Comparative Study of Ammonium and Orthophosphate Removal Efficiency with Natural and Modified Clay-Based Materials, for Sustainable Management of Eutrophic Water Bodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Materials
2.2. Preparation of the Modified Materials
2.3. Kinetic Adsorption
2.4. Adsorption Isotherms
2.5. Experimental Procedures
3. Results
3.1. Ammonium Ion Uptake by Natural Materials in Seawater and Freshwater
3.2. Orthophosphate Ion Uptake by Natural Materials in Seawater and Freshwater
3.3. Ammonium Ion Uptake by Modified Materials in Seawater and Freshwater
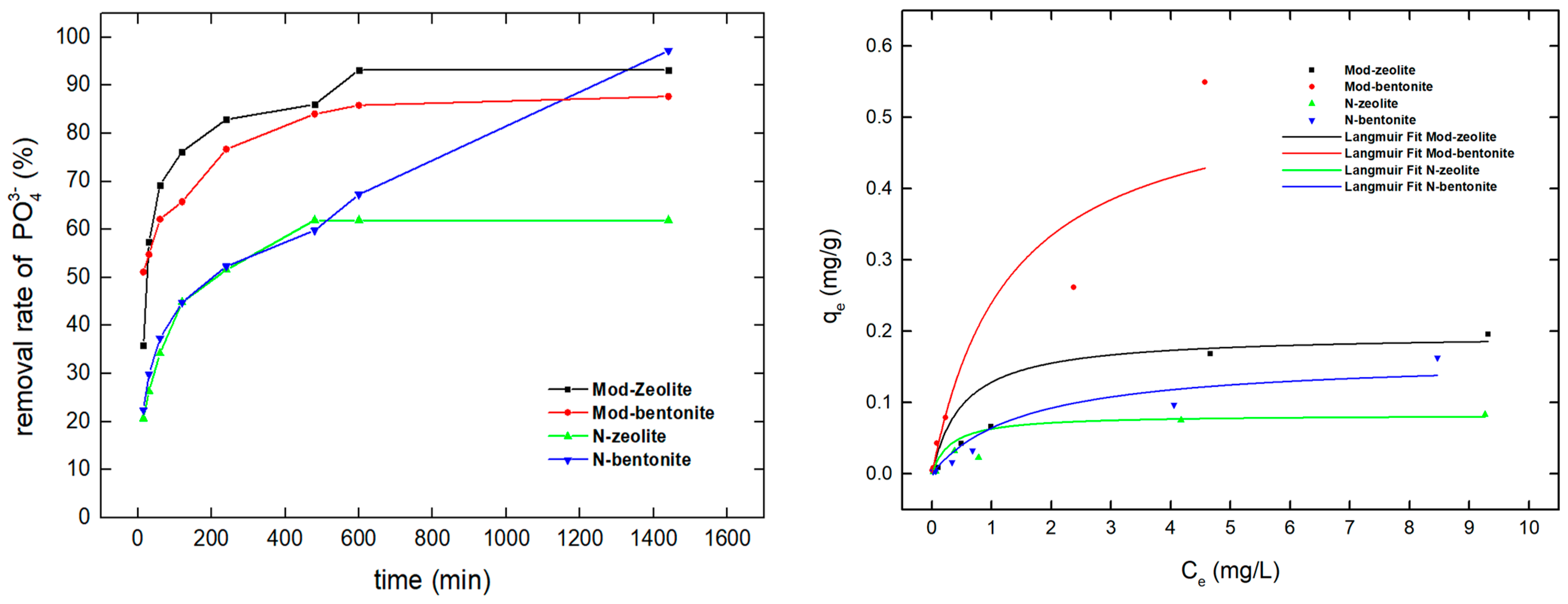
3.4. Orthophosphate Ion Uptake by Modified Materials in Seawater and Freshwater
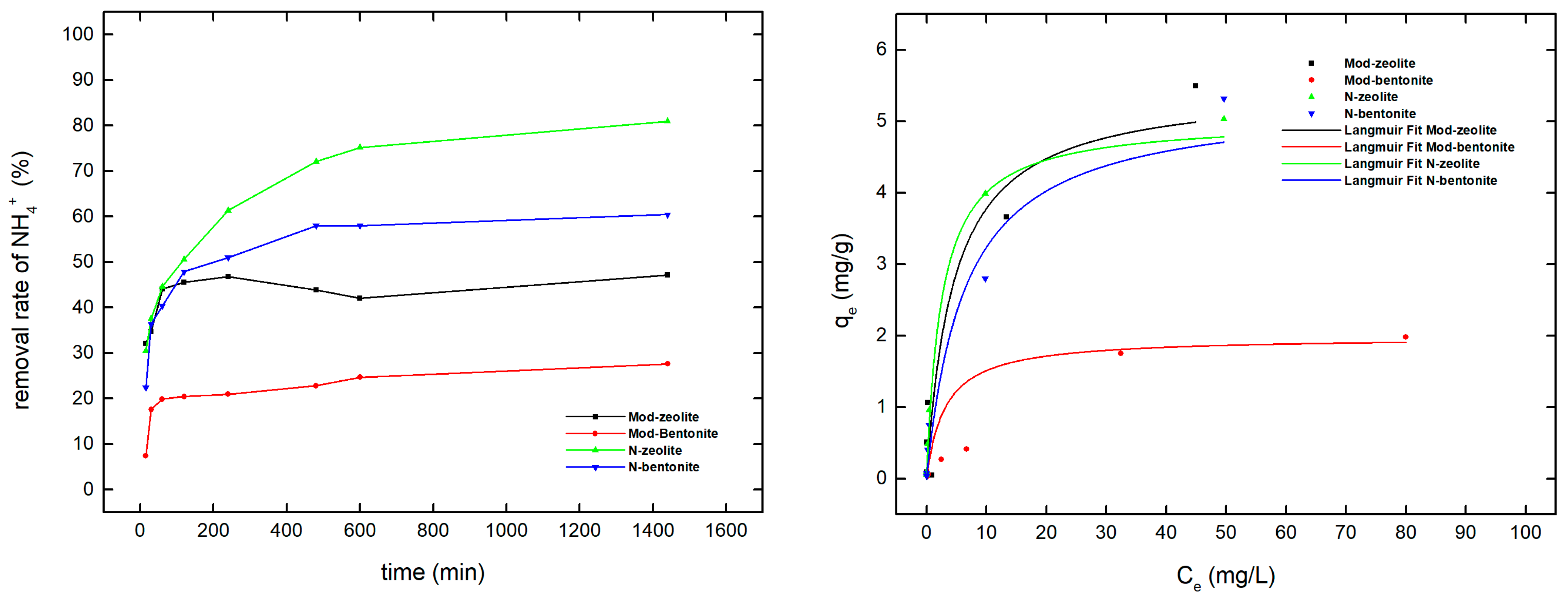
3.5. Ammonium Isotherm Results
3.6. Orthophosphate Isotherm Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dotto, G.L.; McKay, G. Current Scenario and Challenges in Adsorption for Water Treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Gupta, G.; Khan, J.; Singh, N.K. Application and Efficacy of Low-Cost Adsorbents for Metal Removal from Contaminated Water: A Review. Mater. Today Proc. 2021, 43, 2958–2964. [Google Scholar] [CrossRef]
- Duan, P.; Ding, S.; Jiao, L.; Wang, M.; Zhang, Y.; Qian, C. Simultaneous Immobilization of Ammonia and Phosphorous by Thermally Treated Sediment Co-Modified with Hydrophilic Organic Matter and Zeolite. J. Environ. Manag. 2023, 339, 117800. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Cadar, O. Modification of Natural Zeolites and Their Applications for Heavy Metal Removal from Polluted Environments: Challenges, Recent Advances, and Perspectives. Heliyon 2024, 10, e25303. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, L.; Shi, M.; Gu, C.; Zhang, J.; Lv, J.; Xuan, L. Ca/Fe-Layered Double Hydroxide–Zeolite Composites for the Control of Phosphorus Pollution in Sediments: Performance, Mechanisms, and Microbial Community Response. Chem. Eng. J. 2022, 450, 138277. [Google Scholar] [CrossRef]
- Han, M.; Wang, Y.; Zhan, Y.; Lin, J.; Bai, X.; Zhang, Z. Efficiency and Mechanism for the Control of Phosphorus Release from Sediment by the Combined Use of Hydrous Ferric Oxide, Calcite and Zeolite as a Geo-Engineering Tool. Chem. Eng. J. 2022, 428, 131360. [Google Scholar] [CrossRef]
- Kang, L.; Haasler, S.; Mucci, M.; Korving, L.; Dugulan, A.I.; Prot, T.; Waajen, G.; Lürling, M. Comparison of Dredging, Lanthanum-Modified Bentonite, Aluminium-Modified Zeolite, and FeCl2 in Controlling Internal Nutrient Loading. Water Res. 2023, 244, 120391. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Chen, Y.; Li, Y.; Zhang, R.; Tang, C. Xi Hu Cd(II) and Pb(II) Absorbed on Humic Acid-Iron-Pillared Bentonite: Kinetics, Thermodynamics and Mechanism of Adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 612. [Google Scholar] [CrossRef]
