Carbon Dioxide-Based Neutralization of High-Density Sludge Effluents as a Sustainable Climate and Water Quality Alternative to the Use of Strong Mineral Acids
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Choloque Subdrainage (Feeding) | Alcalinization (Lime Milk) | Flocculation (Floc. MT-6506) | Overflow | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Flow (m3/h) | pH | NTU | mL/min | pH | Kg/m3 | mL/min | g/m3 | pH | NTU |
| 3 | 5.78 | 61.2 | 130 | 10.4 | 0.434 | 50 | 0.99 | 10 | 14.2 |
| 4 | 5.1 | 59.9 | 150 | 10.6 | 0.376 | 57 | 0.84 | 10.25 | 17.64 |
| 3.5 | 4.17 | 74.7 | 200 | 10.6 | 0.573 | 57 | 0.96 | 10.17 | 11.3 |
| 4 | 3.8 | 70.2 | 190 | 10.55 | 0.476 | 57 | 0.84 | 10.09 | 13.33 |
| 4 | 3.71 | 98.7 | 142 | 10.48 | 0.356 | 55 | 0.81 | 10.2 | 16.9 |
| 5 | 3.48 | 111.5 | 200 | 10.45 | 0.401 | 57 | 0.68 | 10.41 | 11.47 |
| 4 | 3.65 | 90.4 | 200 | 10.87 | 0.501 | 57 | 0.84 | 10.4 | 17.72 |
| 4 | 3.62 | 90.2 | 200 | 10.75 | 0.501 | 57 | 0.84 | 10.72 | 17.00 |
Appendix B
| Parameter | V | Unit | MPL | ECA-3 | HLC-P1-02 | HLC-P1-04 | HLC-P1-05 | HLC-P1-07 |
|---|---|---|---|---|---|---|---|---|
| pH | - | 6–9 | 6.5–8.5 | 5.1 | 3.8 | 3.7 | 3.7 | |
| Alkalinity | mg/L CaCO3 | <1.0 | <1.0 | <1.0 | <1.0 | |||
| Bicarbonate | mg/L HCO3 | <1.2 | <1.2 | <1.2 | <1.2 | |||
| Carbonate | mg/L CO3 | <0.6 | <0.6 | <0.6 | <0.6 | |||
| TSS | mg/L | 25 | - | 50 | 63 | 46 | 52 | |
| Cr (VI) | 2 | mg/L | 0.08 | - | <0.005 | <0.005 | <0.005 | <0.005 |
| AG | mg/L | 16 | 5 | <0.4 | 0.4 | <0.4 | <0.4 | |
| Total CN | mg/L | 0.8 | - | <0.0008 | <0.0008 | <0.0008 | <0.0008 | |
| CN WAD | mg/L | - | 0.1 | <0.0008 | <0.0008 | <0.0008 | <0.0008 | |
| Total Al | 3 | mg/L | - | 5 | 1.93 | 1.831 | 0.747 | 1.307 |
| Total As | mg/L | 0.08 | 0.1 | 0.00301 | 0.02279 | 0.01936 | 0.02172 | |
| Total Cd | mg/L | 0.04 | 0.01 | 0.00024 | 0.00906 | 0.00083 | 0.00981 | |
| Total Cu | 2, 1 | mg/L | 0.4 | 0.2 | 0.02375 | 0.02859 | 0.02511 | 0.02393 |
| Total Hg | mg/L | 0.0016 | 0.001 | 0.00015 | 0.00032 | 0.00026 | 0.00039 | |
| Total Mn | 7, 4, 2 | mg/L | - | 0.2 | 37.496 | 38.388 | 37.762 | 32.411 |
| Total Pb | mg/L | 0.16 | 0.05 | 0.0064 | 0.0053 | 0.0041 | 0.0076 | |
| Total Zn | 2 | mg/L | 1.2 | 2 | 7.791 | 6.631 | 6.455 | 6.580 |
| Diss. Fe | mg/L | 1.6 | - | 28.670 | 21.057 | 23.516 | 32.946 |
Appendix C
| Parameter | V | Unit | MPL | ECA-3 | HLC-P2-02 | HLC-P2-04 | HLC-P2-05 | HLC-P2-07 |
|---|---|---|---|---|---|---|---|---|
| pH | - | 6 to 9 | 6.5–8.5 | 10 | 10.2 | 10.1 | 10.4 | |
| Alkalinity | mg/L CaCO3 | 16.1 | 7.3 | 14.6 | 9.8 | |||
| Bicarbonate | mg/L HCO3 | 19.7 | 8.9 | 17.8 | 12 | |||
| Carbonate | mg/L CO3 | 1.6 | 5 | 5 | 1.2 | |||
| TSS | mg/L | 25 | 518 | 6 | 22 | 17 | 17 | |
| Cr(VI) | 2 | mg/L | 0.08 | - | <0.005 | <0.005 | <0.005 | <0.005 |
| AG | mg/L | 16 | - | <0.4 | <0.4 | <0.4 | <0.4 | |
