Biochar Application Reduces Saline–Alkali Stress by Improving Soil Functions and Regulating the Diversity and Abundance of Soil Bacterial Community in Highly Saline–Alkali Paddy Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Experimental Soil’s Properties
2.2. The Properties of Biochar
2.3. Design and Field Practices for the Experiment
2.4. Examination of Soil Chemical and Nutrient Characteristics
2.5. Extraction of DNA and High-Throughput Sequencing
2.6. Data Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Soil Nutrient Status
3.3. Alpha Diversity of the Bacterial Communities
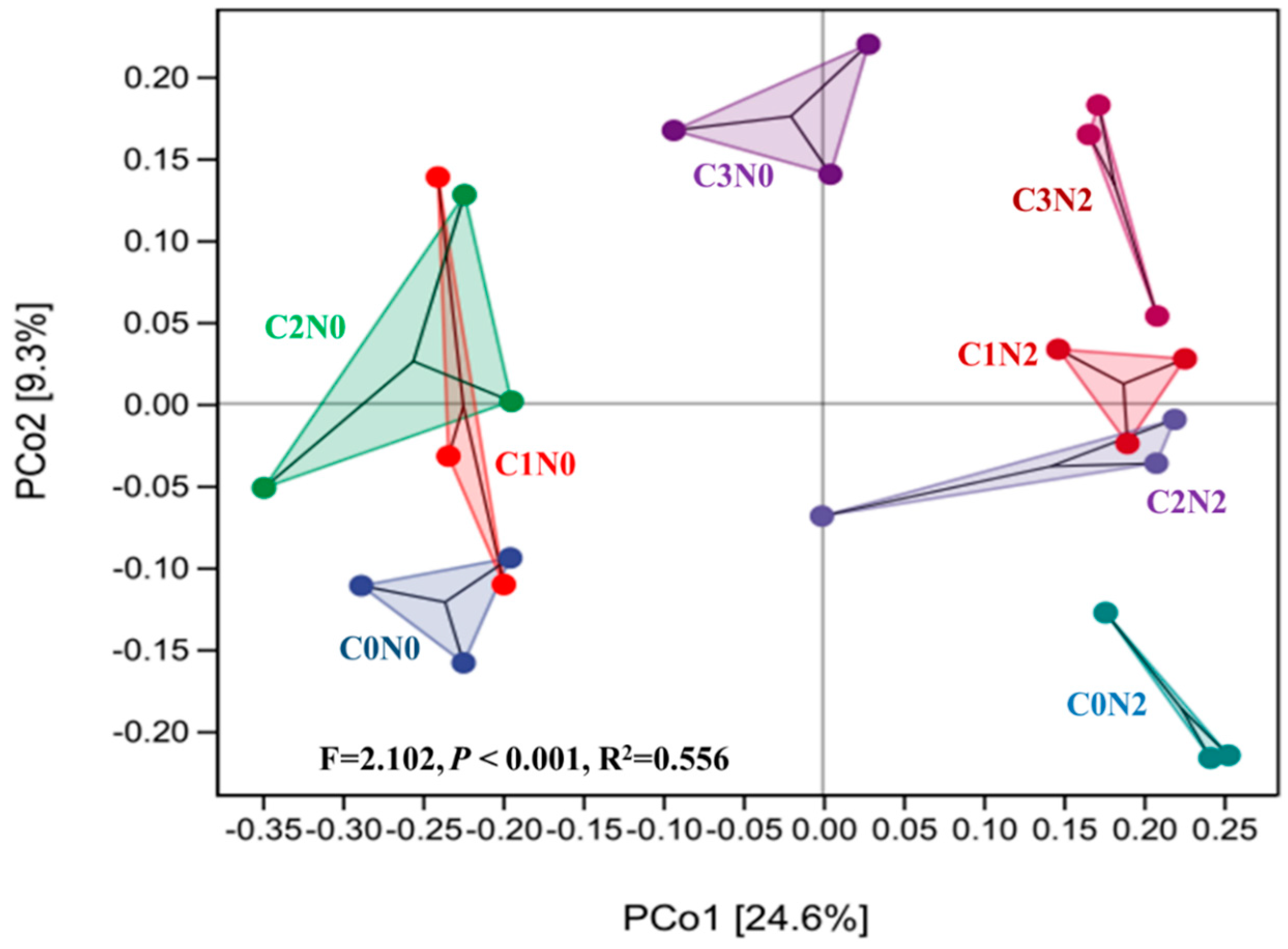
3.4. Beta Diversity of the Bacterial Communities
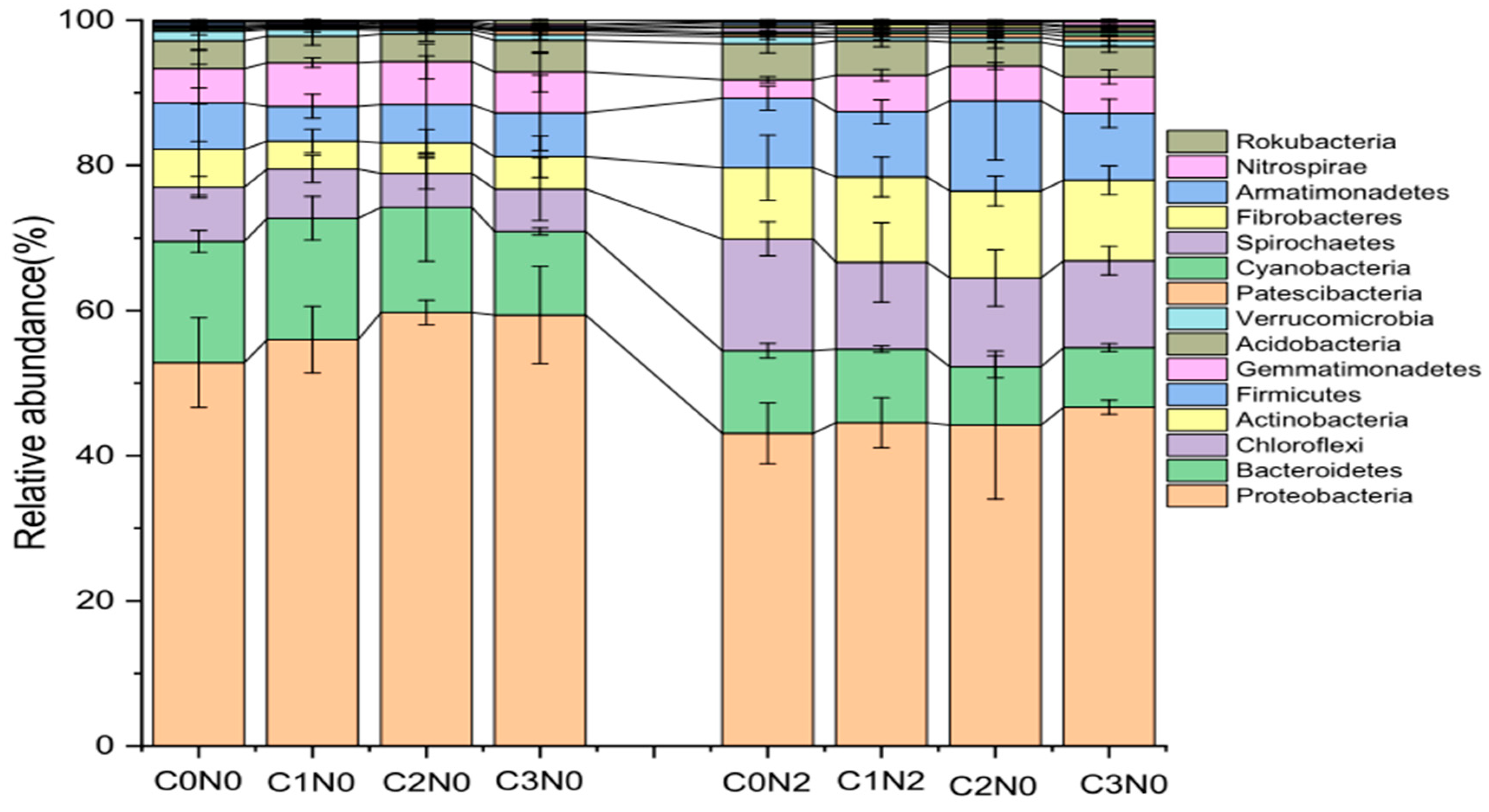
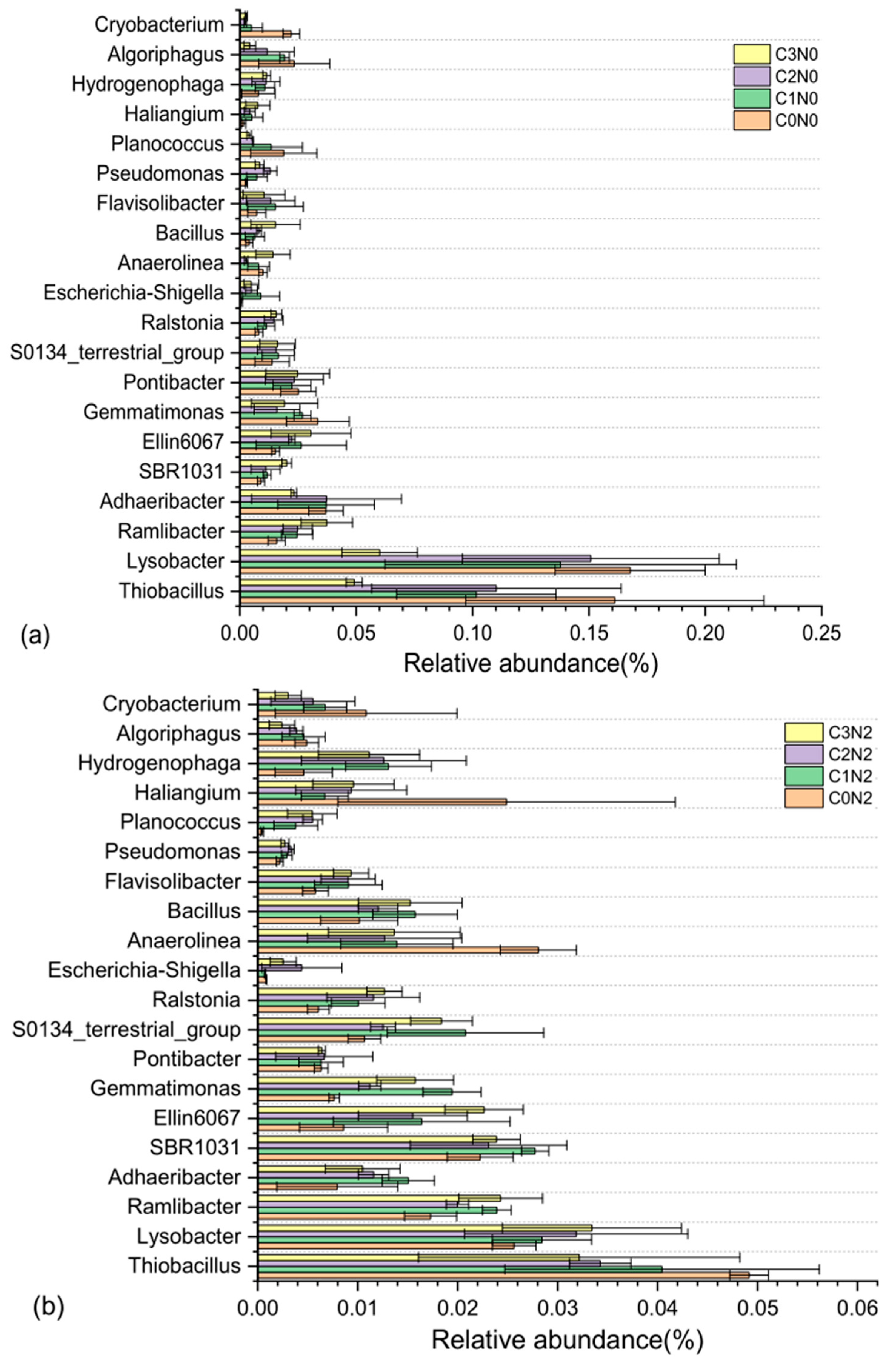
3.5. Relative Abundance of the Bacterial Communities
3.6. Correlations between the Relative Abundance of Bacterial Communities and Soil Environmental Factors
4. Discussion
4.1. Effect of Biochar Application on the Functions of Highly Saline–Alkali Paddy Field Soils
4.2. Effect of Biochar Application on Bacterial Community Diversity and Abundance in Highly Saline–Alkali Paddy Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Seki, K.; Miyazaki, T.; Ishihama, Y. The causes of soil alkalinization in the Songnen Plain of Northeast China. Paddy Water Environ. 2009, 7, 259–270. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. In USDA Handbook No. 60; USDA: Washington, DC, USA, 1954. [Google Scholar]
- Kamran, M.; Wang, D.; Xie, K.Z.; Lu, Y.S.; Shi, C.H.; Sabagh, A.E.L.; Gu, W.J.; Xu, P.Z. Pre-sowing seed treatment with kinetin and calcium mitigates salt induced inhibition of seed germination and seedling growth of choysum. (Brassica rapa var. parachinensis). Ecotoxicol. Environ. Saf. 2021, 227, 112921. [Google Scholar] [CrossRef] [PubMed]
- Morsy, S.; Elbasyoni, I.S.; Baenziger, S.; Abdallah, A.M. Gypsum amendment influences performance and mineral absorption in wheat cultivars grown in normal and saline-sodic soils. J. Agron. Crop Sci. 2022, 208, 675–692. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.M.; Liu, Z.Z.; Liu, K.S.; Wang, X.; Shang, J.Y. Salt-affected marginal lands: A solution for biochar production. Biochar 2023, 5, 21. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Sun, J.L.; Li, H.B.; Wang, Y.N.; Du, Z.L.; Rengel, Z.; Zhang, A.P. Biochar and nitrogen fertilizer promote rice yield by altering soil enzyme activity and microbial community structure. GCB Bioenergy 2022, 14, 1266–1280. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Manjunath, B.L.; Latare, A.M.; D’Souza, R.; Vishwakarma, S.; Singh, N.P. Spatial and temporal variability in microbial activities of coastal acid saline soils of Goa, India. Solid Earth Discuss 2015, 7, 3087–3115. [Google Scholar]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P. Response of soil respiration and microbial biomass to changing EC in saline soils. Soil Biol. Biochem. 2013, 65, 322–328. [Google Scholar] [CrossRef]
