Abstract
Microbial deterioration poses a significant threat to built heritage, particularly mural paintings, where traditional synthetic biocides can have adverse environmental and material impacts. This study evaluates the effectiveness of essential oils derived from four aromatic plants—thyme (Thymus mastichina L.), fennel (Foeniculum vulgare Mill.), pennyroyal (Mentha pulegium L.), and green lavender (Lavandula viridis L’Hér.)—as natural biocides against microorganisms isolated from mural paintings in the House of Moscadim, an 18th-century manor house in Portugal. The antimicrobial activity of the essential oils was assessed using both direct contact and micro-atmosphere methods. Four microorganisms were isolated, including two bacteria, Bacillus wiedmannii and Bacillus mobilis, and two fungi, Penicillium brevicompactum and Cladosporium cladosporioides. Fennel essential oil exhibited the strongest antifungal activity against both fungal species, surpassing the efficacy of the commercial biocide Biotin T® in some cases. Pennyroyal and lavender essential oils also showed significant inhibitory effects. The micro-atmosphere method demonstrated the potential for noninvasive application of essential oils while preserving the integrity of delicate mural surfaces. These findings suggest that essential oils, particularly fennel oil, constitute a promising natural alternative to synthetic biocides for the sustainable conservation of cultural heritage. Further research is recommended to explore their long-term effects and to optimize application techniques.
1. Introduction
Biological agents such as fungi, bacteria, algae, and lichens play a significant role in the deterioration of built heritage [1,2,3]. Most of the microbial genera found in these environments originate from natural air and soil microbiota, but some can be pathogenic to humans or cause opportunistic infections [4,5,6,7,8]. Once these microorganisms colonize indoor and outdoor surfaces, they excrete biotic compounds, including organic and inorganic acids as well as enzymes, which dissolve minerals and degrade organic materials. This activity results in both structural and aesthetic damage to heritage surfaces [5,6,9,10,11,12,13,14].
Fungi, for instance, can penetrate stone and plaster with their hyphae. Many species also produce melanin pigments that darken facades and wall paintings, leaving them with a dirty appearance. Bacteria, on the other hand, excrete polymeric substances that enhance microbial adhesion and promote the formation of symbiotic biofilms. These biofilms not only protect microorganisms from ultraviolet radiation but also retain moisture, trap dirt, and accumulate organic molecules, contributing to surface blackening. This black discoloration is a common issue on historic buildings, statues, and artifacts worldwide, which significantly diminishes their aesthetic value [5,7,10,11,12,14,15,16].
Cultural heritage conservation is a multidisciplinary field that aims to preserve art, architecture, and historical artifacts for future generations. Research has emphasized the importance of environmental control, such as regulating humidity and temperature, as well as the use of biocides and noninvasive cleaning methods to prevent microbial growth and preserve built heritage [11,16,17,18,19].
Synthetic chemical biocides based on formaldehyde, phenols, and quaternary ammonium compounds have been commonly used to control microbial growth on built heritage. While effective, these substances pose significant risks due to their toxicity to humans and the environment, and their repeated application can lead to the development of resistant microbial strains [4,20]. Furthermore, synthetic biocides can have deleterious effects on the artworks themselves, causing discoloration or weakening of the materials [21,22]. These issues have spurred interest in exploring more sustainable, less harmful alternatives for heritage conservation, such as natural biocides derived from essential oils (EOs) and plant extracts [4,15,23,24,25]. EOs extracted from aromatic plants are traditionally known for their antimicrobial, antifungal, and insecticidal properties with applications in other areas such as food preservation, cosmetics, and pharmaceuticals [24,26,27].
Essential oils are noted for presenting lower toxicity to humans and the environment compared with synthetic biocides [4] and are generally considered more environmentally friendly than many synthetic compounds used in conservation and restoration. They are biodegradable and are not known to persist in the environment or accumulate in living organisms. Additionally, the natural origin of plant EOs allows for sustainable production as the plants are a renewable resource. This is particularly important as conservation and restoration practices increasingly adopt ecological engineering principles, aiming to reduce their environmental footprint in line with global sustainability goals. EOs can also be obtained by recycling vegetable residues from the aromatic plant supply chain [28].
Despite the promising potential of EOs as natural biocides, several challenges to their application in the cultural heritage conservation remain. It is crucial to assess how these oils interact with the materials over time and whether repeated applications could result in unintended consequences, such as chemical degradation or discoloration [20]. There is also a lack of research on application methods for essential oil-based biocides. Enhancing resource efficiency in conservation practices requires developing application methods that minimize material use and waste. Most existing studies focus on the use of direct contact methods, which may be unsuitable for delicate works such as paintings due to potential interference with pigments and substrates. Given that EOs are volatile, this property could be leveraged to develop noninvasive biocidal atmospheres, a method particularly practical for smaller objects that can be placed in controlled atmosphere chambers or for fumigation treatments in indoor environments.
The conservation of mural paintings is often complicated by biological deterioration, and their integration into architectural structures, which exposes them directly to environmental conditions that favor microbial growth. Common microorganisms found on mural surfaces include fungi such as genera Aspergillus and Penicillium and bacteria like Bacillus species, all of which contribute to the deterioration of pigments and binding materials [29,30,31]. These microorganisms produce acids and enzymes that degrade the organic components of the paintings, leading to discoloration, cracking, and flaking of the surface. Addressing these challenges is essential for the sustainability of cultural heritage preservation. As these biodeteriorative agents are persistent and difficult to completely eradicate, continuous intervention is required to maintain the integrity of these cultural assets [19]. Regarding the use of EOs as biocides in the preservation of mural paintings, significant challenges persist. Most research has concentrated on their application in conserving stone artworks and archival materials like paper, photographs, and graphic works. However, few studies have focused on mural paintings [4,24,32,33,34], underscoring the need for further exploration in this area. This limited scope indicates a gap in resource efficiency and eco-efficiency within conservation practices as the potential benefits of EOs in mural conservation remain underexplored.
This study aims to provide insights into the use of EOs extracted from four aromatic plants—thyme (Thymus mastichina L.), fennel (Foeniculum vulgare Mill.), pennyroyal (Mentha pulegium L.), and green lavender (Lavandula viridis L’Hér.)—as natural biocides for the preservation of mural paintings. EOs, extracted from endemic aromatic Portuguese plants, and their mixtures were evaluated for their antimicrobial properties, specifically targeting microorganisms isolated from the deteriorated murals of the House of Moscadim. In addition to assessing their effectiveness in inhibiting microbial growth, the research also explored the possibility of using these EOs in noninvasive conservation techniques. By doing so, the study not only highlights the essential oils’ potential for reducing microbial deterioration but also examines their suitability for practical application in cultural heritage preservation, with a focus on minimal intervention and safeguarding the integrity of the original artworks. This approach enhances the relevance of EOs as a solution for the ecological conservation of mural art, promoting sustainability in heritage preservation practices.