| CN total | mg/L | 0.8 | 5 | <0.0008 | <0.0008 | <0.0008 | <0.0008 | |
| CN WAD | mg/L | - | - | <0.0008 | <0.0008 | <0.0008 | <0.0008 | |
| Total Al | 3 | mg/L | - | 0.1 | 0.075 | 0.18 | 0.216 | 0.111 |
| Total As | mg/L | 0.08 | 5 | 0.00075 | 0.00215 | 0.00141 | 0.00301 | |
| Total Cd | mg/L | 0.04 | 0.1 | 0.00021 | 0.00046 | 0.00052 | 0.00071 | |
| Total Cu | 2, 1 | mg/L | 0.4 | 0.01 | 0.00377 | 0.00391 | 0.00403 | 0.00341 |
| Total Hg | mg/L | 0.0016 | 0.2 | 0.00019 | 0.00025 | 0.00032 | 0.00039 | |
| Total Mn | 7, 4, 2 | mg/L | - | 0.001 | 2.60498 | 1.55266 | 1.65068 | 0.91863 |
| Total Pb | mg/L | 0.16 | 0.2 | 0.0006 | 0.0006 | 0.0006 | 0.0012 | |
| Total Zn | 2 | mg/L | 1.2 | 0.05 | 0.0587 | 0.2205 | 0.2613 | 0.2156 |
| Diss. Fe | mg/L | 1.6 | 2 | 0.3036 | 0.8695 | 1.4928 | 1.0198 |
Appendix D
| Parameter | V | Unit | MPL | ECA-3 | HLC-P3-02 | HLC-P3-04 | HLC-P3-05 | HLC-P3-07 |
|---|---|---|---|---|---|---|---|---|
| pH | pH | 6 to 9 | 6.5–8.5 | 6.6 | 7.8 | 7.4 | 7.5 | |
| Alkalinity | mg/L CaCO3 | 26.6 | 18.8 | 21.9 | 34.0 | |||
| Bicarbonate | mg/L HCO3 | 32.4 | 22.9 | 26.7 | 41.5 | |||
| Carbonate | mg/L CO3 | <0.6 | <0.6 | <0.6 | <0.6 | |||
| TSS | mg/L | 25 | - | <3 | <3 | <3 | <3 | |
| Cr(VI) | 2 | mg/L | 0.08 | - | <0.005 | <0.005 | <0.005 | <0.005 |
| Oils and fats | Mg/L | 16 | 5 | <0.4 | <0.4 | <0.4 | <0.4 | |
| Total CN | mg/L | 0.8 | - | <0.0008 | <0.0008 | <0.0008 | <0.0008 | |
| CN WAD | mg/L | - | 0.1 | <0.0008 | <0.0008 | <0.0008 | <0.0008 | |
| Total Al | 3 | mg/L | - | 5 | 0.134 | 0.122 | 0.104 | 0.081 |
| Total As | mg/L | 0.08 | 0.1 | 0.00045 | 0.00339 | 0.00106 | 0.00105 | |
| Total Cd | mg/L | 0.04 | 0.01 | 0.00018 | 0.00034 | 0.00026 | 0.00049 | |
| Total Cu | 2, 1 | mg/L | 0.4 | 0.2 | 0.01583 | 0.00882 | 0.00508 | 0.01506 |
| Total Hg | mg/L | 0.0016 | 0.001 | 0.00024 | 0.0003 | 0.00032 | 0.00034 | |
| Total Mn | 7, 4, 2 | mg/L | - | 0.2 | 0.60192 | 0.57469 | 0.5936 | 0.16357 |
| Total Pb | mg/L | 0.16 | 0.05 | 0.0008 | 0.0006 | 0.0007 | 0.001 | |
| Total Zn | 2 | mg/L | 1.2 | 2 | 0.092 | 0.1034 | 0.0916 | 0.0802 |
| Diss. Fe | mg/L | 1.6 | - | 0.2881 | 0.3965 | 0.305 | 0.2612 | |
| Bicarbonates | mg/L | - | 518 | 24.1 | 22.9 | 26.7 | 41.5 | |
| Clorides | mg/L | - | 500 | 4.78 | 4.764 | 5.132 | 2.851 | |
| Conductivity | uS/cm | - | 2500 | 796 | 753 | 766 | 697 | |
| Fluorides | mg/L | - | 1 | 0.018 | 0.112 | 0.112 | 0.158 | |
| Nitrates | mg/L | - | 10 | <0.006 | <0.006 | <0.006 | <0.006 | |
| Nitrites + nitrates | mg/L | - | 100 | <0.0062 | <0.0062 | <0.0062 | 0.434 | |
| Sulfates | mg/L | - | 1000 | 893.31 | 819.13 | 537.5 | 702.24 | |
| Total Ba | mg/L | - | 0.7 | 0.0226 | 0.0249 | 0.0225 | 0.0163 | |
| Total Be | 2 | mg/L | - | 0.1 | 0.000019 | 0.00017 | 0.00013 | <0.00006 |
| Total B | 3 | mg/L | - | 1 | 0.091 | 0.059 | 0.055 | 0.066 |
| Total Li | 1 | mg/L | - | 2.5 | 0.0201 | 0.0222 | 0.0218 | 0.0208 |