- Singh, A.; Panda, S.N. Effect of saline irrigation water on mustard (Brassica juncea) crop yield and soil salinity in a semi-arid area of North India. Exp. Agric. 2012, 48, 99–110. [Google Scholar] [CrossRef]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van, Z.L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Saifullah, D.S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Gunarathne, V.; Senadeera, A.; Gunarathne, U.; Biswas, J.K.; Almaroai, Y.A.; Vithanage, M. Potential of biochar and organic amendments for reclamation of coastal acidic salt affected soil. Biochar 2020, 2, 107–120. [Google Scholar] [CrossRef]
- Ndiaye, I.N.; Qamar, U.Z.; Imade, N.F.; Oyeyemi, A.D.; Abdul, R.; Muhammad, A.; Deborah, G.; Aboubacry, K.; Cai, L.Q.; Fasih, U.H. Soil Amendment with Arbuscular Mycorrhizal Fungi and Biochar Improves Salinity Tolerance, Growth, and Lipid Metabolism of Common Wheat (Triticum aestivum L.). Sustainability 2022, 14, 3210. [Google Scholar] [CrossRef]
- Nan, Q.; Fang, C.X.; Cheng, L.Q.; Wang, H.; Wu, W.X. Elevation of NO3-N from biochar amendment facilitates mitigating paddy CH4 emission stably over seven years. Environ. Pollut. 2022, 295, 118707. [Google Scholar] [CrossRef]
- Li, X.; Yao, T.X.; Huang, X.X.; Li, X.B.; Li, P.Y.; Du, S.; Wang, W.; Miao, S.H.; Wang, D.; Jin, F.; et al. Biochar increases rice yield by improving root morphological and root physiological functions in heavily saline-sodic paddy soil of northeast China. BioResources 2022, 17, 1421–1456. [Google Scholar] [CrossRef]
- Yao, T.X.; Zhang, W.T.; Gulaqa, A.; Cui, Y.F.; Zhou, Y.M.; Weng, W.A.; Wang, X.; Jin, F. Effects of peanut shell biochar on soil nutrients, soil enzyme activity, and rice yield in heavily saline-sodic paddy field. J. Soil Sci. Plant Nutr. 2021, 21, 655–664. [Google Scholar] [CrossRef]
- Li, X.B.; Che, W.K.; Piao, J.L.; Li, X.; Jin, F.; Yao, T.X.; Li, P.Y.; Wang, W.; Tan, T.; Shao, X.W. Peanut Shell Biochar’s Effect on Soil Physicochemical Properties and Salt Concentration in Highly Saline-Sodic Paddy Fields in Northeast China. BioResources 2022, 17, 5936–5957. [Google Scholar] [CrossRef]
- Piao, J.L.; Che, W.K.; Li, X.; Li, X.B.; Zhang, C.B.; Wang, Q.S.; Hua, S. Application of peanut shell biochar increases rice yield in saline-alkali paddy fields by regulating leaf ion concentrations and photosynthesis rate. Plant Soil 2022, 483, 589–606. [Google Scholar] [CrossRef]
