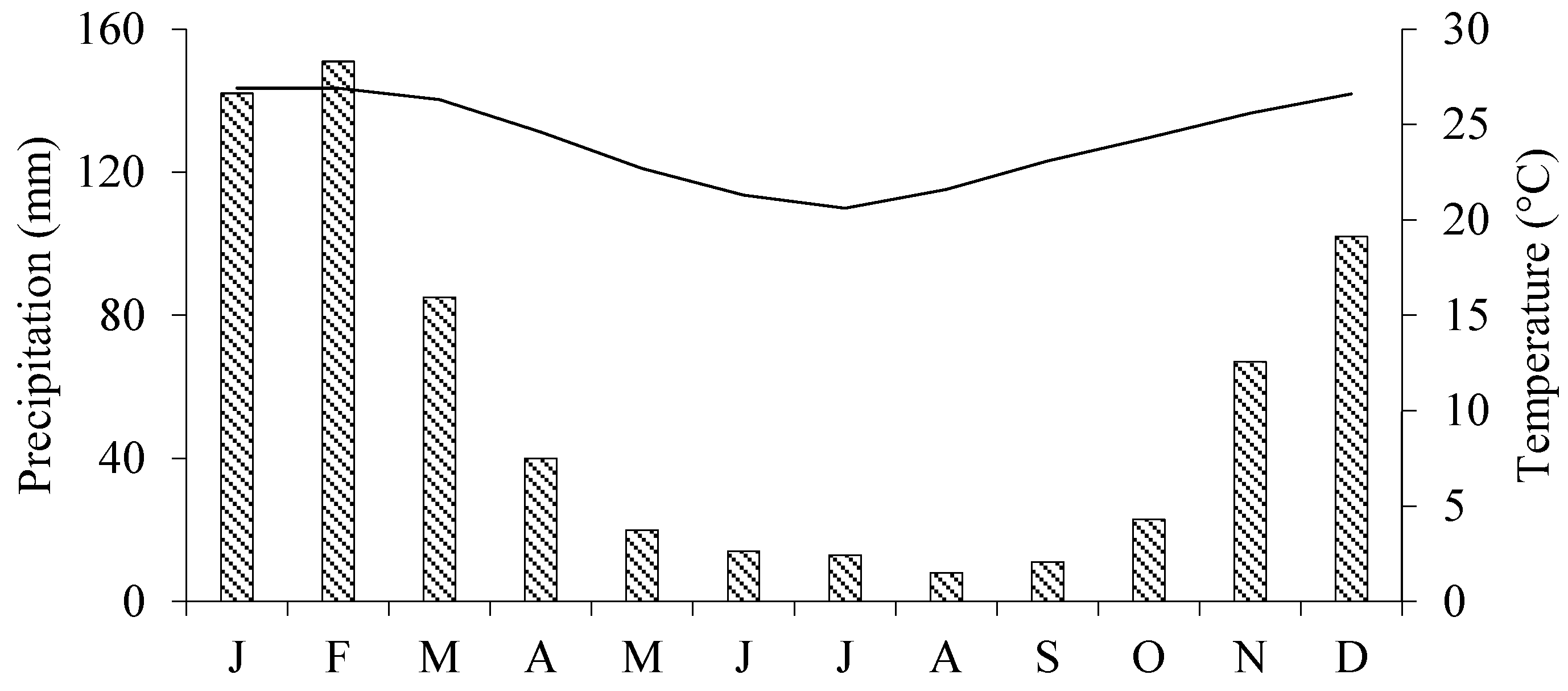
Vilankulo is characterized by a semi-arid climate. Under the Köppen classification system, it lies in the Aw type (dry winter) [
28]. Vilankulo experiences two distinct seasons throughout the year. The first, from October to March, is characterized by hot temperatures and abundant rainfall. The second season, lasting from April to September, brings cooler and drier conditions. The rainfall in this region is erratic, with a long-term (normal) annual precipitation of 677 mm and an average temperature of 24.2 °C [
29]. The monthly variations in precipitation and temperature are shown in
Figure 1.
The soil is a haplic Lixosol [
30], developed from limestone. The soil properties of the plots where the experiments were conducted were determined from soil samples taken at the outset of the experiments from a depth of 0–0.20 m (
Table 1).
2.2. Experimental Design and Crop Management
The field experiments were carried out during the growing seasons of 2017/2018 and 2019. The experiment was laid out as a completely randomized design with three treatments and three replicates. The treatments consisted of the following cropping systems: intercropping of maize and cowpea (M+C) and sole cropping of maize (M) and cowpea (C). The treatments were arranged in plots measuring 6.0 × 2.4 m and the crops were grown in the hot season under rainfed conditions.