2. Materials and Methods
2.1. Site Description
The House of Moscadim, located on the main street of Vila of Chamusca in the Ribatejo region of Portugal, is an 18th-century manor house (Figure 1). It is considered unique in the village and one of the most important in the region. The house is distinguished by its historical and cultural significance, particularly due to the notable collection of tiles found inside. Additionally, it is unique among the local residences for its Pillement-style mural paintings. The diverse decorative connection between fresco, dry mural paintings, and tiles with the house’s structure reflects its well-preserved state and the trends of a residence that has been inhabited continuously from the 18th century to the present day.

Figure 1.
House of Moscadim: (a) main facade (b); tile representation of a male “invitation” figure, a “muscadine” (a typical figure from the post-French Revolution era, distinguished by their elegant style of dress and the use of musk fragrance), origin of the current name of the house.
Currently, the house is safeguarded by the company Patrimonium and has been the focus of a conservation and musealization project aimed at turning it into a public-access laboratory. These actions include cultural mediation, conservation research, musealization, and historical studies, involving a multidisciplinary team in collaboration with higher educational institutions such as the Polytechnic Institute of Tomar. The distinctive measures for preservation and musealization not only highlight the architectural and artistic uniqueness of the house but also enrich the understanding of local, national, and international history. In this context, the project aims to achieve the restoration and conservation of the House of Moscadim using an ecological engineering approach. One specific goal is to test natural biocides in this unique environment, particularly in the mural painting of a central room on the upper floor, known as the Pillement Room.
The Pillement Room (Figure 2) is adorned with tiles up to a height of one meter and features dry painting achieved through oil paint applied over the tile composition. The painting, which has a monochromatic tone and a narrative style reminiscent of the artist Jean Pillement’s atelier, consists of five medallions framed with floral motifs, both worked in oil on a thin layer of pre-dried lime. This artwork has been restored, covered, and outlined with plastic paint.
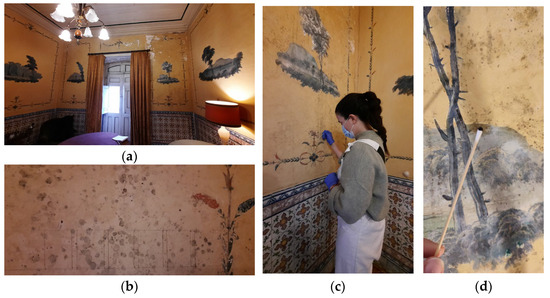
Figure 2.
Pillement Room: (a) general view; (b) in situ test of biocides; (c,d) sample collection for laboratory tests.
Aesthetical and structural damage was observed on the wall near the windows, indicating that the structure suffered physical issues due to previous localized roof leaks, which caused cracks, fissures, and conditions conducive to the development of biofilms. Microorganism samples were collected from this area for laboratory testing (Figure 2).
2.2. Microorganisms Isolation: Morphological Characterization and Molecular Identification
The collection of microorganisms was conducted in situ at the House of Moscadim using the swabbing technique (Figure 2c,d) [35]. Samples were taken from pigmented biofilms formed on the wall paintings near the windows of the Pillement Room. The biological material was maintained at 4 °C in a sterile Ringer solution until further use. Cultivable microorganisms were isolated by subculturing onto appropriate solid culture media and incubated in the dark for 3–7 days at 22 ± 1 °C. Bacteria were isolated using tryptic soy agar (TSA, HiMedia, Mumbai, India), while fungi were isolated using potato dextrose agar (PDA, HiMedia, Mumbai, India) supplemented with chlortetracycline.
The morphological characteristics of each colony were observed, and their size, shape, surface gloss, edge morphology, and color were recorded. After Gram’s staining, the single-cell morphology of the bacteria was observed under a 1000× microscope (Olympus, CH30, Tokyo, Japan). Additionally, the fruiting bodies and conidia produced by the filamentous fungi cultures growing on the PDA plates were examined under a 400× microscope (Olympus CH30, Tokyo, Japan).
To genetically identify the isolated bacterial and fungal species, a colony was selected from each culture medium, suspended in 10 µL of PBS (phosphate-buffered saline; 1X, pH 7.4), applied to an FTA card, and allowed to dry for 2 h at room temperature. The samples prepared were then sent to the STABVIDA genetics laboratory https://www.stabvida.com/ (accessed on 1 November 2024) for identification, according to the following summarized procedure. For fungi, DNA was purified from cell culture stored on the FTA card, followed by PCR amplification of the rDNA segment containing ITS and D1/D2 regions using ITS5/LR6 primers; amplified products were then subjected to Sanger sequencing (sense and antisense), aligned for consensus sequence generation, and BLASTed against the NCBI database. For bacteria, DNA was purified from cell culture stored on the FTA-indicating card, followed by PCR amplification of the 16S rDNA gene segment containing variable regions V1 to V9; amplified products were then subjected to Sanger sequencing (sense and antisense), aligned for consensus sequence generation, and BLASTed against the NCBI database.
2.3. Essential Oil Obtention and Stock Biocide Solution Preparation
The EOs were obtained from D’Alenguadiana Company (Alentejo, Portugal). The EOs were extracted by hydrodistillation from the aerial parts of four aromatic plants: thyme (Thymus mastichina L.), fennel (Foeniculum vulgare Mill.), pennyroyal (Mentha pulegium L.), and green lavender (Lavandula viridis L’Hér.). These plants are native to the Portuguese territory and are produced organically and hand harvested, promoting sustainability and resource efficiency in their cultivation and processing. The EOs were stored at room temperature and protected from light until use.
The EOs were previously characterized by gas chromatography–mass spectrometry (GC-MS) using an Agilent 7890 GC (Santa Clara, CA, USA) coupled with an Agilent 5975 C inert XL mass selective detector (MSD) [36]. The identification of EO components was carried out by comparing the mass spectra with those in the NIST and Wiley data system libraries. Retention indexes were calculated relative to C7–C25 n-alkanes and some components were also compared with pure standard compounds [36].
Essential oil-based biocides, at a final concentration of 20% (v/v), were prepared by emulsifying the corresponding EO with an aqueous solution of SDS (sodium dodecyl sulfate, 1% m/v in deionized sterilized water). Additionally, a biocide containing a mixture of the four EOs, with each EO at a concentration of 5% for a total EO concentration of 20%, was also prepared.
2.4. Biocidal Potential Assessment
The biocidal potential of essential oil emulsions was evaluated using two methods, a direct contact method and a micro-atmosphere method. Both methods were used for the antifungal assays and only the contact method was used for the antibacterial assays.
The tests were conducted in Petri dishes (10 cm) with PDA culture medium for fungi and TSA medium for bacteria; some examples are shown in Figure 3.
