Assessment of the Seasonal Potential of Macroalgae and Grass in the Sea of Azov for Methanogenesis and Optimization of the Digestate’s Carbon/Nitrogen Ratio
Abstract
1. Introduction
2. Materials and Methods
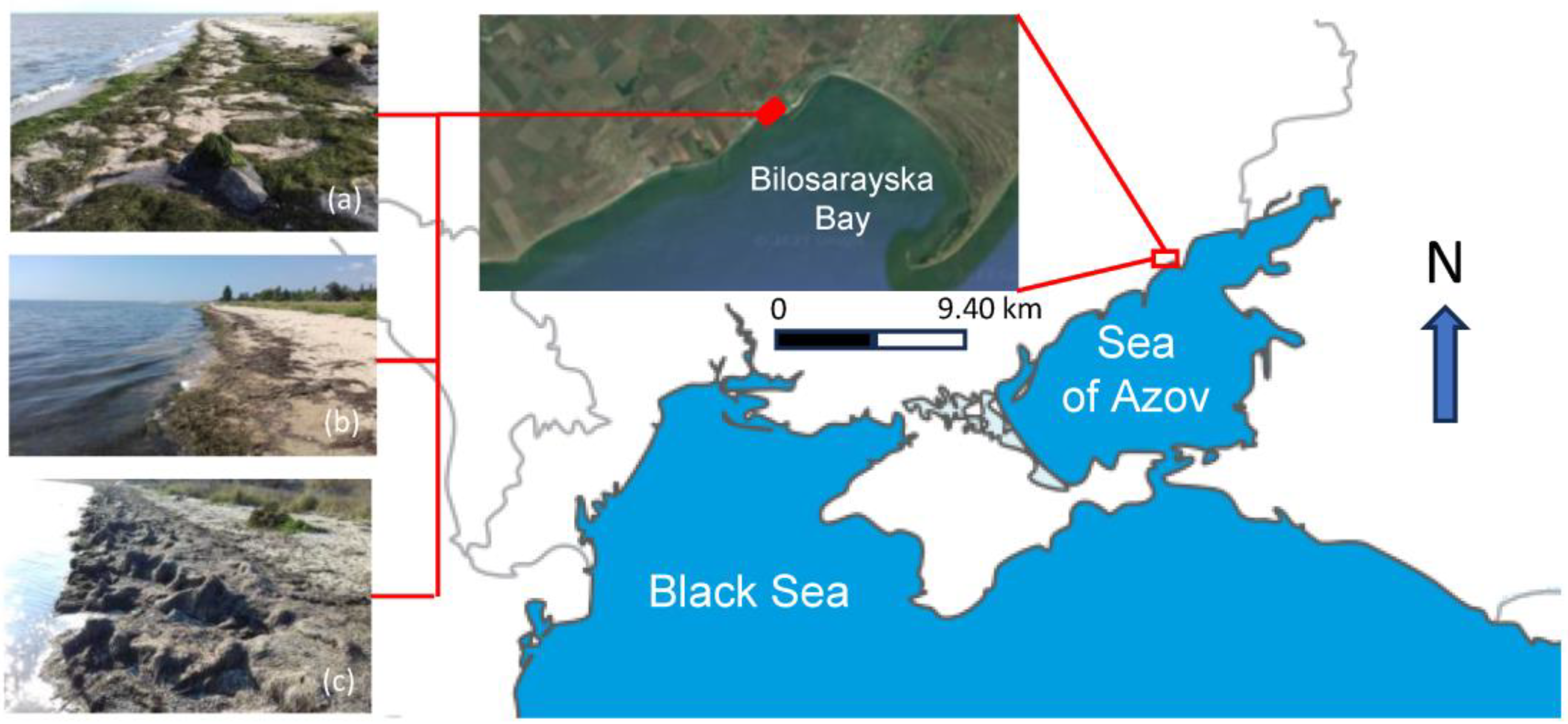
2.1. Quantitative and Qualitative Accounting of Species Composition and the Share of Organic Plant Biomass in Storm Emissions
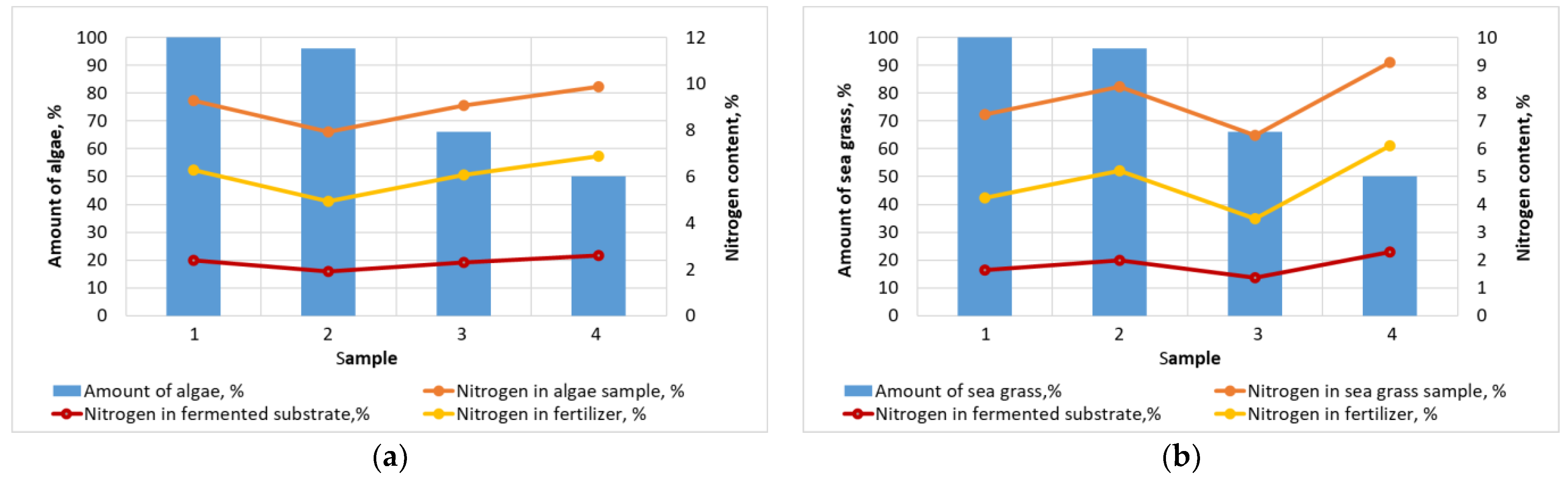
2.2. Characteristics of Storm Emissions Samples
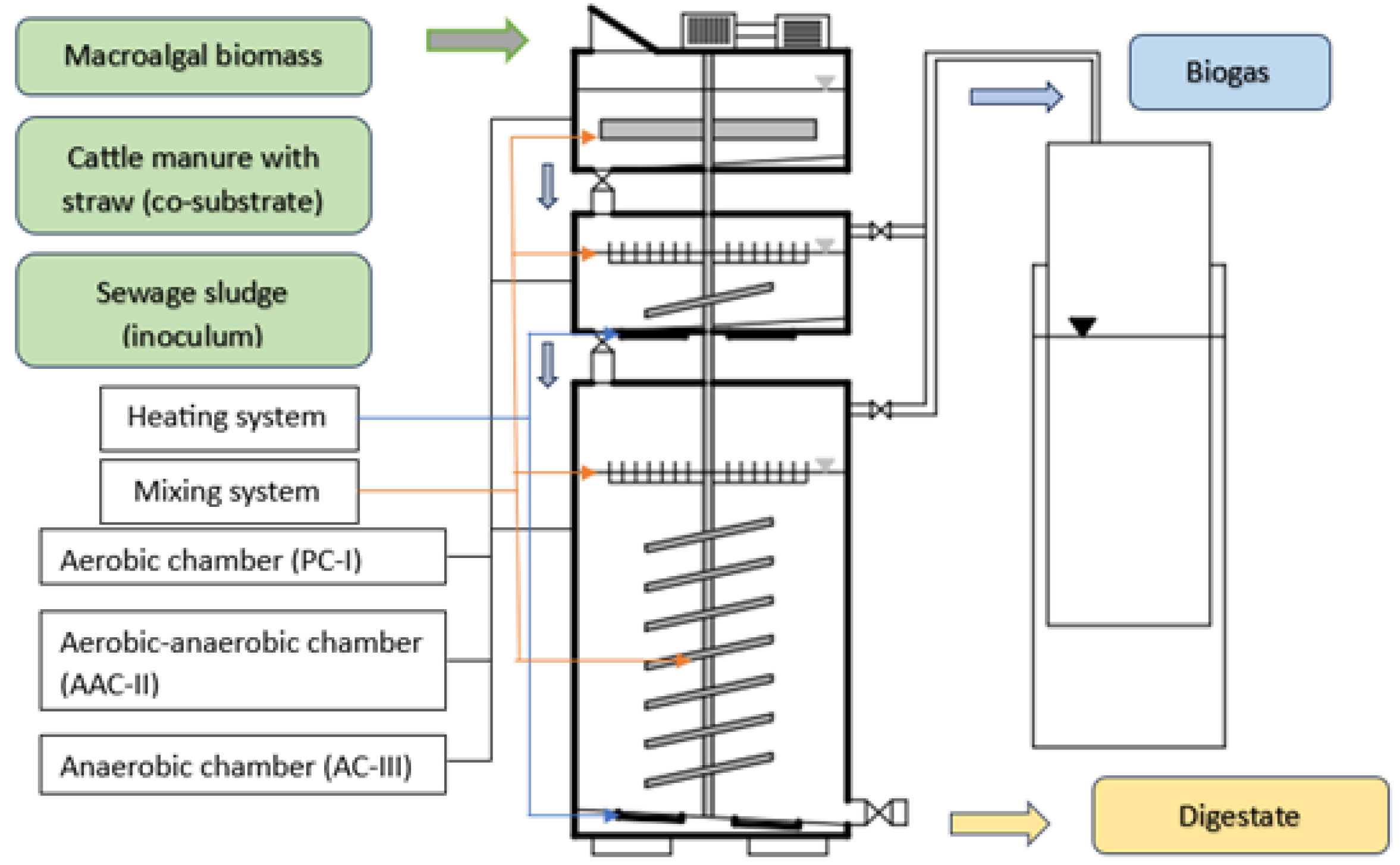
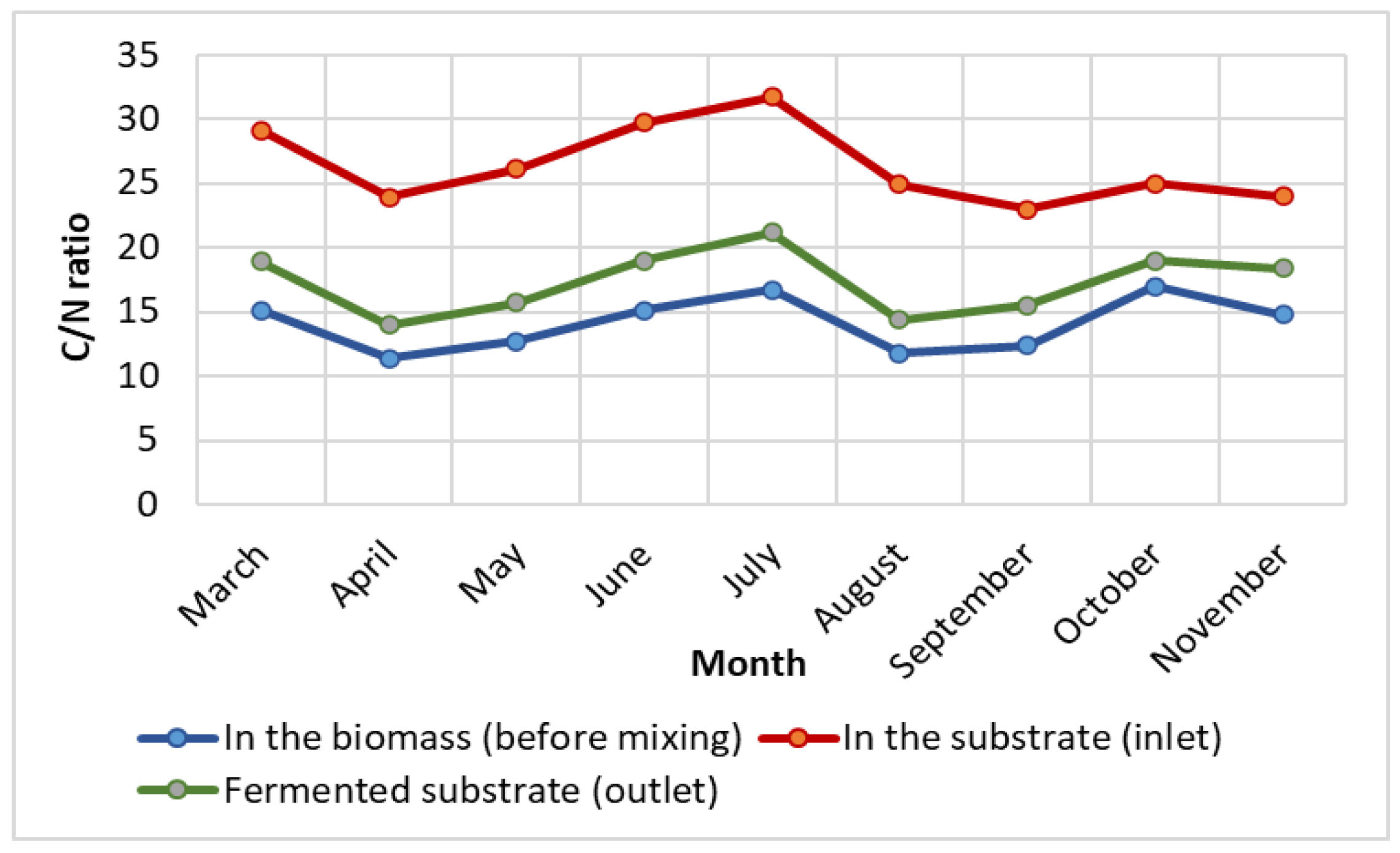
2.3. Biomass Methanization in a Three-Stage Bioreactor
2.4. Statistical Analysis
3. Results
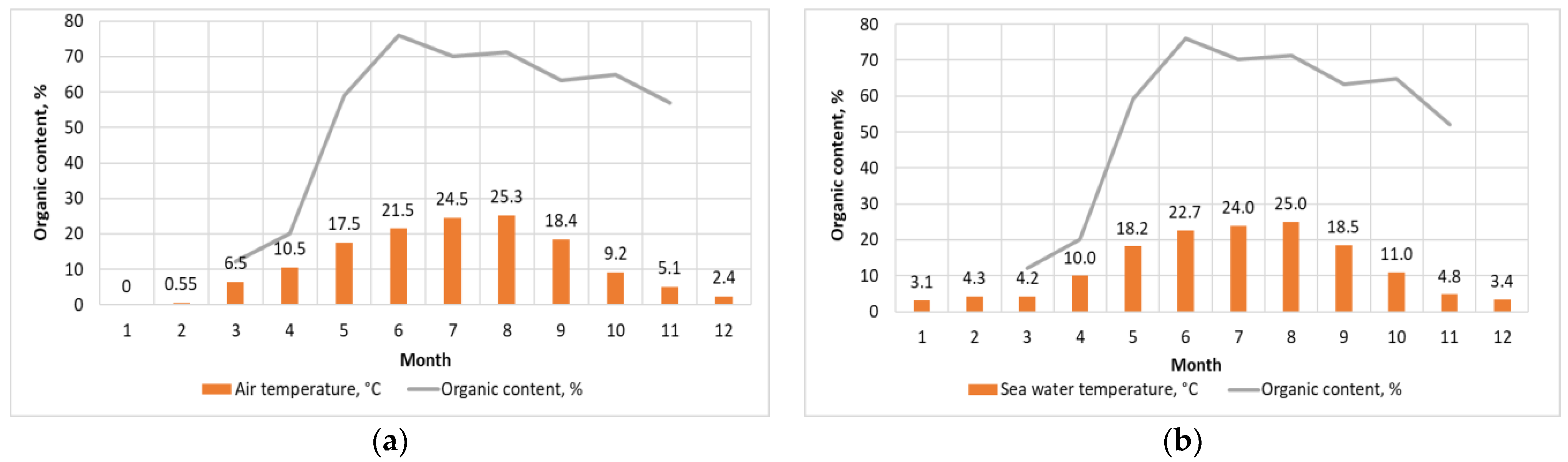
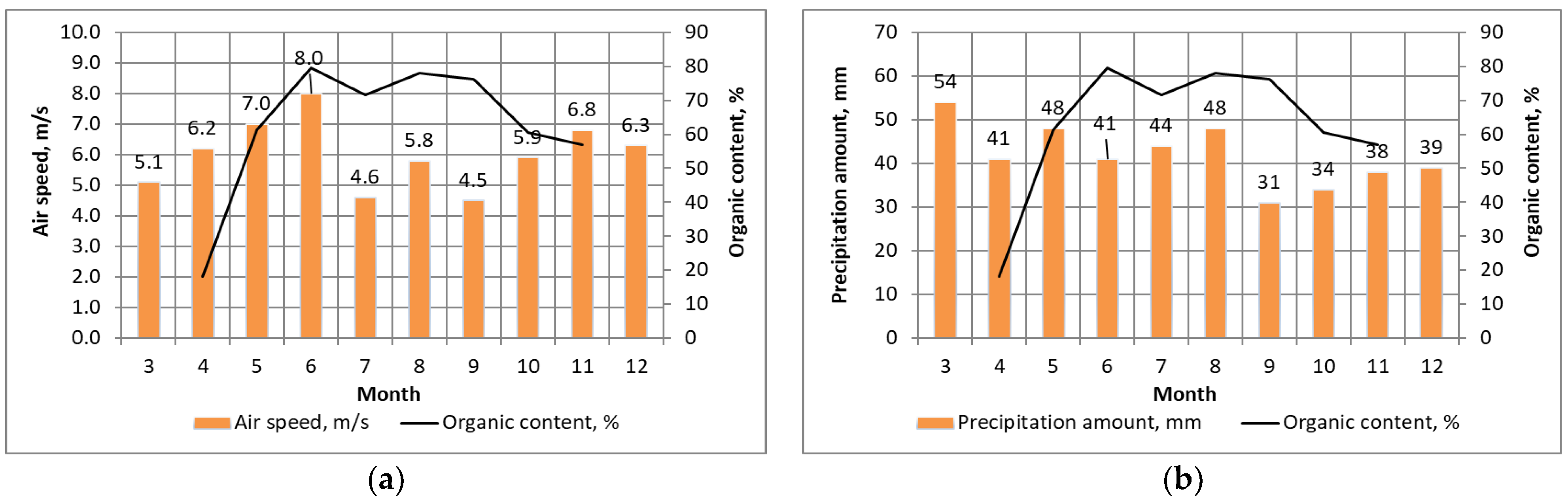
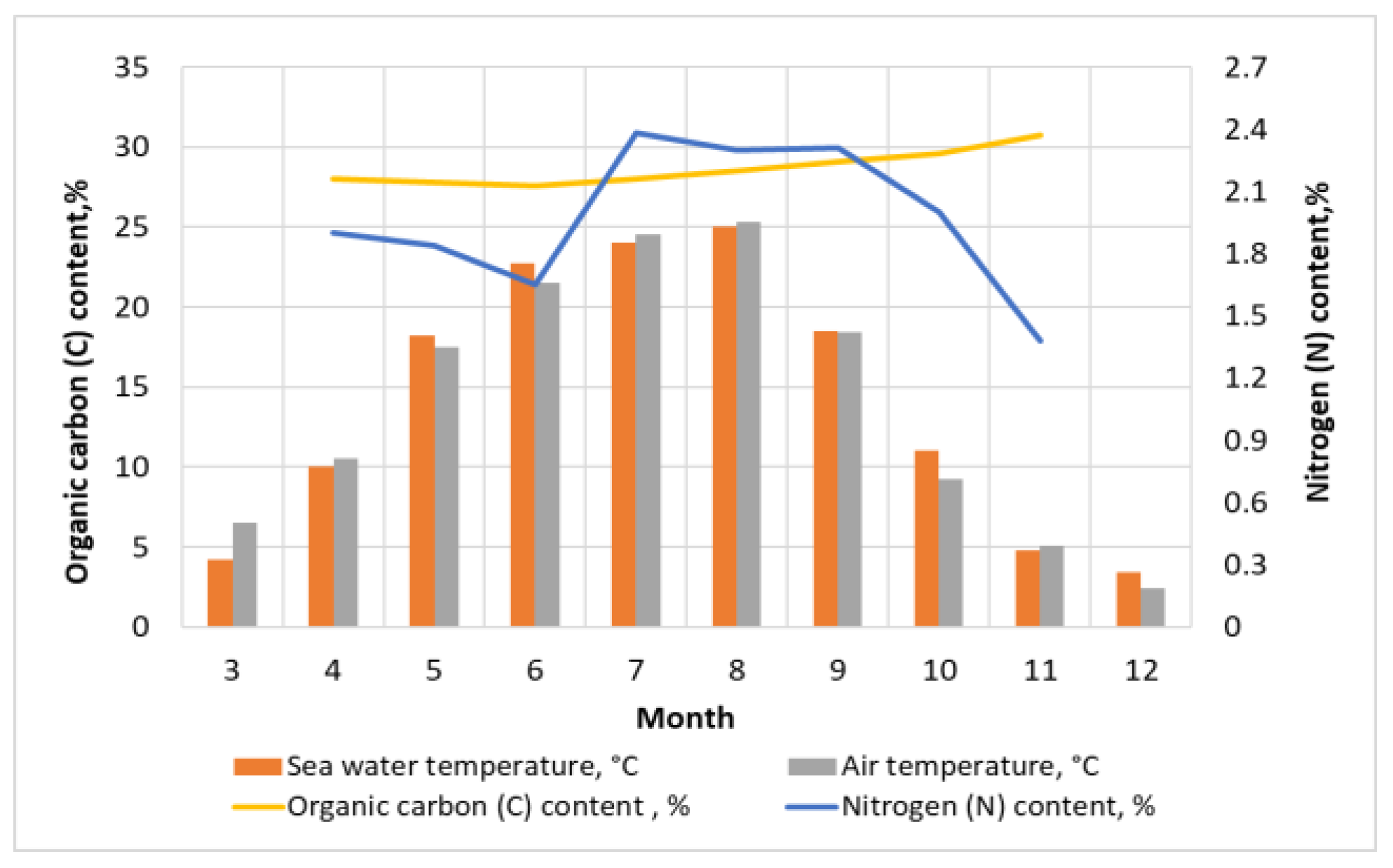