The plot where the experiment took place was fallow, having not been cultivated the previous year. At the beginning of the hot season in October, the soil was manually prepared using a hoe. Fertilizers were not applied in the plots, as the objective was to emphasize the intercropping role in the efficient use of nutrients available in the soil and in the N fixed from the atmosphere by legume species.
In the growing season of 2017/2018, the seeding of maize and cowpea took place on 9 November 2017. Cowpea was harvested on 25 January 2018, and maize was harvested on 11 March 2018. In the 2018/2019 growing season, the experiment followed a similar procedure to the first season, with the sowing of maize and cowpea on 26 February 2019. Cowpea was harvested on 30 April 2019, and maize on 27 May 2019. The maize and cowpea crops were not irrigated; they were rainfed during the two years of this study.
The seeds of the open-pollinated cultivar of cowpea (IT16) and the maize hybrid (MRI 514) were sown in rows 0.8 m apart and spaced 0.30 m within rows. The consortium was arranged in alternating rows of cereal and legume with the same seed spacing.
After harvesting sole crops and the intercrop, crop residues were left on the ground as soil amendments. Plots were then cultivated with cabbage, simulating a rotational cropping sequence. The open-pollinated cultivar of cabbage, ‘Tronchuda’, was grown in the fresh season under furrow irrigation. Cabbage was transplanted at the phenological stage 13 of the Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie (BBCH) scale [
31], corresponding to the third true leaf unfolded, with an inter-row and row spacing of 0.25 × 0.25 m. In 2018, cabbage was transplanted on 25 May and harvested on 30 July, and in 2019, it was planted on 24 August and harvested on September 13.
2.3. Field Measurements and Plant and Soil Sampling
Leaf samples were collected at the end of the vegetative stage before flowering to monitor the nutritional status of the plants. Using the BBCH scale [
31], maize leaves were collected at phenological stage 39, during stem elongation; cowpea was sampled at stage 39 during stem elongation when more than nine extended internodes were visible. Twelve fully expanded leaves were taken according to the procedure reported by Bryson et al. [
32].
Maize and cowpea were harvested by hand. The maize harvest occurred at phenological stage 89, when the maize was fully ripe, with hard and shiny grains, and cowpea was harvested when fully ripe, with most pods dark and seeds dry and hard. Cabbage was also manually harvested at phenological stage 48, when it reached 80% of the maximum size for the variety. The grain of maize and cowpea was separated from the husk, and this part was added to the straw to obtain grain yield and total aboveground biomass.
Soil sampling was conducted at the beginning of the experiments to initially characterize the plots (
Table 1). At the end of the second intercropping trial, composite soil samples were taken again (5 individual cores per composite sample) at the same depth to assess the effects of the treatments on soil properties.
2.4. Laboratory Analyses
After air-dried and sieved (2 mm mesh), soil samples underwent various analytical procedures. Soil pH (H
2O and KCl) at a soil-to-solution ratio of 1:2.5 was determined by potentiometry. Exchangeable bases were extracted by ammonium acetate (pH 7.0) and organic C determined by wet digestion (Walkley–Black method). P and potassium (K) were extracted by ammonium lactate (Egner–Riehm method) and boron (B) by hot water (azomethine-H method). For further details on these analytical procedures, please refer to Van Reeuwijk [
33]. The availability of other micronutrients [Copper (Cu), iron (Fe), zinc (Zn), and manganese (Mn)] in the soil was determined by atomic absorption spectrometry after extraction with ammonium acetate and EDTA [
34].
Tissue samples (maize and cowpea grain, leaves and stalks, and whole cabbage) were oven-dried at 70 °C, ground through a 1 mm mesh, and subjected to elemental tissue analyses. The analyses involved the use of Kjeldahl method for N, colorimetry for B and P, flame emission spectrometry for K, and atomic absorption spectrophotometry for calcium (Ca), magnesium (Mg), Cu, Fe, Zn, and Mn, as described by [
35].
2.5. Competition Indices and Apparent Nitrogen Fixation
Grain yield and aboveground biomass of maize and cowpea were obtained after harvesting the crops. The yields were used to compare the different treatments and to calculate competition indices, namely LER and competitive ratio (CR). LER and CR were estimated according to the procedure first described by Mead and Willey [
22] and subsequently adopted by other authors [
16,
23] using the following equations:
where
and
are the yields of maize and cowpea as sole crops and
and
are the yields of maize and cowpea, respectively, as intercrops, and
where
is the sown proportion of legume in mixture with maize and
is the sown proportion of maize in the mixture.