| Total Mg | 2 | mg/L | - | 250 | 28.305 | 23.916 | 23.37 | 18.01 |
| Total Ni | 2 | mg/L | - | 0.2 | 0.0023 | 0.0038 | 0.0038 | 0.0013 |
| Total Se | mg/L | - | 0.02 | <0.0013 | 0.0021 | 0.0014 | 0.0025 | |
| Phosphates | mg/L | 1 | <0.038 | <0.038 | 0.601 | <0.038 |
Appendix E
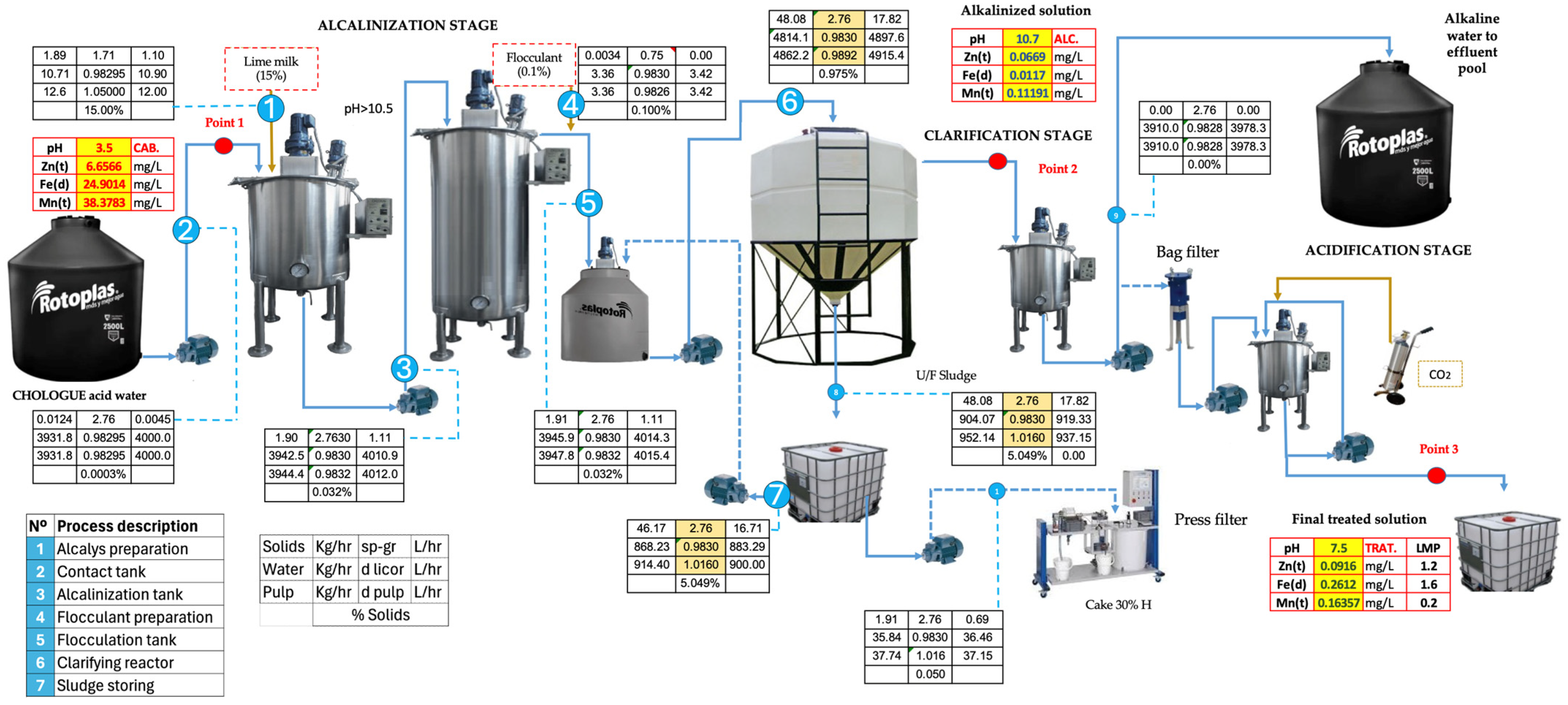
Appendix F
| Parameter | Method of Analysis |
|---|---|
| Alkalinity | SMEWW-APHA-AWWA-WEF Part 2320 B, 24th Ed., 2023. Alkalinity Titration Method. |
| pH | APERA PH850 portable pH, accuracy +/− 0.01 pH |
| Weight | A&D Weighing GXK model GX-12K, max 12 Kg, 0.1 g accuracy |
| TSS | SMEWW-APHA-AWWA-WEF Part 2540-D: 23rd: 2017. Solids: Total Suspended Solids dried at 103–105 °C. |
| Cr(VI) | SMEWW-APHA-AWWA-WEF Part 3500-Cr-B, 23rd Ed., 2017. Chromium. Colorimetric Method. |
| Oils and fats | ASTM D3921—96 (Reapproved 2011).Standard Test Method for Oil and Grease and Petroleum Hydrocarbons in Water. |
| Total CN | ASTM D7511-12 (Reapproved 2017) E01. Standard Test Method for Total Cyanide by Segmented Flow Injection Analysis, In-Line Ultraviolet Digestion and Amperometric Detection |
| CN WAD | EPA Method OIA-1677-09:2010, Avaliable Cyanide by Ligand Exchange and Flow Injection Analysis (FIA) |
| Total Metals | EPA-Method 200.8 Rev. 5.4, 1994. Determination of trace elements in water and wastes by Inductively Coupled Plasma-Mass spectrometry. 2015 |
| Clorides | EPA 300.0. Rev. 2.1:1993. Determination of Inorganic Anions by Ion Chromatography. |
| Fluorides | EPA 300.0. Rev. 2.1:1993. Determination of Inorganic Anions by Ion Chromatography. |
| Nitrates | EPA 300.0. Rev. 2.1:1993. Determination of Inorganic Anions by Ion Chromatography. |
| Nitrites + nitrates | EPA 300.0. Rev. 2.1:1993. Determination of Inorganic Anions by Ion Chromatography. |
| Sulfates | EPA 300.0. Rev. 2.1:1993. Determination of Inorganic Anions by Ion Chromatography. |
| Phosphates | EPA 300.0. Rev. 2.1:1993. Determination of Inorganic Anions by Ion Chromatography. |
References
- Equeenuddin, S.M.; Tripathy, S.; Sahoo, P.K.; Panigrahi, M.K. Hydrogeochemical characteristics of acid mine drainage and water pollution at Makum Coalfield, India. J. Geochem. Explor. 2010, 105, 75–82. [Google Scholar] [CrossRef]
- Córdoba, F.; Luís, A.T.; Leiva, M.; Sarmiento, A.M.; Santisteban, M.; Fortes, J.C.; Dávila, J.M.; Álvarez-Bajo, O.; Grande, J.A. Biogeochemical indicators (waters/diatoms) of acid mine drainage pollution in the Odiel river (Iberian Pyritic Belt, SW Spain). Environ. Sci. Pollut. Res. Int. 2022, 29, 31749–31760. [Google Scholar] [CrossRef] [PubMed]
- Indra, T.L.; Amalia, R.P.; Damayanti, A. GIS and RS-Based Analysis of Water Pollution Potential Caused by Acid Mine Drainage in Samarinda, Indonesia. J. Settl. Spat. Plan. 2021, 9, 5–13. [Google Scholar] [CrossRef]