- Zhan, Q.; Teurlincx, S.; van Herpen, F.; Raman, N.V.; Lürling, M.; Waajen, G.; de Senerpont Domis, L.N. Towards Climate-Robust Water Quality Management: Testing the Efficacy of Different Eutrophication Control Measures during a Heatwave in an Urban Canal. Sci. Total Environ. 2022, 828, 154421. [Google Scholar] [CrossRef]
- Han, Y.; Jeppesen, E.; Lürling, M.; Zhang, Y.; Ma, T.; Li, W.; Chen, K.; Li, K. Combining Lanthanum-Modified Bentonite (LMB) and Submerged Macrophytes Alleviates Water Quality Deterioration in the Presence of Omni-Benthivorous Fish. J. Environ. Manag. 2022, 314, 115036. [Google Scholar] [CrossRef]
- Baie, M.S.; Esfandian, H.; Nesheli, A.A. Removal of Nitrate from Aqueous Solutions in Batch Systems Using Activated Perlite: An Application of Response Surface Methodology. Asia-Pac. J. Chem. Eng. 2016, 11, 437–447. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M. Adsorption Characteristics of Cu(II) and Pb(II) onto Expanded Perlite from Aqueous Solution. J. Hazard. Mater. 2007, 148, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Fan, C.; Meng, J.; Yang, Y. The Comparative Study of a Nano-Fe0-Loaded and a Nano-Fe/Ce-Loaded Expanded-Perlite on Removal of Phosphate from Water. J. Water Process Eng. 2023, 56, 104336. [Google Scholar] [CrossRef]
- Spears, B.M.; Lürling, M.; Yasseri, S.; Castro-Castellon, A.T.; Gibbs, M.; Meis, S.; McDonald, C.; McIntosh, J.; Sleep, D.; Van Oosterhout, F. Lake Responses Following Lanthanum-Modified Bentonite Clay (Phoslock®) Application: An Analysis of Water Column Lanthanum Data from 16 Case Study Lakes. Water Res. 2013, 47, 5930–5942. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhang, K.; Shen, J.; Liu, E. Integrating Long-Term Dynamics of Ecosystem Services into Restoration and Management of Large Shallow Lakes. Sci. Total Environ. 2019, 671, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Zamparas, M.; Drosos, M.; Deligiannakis, Y.; Zacharias, I. Eutrophication Control Using a Novel Bentonite Humic-Acid Composite Material BephosTM. J. Environ. Chem. Eng. 2015, 3, 3030–3036. [Google Scholar] [CrossRef]
- Trujillo-Reyes, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Supported and Unsupported Nanomaterials for Water and Soil Remediation: Are They a Useful Solution for Worldwide Pollution? J. Hazard. Mater. 2014, 280, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Y. Natural Zeolites as Effective Adsorbents in Water and Wastewater Treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- De Velasco-Maldonado, P.S.; Hernández-Montoya, V.; Montes-Morán, M.A.; Vázquez, N.A.R.; Pérez-Cruz, M.A. Surface Modification of a Natural Zeolite by Treatment with Cold Oxygen Plasma: Characterization and Application in Water Treatment. Appl. Surf. Sci. 2018, 434, 1193–1199. [Google Scholar] [CrossRef]