- Ran, C.; Gulaqa, A.; Zhu, J.; Wang, W.W.; Zhang, S.Q.; Geng, Y.Q.; Guo, L.Y.; Jin, F.; Shao, X.W. Benefits of biochar for improving ion contents, cell membrane permeability, leaf water status and yield of rice under saline-sodic paddy field condition. J. Plant Growth Regul. 2019, 39, 370–377. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’–does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zong, H.Y.; Zheng, H.; Liu, G.C.; Chen, L.; Xing, B.S. Reduced nitrification and abundance of ammonia-oxidizing bacteria in acidic soil amended with biochar. Chemosphere 2015, 138, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.Y.; Xia, Y.; Zhang, Y.P.; Wang, H.F.; Luo, X.X.; Xing, B.S. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, C.Q.; Kong, Y.L.; Cao, X.C.; Zhu, L.F.; Zhang, Y.C.; Ning, Y.W.; Tian, W.H.; Zhang, H.; Yu, Y.J.; et al. Biochar Application Alleviated Rice Salt Stress via Modifying Soil Properties and Regulating Soil Bacterial Abundance and Community Structure. Agronomy 2022, 212, 409. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Microscopy observations of habitable space in biochar for colonization by fungal hyphae from soil. J. Integr. Agric. 2014, 13, 483–490. [Google Scholar] [CrossRef]
- Brewer, C.E.; Brown, R.C. Biochar. In Comprehensive Renewable Energy; Sayigh, A.A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 257–384. [Google Scholar]
- Norton, J.M.; Firestone, M.K. N dynamics in the rhizosphere of Pinus ponderosa seedlings. Soil Biol. Biochem. 1996, 28, 351–362. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Castaldi, S.; Riondino, M.; Baronti, S.; Esposito, F.R.; Marzaioli, R.; Rutigliano, F.A.; Vaccari, F.P.; Miglietta, F. Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 2011, 85, 1464–1471. [Google Scholar] [CrossRef]
- Zavalloni, C.; Alberti, G.; Biasiol, S.; Delle, V.G.; Fornasier, F.; Liu, J.; Peressotti, A. Microbial mineralization of biochar and wheat straw mixture in soil: A short-term study. Appl. Soil Ecol. 2011, 50, 45–51. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M.; Šimůnek, J. Leaching and reclamation of a biochar and compost amended saline–sodic soil with moderate SAR reclaimed water. Agric. Water Manag. 2015, 158, 255–265. [Google Scholar] [CrossRef]
- Jing, C.L.; Xu, Z.C.; Zou, P.; Tang, Q.; Li, Y.Q.; You, X.W.; Zhang, C.S. Coastal halophytes alter properties and microbial community structure of the saline soils in the Yellow River Delta China. Appl. Soil Ecol. 2019, 134, 1–7. [Google Scholar] [CrossRef]