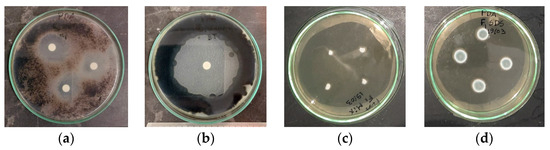
Figure 3.
The disk diffusion contact method for Cladosporium cladosporioides: (a) pennyroyal essential oil; (b) positive control (BT, 1% v/v), and micro-atmosphere tests for Penicillium brevicompactum; (c) mixture of essential oils; and (d) negative control (SDS, 1% w/v).
For the contact method, the disk diffusion test [37] was employed. Microbial suspensions were prepared in sterilized water and 400 µL were spread onto the agar media. Then, 6 mm diameter sterilized filter paper discs (Macherey–Nagel) were placed onto the Petri dishes and imbibed with 12 µL of biocide. The samples were incubated in a thermostatic chamber at 22 °C for four days, after which the radius of the zones of growth inhibition (inhibition halo, IH) was measured (Figure 3a,b).
In the second procedure, micro-atmosphere tests [38,39] were conducted. Mycelium plugs of filamentous fungi were inoculated on the agar media, and 40 µL of biocides and controls were applied to the lid of the inverted Petri plates. This method allowed for the evaporation of volatile components without direct contact with the biological material. The plates were sealed with Parafilm® M to create a modified micro-atmosphere. Samples were incubated in a thermostatic chamber at 22 °C for seven days, and colony diameters were measured on days 3, 4, 5, and 7 (Figure 3c,d). The colony’s growth rate was calculated from the slope of the linear plot of colony diameter versus time.
As a positive control, a 1% v/v solution of the commercial biocide Biotin T® (CTS SRL) was used. Biotin T® (BT) is a commercially available mixture with synergistic action of synthetic active ingredients, including n-octyl-isothiazolinone and a quaternary ammonium salt, designed to preserve and repair surfaces from microbial attacks. For the negative control, a 1% solution of SDS in deionized sterile water was used to ensure that the solvent did not interfere with the activity of the EOs.
The tests were performed in triplicate, with ten random measurements of inhibition halo (IH) and colony diameter recorded for each test, resulting in a total of 30 measurements for each biocide–microorganism pair.
2.5. Statistical Analysis
The experimental data were organized and analyzed using Microsoft Excel® spreadsheet software, version 2206, 64-bit. SPSS® software was employed to evaluate the biocidal effects of the EOs emulsions, namely, to calculate the means and the 95% confidence intervals of the colonies’ radius and the inhibition halos (IH). Analysis of variance (ANOVA), and Tukey’s HSD post hoc comparison tests with a confidence level of 95% (p = 0.05) were used to identify homogeneous subsets, after checking normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests.
3. Results and Discussion
3.1. Microorganism Isolation and Identification
As previously mentioned, biological samples were collected from a wall near a window of Pillement Room, an area that had experienced physical damage due to past roof infiltrations, which led to cracks and created conditions conducive to biofilm development, causing dark spots, as illustrated in Figure 2.
The experimental approach led to the isolation and identification of four cultivable microorganisms: two bacterial species (B1 and B2) and two filamentous fungi (F1 and F2). The morphology of the colonies and cells for each species is shown in Figure 4.
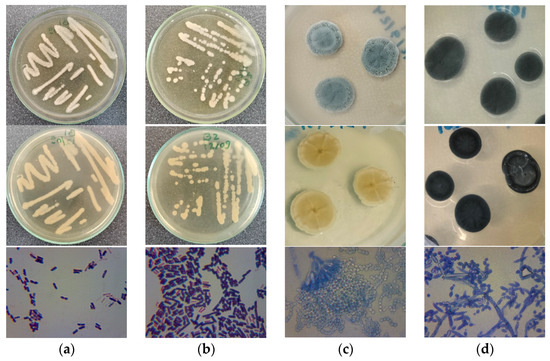
Figure 4.
The colony morphology (surface and reverse) and microstructure after 7 days of incubation for fungi and 2 days for bacteria at 22 °C: (a) Bacillus wiedmannii; (b) Bacillus mobilis; (c) Penicillium brevicompactum; (d) Cladosporium cladosporioides.
The Gram staining test revealed that both bacterial species are endospore-forming, Gram-positive Bacillus. The B1 colonies are medium to large, reaching several millimeters in diameter after 24 to 48 h of incubation at 22 °C. They are circular with wavy, slightly irregular edges, opaque, and cream-colored, with a smooth to slightly wrinkled surface. This morphology is consistent with the species Bacillus wiedmannii (with accession number NR152692.1), which was confirmed through molecular analysis with an identified similarity of 100%. B2 colonies are medium-sized, reaching approximately 2–5 mm in diameter after 24–48 h of incubation at 22 °C. They are circular with smooth, well-defined edges and exhibit an opaque, white to off-white color. The colony surface is smooth and has a slightly glossy appearance. This morphology is consistent with the species Bacillus mobilis (with accession number NR157731.1), which was confirmed through molecular analysis with an identified similarity of 99.92%.
The F1 colonies are consistent with the genus Penicillium, exhibiting moderate to rapid growth on PDA at 22 °C, and have radial striae and a velvety to downy texture. The colonies are initially white and over time become bluish-green, with a white border. The reverse of the plaque is pale yellowish with a lighter border. Microscopic examination of F1 is also compatible with Penicillium sp. with conidia on conidiophores. The conidia are arranged in long chains of globose shapes. This fungus was identified by molecular analysis as Penicillium brevicompactum (identified similarity 99.92%; with accession number MH047201.1).
Colonies of F2 are consistent with the genus Cladosporium, showing moderate growth. The colonies range from olive-green to blackish as they mature, with a velvety texture. The surface appears slightly raised and uneven, and the margins are well defined. The reverse side of the colonies displays a dark brown to black coloration. Microscopic observation of F2 revealed branched, erect conidiophores that produce chains of small, oval conidia. This fungus was identified by molecular analysis as Cladosporium cladosporioides (identified similarity 97.69%; with accession number OR243761.1).
Figure 5 presents the phylogenetic trees of the microbial species isolated, in comparison with species reported in other studies focusing on built heritage biodeterioration [22]. Panel (a) illustrates the phylogenetic relationships among bacterial species, while panel (b) depicts those among fungal species.