- Dizadji, N.; Dehpouri, S.; Vossoughi, S.S.S. Experimental Investigation of Adsorption of Heavy Metals (Copper (II)) from Industrial Wastewater with Clinoptilolite. Chem. Eng. Trans. 2012, 29, 1309–1314. [Google Scholar] [CrossRef]
- Wan, C.; Ding, S.; Zhang, C.; Tan, X.; Zou, W.; Liu, X.; Yang, X. Simultaneous Recovery of Nitrogen and Phosphorus from Sludge Fermentation Liquid by Zeolite Adsorption: Mechanism and Application. Sep. Purif. Technol. 2017, 180, 1–12. [Google Scholar] [CrossRef]
- Roshanfekr Rad, L.; Anbia, M. Zeolite-Based Composites for the Adsorption of Toxic Matters from Water: A Review. J. Environ. Chem. Eng. 2021, 9, 106088. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.; Pantazidis, A.; Oikonomou, G.; Baziotis, I. Phosphate Recovery from Real Fresh Urine by Ca(OH)2 Treated Natural Zeolite. Chem. Eng. J. 2018, 347, 618–630. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.; Liu, T.; Zhang, N.; Wang, M.; Yang, S.; Wang, W.; Jin, P. Evaluation of the Adsorption of Ammonium-Nitrogen and Phosphate on a Granular Composite Adsorbent Derived from Zeolite. Environ. Sci. Pollut. Res. 2019, 26, 17632–17643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lyu, T.; Liu, L.; Hu, Z.; Chen, J.; Su, B.; Yu, J.; Pan, G. Exploring a Multifunctional Geoengineering Material for Eutrophication Remediation: Simultaneously Control Internal Nutrient Load and Tackle Hypoxia. Chem. Eng. J. 2021, 406, 127206. [Google Scholar] [CrossRef]
- Kasprzyk, M.; Gajewska, M. Phosphorus Removal by Application of Natural and Semi-Natural Materials for Possible Recovery According to Assumptions of Circular Economy and Closed Circuit of P. Sci. Total Environ. 2019, 650, 249–256. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, T.A. Clay-Hydrogel Nanocomposites for Adsorptive Amputation of Environmental Contaminants from Aqueous Phase: A Review. J. Environ. Chem. Eng. 2021, 9, 105575. [Google Scholar] [CrossRef]
- Perassi, I.; Borgnino, L. Adsorption and Surface Precipitation of Phosphate onto CaCO3-Montmorillonite: Effect of PH, Ionic Strength and Competition with Humic Acid. Geoderma 2014, 232–234, 600–608. [Google Scholar] [CrossRef]
- El Bouraie, M.; Masoud, A.A. Adsorption of Phosphate Ions from Aqueous Solution by Modified Bentonite with Magnesium Hydroxide Mg(OH)2. Appl. Clay Sci. 2017, 140, 157–164. [Google Scholar] [CrossRef]
- Xi, H.; Zhang, X.; Hua Zhang, A.; Guo, F.; Yang, Y.; Lu, Z.; Ying, G.; Zhang, J. Concurrent Removal of Phosphate and Ammonium from Wastewater for Utilization Using Mg-Doped Biochar/Bentonite Composite Beads. Sep. Purif. Technol. 2021, 285, 120399. [Google Scholar] [CrossRef]
- Shah, G.M.; Nasir, M.; Imran, M.; Bakhat, H.F.; Rabbani, F.; Sajjad, M.; Farooq, A.B.U.; Ahmad, S.; Song, L. Biosorption Potential of Natural, Pyrolysed and Acid-Assisted Pyrolysed Sugarcane Bagasse for the Removal of Lead from Contaminated Water. PeerJ 2018, 2018, e5672. [Google Scholar] [CrossRef] [PubMed]