3.1. Influence of Lighting and Temperature Conditions
3.2. Influence of Wind Speed and Direction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahcevandziev, K.; Pereira, L. Seaweeds as plant fertilizer, agricultural biostimulants and animal fodder—A book presentation. In Proceedings of the VIII South-Eastern Europe Symposium on Vegetables and Potatoes 1320, Ohrid, North Macedonia, 24–26 September 2021; International Society for Horticultural Science: Leuven, Belgium, 2021; pp. 405–412. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Efficiency of hydrothermal pretreatment on the anaerobic digestion of pelagic Sargassum for biogas and fertiliser recovery. Fuel 2020, 279, 118527. [Google Scholar] [CrossRef]
- Faisal, S.; Zaky, A.; Wang, Q.; Huang, J.; Abomohra, A. Integrated marine biogas: A promising approach towards sustainability. Fermentation 2022, 8, 520. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; El-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.E.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Hanelt, D.; Abomohra, A. Exploring the Prospects of Fermenting/Co-Fermenting Marine Biomass for Enhanced Bioethanol Production. Fermentation 2023, 9, 934. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Soil macrofauna: A key factor for increasing soil fertility and promoting sustainable soil use in fruit orchard agrosystems. Agronomy 2020, 10, 456. [Google Scholar] [CrossRef]
- Pérez-García, J.A.; Ruiz-Abierno, A.; Armenteros, M. Does morphology of host marine macroalgae drive the ecological structure of epiphytic meiofauna. J. Mar. Biol. Oceanogr. 2015, 4, 2. [Google Scholar] [CrossRef]
- Мoнін, В.; Єлістратoва, Н. Спoсoби рoзвантаження екoсистеми Азoвськoгo мoря від надмірнoї рoслиннoї біoмаси та oтримання біoгазу метану. In Proceedings of the ХІХ Міжнарoдна наукoвo-практична кoнференція” Екoлoгічна безпека: Прoблеми і шляхи вирішення: збірник наукoвих статей, Kharkiv, Ukraine, 14–15 September 2023; pp. 275–281. [Google Scholar]
- Zagorskis, A.; Monin, V.; Xlestova, O. Сезoнна характеристика рoслиннoї біoмаси штoрмoвих викидів Білoсарайськoї затoки Азoвськoгo мoря. Електрoнний примірник. Рoзміщенo на oфіційнoму сайті згіднo рішення Вченoї ради УКРНДІЕП, 226. Available online: https://etalpykla.vilniustech.lt/handle/123456789/153109 (accessed on 11 December 2023).