- Singovszka, E.; Balintova, M.; Demcak, S.; Pavlikova, P. Metal Pollution Indices of Bottom Sediment and Surface Water Affected by Acid Mine Drainage. Metals 2017, 7, 284. [Google Scholar] [CrossRef]
- Candeias, C.; Avila, P.F.; Ferreira da Silva, E.; Ferreira, A.; Salgueiro, A.R.; Teixeira, J.P. Acid mine drainage from the Panasqueira Mine and its influence on Zezere River (central Portugal). J. Afr. Earth Sci. 2014, 99, 705–712. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Bonotto, D.M. Hydrogeochemical features of surface water and groundwater contaminated with acid mine drainage (AMD) in coal mining areas: A case study in southern Brazil. Environ. Sci. Pollut. Res. Int. 2016, 23, 18911–18927. [Google Scholar] [CrossRef]
- Herbst, D.B.; Medhurst, R.B.; Black, N.J.P. Long-term effects and recovery of streams from acid mine drainage and evaluation of toxic metal threshold ranges for macroinvertebrate community reassembly. Environ. Toxicol. Chem. 2018, 37, 2575–2592. [Google Scholar] [CrossRef]
- Grande, J.A.; Loayza-Muro, R.; Alonso-Chaves, F.M.; Fortes, J.C.; Willems, B.; Sarmiento, A.M.; Santisteban, M.; Dávila, J.M.; de la Torre, M.L.; Durães, N.; et al. The Negro River (Ancash-Peru): A unique case of water pollution, three environmental scenarios and an unresolved issue. Sci. Total Environ. 2019, 648, 398–407. [Google Scholar] [CrossRef]
- Sevink, J.; Verstraten, J.M.; Kooijman, A.M.; Loayza-Muro, R.A.; Hoitinga, L.; Palomino, E.J.; Jansen, B. Rare Moss-Built Microterraces in a High-Altitude, Acid Mine Drainage-Polluted Stream (Cordillera Negra, Peru). Water Air Soil Pollut. 2015, 226, 201–220. [Google Scholar] [CrossRef][Green Version]
- Vriens, B.; Peterson, H.; Laurenzi, L.; Smith, L.; Aranda, C.; Mayer, K.U.; Beckie, R.D. Long-term monitoring of waste-rock weathering at the Antamina mine, Peru. Chemosphere 2019, 215, 858–869. [Google Scholar] [CrossRef]
- Mosai, A.K.; Ndlovu, G.; Tutu, H. Improving acid mine drainage treatment by combining treatment technologies: A review. Sci. Total Environ. 2024, 919, 170806. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, H.; Xu, Y.; Zhong, F.; Dong, H.; Wang, M. Enhanced Microbial Oxidation-Neutralization Treatment of Acid Mine Drainage Rich in Ferrous Ions (Fe2+). Int. J. Environ. Res. Public Health 2022, 19, 6543. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Naidu, G.; Hasan Johir, M.A.; Choi, Y.; Jeong, S.; Vigneswaran, S. Acid mine drainage treatment by integrated submerged membrane distillation–sorption system. Chemosphere 2019, 218, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Pat-Espadas, A.; Loredo Portales, R.; Amabilis-Sosa, L.; Gómez, G.; Vidal, G. Review of Constructed Wetlands for Acid Mine Drainage Treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Cost of Remediation at Mine Sites. Available online: https://archive.epa.gov/epawaste/hazard/web/pdf/costs.pdf (accessed on 6 October 2024).