- Samar, M.; Saxena, S. Study of Chemical & Physical of Perlite & Its Application in India. Int. J. Sci. Manag. 2016, 5, 70–80. [Google Scholar]
- Engstrom, D.R. Long-Term Changes in Iron and Phosphorus Sedimentation in Vadnais Lake, Minnesota, Resulting from Ferric Chloride Addition and Hypolimnetic Aeration. Lake Reserv. Manag. 2005, 21, 95–105. [Google Scholar] [CrossRef]
- Funes, A.; del Arco, A.; Álvarez-Manzaneda, I.; de Vicente, J.; de Vicente, I. A Microcosm Experiment to Determine the Consequences of Magnetic Microparticles Application on Water Quality and Sediment Phosphorus Pools. Sci. Total Environ. 2017, 579, 245–253. [Google Scholar] [CrossRef] [PubMed]
- del Arco, A.; Álvarez-Manzaneda, I.; Funes, A.; Pérez-Martínez, C.; de Vicente, I. Assessing the Toxic Effects of Magnetic Particles Used for Lake Restoration on Phytoplankton: A Community-Based Approach. Ecotoxicol. Environ. Saf. 2021, 207, 111288. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Zhou, J.; Wang, H.; Du, C.; Chen, X. Removal of Phosphate from Aqueous Solution by Thermally Treated Natural Palygorskite. Water Res. 2009, 43, 2907–2915. [Google Scholar] [CrossRef]
- Yin, H.; Yan, X.; Gu, X. Evaluation of Thermally-Modified Calcium-Rich Attapulgite as a Low-Cost Substrate for Rapid Phosphorus Removal in Constructed Wetlands. Water Res. 2017, 115, 329–338. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Liu, Y.; Tai, K.; Dillon, S.J. Growth Kinetics and Morphological Evolution of ZnO Precipitated from Solution. Chemiistru Mater. 2013, 25, 2927–2933. [Google Scholar] [CrossRef]
- Pandey, S. A Comprehensive Review on Recent Developments in Bentonite-Based Materials Used as Adsorbents for Wastewater Treatment. J. Mol. Liq. 2017, 241, 1091–1113. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Gandi, G.K.; Silva, V.M.T.M.; Rodrigues, A.E. Acetaldehyde Dimethylacetal Synthesis with Smopex 101 Fibres as Catalyst/Adsorbent. Chem. Eng. Sci. 2007, 62, 907–918. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, J.; Yu, G.; Núñez-delgado, A.; Kornaros, M. A Review on Halloysite-Based Adsorbents to Remove Pollutants in Water and Wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Biliani, S.E.; Vakros, J.; Manariotis, I.D. Screening of Raw and Modified Biochars from Food Processing Wastes for the Removal of Phosphates, Nitrates, and Ammonia from Water. Sustainability 2022, 14, 16483. [Google Scholar] [CrossRef]
- Dada, A. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn 2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Method 4500-P E; Phosphorus (Orthophosphate) by Ascorbic Acid Method. Standard Methods for the Examination of Water and Wastewater. APHA, APHAAWWA, WEF; American Public Health Association: Washington, DC, USA, 2017; pp. 4.146–4.148.