- Dempster, D.N.; Gleeson, D.B.; Solaiman, Z.I.; Jones, D.L.; Murphy, D.V. Decreased soil microbial biomass and nitrogen mineralisation with eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Chi, C.M.; Wang, Z.C. Characterizing salt-affected soils of Songnen Plain using saturated paste and 1:5 soil-to-water extraction methods. Arid Soil Res. Rehabil. 2010, 24, 1–11. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Analysis for Agronomy, 3rd ed.; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Jin, V.L.; Potter, K.N.; Johnson, M.V.V.; Harmel, R.D.; Arnold, J.G. Surface-applied biosolids enhance soil organic carbon and nitrogen stocks but have contrasting effects on soil physical quality. Appl. Environ. Soil Sci. 2015, 2015, 9–11. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Li, Z.Y.; Zhang, Z.Q.; You, L.R.; Xu, L.F.; Huang, H.Y.; Wang, X.P.; Gao, Y.; Cui, X.J. Treatment of the saline-alkali soil with acidic corn stalk biochar and its effect on the sorghum yield in western Songnen Plain. Sci. Total Environ. 2021, 797, 149190. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhang, X.C. Effect of biochar on pH of alkaline soils in the loess plateau: Results from incubation experiments. Int. J. Agric. Biol. 2012, 14, 745–750. [Google Scholar]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G.X. Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- Sun, C.X.; Chen, X.; Cao, M.M.; Li, M.Q.; Zhang, Y.L. Growth and metabolic responses of maize roots to straw biochar application at different rates. Plant Soil 2017, 416, 487–502. [Google Scholar] [CrossRef]
- Abrishamkesh, S.; Gorji, M.; Asadi, H.; Bagheri-Marand, G.H.; Pourbabaee, A.A. Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ. 2015, 11, 475–482. [Google Scholar] [CrossRef]
- Liu, D.F.; Feng, Z.Y.; Zhu, H.D.; Yu, L.H.; Yang, K.J.; Yu, S.; Zhang, Y.F.; Guo, W. Effects of corn straw biochar application on soybean growth and alkaline soil properties. BioResources 2020, 15, 1463–1481. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M. Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline-sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 2015, 259, 45–55. [Google Scholar] [CrossRef]
- Downie, H.F.; Adu, M.O.; Schmidt, S.; Otten, W.; Dupuy, L.X.; White, P.J.; Valentine, T.A. Challenges and opportunities for quantifying roots and rhizosphere interactions through imaging and image analysis. Plant Cell Environ. 2015, 38, 1213–1232. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, K.R.; Yang, J.E.; Ok, Y.S.; Owens, G.; Nehls, T.; Wessolek, G.; Kim, K.H. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Guo, W.N.; Lin, Q.M.; Li, G.T.; Zhao, X.R. Improving salt leaching in a simulated saline soil column by three biochars derived from rice straw (Oryza sativa L.), sunflower straw (Helianthus annuus), and cow manure. J. Soil Water Conserv. 2016, 71, 467–475. [Google Scholar] [CrossRef]