- Black, K.J.; Weber, J.G. Treating abandoned mine drainage can protect streams cost effectively and benefit vulnerable communities. Commun. Earth Environ. 2024, 5, 508. [Google Scholar] [CrossRef]
- Seo, E.Y.; Cheong, Y.W.; Yim, G.J.; Min, K.W.; Geroni, J.N. Recovery of Fe, Al and Mn in acid coal mine drainage by sequential selective precipitation with control of pH. Catena 2017, 148, 11–16. [Google Scholar] [CrossRef]
- Abd Al-Hussein, A.S. Using sulfuric acid (H2SO4) for pH adjustment in water treatment. J. Pet. Res. Stud. 2021, 11, 19–27. [Google Scholar] [CrossRef]
- Perutz, M.F.; Shih, D.T.; Williamson, D. The Chloride Effect in Human Haemoglobin: A New Kind of Allosteric Mechanism. J. Mol. Biol. 1994, 239, 555–560. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Nayak, D.K.; Kumar, R.; Kumar, V. Corrosion of reinforced steel in concrete and its control: An overview. J. Steel Struct. Constr. 2017, 2, 124. [Google Scholar] [CrossRef]
- Rahmania, H.; Asbaghb, Y.I. Effects of sulphate ions on the corrosion resistance of Montmorillonite-modified dense concretes. Aust. J. Struct. Eng. 2018, 19, 248–255. [Google Scholar] [CrossRef]
- Lomba, L.; Errazquin, D.; Garralaga, P.; López, N.; Giner, B. Ecotoxicological study of glucose:choline chloride and sorbitol:choline chloride at different contents of water. Environ. Sci. Pollut. Res. Int. 2023, 30, 46427–46434. [Google Scholar] [CrossRef] [PubMed]
- Mukwevho, M.; Chirwa, E.; Maharajh, D. The effect of pH and temperature on biological sulphate reduction. Chem. Eng. Trans. 2019, 74, 517–522. [Google Scholar]
- Kartic, D.N.; Narayana, B.C.h.A.; Arivazhagan, M. Removal of high concentration of sulfate from pigment industry effluent by chemical precipitation using barium chloride: RSM and ANN modeling approach. J. Environ. Manag. 2018, 206, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Oztemur, G.; Basaran, S.T.; Tayran, Z.; Sahinkaya, E. Fluidized bed membrame bioreactor achieves high sulphate reduction and filtration performances at moderate temperatures. Chemosphere 2020, 252, 126587. [Google Scholar] [CrossRef] [PubMed]
- van Rooyen, M.; van Staden, P.J.; du Preez, K.A. Sulphate removal technologies for the treatment of mine-impacted water. J. S. Afr. Inst. Min. Metall. 2021, 1221, 523–529. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Olson, A.R.; Youle, P.V. The Strength of Carbonic Acid. The Rate. of Reaction of Carbon Dioxide with Water and Hydroxyl Ion. J. Am. Chem. Soc. 1940, 62, 1027. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Silva, A.M.T.; Fonseca, N.N.C.; Vaz, J.R.; Figueiredo, J.L.; Faria, J.L. Photocatalytic Reduction of CO2 with Water into Methanol and Ethanol Using Graphene Derivative–TiO2 Composites: Effect of pH and Copper(I) Oxide. Top. Catal. 2016, 59, 1279–1291. [Google Scholar] [CrossRef]
- Yang, B.; Bai, Z.; Zhang, J. Environmental impact of mining-associated carbon emissions and analysis of cleaner production strategies in China. Environ. Sci. Pollut. Res. 2021, 28, 13649–13659. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Rezaee, M.; Pisupati, S.V. Precipitation of rare earth elements from acid mine drainage by CO2 mineralization process. Chem. Eng. J. 2020, 399, 125716. [Google Scholar] [CrossRef]
- Masindi, V.; Madzivire, G.; Tekere, M. Reclamation of water and the synthesis of gypsum and limestone from acid mine drainage treatment process using a combination of pre-treated magnesite nanosheets, lime, and CO2 bubbling. Water Resour. Ind. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Lee, H.C.; Min, K.W.; Seo, E.Y. A feasibility study on CO2 sequestration using the neutralization process of acid mine drainage. Geosystem Eng. 2016, 19, 293–301. [Google Scholar] [CrossRef]
- Praxair. Case Study: Cost and Maintenance Reduction with CO2 to Replace Sulfuric Acid for pH Control at Steel Dynamics, Inc. (Butler, IN). 3 p. 2015. Available online: https://www.tpomag.com/uploads/downloads/P-40-4099_CO²_replaces_Sulfuric_for_pH_control_at_SDI_180119_134148.pdf (accessed on 3 September 2024).