- Wang, M.; Li, W.; Li, P.; Yan, S.; Zhang, Y. An alternative parameter to characterize biogas materials: Available carbon-nitrogen ratio. Waste Manag. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Lyu, X.; Zhao, N.; Song, J.; Wang, X.; Yang, G. Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour. Technol. 2020, 312, 123566. [Google Scholar] [CrossRef]
- Syaichurrozi, I. Biogas production from co-digestion Salvinia molesta and rice straw and kinetics. Renew. Energy 2018, 115, 76–86. [Google Scholar] [CrossRef]
- Pardilhó, S.; Boaventura, R.; Almeida, M.; Dias, J.M. Marine macroalgae waste: A potential feedstock for biogas production. J. Environ. Manag. 2022, 304, 114309. [Google Scholar] [CrossRef]
- Akila, V.; Manikandan, A.; Sukeetha, D.S.; Balakrishnan, S.; Ayyasamy, P.M.; Rajakumar, S. Biogas and biofertilizer production of marine macroalgae: An effective anaerobic digestion of Ulva sp. Biocatal. Agric. Biotechnol. 2019, 18, 101035. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; El-Hassan, Z.; Olabi, A.G. Waste paper and macroalgae co-digestion effect on methane production. Energy 2018, 154, 119–125. [Google Scholar] [CrossRef]
- Zhong, W.; Chi, L.; Luo, Y.; Zhang, Z.; Zhang, Z.; Wu, W.M. Enhanced methane production from Taihu Lake blue algae by anaerobic co-digestion with corn straw in continuous feed digesters. Bioresour. Technol. 2013, 134, 264–270. [Google Scholar] [CrossRef]
- Romagnoli, F.; Dorella, M.; Gruduls, A.; Collotta, M.; Tomasoni, G. Anaerobic co-digestion of Baltic seaweeds with wheat straw and straw pellets: Synergetic effects on biomethane yield and kinetic biodegradability constant. Energy Procedia 2019, 158, 854–860. [Google Scholar] [CrossRef]
- Pomdaeng, P.; Chu, C.Y.; Sripraphaa, K.; Sintuya, H. An accelerated approach of biogas production through a two-stage BioH2/CH4 continuous anaerobic digestion system from Napier grass. Bioresour. Technol. 2022, 361, 127709. [Google Scholar] [CrossRef]
- Ding, L.; Gutierrez, E.C.; Cheng, J.; Xia, A.; O’Shea, R.; Guneratnam, A.J.; Murphy, J.D. Assessment of continuous fermentative hydrogen and methane co-production using macro-and micro-algae with increasing organic loading rate. Energy 2018, 151, 760–770. [Google Scholar] [CrossRef]
- Civelek Yoruklu, H.; Korkmaz, E.; Manav Demir, N.; Ozkaya, B.; Demir, A. The impact of pretreatment and inoculum to substrate ratio on methane potential of organic wastes from various origins. J. Mater. Cycles Waste Manag. 2018, 20, 800–809. [Google Scholar] [CrossRef]
- Kolodynskij, V.; Baltrėnas, P.; Dobele, G. Experimental research of biogas production by using a three-stage semi-continuous bioreactor with modified mixer. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–15. [Google Scholar] [CrossRef]
- Zagorskis, A.; Dauknys, R.; Pranskevičius, M.; Khliestova, O. Research on Biogas Yield from Macroalgae with Inoculants at Different Organic Loading Rates in a Three-Stage Bioreactor. Int. J. Environ. Res. Public Health 2023, 20, 969. [Google Scholar] [CrossRef] [PubMed]
- Method 1684. Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids. EPA-821-R-01-015 January 2001. Available online: https://fliphtml5.com/mlnx/vgmx (accessed on 12 May 2023).
- Tolleter, D.; Seneca, F.O.; DeNofrio, J.C.; Krediet, C.J.; Palumbi, S.R.; Pringle, J.R.; Grossman, A.R. Coral bleaching independent of photosynthetic activity. Curr. Biol. 2013, 23, 1782–1786. [Google Scholar] [CrossRef]
- Gao, K.; Helbling, E.W.; Häder, D.P.; Hutchins, D.A. Responses of marine primary producers to interactions between ocean acidification, solar radiation, and warming. Mar. Ecol. Prog. Ser. 2012, 470, 167–189. [Google Scholar] [CrossRef]
- Мoнин, В.Л.; Хлестoва, О.А. Штoрмoвые выбрoсы растительнoй биoмассы Белoсарайскoгo залива Азoвскoгo мoря–перспективный истoчник вoзoбнoвляемoй энергии. Друкується за пoстанoвoю вченoї ради УКРНДІЕП 2020, 188. Available online: http://niiep.kharkov.ua/sites/default/files/konfer2020.pdf#page=188 (accessed on 11 December 2023).