- Xu, M.; Ma, J.; Zhang, X.H.; Yang, G.; Long, L.L.; Chen, C.; Song, C.; Wu, J.; Gao, P.; Guan, D.X. Biochar-bacteria partnership based on microbially induced calcite precipitation improves Cd immobilization and soil function. Biohcar 2023, 5, 20. [Google Scholar] [CrossRef]
- Reitz, D.N.; Haynes, R.J. Effect of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Lakhdar, A.; Rabhi, M.; Ghnaya, T.; Montemurro, F.; Jedidi, N.; Abdelly, C. Effectiveness of compost use in salt-affected soil. J. Hazard. Mater. 2009, 171, 29–37. [Google Scholar] [CrossRef]
- Bohara, H.; Dodla, S.; Wang, J.J.; Darapuneni, M.; Kongchum, M.; Fromme, D.D.; Harrell, D. Impacts of N-stabilizers and biochar on nitrogen losses, nitrogen phytoavailability, and cotton yield in poultry litter-fertilized soils. Agron J. 2018, 110, 2016–2024. [Google Scholar] [CrossRef]
- Gong, H.Y.; Li, Y.F.; Li, S.J. Effects of the interaction between biochar and nutrients on soil organic carbon sequestration in soda saline-alkali grassland: A review. Glob. Ecol. Conserv. 2021, 26, e01449. [Google Scholar] [CrossRef]
- Rasse, D.P.; Weldon, S.; Joner, E.J.; Kammann, C.I.; Liu, X.Y.; O’Toole, A.; Pan, G.X.; Kocatürk-Schumacher, N.P. Enhancing plant N uptake with biochar-based fertilizers: Limitation of sorption and prospects. Plant Soil 2022, 475, 213–236. [Google Scholar] [CrossRef]
- Luo, X.; Wang, L.; Liu, G.; Wang, X.; Wang, Z.; Zheng, H. Effects of biochar on carbon mineralization of coastal wetland soils in the Yellow River Delta, China. Ecol. Eng. 2016, 94, 329–336. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015, 8, 108–123. [Google Scholar] [CrossRef]
- Tripathi, S.; Chakraborty, A.; Chakrabarti, K.; Bandyopadhyay, B.K. Enzyme activities and microbial biomass in coastal soils of India. Soil Biol. Biochem. 2007, 39, 2840–2848. [Google Scholar] [CrossRef]
- Marina, M.A.C.; Ana, P.C.L.; Angelita, M.O.J.; Ana, K.V.C.; Aurora, M.P.E. Impact of Soil Amendment with Biochar on Greenhouse Gases Emissions, Metals Availability and Microbial Activity: A Meta-Analysis. Sustainability 2022, 14, 15648. [Google Scholar]
- Sun, K.; Han, L.F.; Yang, Y.; Xia, X.H.; Yang, Z.F.; Wu, F.C.; Li, F.B.; Feng, Y.F.; Xing, B.S. Application of hydrochar altered soil microbial community composition and the molecular structure of native soil organic carbon in a paddy soil. Environ. Sci. Technol. 2020, 54, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.S.; Liu, X.X.; Qiao, L.K.; Zhang, S.; Su, K.W.; Qiu, Z.L.; Li, X.H.; Zhao, Q.C.; Yu, C.H. Study on the spatial distribution of ureolytic microorganisms in farmland soil around tailings with different heavy metal pollution. Sci. Total Environ. 