- APHA; WEF; AWWA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; Clesceri, L.S., Greenberg, A.E., Eaton, A.D.A., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Pai, S.C.; Tsau, Y.J.; Yang, T.I. PH and Buffering Capacity Problems Involved in the Determination of Ammonia in Saline Water Using the Indophenol Blue Spectrophotometric Method. Anal. Chim. Acta 2001, 434, 209–216. [Google Scholar] [CrossRef]
- Fan, T.; Wang, M.; Wang, X.; Chen, Y.; Wang, S.; Zhan, H.; Chen, X.; Lu, A.; Zha, S. Experimental Study of the Adsorption of Nitrogen and Phosphorus by Natural Clay Minerals. Adsorpt. Sci. Technol. 2021, 2021, 4158151. [Google Scholar] [CrossRef]
- Song, P.N.; Mahy, J.G.; Farcy, A.; Calberg, C.; Fagel, N.; Lambert, S.D. Development of novel composite materials based on kaolinitic clay modified with ZnO for the elimination of azo dyes by adsorption in water. Results Surf. Interfaces 2024, 16, 100255. [Google Scholar] [CrossRef]
- Han, B.; Weatherley, A.J.; Mumford, K.; Bolan, N.; He, J.Z.; Stevens, G.W.; Chen, D. Modification of Naturally Abundant Resources for Remediation of Potentially Toxic Elements: A Review. J. Hazard. Mater. 2022, 421, 126755. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, I.; Biliani, I. Cultural Eutrophication Treatment with Innovative Clay-Based Materials. In Proceedings of the 3rd International Conference of Ecological and Environmental Engineering (3rd COEE), Poznań, Poland, 28 June 6–1 July 2022. [Google Scholar]