- Veolia. WSTP South End Plant Process Selection Report. Appendix H: CO2 Emission Factors Database. Winnipeg. Available online: https://legacy.winnipeg.ca/finance/findata/matmgt/documents/2012/682-2012/682-2012_appendix_h-wstp_south_end_plant_process_selection_report/appendix%207.pdf (accessed on 6 October 2024).
- Global Solar Atlas. Global Photovoltaic Power Potential. Country Factsheet Peru. Available online: https://globalsolaratlas.info/global-pv-potential-study (accessed on 8 October 2024).
- International Renewable Energy Agency. Energy Profile Peru. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Statistics/Statistical_Profiles/South%20America/Peru_South%20America_RE_SP.pdf (accessed on 8 October 2024).
- Smith, B.L.; Sekar, A.; Mirletz, H.; Heath, G.; Margolis, R. An Updated Lifecycle Assessment of Utility-Scale Solar Photovoltaic Systems Installed in the United States. National Renewable Energy Laboratory. Available online: https://www.nrel.gov/docs/fy24osti/87372.pdf (accessed on 8 October 2024).
- Business Analitiq. All Comodities Index. 2024. Available online: https://businessanalytiq.com/procurementanalytics/index/all-commodities-index/ (accessed on 3 September 2024).
- Environmental Protection Agency. Carbon Dioxide Supply Chain. Executive Summary. Available online: https://www.epa.gov/system/files/documents/2023-03/Carbon%20Dioxide%20Supply%20Chain%20Profile.pdf (accessed on 7 October 2024).
- Friedman, J.; Fan, Z.; Byrum, Z.; Ochu, E.; Bhardwaj, A.; Sheerazi, H. Levelized Cost of Carbon Abatement: An Improved Cost-Assessment Methodology for a Net-Zero Emission World. 2020. Available online: https://www.energypolicy.columbia.edu/publications/levelized-cost-carbon-abatement-improved-cost-assessment-methodology-net-zero-emissions-world/ (accessed on 7 October 2024).
- Chen, S.; Lu, X.; Nielsen, C.P.; McElroy, M.B.; He, G.; Zhang, S.; He, K.; Yang, X.; Zhang, F.; Hao, J. Deploying solar photovoltaic energy first in carbon-intensive regions brings gigatons more carbon mitigations to 2060. Commun. Earth Environ. 2023, 4, 369. [Google Scholar] [CrossRef]
- IPCC 5th Assessment Report. Annex III. Technology Specific Cost and Performance Parameters (PDF). Available online: https://archive.ipcc.ch/pdf/assessment-report/ar5/wg3/drafts/fgd/ipcc_wg3_ar5_final-draft_fgd_annex-iii.pdf (accessed on 8 October 2024).
- Unger-Lindig, Y.; Merkel, B.; Schipek, M. Carbon dioxide treatment of low-density sludge: A new remediation strategy for acidic mining lakes? Environ. Earth Sci. 2010, 60, 1711–1722. [Google Scholar] [CrossRef]
- de Vries, A.; Goloviznina, K.; Reiter, M.; Salanne, M.; Lukatskaya, M.R. Solvation-Tuned Photoacid as a Stable Light-Driven pH Switch for CO2 Capture and Release. Chem. Mater. 2024, 36, 1308–1317. [Google Scholar] [CrossRef]
- Campbell, K.M.; Alpers, C.N.; Nordstrom, D.K. Formation and prevention of pipe scale from acid mine drainage at Iron Mountain and Leviathan Mines, California, USA. Appl. Geochem. 2020, 115, 104521. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). CCS in the Mining Sector: A Pathway to Decarbonization. 2021. Available online: https://www.iea.org (accessed on 12 November 2024).
- Global CCS Institute. Global Status of CCS 2021. 2021. Available online: https://www.globalccsinstitute.com (accessed on 12 November 2024).
- Carbon Pricing Leadership Coalition (CPLC). The Role of Carbon Pricing in Achieving Net Zero Emissions. 2020. Available online: https://www.carbonpricingleadership.org (accessed on 12 November 2024).
- U.S. Department of Energy. 45Q Tax Credit for Carbon Capture. 2021. Available online: https://www.energy.gov (accessed on 12 November 2024).
- Department of Energy. Carbon Utilization Procurement Grants. 2024. Available online: https://www.energy.gov/fecm/funding-notice-bipartisan-infrastructure-law-carbon-utilization-procurement-grants#:~:text=The%20Carbon%20Utilization%20Procurement%20Grants,dioxide%20and%20carbon%20monoxide%20emissions (accessed on 12 November 2024).
- European Commission. The Innovation Fund. 2020. Available online: https://ec.europa.eu (accessed on 12 November 2024).
- Institute for Energy, Economics, and Financial Analysis. Carbon Capture Is About Reputation, Not Economics. 2020. Available online: https://ieefa.org/wp-content/uploads/2020/07/CCS-Is-About-Reputation-Not-Economics_July-2020.pdf (accessed on 12 November 2024).