2021, 775, 144946. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar Increases Soil Microbial Biomass but Has Variable Effects on Microbial Diversity: A Meta-Analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Wang, X.J.; Yao, G.W.; Lin, Z.H.; Xu, L.Y.; Jiang, Y.L.; Jin, Z.W.; Ping, L.F. Advances in the Effects of Biochar on Microbial Ecological Function in Soil and Crop Quality. Sustainability 2022, 14, 10411. [Google Scholar] [CrossRef]
- Prayogo, C.; Jones, J.E.; Baeyens, J.; Bending, G.D. Impact of biochar on mineralization of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol. Fertil. Soils 2014, 50, 695–702. [Google Scholar] [CrossRef]
| Properties | Experimental Soil |
|---|---|
| Bulk density (g cm−3) | 1.61 ± 0.13 |
| ECe (μs m−1) | 24.08 ± 0.71 |
| pH | 10.10 ± 0.24 |
| SARe (mmolc L−1)1/2 | 368.11 ± 14.03 |
| ESP (%) | 77.11 ± 2.17 |
| CEC (cmol kg−1) | 10.99 ± 0.34 |
| Organic matter (%) | 0.64 ± 0.04 |
| Total N (g kg−1) | 0.27 ± 0.01 |
| Alkali-hydrolysable N (mg kg−1) | 16.30 ± 1.11 |
| Available P (mg kg−1) | 9.13 ± 0.68 |
| Available K (mg kg−1) | 107.25 ± 5.68 |
| pH and Elemental Component | Biochar |
|---|---|
| pH | 7.94 ± 0.32 |
| CEC (cmol kg−1) | 78.69 ± 11.32 |
| EC (dS m−1) | 7.88 ± 0.59 |
| SA (m2 g−1) | 7.41 ± 0.12 |
| C (mg g−1) | 540.64 ± 26.58 |
| N (mg g−1) | 15.93 ± 1.01 |
| S (mg g−1) | 6.85 ± 0.34 |
| P (mg g−1) | 0.74 ± 0.03 |
| Mg (mg g−1) | 0.25 ± 0.00 |
| K (mg g−1) | 12.53 ± 0.51 |
| Ca (mg g−1) | 2.01 ± 0.02 |
| Na (mg g−1) | 1.17 ± 0.21 |
| Nitrogen Level | Biochar Treatment | pH | ESP (%) | ECe (ds m−1) | SARe (mmolc L−1)1/2 | CEC (cmol kg−1) |
|---|---|---|---|---|---|---|
| N0 | C0 | 9.79 ± 0.03 a | 40.78 ± 0.23 a | 22.39 ± 0.20 a | 317.86 ± 15.42 a | 11.44 ± 0.50 d |
| C1 | 9.63 ± 0.02 b | 21.56 ±0.16 b | 11.47 ± 0.35 b | 165.02 ± 5.57 b | 13.32 ± 0.34 c | |
| C2 | 9.61 ± 0.02 b | 16.79 ± 0.46 c | 8.96 ± 0.07 c | 150.84 ± 2.59 c | 15.01 ± 0.20 b | |
| C3 | 9.56 ± 0.02 b | 13.48 ± 0.44 d | 6.79 ± 0.18 d | 122.43 ± 3.38 d | 16.19 ± 0.33 a | |
| N2 | C0 | 9.70 ± 0.02 a | 36.78 ± 0.76 a | 20.87 ± 0.61 a | 300.86 ± 14.13 a | 11.46 ± 0.49 d |
| C1 | 9.50 ± 0.04 b | 18.17 ± 0.20 b | 9.48 ± 0.39 b | 139.02 ± 5.15 b | 14.88 ± 0.15 c | |
| C2 | 9.41 ± 0.02 b | 14.09 ± 0.12 c | 6.97 ± 0.14 c | 122.84 ± 4.46 bc | 16.23 ± 0.34 b | |
| C3 | 9.41 ± 0.01 b | 11.55 ± 0.25 d | 4.46 ± 0.21 d | 107.43 ± 4.17 c | 16.97 ± 0.27 a | |
| Source of variation | ||||||
| Biochar treatment (C) | ** | ** | ** | ** | ** | |
| Nitrogen level (N) | ** | ** | ** | ** | ** | |
| C × N | * | ** | ** | ** | ** | |