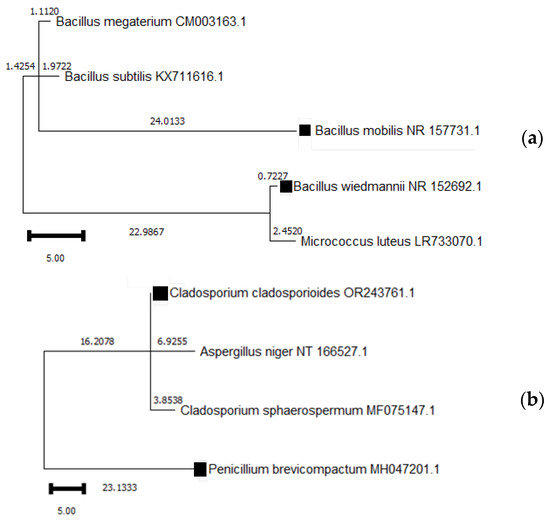
Figure 5.
Phylogenetic tree of species identified in the Moscadim house in relation to species found in other similar projects; (a) represents bacterial species and (b) represents fungal species. The species marked with a square symbol (■) are those analyzed in this study, along with their respective accession numbers.
Considering the specific context from where the fungi were isolated and considering the information provided by Trovão and Portugal [31], these species might display the ensuing putative biodeterioration abilities: P. brevicompactum—mineralization, fibrinolytic, proteolytic and cellulolytic; C. cladosporioides—mineralization, ligninolytic, keratinolytic, lipolytic, proteolytic and cellulolytic. These genera are known for colonizing various substrates, including stone, plaster and wood, especially in environments with high humidity, such as walls damaged by water infiltration and poor ventilation. These fungi are associated with the production of secondary metabolites, which can cause discoloration and further material weakening, leading to both aesthetic and structural damage [5,7,12,15,23,31,32]. Cladosporium species produce dark pigments that stain surfaces, while their hyphal growth can cause mechanical damage by penetrating building materials. Additionally, their metabolic activity leads to the production of organic acids that contribute to the chemical degradation of materials like limestone and gypsum. Penicillium species, on the other hand, can produce both enzymes and organic acids that accelerate the degradation of organic substrates like wood, textiles, and paper, as well as inorganic materials.
Concerning the isolated bacteria, Bacillus species pose a significant threat to cultural heritage due to their ability to excrete enzymes and acid byproducts that impact both organic and inorganic materials like paper, leather, coatings, stone, and metal [40,41,42]. The Gram-positive, endospore-forming Bacillus species can endure extreme conditions, allowing them to survive much longer than vegetative cells. This spore-forming capability provides Bacillus bacteria with high resistance to harsh environments. Bacillus wiedmannii, for instance, has been identified within bacterial communities that form bioweathering crusts on limestone sculptures, contributing to their gradual deterioration [42]. The Bacillus mobilis species has also been recognized for its role in marble biodeterioration, by precipitating and metabolizing calcium carbonate, which can lead to damage in marble sculptures [43].
3.2. Biocidal Potential Assessment
The antimicrobial efficacy of EOs is fundamentally influenced by the molecular mechanisms of their bioactive compounds. The EOs are rich in terpenoids and phenylpropenoids—hydrophobic molecules such as limonene, α-pinene, and β-pinene—[36] that can integrate into microbial cell membranes due to their lipophilic nature [26]. This integration disrupts membrane structure, increases permeability, and causes leakage of cellular components, ultimately leading to cell death. Additionally, phenolic compounds in EOs can interact with membrane proteins and enzymes, impairing essential cellular processes. These key structural features are crucial for enhanced antimicrobial activity and the potential application of EOs as natural biocides.
Direct contact methods can significantly alter the growth environment of microorganisms and, in the case of EO application, long-term effectiveness can also be compromised due to the volatility of its main components. On the other hand, micro-atmosphere methods minimize the direct impact on substrates and capitalize on the volatility of EOs. However, micro-atmosphere methods may not be suitable for all practical applications, such as outdoor environments found on built heritage facades. They are particularly practical for smaller objects that may be placed in controlled atmosphere chambers or used in indoor fumigation treatments.
3.2.1. Antifungal Activity Assessed by Micro-Atmosphere Method
The antifungal efficacy of EOs was assessed against P. brevicompactum and C. cladosporioides using the micro-atmosphere method. This method allows for the evaluation of volatile compounds’ ability to inhibit fungal growth without direct contact, enhancing eco-efficiency by minimizing material use and waste. This approach is particularly relevant in the context of heritage conservation where noninvasive techniques are critical to preserving fragile surfaces.
Figure 6 shows the growth of P. brevicompactum and C. cladosporioides colonies over time in response to the different essential oil-based emulsions. The graphs present the mean colony radius measured at 3, 4, 5, and 7 days. Table 1 presents the initial colony radius (measured at 3 days and the colony growth rate G.R.) for each biocide and controls.
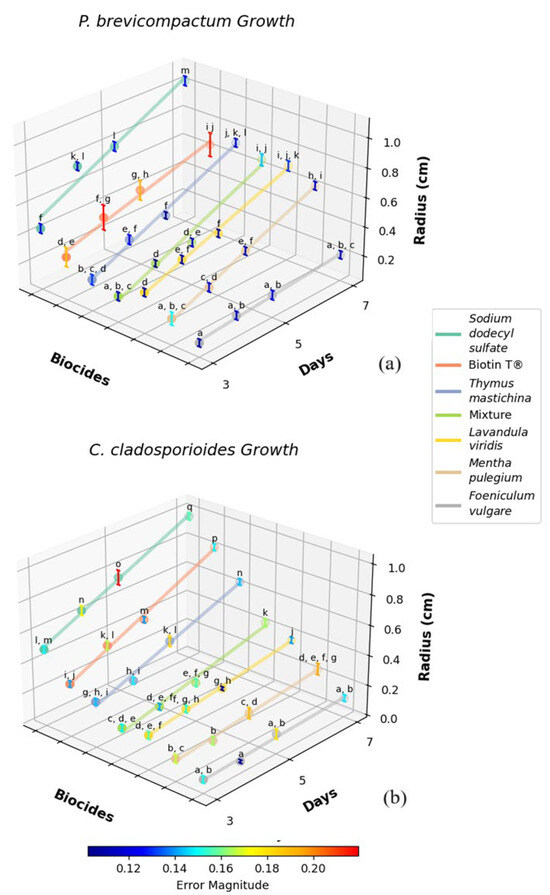
Figure 6.
Growth of Penicillium brevicompactum (a) and Cladosporium cladosporioides (b) colonies over time in response to different essential oil-based biocides. The graph presents the mean colony radius (cm) measured at 3, 4, 5, and 7 days, with confidence intervals at significance level α of 0.05 included for each measurement. The essential oils tested include fennel (Foeniculum vulgare), lavender (Lavandula viridis), pennyroyal (Mentha pulegium), thyme (Thymus mastichina), a mixture of EOs, sodium dodecyl sulfate as a control, and the commercial biocide Biotin T®. Means marked with different letters within the same species are significantly different at a statistical level of 0.05.

Table 1.