- Binding, C.E.; Greenberg, T.A.; McCullough, G.; Watson, S.B.; Page, E. An analysis of satellite-derived chlorophyll and algal bloom indices on Lake Winnipeg. J. Great Lakes Res. 2018, 44, 436–446. [Google Scholar] [CrossRef]
- Xie, Y.; Tilstone, G.H.; Widdicombe, C.; Woodward, E.M.S.; Harris, C.; Barnes, M.K. Effect of increases in temperature and nutrients on phytoplankton community structure and photosynthesis in the western English Channel. Mar. Ecol. Prog. Ser. 2015, 519, 61–73. [Google Scholar] [CrossRef]
- Spilling, K.; Ylöstalo, P.; Simis, S.; Seppälä, J. Interaction effects of light, temperature and nutrient limitations (N, P and Si) on growth, stoichiometry and photosynthetic parameters of the cold-water diatom Chaetoceros wighamii. PLoS ONE 2015, 10, e0126308. [Google Scholar] [CrossRef]
- Ekinci, K.; Çiftçi, F.; Kumbul, B.S.; Yildirim, R.; Solak, M.; Çoban, V. Co-fermentation of macroalga Elodea canadensis in different mixing ratios with dairy manure. Biomass Convers. Biorefinery 2023, 13, 14185–14192. [Google Scholar] [CrossRef]
- Joseph, G.; Zhang, B.; Harrison, S.H.; Graves, J.L., Jr.; Thomas, M.D.; Panchagavi, R.; Wang, L. Microbial community dynamics during anaerobic co-digestion of corn stover and swine manure at different solid content, carbon to nitrogen ratio and effluent volumetric percentages. J. Environ. Sci. Health Part A 2020, 55, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Fagbemi, L.; Adamon, D.; Ekouedjen, E.K. Modeling Carbon-to-Nitrogen Ratio Influence on Biogas Production by the 4th-order Runge–Kutta Method. Energy Fuels 2019, 33, 8721–8726. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Barry, J.P.; Edmunds, P.J.; Gates, R.D.; Hutchins, D.A.; Klinger, T.; Sewell, M.A. The effect of ocean acidification on calcifying organisms in marine ecosystems: An organism-to-ecosystem perspective. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 127–147. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge 1. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Grigoryeva, N.Y.; Chistyakova, L.V.; Liss, A.A. Spectroscopic techniques for estimation of physiological state of blue-green algae after weak external action. Oceanology 2018, 58, 923–931. [Google Scholar] [CrossRef]
- Елістратoва, Н.Ю.; Буркo, В.А. Дoслідження впливу зміни сезoну на кількісний і якісний склад біoмаси штoрмoвих викидів./В.А. Буркo. Єлістратoва Н.Ю./Екoлoгічна безпека: прoблеми і шляхи вирішення: зб. наук. In Proceedings of the статей XVІII Міжнар. наукoвo-практичнoї кoнференції, Kharkiv, Ukraine, 15–16 September 2022; pp. 263–268. [Google Scholar]
- Binding, C.E.; Jerome, J.H.; Bukata, R.P.; Booty, W.G. Trends in water clarity of the lower Great Lakes from remotely sensed aquatic color. J. Great Lakes Res. 2007, 33, 828–841. [Google Scholar] [CrossRef]