| Nitrogen Level | Biochar Treatment | TN (g kg−1) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | SOM (%) | C/N Ratio |
|---|---|---|---|---|---|---|---|
| N0 | C0 | 0.18 ± 0.01 d | 17.79 ± 0.33 c | 6.69 ± 0.37 d | 99.27 ± 1.79 d | 0.81 ± 0.14 d | 15.29 ± 1.78 d |
| C1 | 0.31 ± 0.03 c | 20.71 ± 0.30 b | 10.76 ± 0.25 c | 187.54 ± 6.48 c | 1.15 ± 0.22 c | 27.57 ± 0.77 c | |
| C2 | 0.35 ± 0.01 b | 23.52 ± 0.43 a | 14.58 ± 0.40 b | 349.72 ± 20.52 b | 1.70 ± 0.24 b | 34.90 ± 1.78 b | |
| C3 | 0.43 ± 0.03 a | 21.58 ± 0.88 a | 20.32 ± 0.53 a | 416.96 ± 11.56 a | 2.43 ± 0.16 a | 39.39 ± 0.46 a | |
| N2 | C0 | 0.21 ± 0.03 d | 28.92 ± 1.08 c | 8.15 ± 0.25 d | 105.27 ± 5.37 d | 0.88 ± 0.05 d | 18.39 ± 2.56 d |
| C1 | 0.39 ± 0.01 c | 34.50 ± 0.95 b | 12.76 ± 0.29 c | 227.54 ± 8.51 c | 1.19 ± 0.14 c | 24.66 ± 1.57 c | |
| C2 | 0.48 ± 0.01 b | 45.33 ± 1.23 a | 18.58 ± 0.50 b | 379.76 ±31.65 b | 1.94 ± 0.28 b | 27.01 ± 0.72 b | |
| C3 | 0.55 ± 0.01 a | 42.58 ± 1.53 a | 25.20 ± 0.19 a | 476.96 ± 23.39 a | 2.79 ± 0.07 a | 32.42 ± 1.98 a | |
| Source of variation | |||||||
| Biochar treatment (C) | ** | ** | ** | ** | ** | ** | |
| Nitrogen level (N) | ** | ** | ** | ** | ** | ** | |
| C × N | * | ** | ** | ** | ** | ** | |
| Parameters | Nitrogen Level | Biochar Treatment | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| C0 | C1 | C2 | C3 | Biochar Treatment (C) | Nitrogen Levels (N) | C × N | ||
| Chao 1 | N0 | 4056.77 ± 222.60 a | 3659.24 ± 287.04 a | 3793.70 ± 432.81 a | 4361.17 ± 673.99 a | NS | ** | * |
| N2 | 4162.52 ± 209.58 b | 4229.02 ± 111.52 b | 4596.18 ± 206.40 a | 4616.04 ± 254.24 a | ||||
| Simpson | N0 | 7.99 ± 0.40 b | 8.10 ± 0.26 b | 8.00 ± 0.32 b | 9.40 ± 0.30 a | * | ** | NS |
| N2 | 9.73 ± 0.19 ab | 9.61 ± 0.21 ab | 9.46 ± 0.43 b | 9.97 ± 0.04 a | ||||
| Shannon | N0 | 0.97 ± 0.01 a | 0.97 ± 0.01 a | 0.95 ± 0.06 a | 0.99 ± 0.00 a | NS | * | NS |
| N2 | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 0.99 ± 0.01 a | 1.00 ± 0.00 a | ||||
| The Number of OTUs | N0 | 2332.00 ± 292.32 c | 2543.00 ± 135.70 bc | 2751.33 ± 118.71 ab | 3198.33 ± 162.20 a | * | ** | * |
| N2 | 2865.00 ± 75.72 b | 3093.33 ± 57.74 ab | 3188.00 ± 113.33 a | 3304.00 ± 155.96 a | ||||
| Parameters | pH | ESP | ECe | SARe | CEC | TN | AN | AP | AK | SOM | C/N Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteobacteria | 0.397 | 0.501 | 0.503 | 0.423 | −0.630 | −0.531 | −0.261 | −0.660 | −0.767 * | −0.723 * | −0.614 |
| Bacteroidetes | 0.847 ** | 0.934 ** | 0.942 ** | 0.911 ** | −0.985 ** | −0.942 ** | −0.507 | −0.945 ** | −0.962 ** | −0.925 ** | −0.847 ** |
| Chloroflexi | −0.389 | −0.545 | −0.542 | −0.466 | 0.640 | 0.527 | 0.218 | 0.652 | 0.776 * | 0.714 * | 0.683 |