Antifungal activity of essential oil-based biocides against Penicillium brevicompactum and Cladosporium cladosporioides using the micro-atmosphere method.
The results obtained reveal substantial differences in the antifungal performance of various biocides. This variability is also reported in the literature and can be partly attributed to differences in the composition of EOs [4,15,44,45]. In general, the major constituent confers the biological activity of the essential oil, although this activity often results from a synergistic action between several minor constituents. Additionally, it is worth noting that the composition of EOs from the same plant species often varies depending on the region, as well as environmental, cultivation, and harvesting conditions [36,46,47,48,49,50].
For P. brevicompactum, fennel EO demonstrated the most pronounced inhibitory effect, with an initial colony radius of 0.12 ± 0.03 cm after 3 days and a low growth rate of 0.031 cm/day. These findings suggest that fennel EO contains highly effective antifungal compounds that act rapidly to suppress fungal growth. Pennyroyal EO also exhibited significant inhibition, with an initial radius of 0.21 ± 0.04 cm and a growth rate of 0.088 cm/day, indicating its potential utility in similar applications.
Lavender EO, while associated with a larger initial colony radius (0.30 ± 0.03 cm), maintained a strong inhibitory effect over time, as evidenced by its growth rate of 0.096 cm/day. This raises an important consideration regarding the mode of action of lavender EO: despite its slower initial effect, its ability to inhibit fungal growth over a longer duration may be advantageous in scenarios requiring sustained antimicrobial activity. The EO mixture, which combines the properties of multiple oils, showed an initial radius of 0.20 ± 0.03 cm and a growth rate of 0.090 cm/day, suggesting that this formulation could serve as an effective broad-spectrum antifungal agent.
The performance of the SDS control, with a much larger initial radius of 0.45 ± 0.03 cm and a high growth rate of 0.150 cm/day, confirms that the antifungal activity observed with the EOs is not attributable to the solvent system. In comparison, the commercial biocide Biotin T® produced an initial radius of 0.32 ± 0.07 cm and a growth rate of 0.109 cm/day, indicating that fennel and pennyroyal EOs may offer comparable, if not superior, antifungal efficacy under similar conditions.
In the case of C. cladosporioides, fennel EO once again demonstrated the most significant inhibitory activity, with a small initial radius of 0.08 ± 0.02 cm and a growth rate of 0.017 cm/day. This consistency across different fungal species highlights fennel EO as a particularly potent antifungal agent with broad-spectrum potential. Pennyroyal EO also exhibited effective inhibition, with an initial radius of 0.14 ± 0.03 cm and a growth rate of 0.034 cm/day, further supporting its role as a viable natural biocide.
Lavender EO demonstrated an initial radius of 0.21 ± 0.02 cm and a growth rate of 0.053 cm/day, similar to its performance against Penicillium. The EO mixture, with an initial radius of 0.19 ± 0.02 cm and a growth rate of 0.058 cm/day, reinforces the potential of EO combinations to provide effective control over diverse fungal species, potentially increasing the breadth of antifungal activity.
The SDS control, which exhibited the highest initial radius of 0.50 ± 0.02 cm and a growth rate of 0.139 cm/day, further confirmed that the antifungal effects observed are attributable to the EOs themselves. The commercial biocide Biotin T® (initial radius of 0.33 ± 0.02 cm and growth rate of 0.115 cm/day) also performed as expected, yet the comparable efficacy of natural EOs, particularly fennel EO, suggests that these natural alternatives could effectively replace or complement synthetic biocides in certain contexts.
These results indicate that fennel EO consistently exhibited the strongest antifungal activity across both fungal species, characterized by rapid suppression of growth and sustained inhibition. Pennyroyal and lavender EOs also demonstrated considerable efficacy. The EO mixture, combining the properties of multiple oils, presents a promising broad-spectrum alternative to both individual EOs and synthetic biocides. Taken together, these findings highlight the potential for EOs to serve as sustainable, environmentally friendly alternatives to traditional biocides in the conservation of heritage materials, offering effective microbial control while minimizing potential harm to delicate surfaces.
3.2.2. Antimicrobial Activity Assed by Contact Method
The antimicrobial efficacy of the essential oil emulsions was evaluated against the two filamentous fungi P. brevicompactum and C. cladosporioides and the two bacterial strains B. wiedmannii and B. mobilis using the disk diffusion method. This method assesses the direct inhibitory effect of the EO-based biocides.
Figure 7 illustrates the inhibition halos formed after 4 days of incubation at 22 °C for each microbial species in response to the different biocides. Table 2 presents the mean inhibition halo radius along with their respective confidence intervals at significance level of α = 0.05.
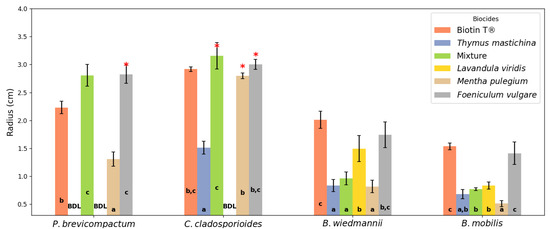
Figure 7.
Inhibition halos (radius, in cm) measured after 4 days of exposure to different essential oil-based biocides and the commercial biocide Biotin T® against the fungi Penicillium brevicompactum and Cladosporium cladosporioides, and the bacteria Bacillus wiedmannii and Bacillus mobilis. The EOs tested include Foeniculum vulgare (fennel), Lavandula viridis (lavender), Mentha pulegium (pennyroyal), Thymus mastichina (thyme), and a mixture of these EOs. Biotin T® served as the positive control. a–c Means with various superscript letters within the same treatment indicate a significant difference. “BDL” indicates inhibition below the detection limit. Error bars represent the confidence intervals at significance level α of 0.05 of the mean. An asterisk (*) above a bar indicates that the inhibition halo radius was larger than measured due to the distance between the disk and the Petri dish.

Table 2.
Mean inhibition halos (cm) of essential oils and Biotin T® against the microorganisms isolated.
Like the biocidal effect obtained by the micro-atmosphere method, the results observed by the contact method also reveal significant differences in the antimicrobial performance of the various EOs and the commercial biocide.
Fennel EO demonstrated the most pronounced inhibitory effect against P. brevicompactum, with a mean inhibition radius halo of 2.83 ± 0.16 cm. Notably, in some instances, the inhibition halo produced by fennel EO extended to the edge of the Petri dish, exceeding the measurable area due to the proximity between the disk and the dish wall. This indicates that the actual inhibitory effect could be even greater than recorded. The EO mixture also showed substantial antifungal activity, producing an inhibition halo of 2.81 ± 0.20 cm. The commercial biocide Biotin T® resulted in an inhibition halo of 2.23 ± 0.11 cm, slightly lower than that observed for fennel EO and the EO mixture. Pennyroyal EO exhibited moderate antifungal activity against P. brevicompactum, with an inhibition halo of 1.31 ± 0.13 cm. In contrast, lavender EO and thyme EO did not display detectable inhibition against P. brevicompactum, indicating limited efficacy against this fungal species.