- Abukhadra, M.R.; Abukhadra, M.R.; Ali, S.M.; Ali, S.M.; Nasr, E.A.; Nasr, E.A.; Mahmoud, H.A.A.; Mahmoud, H.A.A.; Awwad, E.M. Effective Sequestration of Phosphate and Ammonium Ions by the Bentonite/Zeolite Na-P Composite as a Simple Technique to Control the Eutrophication Phenomenon: Realistic Studies. ACS Omega 2020, 5, 14656–14668. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of Phosphate from Aqueous Solution Using Iron-Zirconium Modified Activated Carbon Nanofiber: Performance and Mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, C.; Zhao, J.; Zhang, Z.; Wang, H.; Zhou, S.; Wu, L. Synthesis of Zeolite P1 from Fly Ash under Solvent-Free Conditions for Ammonium Removal from Water. J. Clean. Prod. 2018, 202, 11–22. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Ayati, B. Remarkable Phosphate Removal from Saline Solution by Using a Novel Trimetallic Oxide Nanocomposite. Water Reuse 2021, 11, 410–424. [Google Scholar] [CrossRef]
- Xu, Q.; Li, W.; Ma, L.; Cao, D.; Owens, G.; Chen, Z. Simultaneous Removal of Ammonia and Phosphate Using Green Synthesized Iron Oxide Nanoparticles Dispersed onto Zeolite. Sci. Total Environ. 2020, 703, 135002. [Google Scholar] [CrossRef]
- Zamparas, M.; Drosos, M.; Georgiou, Y.; Deligiannakis, Y.; Zacharias, I. A Novel Bentonite-Humic Acid Composite Material BephosTM for Removal of Phosphate and Ammonium from Eutrophic Waters. Chem. Eng. J. 2013, 225, 43–51. [Google Scholar] [CrossRef]
- Kalla, N.; Khan, S. Effect of Nitrogen, Phosphorus Concentration, PH and Salinity Ranges on Growth, Biomass and Lipid Accumolation of Chlorella Vulgaris. Int. J. Pharm. Sci. Res. 2016, 7, 397–405. [Google Scholar] [CrossRef]
- Zhi, Y.; Zhang, C.; Hjorth, R.; Baun, A.; Duckworth, O.W.; Call, D.F.; Knappe, D.R.U.; Jones, J.L.; Grieger, K. Emerging Lanthanum (III)-Containing Materials for Phosphate Removal from Water: A Review towards Future Developments. Environ. Int. 2020, 145, 106115. [Google Scholar] [CrossRef]