| Actinobacteria | −0.625 | −0.716 * | −0.724 * | −0.657 | 0.829 * | 0.762 * | 0.392 | 0.858 ** | 0.918 ** | 0.896 ** | 0.751 * |
| Firmicutes | −0.439 | −0.657 | −0.656 | −0.603 | 0.749 * | 0.625 | 0.095 | 0.757 * | 0.838 ** | 0.829 * | 0.829 * |
| Gemmatimonadetes | −0.017 | 0.242 | 0.232 | 0.192 | −0.273 | −0.109 | 0.173 | −0.191 | −0.384 | −0.264 | −0.438 |
| Acidobacteria | −0.426 | −0.374 | −0.369 | −0.365 | 0.367 | 0.337 | 0.475 | 0.196 | 0.319 | 0.125 | 0.128 |
| Verrucomicrobia | 0.625 | 0.654 | 0.631 | 0.673 | −0.525 | −0.562 | −0.381 | −0.449 | −0.421 | −0.384 | −0.492 |
| Patescibacteria | −0.891 ** | −0.847 ** | −0.863 ** | −0.826 * | 0.934 ** | 0.945 ** | 0.663 | 0.936 ** | 0.891 ** | 0.889 ** | 0.680 |
| Cyanobacteria | −0.513 | −0.559 | −0.597 | −0.512 | 0.723 * | 0.704 | 0.324 | 0.846 ** | 0.838 ** | 0.894 ** | 0.596 |
| Spirochaetes | −0.412 | −0.597 | −0.589 | −0.524 | 0.653 | 0.539 | 0.210 | 0.637 | 0.777 * | 0.693 | 0.718 * |
| Fibrobacteres | −0.728 * | −0.725 * | −0.738 * | −0.676 | 0.845 ** | 0.816 * | 0.559 | 0.865 ** | 0.901 ** | 0.871 ** | 0.648 |
| Armatimonadetes | 0.714 * | 0.594 | 0.596 | 0.602 | −0.591 | −0.675 | −0.571 | −0.612 | −0.511 | −0.548 | −0.382 |
| Nitrospirae | −0.686 | −0.577 | −0.621 | −0.589 | 0.635 | 0.752 * | 0.540 | 0.756 * | 0.604 | 0.704 | 0.384 |
| Rokubacteria | −0.402 | −0.285 | −0.283 | −0.346 | 0.248 | 0.277 | 0.331 | 0.081 | 0.020 | −0.041 | −0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Miao, S.; Song, Y.; Wang, X.; Jin, F. Biochar Application Reduces Saline–Alkali Stress by Improving Soil Functions and Regulating the Diversity and Abundance of Soil Bacterial Community in Highly Saline–Alkali Paddy Field. Sustainability 2024, 16, 1001. https://doi.org/10.3390/su16031001
Zhang Y, Miao S, Song Y, Wang X, Jin F. Biochar Application Reduces Saline–Alkali Stress by Improving Soil Functions and Regulating the Diversity and Abundance of Soil Bacterial Community in Highly Saline–Alkali Paddy Field. Sustainability. 2024; 16(3):1001. https://doi.org/10.3390/su16031001
Chicago/Turabian StyleZhang, Yue, Shihao Miao, Yang Song, Xudong Wang, and Feng Jin. 2024. "Biochar Application Reduces Saline–Alkali Stress by Improving Soil Functions and Regulating the Diversity and Abundance of Soil Bacterial Community in Highly Saline–Alkali Paddy Field" Sustainability 16, no. 3: 1001. https://doi.org/10.3390/su16031001
APA StyleZhang, Y., Miao, S., Song, Y., Wang, X., & Jin, F. (2024). Biochar Application Reduces Saline–Alkali Stress by Improving Soil Functions and Regulating the Diversity and Abundance of Soil Bacterial Community in Highly Saline–Alkali Paddy Field. Sustainability, 16(3), 1001. https://doi.org/10.3390/su16031001