Against C. cladosporioides, the EO mixture produced the largest inhibition halo of 3.16 ± 0.24 cm. Both fennel EO and pennyroyal EO exhibited strong inhibitory effects with inhibition halos of 3.00 ± 0.09 cm and 2.80 ± 0.05 cm, respectively. Similar to the observations with P. brevicompactum, the inhibition halos for fennel EO and pennyroyal EO occasionally extended beyond the measurable area due to reaching the edge of the Petri dish. This suggests an even greater antifungal potency against C. cladosporioides. The commercial biocide Biotin T® generated an inhibition halo of 2.92 ± 0.04 cm, comparable to the efficacy of fennel EO. Thyme EO showed a smaller inhibition halo of 1.51 ± 0.11 cm, while lavender EO did not exhibit detectable inhibition against this fungus.
Regarding the bacterial strains, Biotin T® exhibited the largest inhibition halos, measuring 2.01 ± 0.15 cm for B. wiedmannii and 1.53 ± 0.06 cm for B. mobilis, confirming its established efficacy as a synthetic biocide. Fennel EO showed good antibacterial activity against B. wiedmannii, with an inhibition halo of 1.74 ± 0.23 cm, and moderate activity against B. mobilis, with a halo of 1.41 ± 0.20 cm. Lavender EO demonstrated antibacterial activity against B. wiedmannii, with an inhibition halo of 1.49 ± 0.23 cm, but it was less effective against B. mobilis, producing a smaller halo of 0.83 ± 0.06 cm. Pennyroyal EO exhibited lower antibacterial activity, with inhibition halos of 0.82 ± 0.11 cm against B. wiedmannii and 0.51 ± 0.05 cm against B. mobilis. Thyme EO showed similar low activity, with inhibition halos of 0.83 ± 0.11 cm against B1 and 0.68 ± 0.08 cm against B. mobilis. The EO mixture demonstrated moderate antibacterial activity, with inhibition halos of 0.96 ± 0.11 cm for B. wiedmannii and 0.77 ± 0.03 cm for B. mobilis.
The negative control, consisting of an SDS solution, did not exhibit any inhibitory effect against any of the tested microorganisms. The inhibition halo radius was always less than that of the disk itself (0.3 cm), indicating no measurable antimicrobial activity. This confirms that the observed inhibition zones for the EOs and Biotin T® are attributable to their inherent antimicrobial properties rather than the solvent used in the emulsions.
Compared with conventional strategies—such as synthetic fungicides, UV-protective films, and other physical barriers—the use of EOs as biocides in mural conservation offers several noteworthy advantages though some challenges remain. On one hand, EOs are environmentally sustainable, generally nontoxic, and can effectively counteract microbial resistance due to their complex chemical profiles and natural biodegradability, thereby preserving both the artwork and human health [51]. Their targeted activity helps maintain the original aesthetic and structural integrity of murals without introducing harmful residues that could compromise future treatments [24]. Conversely, their volatility, often limited durability, and the variability in their antimicrobial efficacy—stemming from factors like plant source, seasonality, and extraction methods—may increase long-term costs and necessitate repeated applications [21].
To maximize the potential of EOs in heritage conservation, further research is essential. Long-term studies are needed to evaluate their impact on different mural substrates, to monitor potential chemical interactions or alterations over time and to evaluate their ecotoxicity. Further exploration is also needed into whether long-term use of EOs will alter the microbial community structure in the microenvironment where murals are located, thereby affecting the global ecological balance. Another challenge lies in the degradation of EOs. Composed primarily of volatile and thermolabile terpenoids, EOs are susceptible to oxidative deterioration. Elevated temperatures, the presence of oxygen, and light can synergistically enhance these oxidative reactions, leading to changes in their chemical composition, which may compromise their pharmacological properties, including biocidal activity [26]. Understanding these degradation mechanisms is crucial for developing effective strategies to preserve the quality, efficacy, and antimicrobial activity of EOs in both storage and application.
Beyond their proven efficacy as biocides on inorganic substrates, essential oils hold significant promise for broader applications in cultural heritage conservation, including the treatment of organic materials such as wood carvings, textiles, and paper-based artifacts [4]. The inherent variability in porosity, composition, and surface characteristics of these substrates influences the absorption and penetration of oils, requiring careful adjustment of formulas and application methods. For instance, certain EOs, due to their nontoxic and eco-friendly profile, can be microencapsulated or combined with consolidants, water repellents, or hydrogel carriers to ensure a controlled release of antimicrobial compounds and prolonged activity [21]. This approach not only targets biodeteriogens more selectively but also respects the substrate’s integrity, reducing risks of discoloration, weakening, or chemical alteration [4]. As research progresses, refining essential oil blends and tailoring their use to diverse materials will expand their protective scope, positioning them as viable, sustainable, and human-safe alternatives to conventional synthetic biocides for multiple fields of cultural heritage preservation.
Finally, scaling up the production of these EOs for industrial applications is both feasible and advantageous. These aromatic plants are endemic or naturalized in the Mediterranean region and are already being harvested sustainably in Portugal by companies like D’Alenguadian, for extraction of EOs through hydrodistillation methods [36]. The extraction process is well established and suitable for large-scale production without significant challenges. Moreover, the exploitation and production of these essential oils can positively impact local communities by promoting organic cultivation and providing economic opportunities for small and local enterprises. Implementing sustainable practices, such as harvesting methods that allow for plant regeneration and crop rotation, enhances environmental stewardship. Therefore, utilizing these EOs not only offers an effective natural solution for the conservation of mural paintings but also contributes to ecological sustainability and supports the local economy.
4. Conclusions
This study demonstrates that EOs derived from the aromatic plants thyme, fennel, pennyroyal, and green lavender possess significant antimicrobial properties against microorganisms responsible for the biodeterioration of mural paintings. Fennel essential oil exhibited the most potent antifungal activity, surpassing the efficacy of the commercial biocide Biotin T® in inhibiting the growth of the isolated fungal species. Pennyroyal and lavender EOs also showed considerable inhibitory effects. Notably, a mixture of these EOs demonstrated enhanced antimicrobial efficacy, suggesting potential synergistic effects when combined. The use of the micro-atmosphere method proved effective for the noninvasive application of these EOs, thereby preserving the integrity of delicate painted mural surfaces. These findings suggest that EOs, particularly fennel oil and their mixtures, represent promising natural alternatives to synthetic biocides for the sustainable conservation and restoration of built cultural heritage, meeting global sustainability goals.