- Shaheen, U.; Ye, Z.L.; Abass, O.K.; Zamel, D.; Rehman, A.; Zhao, P.; Huang, F. Evaluation of Potential Adsorbents for Simultaneous Adsorption of Phosphate and Ammonium at Low Concentrations. Microporous Mesoporous Mater. 2024, 379, 113301. [Google Scholar] [CrossRef]
- Koh, K.Y.; Yang, Y.; Chen, J.P. Critical Review on Lanthanum-Based Materials Used for Water Purification through Adsorption of Inorganic Contaminants. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1773–1823. [Google Scholar] [CrossRef]
- Omorogie, M.O.; Agunbiade, F.O.; Alfred, M.O.; Olaniyi, O.T.; Adewumi, T.A.; Bayode, A.A.; Ofomaja, A.E.; Naidoo, E.B.; Okoli, C.P.; Adebayo, T.A.; et al. The Sequestral Capture of Fluoride, Nitrate and Phosphate by Metal-Doped and Surfactant-Modified Hybrid Clay Materials. Chem. Pap. 2018, 72, 409–417. [Google Scholar] [CrossRef]
- Zamparas, M.; Zacharias, I. Restoration of Eutrophic Freshwater by Managing Internal Nutrient Loads. A Review. Sci. Total Environ. 2014, 496, 551–562. [Google Scholar] [CrossRef] [PubMed]

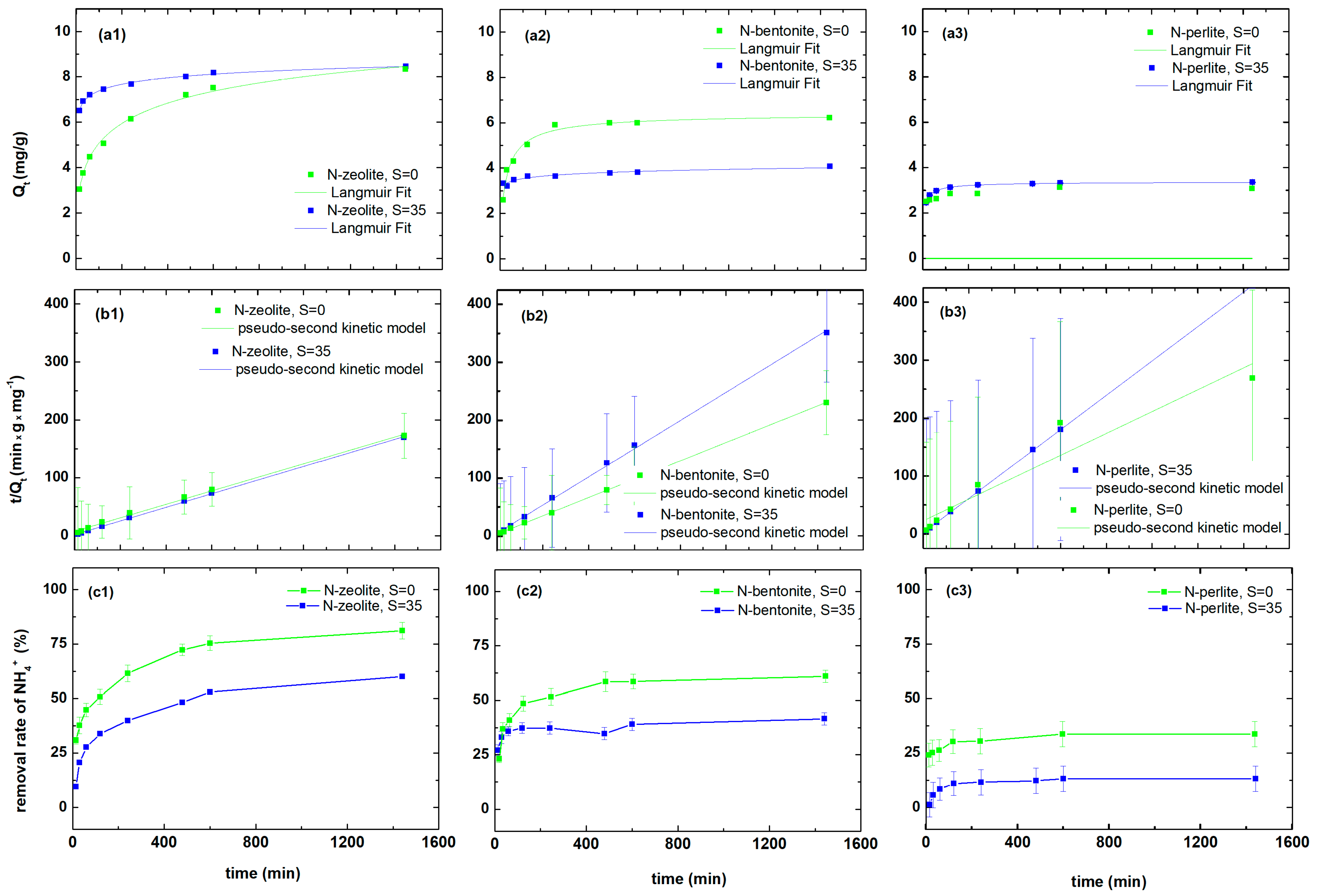

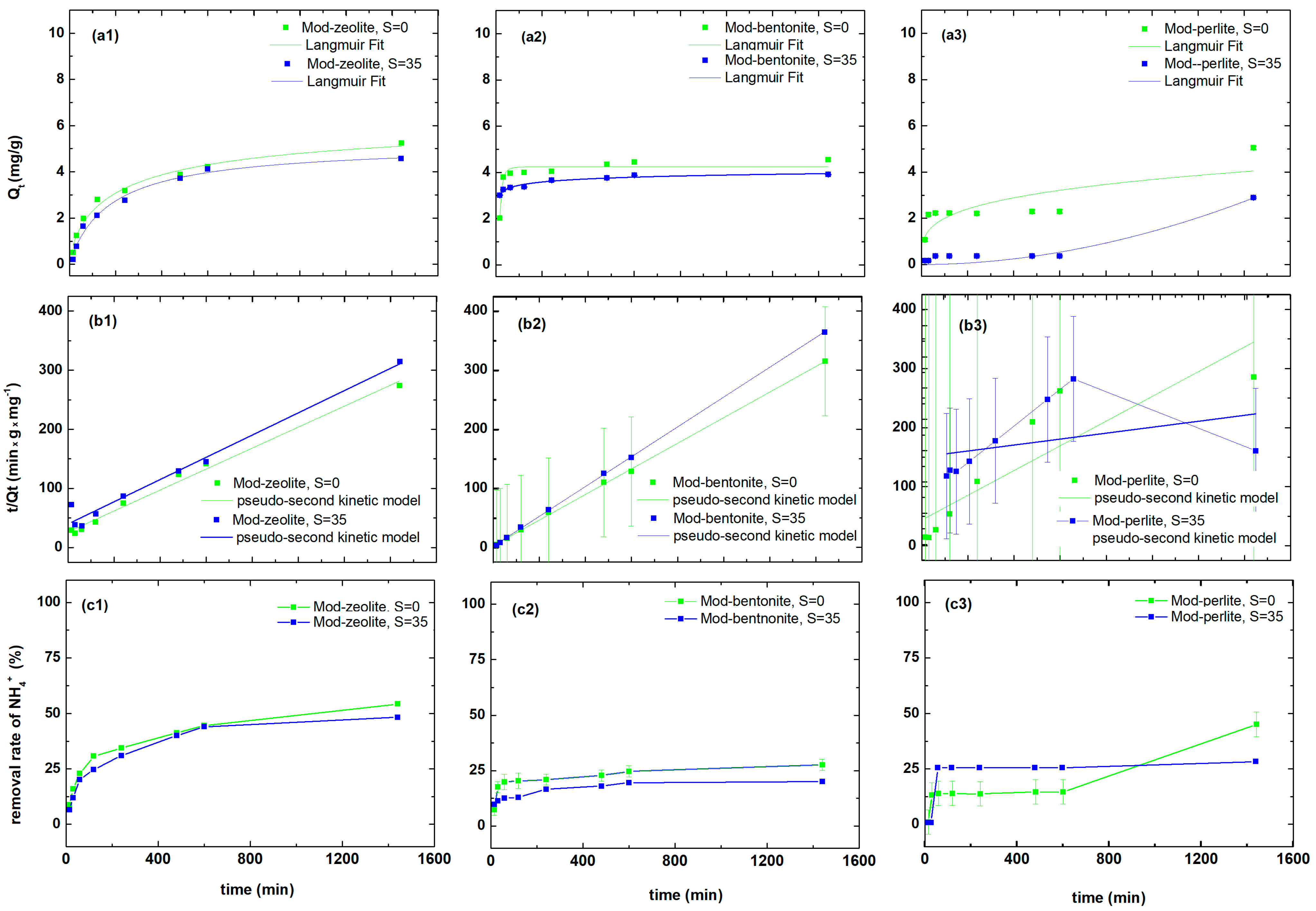
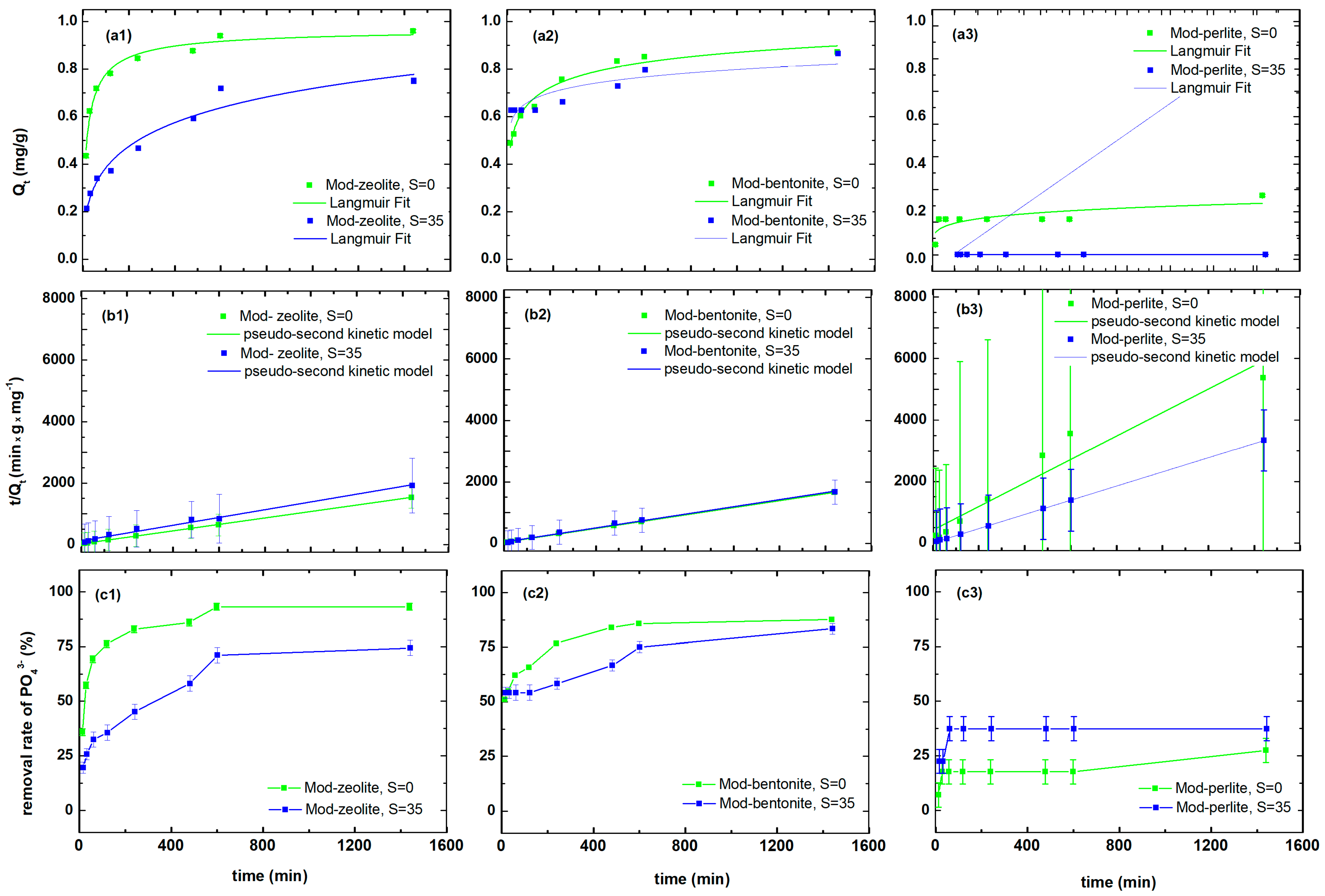
| Pollutant | Material | Qe | k2 | R2 |
|---|---|---|---|---|
| mg/g | g/(min × mg) | |||
| NH4+ | N-zeolite | 8.59 | 0.10 | 0.9963 |
| N-bentonite | 6.33 | 0.10 | 0.9998 | |
| N-perlite | 5.30 | 0.79 | 0.9075 | |
| Mod-zeolite | 5.63 | 0.002 | 0.9941 | |
| Mod-bentonite | 4.61 | 0.013 | 0.9992 | |
| Mod-perlite | 4.81 | 0.001 | 0.7431 | |
| PO4−3 | N-zeolite | 0.53 | 0.07 | 0.999 |
| N-bentonite | 1.00 | 0.016 | 0.937 | |
| N-perlite | 0.28 | 0.009 | 0.555 | |
| Mod-zeolite | 0.95 | 0.046 | 0.9995 | |
| Mod-bentonite | 0.88 | 0.039 | 0.9994 | |