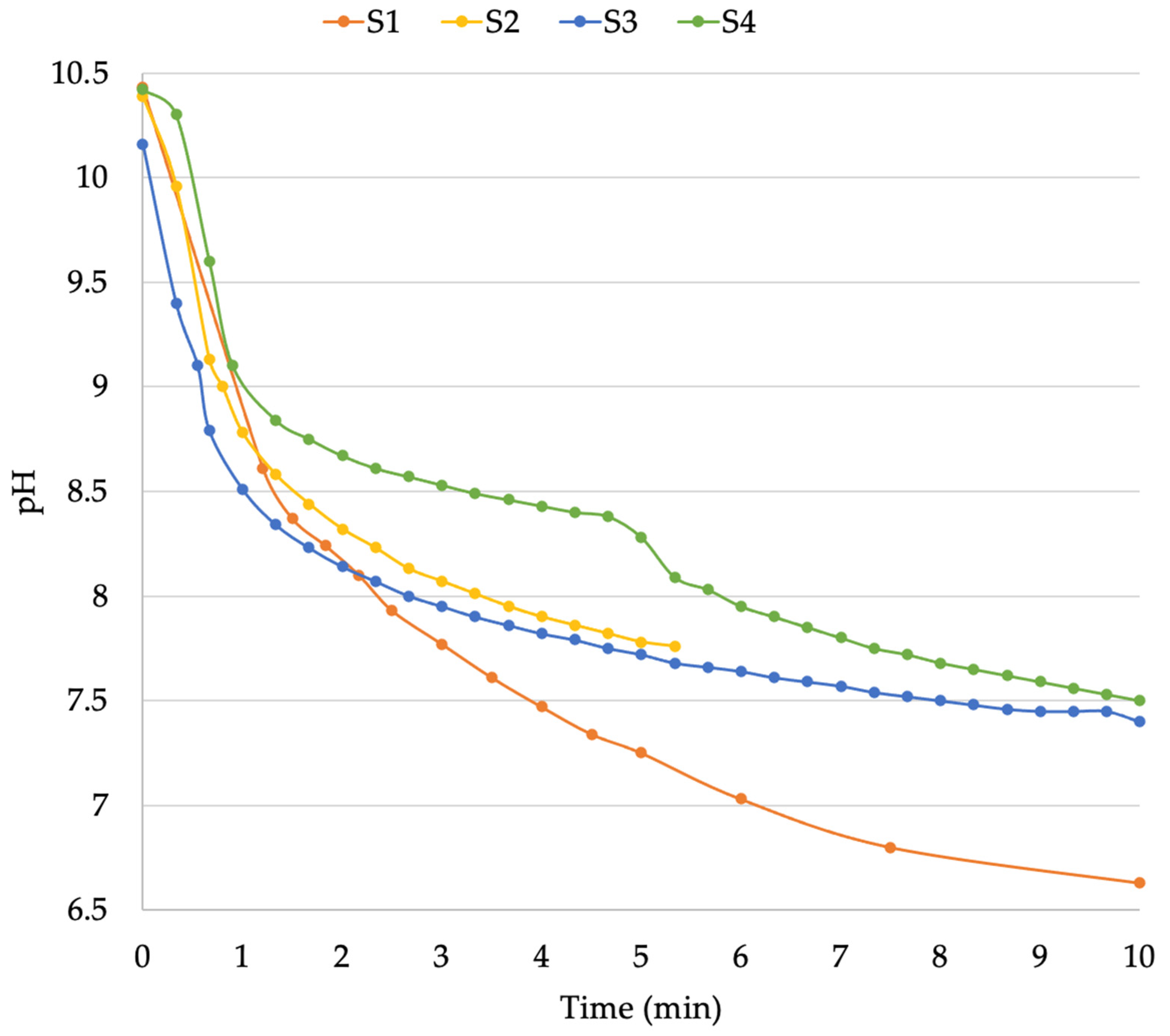
| Sample ID | Solution Weight (kg) | Initial pH | CO2 Flow (L/min) | Reaction Time (min.) | Initial CO2 Tank Weight (kg) | Final CO2 Tank Weight (kg) | CO2 Consumption (g/m3) | Residence Time (min.) | Final pH | pH Drop |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 13.82 | 10.43 | 0.5 | 1.2 | 8.632 | 8.63 | 144.7 | 10 | 6.63 | 3.80 |
| S2 | 16.11 | 10.39 | 0.5 | 0.8 | 8.612 | 8.61 | 124.18 | 5.3 | 7.76 | 2.63 |
| S3 | 15.08 | 10.16 | 0.5 | 0.55 | 8.608 | 8.606 | 132.59 | 10 | 7.40 | 2.76 |
| S4 | 17.05 | 10.42 | 0.5 | 0.9 | 8.612 | 8.61 | 117.32 | 10 | 7.50 | 2.92 |
| Sample ID | CO2 Consumption (g/m3) | CO2 Cost (USD/m3) | HCl Equivalent (g/m3) | HCl Cost (USD/m3) | H2SO4 Equivalent (g/m3) | H2SO4 Cost (USD/m3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave. | Min. | Max. | Ave. | Min. | Max. | Ave. | Min. | Max. | ||||
| S1 | 144.7 | 0.03 | 0.03 | 0.04 | 102.9 | 0.02 | 0.01 | 0.03 | 138.3 | 0.02 | 0.02 | 0.04 |
| S2 | 124.2 | 0.03 | 0.03 | 0.03 | 88.3 | 0.02 | 0.01 | 0.02 | 118.7 | 0.02 | 0.01 | 0.04 |
| S3 | 132.6 | 0.03 | 0.03 | 0.03 | 94.3 | 0.02 | 0.01 | 0.03 | 126.7 | 0.02 | 0.01 | 0.04 |
| S4 | 117.3 | 0.03 | 0.03 | 0.03 | 83.5 | 0.02 | 0.01 | 0.02 | 112.2 | 0.02 | 0.01 | 0.03 |
| Sample ID | CO2 Consumption (g/m3) | CO2 300 m3/h (Ton/Year) | Average CO2 Cost (USD/m3) | Average CO2 Cost with USD 100/Ton CO2 Credit (USD/m3) | Cost 300 m3/h (USD/Year) | Cost 300 m3/h with USD 100/Ton CO2 Credit (USD/Year) | HCl Equivalent (g/m3) | Average HCl Cost (USD/m3) | Cost 300 m3/h (USD/Year) | H2SO4 Equivalent (g/m3) | Average H2SO4 Cost (USD/m3) | Cost 300 m3/h (USD/Year) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 144.7 | 380.3 | 0.034 | 0.020 | 89,744 | 51,717 | 102.926 | 0.019 | 48,688 | 138.3 | 0.022 | 58,527 |
| S2 | 124.2 | 326.3 | 0.029 | 0.017 | 77,017 | 44,383 | 88.330 | 0.016 | 41,783 | 118.7 | 0.019 | 50,227 |
| S3 | 132.6 | 348.4 | 0.031 | 0.018 | 82,233 | 47,389 | 94.312 | 0.017 | 44,613 | 126.7 | 0.020 | 53,629 |
| S4 | 117.3 | 308.3 | 0.028 | 0.016 | 72,763 | 41,931 | 83.450 | 0.015 | 39,475 | 112.2 | 0.018 | 47,452 |
| Average | 129.7 | 340.8 | 0.031 | 0.018 | 80,439 | 46,355 | 92.254 | 0.017 | 43,640 | 124.0 | 0.020 | 52,459 |