To confirm the universality of results, future studies should include tests with samples from different mural painting contexts. It is also recommended that further research be carried out to evaluate the long-term impacts of EO application, with a focus on its effect on mural substrates and on the surrounding environmental ecosystems, and to optimize application techniques.
Author Contributions
Conceptualization, D.M. and F.C.; methodology, D.M., F.C. and L.M.; formal analysis, D.M. and V.d.J.; investigation, D.M., F.C. and L.M.; writing—original draft preparation, D.M., L.M. and V.d.J.; writing—review and editing, D.M., F.C. and V.d.J.; supervision, D.M. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Portuguese Foundation for Science and Technology, project UID/05488/2020 granted to Techn&Art.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available upon request.
Acknowledgments
The authors thank the kind support of Joel Moedas-Miguel which oversees the House of Moscadim.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schröer, L.; Boon, N.; De Kock, T.; Cnudde, V. The Capabilities of Bacteria and Archaea to Alter Natural Building Stones—A Review. Int. Biodeterior. Biodegrad. 2021, 165, 105329. [Google Scholar] [CrossRef]
- Sterflinger, K.; Pinzari, F. The Revenge of Time: Fungal Deterioration of Cultural Heritage with Particular Reference to Books, Paper and Parchment. Environ. Microbiol. 2012, 14, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Crispim, C.A.; Gaylarde, C.C. Cyanobacteria and Biodeterioration of Cultural Heritage: A Review. Microb. Ecol. 2005, 49, 1–9. [Google Scholar] [CrossRef]
- Russo, R.; Palla, F. Plant Essential Oils as Biocides in Sustainable Strategies for the Conservation of Cultural Heritage. Sustainability 2023, 15, 8522. [Google Scholar] [CrossRef]
- Ahmed, E.A.-E.; Mohamed, R.M. Bacterial Deterioration in the Limestone Minaret of Prince Muhammad and Suggested Treatment Methods, Akhmim, Egypt. Geomaterials 2022, 12, 37–58. [Google Scholar] [CrossRef]
- Mateus, D.M.R.; Silva, R.B.; Costa, F.M.C.; Coroado, J.P.F. Microbiological Diversity in the Unfinished Sacristy Building of the Convent of Christ, Tomar, and Evaluation of Its Biocide-Based Control. Conserv. Património 2013, 17, 11–20. [Google Scholar] [CrossRef]
- Di Carlo, E.; Chisesi, R.; Barresi, G.; Barbaro, S.; Lombardo, G.; Rotolo, V.; Sebastianelli, M.; Travagliato, G.; Palla, F. Fungi and Bacteria in Indoor Cultural Heritage Environments: Microbial-Related Risks for Artworks and Human Health. Environ. Ecol. Res. 2016, 4, 257–264. [Google Scholar] [CrossRef]
- Resende, M.A. De Biodeterioração de Monumentos Históricos. In Microbiologia Ambiental; de Azevedo, J.L., de Melo, I.S., Eds.; Embrapa Meio Ambiente: Jaguariúna, SP, Brazil, 2008; pp. 501–520. [Google Scholar]
- Văcar, C.L.; Mircea, C.; Pârvu, M.; Podar, D. Diversity and Metabolic Activity of Fungi Causing Biodeterioration of Canvas Paintings. J. Fungi 2022, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Baptista Neto, J.A.; Gaylarde, C.; Beech, I.; Smith, B.J.; McAlister, J.J. Degradação de Gnaisse e Granito Em Fachadas de Edifícios Históricos No Centro Do Rio de Janeiro. Sistemas Gestão 2020, 15, 80–90. [Google Scholar] [CrossRef]
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.-D. Microbial Deterioration and Sustainable Conservation of Stone Monuments and Buildings. Nat. Sustain. 2020, 3, 991–1004. [Google Scholar] [CrossRef]
- Sterflinger, K.; Piñar, G. Microbial Deterioration of Cultural Heritage and Works of Art—Tilting at Windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef]
- Shirakawa, M.A.; John, V.M.; Cincotto, M.A. Biodeterioração No Ambiente Construído. In Microbiologia Ambiental; de Melo, I.S., de Azevedo, J.L., Eds.; Embrapa Meio Ambiente: Jaguariúna, SP, Brazil, 2008; pp. 478–499. [Google Scholar]
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration; Cambridge University Press: Cambridge, MA, USA, 2004; ISBN 9780521528870. [Google Scholar]
- Mateus, D.M.R.; Costa, F.M.C.; Triães, R.P. Essential Oils of Plants as Biocides Against Microorganisms Isolated from Portuguese Convent of Christ in Tomar; Springer International Publishing: Cham, Switzerland, 2023; pp. 129–139. [Google Scholar]
- Cappitelli, F.; Abbruscato, P.; Foladori, P.; Zanardini, E.; Ranalli, G.; Principi, P.; Villa, F.; Polo, A.; Sorlini, C. Detection and Elimination of Cyanobacteria from Frescoes: The Case of the St. Brizio Chapel (Orvieto Cathedral, Italy). Microb. Ecol. 2009, 57, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential Oils as Natural Biocides in Conservation of Cultural Heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Khan, A.A.; Jain, S.K.; Rai, M.K. Biodeterioration of Archaeological Monuments and Approach for Restoration. In Geomicrobiology; Jain, S.K., Khan, A.A., Rai, M.K., Eds.; CRC Press Taylor and Francis Group Science Publishers: Boca Raton, FL, USA, 2010; pp. 255–302. [Google Scholar]
- Liu, X.; Qian, Y.; Wu, F.; Wang, Y.; Wang, W.; Gu, J.-D. Biofilms on Stone Monuments: Biodeterioration or Bioprotection? Trends Microbiol. 2022, 30, 816–819. [Google Scholar] [CrossRef]
- Cappitelli, F.; Cattò, C.; Villa, F. The Control of Cultural Heritage Microbial Deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Pinna, D. Can We Do without Biocides to Cope with Biofilms and Lichens on Stone Heritage? Int. Biodeterior. Biodegrad. 2022, 172, 105437. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural Biocides for the Conservation of Stone Cultural Heritage: A Review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Mateus, D.M.R.; Ferraz, E.; Perna, V.; Sales, P.; Hipólito-Correia, V. Essential Oils and Extracts of Plants as Biocides against Microorganisms Isolated from the Ruins of the Roman City of Conímbriga (Portugal). Environ. Sci. Pollut. Res. 2024, 31, 40669–40677. [Google Scholar] [CrossRef]