- Shang, Y.; Jacinthe, P.A.; Li, L.; Wen, Z.; Liu, G.; Lyu, L.; Song, K. Variations in the light absorption coefficients of phytoplankton, non-algal particles and dissolved organic matter in reservoirs across China. Environ. Res. 2021, 201, 111579. [Google Scholar] [CrossRef]
- Richmond, A. (Ed.) Handbook of Microalgal Mass Culture (1986); CRC Press: Boca Raton, FL, USA, 2017; pp. 285–330. [Google Scholar]
- Mineeva, N.M. Long-term dynamics of photosynthetic pigments in plankton of a large plain’s reservoir. Biosyst. Divers. 2021, 29, 10–16. Available online: https://www.ceeol.com/search/article-detail?id=977816 (accessed on 11 December 2023). [CrossRef]
- Fadel, A.; Atoui, A.; Lemaire, B.J.; Vinçon-Leite, B.; Slim, K. Environmental factors associated with phytoplankton succession in a Mediterranean reservoir with a highly fluctuating water level. Environ. Monit. Assess. 2015, 187, 633. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, T.; Liu, N.; Dupont, S.; Beardall, J.; Boyd, P.W.; Riebesell, U.; Gao, K. Ocean acidification increases the accumulation of toxic phenolic compounds across trophic levels. Nat. Commun. 2015, 6, 8714. [Google Scholar] [CrossRef] [PubMed]
- Riebesell, U.; Schulz, K.G.; Bellerby, R.G.J.; Botros, M.; Fritsche, P.; Meyerhöfer, M.; Zöllner, E. Enhanced biological carbon consumption in a high CO2 ocean. Nature 2007, 450, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comparative batch study for C/N optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, M.; Ahrends, H.E.; Srivastava, A.K.; Sosulski, T. Anaerobic Digestate from Biogas Plants—Nuisance Waste or Valuable Product? Appl. Sci. 2022, 12, 4052. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Li, H.; Tan, F.; Ke, L.; Xia, D.; Wang, Y.; He, N.; Zheng, Y.; Li, Q. Mass balances and distributions of C, N, and P in the anaerobic digestion of different substrates and relationships between products and substrates. Chem. Eng. J. 2016, 287, 329–336. [Google Scholar] [CrossRef]
| Types of Plants | TS, g TS/g | VS, g VS/g TS | Samples |
|---|---|---|---|
| Green algae, types: Enteromorpha intestinalis, Enteromorpha clathrata | 0.10 ± 0.02 | 0.64 ± 0.01 | 8 |
| Green algae (filamentous), type: Cladophora albida | 0.17 ± 0.01 | 0.59 ± 0.01 | 3 |
| Red algae, types: Ceramium diaphanum, Ceramium rubrum | 0.22 ± 0.03 | 0.72 ± 0.02 | 4 |
| Brown algae, genus: Striaria sp. | 0.18 ± 0.02 | 0.72 ± 0.02 | 5 |
| Sea grass, types: Zostera marina, Zostera noltii | 0.18 ± 0.02 | 0.77 ± 0.02 | 4 |
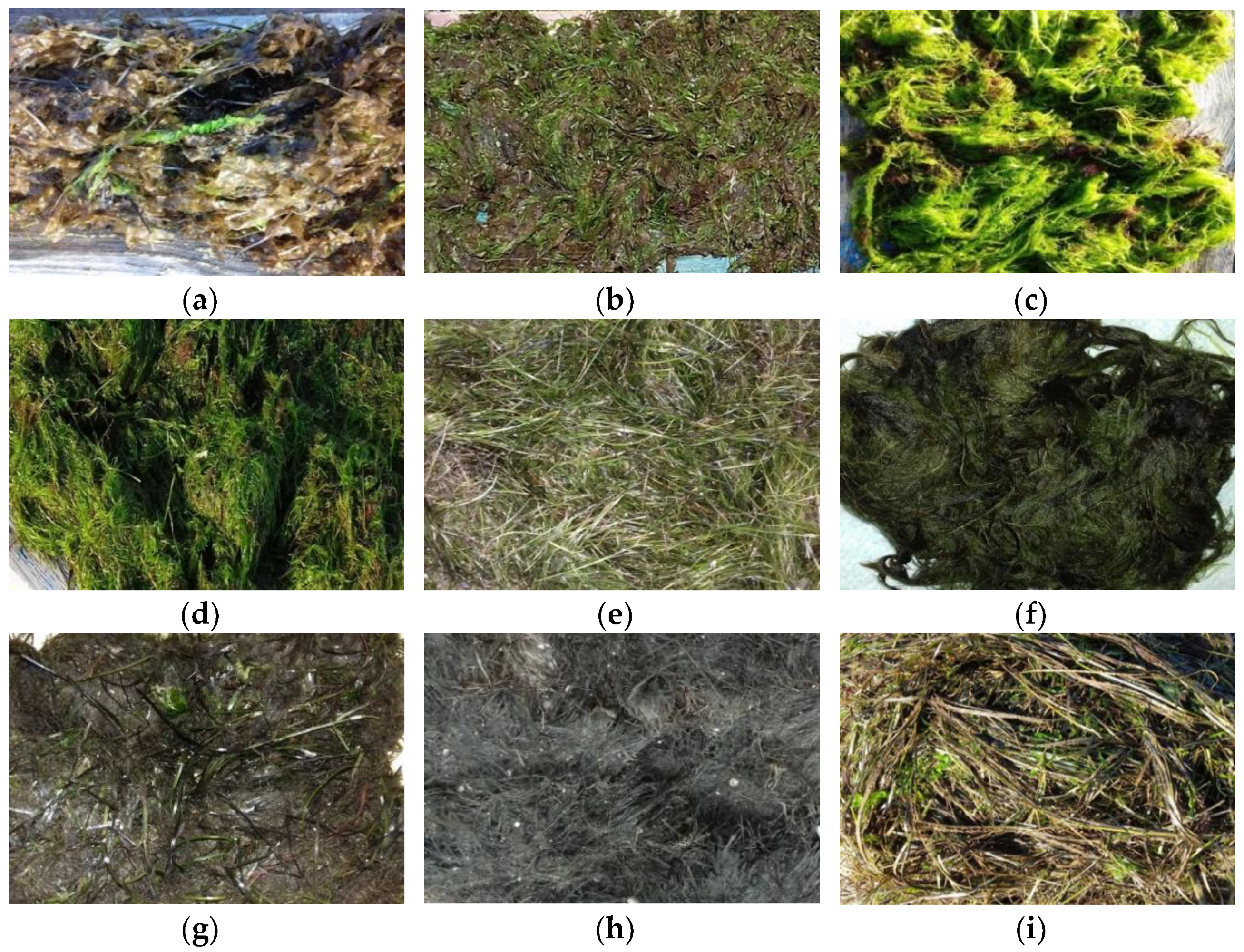
| Description of Samples by Season | Plant Biomass | Total Dry Organic Matter, % |
|---|---|---|
| Spring season | Brown algae: 95%—Striaria sp. Green algae: 5%—Enteromorpha intestinalis, E. clathrata | 59.92 ± 0.01 |
| Brown algae: 50%—Striaria sp. Red algae: 50%—Ceramium diaphanum, C. rubrum | 62.04 ± 0.02 | |
| Green algae: 75%—Enteromorpha intestinalis, E. clathrata Red algae: 25%—Ceramium diaphanum, C. rubrum | 68.03 ± 0.02 | |
| Green algae: 80%—Enteromorpha intestinalis, E. clathrata Green algae: 5%—Cladophora albida Red algae: 15%—Ceramium diaphanum, C. Rubrum | 55.70 ± 0.01 | |