Apparent N fixation was estimated using the difference method, where the N recovered in the legume is subtracted from the N recovered in the grass. The N recovered in the grass is believed to represent the N available in the soil, and the difference is considered the N fixed by the legume [
13]. Thus,
2.6. Data Analysis
The data analysis was performed using the statistical software SPSS Statistics (version 25, IBM SPSS, Armonk, NY, USA). The normality and homogeneity of variances for the data were initially assessed using the Shapiro–Wilk test and Levene’s test, respectively. The effects of the treatments were compared using a one-way analysis of variance (ANOVA). If significant differences were detected (p < 0.05) and there were more than two treatments, means were separated using the Tukey honestly significant difference (HSD) test at a significance level of α = 0.05.
5. Conclusions
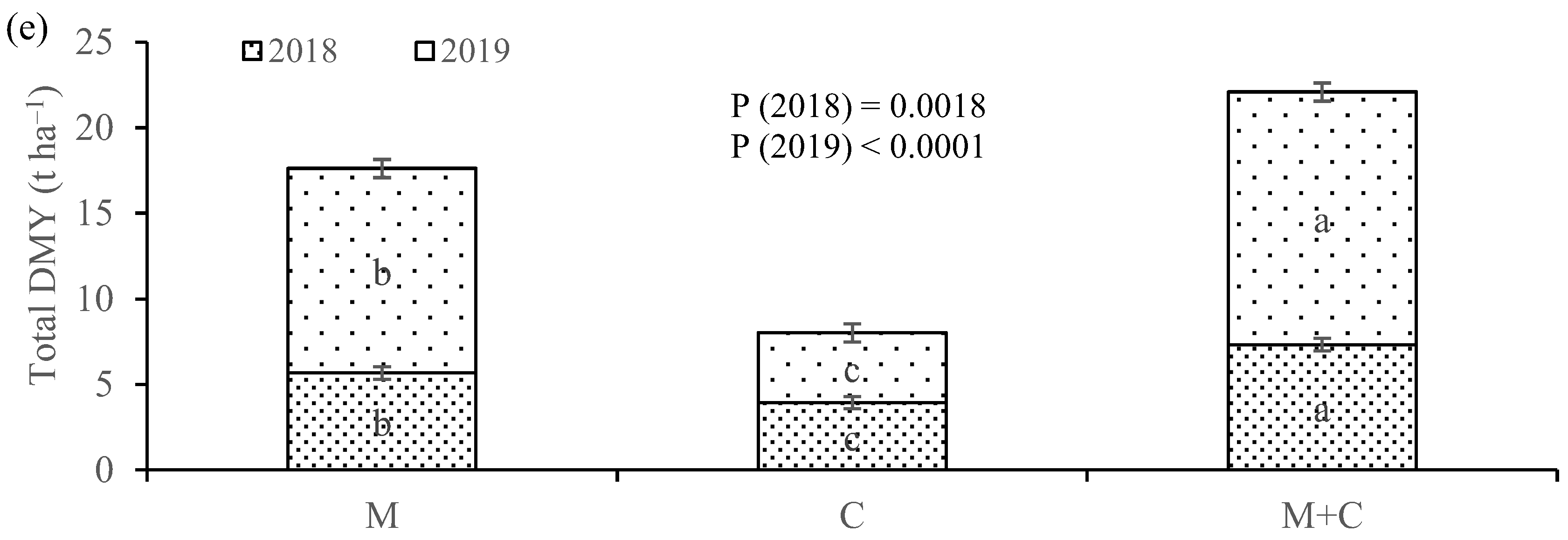
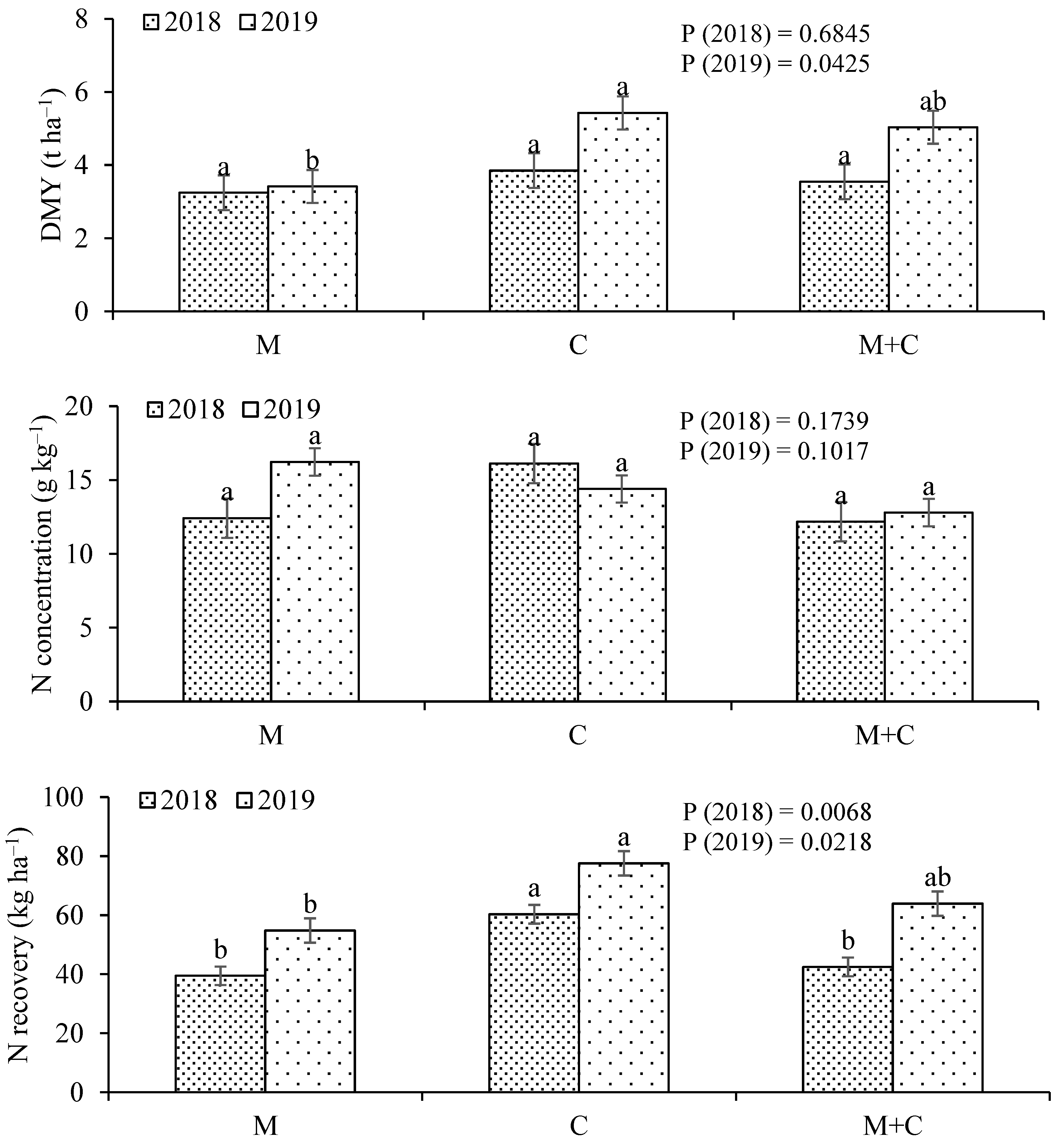
The DMY of maize increased with intercropping with cowpea, while the productivity of cowpea was penalized by intercropping with maize, the result also being stressed by a higher CR of maize in comparison to cowpea. The total DMY increased in intercropping compared to cultivating both species in sole cropping, as also demonstrated by a LER of the consortium higher than 1.
Nutrient acquisition by maize and cowpea showed variations in several essential nutrients, depending on specific traits related to whether the plants are dicotyledonous or monocotyledonous (e.g., Ca, Mg, B, or Mn), and how each species can alter the solubility of nutrients in the rhizosphere (such as P) or possess a greater or lesser capacity to fix N from the atmosphere. The results showed some evidence that cowpea had access to more P, possibly due to its higher capacity to access sparingly soluble P sources. Regarding N, in addition to the legume’s access to atmospheric N, there is an indication that maize may have also had access to atmospheric N, probably through endophytic diazotrophs often associated with tropical grasses, as it contained more N in its tissues than would be expected from cowpea alone.
The residues from cowpea seem to have made a greater contribution than those from maize to the growth of cabbage cultivated as a succeeding crop. This was attributed to the higher concentration in N of cowpea residues, which accelerated their mineralization. Additionally, the process was favored by sandy soils and a warm climate.