- Reale, R.; Medeghini, L.; Botticelli, M. Stealing from Phytotherapy—Heritage Conservation with Essential Oils: A Review, from Remedy to Sustainable Restoration Product. Sustainability 2024, 16, 5110. [Google Scholar] [CrossRef]
- Casorri, L.; Masciarelli, E.; Ficociello, B.; Ietto, F.; Incoronato, F.; Di Luigi, M.; Pacioni, G. Natural Substances as Biocides in the Fungi Treatment on Artistic Products to Protect the Environment and Health of Restoration Workers. Ital. J. Mycol. 2023, 52, 89–111. [Google Scholar]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Zaccardelli, M.; Roscigno, G.; Pane, C.; Celano, G.; Di Matteo, M.; Mainente, M.; Vuotto, A.; Mencherini, T.; Esposito, T.; Vitti, A.; et al. Essential Oils and Quality Composts Sourced by Recycling Vegetable Residues from the Aromatic Plant Supply Chain. Ind. Crops Prod. 2021, 162, 113255. [Google Scholar] [CrossRef]
- Gomoiu, I.; Cojoc, R.L.; Enache, M.I.; Neagu, S.E.; Mohanu, D.; Mohanu, I. Microbial Ability to Colonize Mural Painting and Its Substrate. Acta Phys. Pol. A 2018, 134, 383–386. [Google Scholar] [CrossRef]
- Wu, F.; Gu, J.-D.; Li, J.; Feng, H.; Wang, W. Microbial Colonization and Protective Management of Wall Paintings. In Cultural Heritage Microbiology-Recent Developments; Mitchell, R., Clifford, J., Vasanthakumar, A., Eds.; Archetype Publications: London, UK, 2022; pp. 57–81. [Google Scholar]
- Trovão, J.; Portugal, A. Current Knowledge on the Fungal Degradation Abilities Profiled through Biodeteriorative Plate Essays. Appl. Sci. 2021, 11, 4196. [Google Scholar] [CrossRef]
- Isola, D.; Bartoli, F.; Casanova Municchia, A.; Lee, H.J.; Jeong, S.H.; Chung, Y.J.; Caneva, G. Green Biocides for the Conservation of Hypogeal Mural Paintings Raised from Western and Eastern Traditions: Evaluation of Interference on Pigments and Substrata and Multifactor Parameters Affecting Their Activity. J. Cult. Herit. 2023, 61, 116–126. [Google Scholar] [CrossRef]
- Marco, A.; Santos, S.; Caetano, J.; Pintado, M.; Vieira, E.; Moreira, P.R. Basil Essential Oil as an Alternative to Commercial Biocides against Fungi Associated with Black Stains in Mural Painting. Build. Environ. 2020, 167, 106459. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chung, Y.-J. Antifungal, Antibacterial, and Interference Effects of Plant-Extracted Essential Oils Used for Mural Conservation at Buyeo Royal Tomb No. 1. Appl. Sci. 2023, 13, 3645. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A Review of Indoor Microbial Growth across Building Materials and Sampling and Analysis Methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Baptista, C.; Santos, L.; Emília Amaral, M.; Silva, L. Chemical Characterization of Essential Oils With a Biocide Base for Conservation and Restoration. KnE Mater. Sci. 2022, 7, 80–90. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Stupar, M.; Grbić, M.L.; Džamić, A.; Unković, N.; Ristić, M.; Jelikić, A.; Vukojević, J. Antifungal Activity of Selected Essential Oils and Biocide Benzalkonium Chloride against the Fungi Isolated from Cultural Heritage Objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Paolino, B.; Sorrentino, M.C.; Troisi, J.; Delli Carri, M.; Kiselev, P.; Raimondo, R.; Lahoz, E.; Pacifico, S. Lavandula Angustifolia Mill. for a Suitable Non-Invasive Treatment against Fungal Colonization on Organic-Media Cultural Heritage. Herit. Sci. 2024, 12, 53. [Google Scholar] [CrossRef]
- Zerhouni, K.; Abbouni, B.; Kanoun, K.; Daouadji, K.L.; Tifrit, A.; Benahmed, M.; Chaouche, T.M. Isolation and Identification of Low Density Polythene-Degrading Bacteria from Soil of North West of Algeria. South Asian J. Exp. Biol. 2019, 8, 76–82. [Google Scholar] [CrossRef]
- Ünlü-Yokuş, Y.; Göksay-Kadaifçiler, D.; Ilhan-Sungur, E. Amylolytic and Proteolytic Bacteria in Deteriorated Paper-Based Historical Manuscripts. J. Sci. Ind. Res. 2024, 83, 1012–1021. [Google Scholar] [CrossRef]
- Ma, C.; Fang, Z.; Li, X.; Liu, X. Identification of Bacterial Communities Involved in Bioweathering Crusts on Limestone Sculptures of the Longmen Grottoes. Coatings 2023, 13, 1506. [Google Scholar] [CrossRef]
- Nigro, L.; Mura, F.; Toti, M.P.; Cirigliano, A.; Rinaldi, T. Carbonatogenic Bacteria on the ‘Motya Charioteer’ Sculpture. J. Cult. Herit. 2022, 57, 256–264. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Giovanelli, E.; Deans, S.G.; Eaglesham, E. Antibacterial and Antioxidant Properties of Mediterranean Aromatic Plants. Ind. Crops Prod. 1993, 2, 47–50. [Google Scholar] [CrossRef]
- Mota, A.S.; Martins, M.R.; Arantes, S.; Lopes, V.R.; Bettencourt, E.; Pombal, S.; Gomes, A.C.; Silva, L.A. Antimicrobial Activity and Chemical Composition of the Essential Oils of Portuguese Foeniculum Vulgare Fruits. Nat. Prod. Commun. 2015, 10. [Google Scholar] [CrossRef]
- Rodrigues, M.; Lopes, A.C.; Vaz, F.; Filipe, M.; Alves, G.; Ribeiro, M.P.; Coutinho, P.; Araujo, A.R.T.S. Thymus Mastichina: Composition and Biological Properties with a Focus on Antimicrobial Activity. Pharmaceuticals 2020, 13, 479. [Google Scholar] [CrossRef]
- Yasa, H.; Onar, H.Ç.; Yusufoglu, A.S. Chemical Composition of the Essential Oil of Mentha pulegium L. from Bodrum, Turkey. J. Essent. Oil Bear. Plants 2012, 15, 1040–1043. [Google Scholar] [CrossRef]
- Ouakouak, H.; Chohra, M.; Denane, M. Chemical Composition, Antioxidant Activities of the Essential Oil of Mentha pulegium L, South East of Algeria. Int. Lett. Nat. Sci. 2015, 39, 49–55. [Google Scholar] [CrossRef]
- Yamini, Y.; Sefidkon, F.; Pourmortazavi, S.M. Comparison of Essential Oil Composition of Iranian Fennel (Foeniculum vulgare) Obtained by Supercritical Carbon Dioxide Extraction and Hydrodistillation Methods. Flavour Fragr. J. 2002, 17, 345–348. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Gu, J.-D.; Feng, H.; Shah, K.; Wang, W. Controlling Biodeterioration of Cultural Heritage Objects with Biocides: A Review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).