| Mod-perlite | 0.24 | 0.093 | 0.9231 |
| Pollutant | Material | Qm | kL | R2 |
|---|---|---|---|---|
| mg/g | L/mg | |||
| NH4+ | N-zeolite | 5.52 | 0.39 | 0.9997 |
| N-bentonite | 6.43 | 0.08 | 0.9739 | |
| Mod-zeolite | 7.90 | 0.09 | 0.9106 | |
| Mod-bentonite | 2.12 | 0.01 | 0.98399 | |
| PO4−3 | N-zeolite | 0.68 | 0.05 | 0.99 |
| N-bentonite | 0.92 | 0.00 | 0.9984 | |
| Mod-zeolite | 0.24 | 0.43 | 0.9978 | |
| Mod-bentonite | 0.18 | 0.26 | 0.9665 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biliani, I.; Tsavatopoulou, V.; Zacharias, I. Comparative Study of Ammonium and Orthophosphate Removal Efficiency with Natural and Modified Clay-Based Materials, for Sustainable Management of Eutrophic Water Bodies. Sustainability 2024, 16, 10214. https://doi.org/10.3390/su162310214
Biliani I, Tsavatopoulou V, Zacharias I. Comparative Study of Ammonium and Orthophosphate Removal Efficiency with Natural and Modified Clay-Based Materials, for Sustainable Management of Eutrophic Water Bodies. Sustainability. 2024; 16(23):10214. https://doi.org/10.3390/su162310214
Chicago/Turabian StyleBiliani, Irene, Vasiliki Tsavatopoulou, and Ierotheos Zacharias. 2024. "Comparative Study of Ammonium and Orthophosphate Removal Efficiency with Natural and Modified Clay-Based Materials, for Sustainable Management of Eutrophic Water Bodies" Sustainability 16, no. 23: 10214. https://doi.org/10.3390/su162310214
APA StyleBiliani, I., Tsavatopoulou, V., & Zacharias, I. (2024). Comparative Study of Ammonium and Orthophosphate Removal Efficiency with Natural and Modified Clay-Based Materials, for Sustainable Management of Eutrophic Water Bodies. Sustainability, 16(23), 10214. https://doi.org/10.3390/su162310214