| HCl Avoided Emissions (g CO2/m3) | Avoided CO2 300 m3/h (Ton/Year) | Avoided + Sequestered CO2 (g CO2/m3) | Additional Cost of Using CO2 Over HCl (USD/m3) | Cost Avoided CO2 HCl (USD/Ton CO2) | H2SO4 Avoided CO2 Emissions (gCO2/m3) | Avoided CO2 300 m3/h (Ton/Year) | Avoided + Sequestered CO2 (g CO2/m3) | Additional Cost of Using CO2 Over H2SO4 (USD/m3) | Cost Avoided CO2 H2SO4 (USD/Ton CO2) |
|---|---|---|---|---|---|---|---|---|---|
| −24 | −64 | 105 | 0.014 | 133 | −89 | −234 | 41 | 0.011 | 262 |
| Minimum PV Cost (USD/kWh) | Maximum PV Cost (USD/kWh) | Emission Factor PV (Ton CO2/kWh) | Emission Factor Electricity Grid (Ton CO2/kWh) | Minimum Cost Avoided CO2 PV (USD/Ton CO2) | Maximum Cost Avoided CO2 PV (USD/Ton CO2) |
|---|---|---|---|---|---|
| 0.07 | 0.13 | 23 | 213 | 368 | 684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastañadui-Cruz, J.C.; Reyes-Lopez, I.A.; Cortijo-Garcia, A.; Portilla-Rodriguez, H.R.; Bush, J.A.; Vanneste, J.; Garcia-Chevesich, P.A. Carbon Dioxide-Based Neutralization of High-Density Sludge Effluents as a Sustainable Climate and Water Quality Alternative to the Use of Strong Mineral Acids. Sustainability 2024, 16, 10363. https://doi.org/10.3390/su162310363
Gastañadui-Cruz JC, Reyes-Lopez IA, Cortijo-Garcia A, Portilla-Rodriguez HR, Bush JA, Vanneste J, Garcia-Chevesich PA. Carbon Dioxide-Based Neutralization of High-Density Sludge Effluents as a Sustainable Climate and Water Quality Alternative to the Use of Strong Mineral Acids. Sustainability. 2024; 16(23):10363. https://doi.org/10.3390/su162310363
Chicago/Turabian StyleGastañadui-Cruz, Julio C., Iván A. Reyes-Lopez, Agusberto Cortijo-Garcia, Hans R. Portilla-Rodriguez, John A. Bush, Johan Vanneste, and Pablo A. Garcia-Chevesich. 2024. "Carbon Dioxide-Based Neutralization of High-Density Sludge Effluents as a Sustainable Climate and Water Quality Alternative to the Use of Strong Mineral Acids" Sustainability 16, no. 23: 10363. https://doi.org/10.3390/su162310363
APA StyleGastañadui-Cruz, J. C., Reyes-Lopez, I. A., Cortijo-Garcia, A., Portilla-Rodriguez, H. R., Bush, J. A., Vanneste, J., & Garcia-Chevesich, P. A. (2024). Carbon Dioxide-Based Neutralization of High-Density Sludge Effluents as a Sustainable Climate and Water Quality Alternative to the Use of Strong Mineral Acids. Sustainability, 16(23), 10363. https://doi.org/10.3390/su162310363