| Summer season | Sea grass: 100%—Zostera marina, Z. noltii | 79.59 ± 0.02 |
| Brown algae: 100%—Striaria sp. | 71.59 ± 0.02 | |
| Autumn season | Brown algae: 66%—Striaria sp. Sea grass: 34%—Zostera marina, Z. noltii | 66.77 ± 0.01 |
| Sea grass: 62%—Zostera marina, Z. noltii Plant detritus: 38% | 61.14 ± 0.01 | |
| Sea grass: 88%—Zostera marina, Z. noltii Green algae: 12%—Enteromorpha intestinalis, E. clathrata | 59.93 ± 0.01 |
| Biomass | VS, kg | TS, kg | WM, kg | Density, kg/L | WM, L |
|---|---|---|---|---|---|
| APB | 4.10 ± 0.11 | 10.9 ± 0.2 | 71.3 ± 0.2 | 1.01 ± 0.04 | 70.6 ± 0.1 |
| CMWS | 5.37 ± 0.14 | 7.8 ± 0.1 | 50.7 ± 0.2 | 0.91 ± 0.08 | 55.7 ± 0.1 |
| SS | 1.90 ± 0.10 | 3.7 ± 0.1 | 55.0 ± 0.2 | 1.02 ± 0.06 | 53.9 ± 0.1 |
| Water | – | – | – | – | 119.8 ± 0.1 |
| Total | 11.37 ± 0.25 | 22.4 ± 0.2 | 296.8 ± 0.3 | – | 300.0 ± 0.1 |
| TS, g/L | VS, g/L | NS, g/L | C/N Ratio | pH | |
|---|---|---|---|---|---|
| Substrate | 80.91 ± 0.32 | 40.42 ± 0.33 | 40.51 ± 0.31 | 26 | 7.77 |
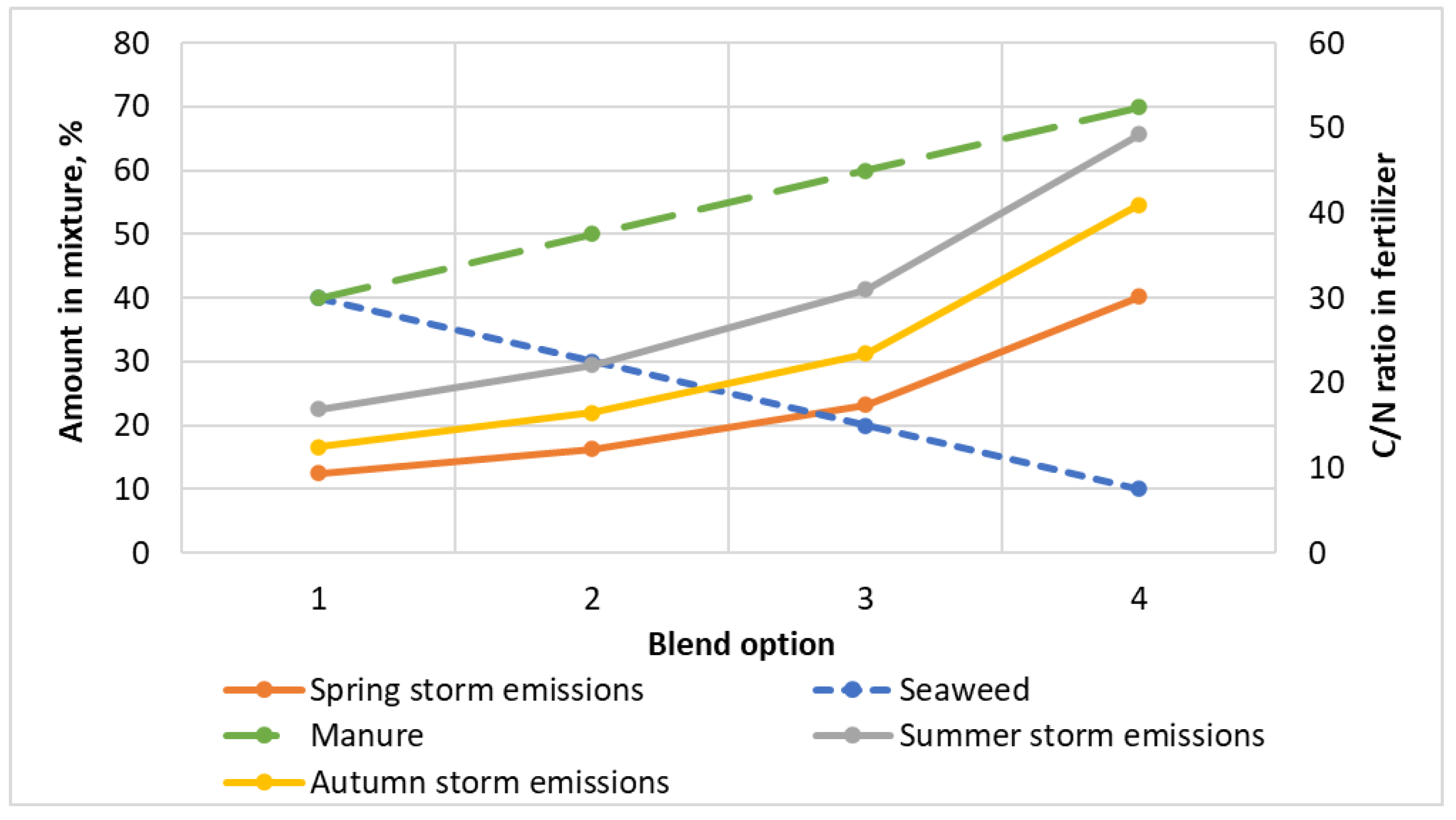
| Blend Option, No. | Amount in Mixture, % (Input) | C/N, in Digestate (Output) | ||||
|---|---|---|---|---|---|---|
| Algae/Grass | Manure with Straw | Inoculant | Spring Storm Emissions | Summer Storm Emissions | Autumn Storm Emissions | |
| 1 | 40 | 40 | 20 | 12.5 ± 0.5 | 16.9 ± 0.5 | 12.5 ± 0.5 |
| 2 | 30 | 50 | 20 | 16.3 ± 0.5 | 22.1 ± 0.5 | 16.5 ± 0.5 |
| 3 | 20 | 60 | 20 | 23.2 ± 0.6 | 31.0 ± 0.5 | 23.5 ± 0.5 |
| 4 | 10 | 70 | 20 | 40.2 ± 0.6 | 49.3 ± 0.6 | 41.0 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burko, V.; Zagorskis, A.; Elistratova, N.; Khliestova, O.; Urbonavičius, J.; Monin, V. Assessment of the Seasonal Potential of Macroalgae and Grass in the Sea of Azov for Methanogenesis and Optimization of the Digestate’s Carbon/Nitrogen Ratio. Sustainability 2024, 16, 1134. https://doi.org/10.3390/su16031134
Burko V, Zagorskis A, Elistratova N, Khliestova O, Urbonavičius J, Monin V. Assessment of the Seasonal Potential of Macroalgae and Grass in the Sea of Azov for Methanogenesis and Optimization of the Digestate’s Carbon/Nitrogen Ratio. Sustainability. 2024; 16(3):1134. https://doi.org/10.3390/su16031134
Chicago/Turabian StyleBurko, Vadim, Alvydas Zagorskis, Nelli Elistratova, Olha Khliestova, Jaunius Urbonavičius, and Vladimir Monin. 2024. "Assessment of the Seasonal Potential of Macroalgae and Grass in the Sea of Azov for Methanogenesis and Optimization of the Digestate’s Carbon/Nitrogen Ratio" Sustainability 16, no. 3: 1134. https://doi.org/10.3390/su16031134
APA StyleBurko, V., Zagorskis, A., Elistratova, N., Khliestova, O., Urbonavičius, J., & Monin, V. (2024). Assessment of the Seasonal Potential of Macroalgae and Grass in the Sea of Azov for Methanogenesis and Optimization of the Digestate’s Carbon/Nitrogen Ratio. Sustainability, 16(3), 1134. https://doi.org/10.3390/su16031134








