Growth and Biomass Yield of Grey Sedge (Lepironia articulata Retz. Domin) under Different Shoot-Cutting Intervals in a Tropical Peatland
Abstract
1. Introduction
2. Materials and Methods
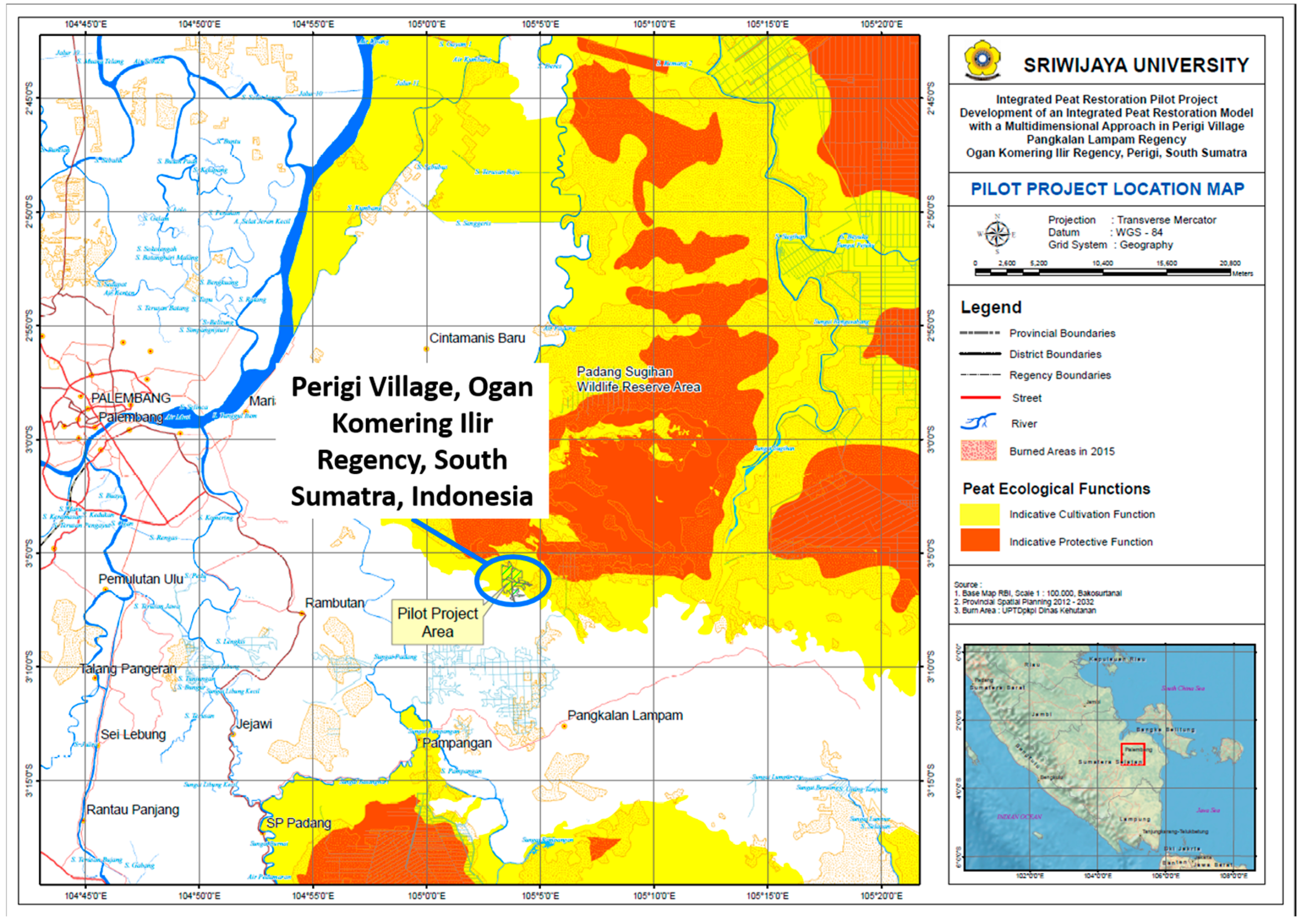
2.1. Study Site
2.2. Experimental Procedure
3. Results
3.1. Preliminary Measurement
3.2. Statistical Results
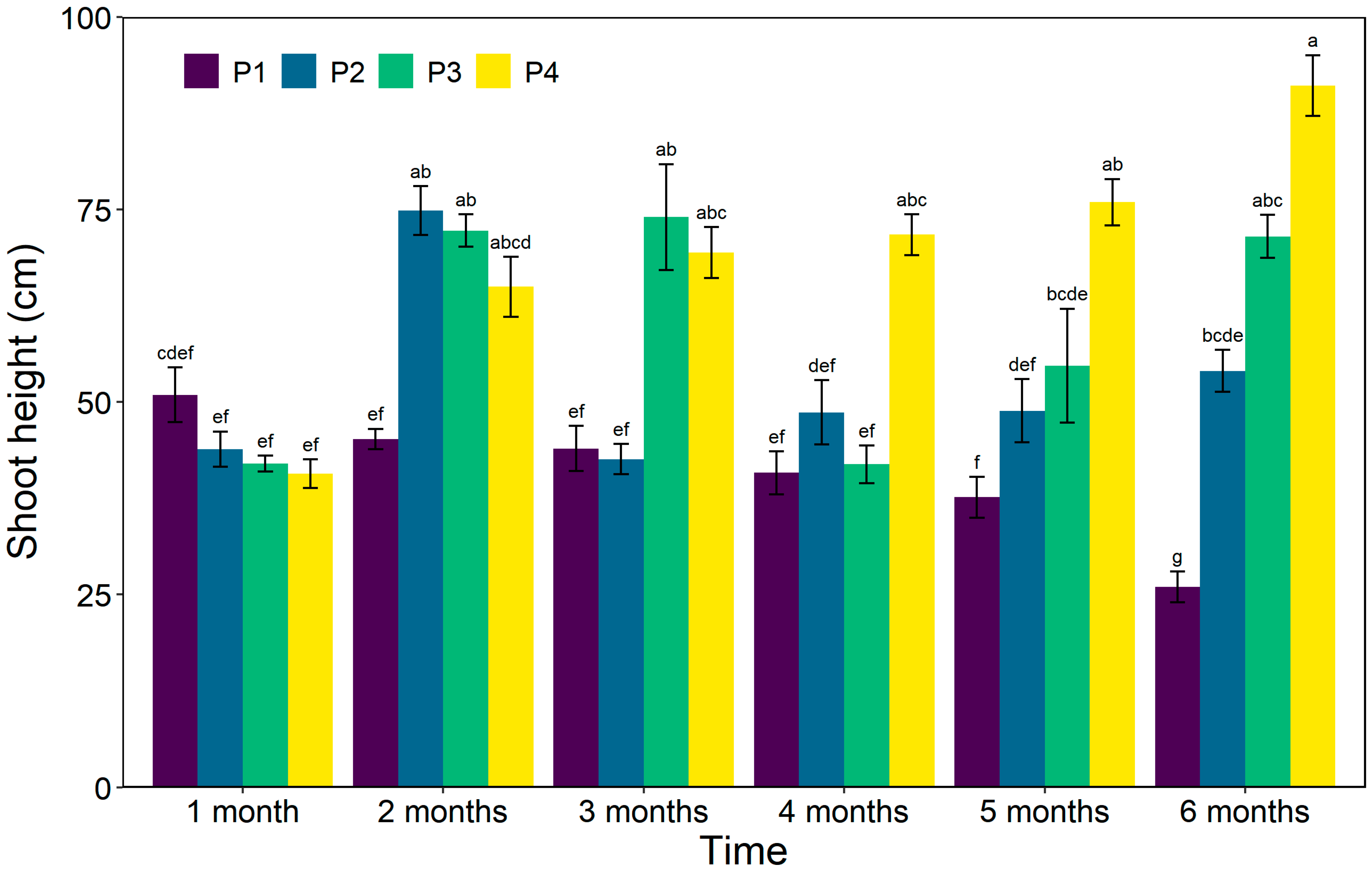
3.3. Shoot Height
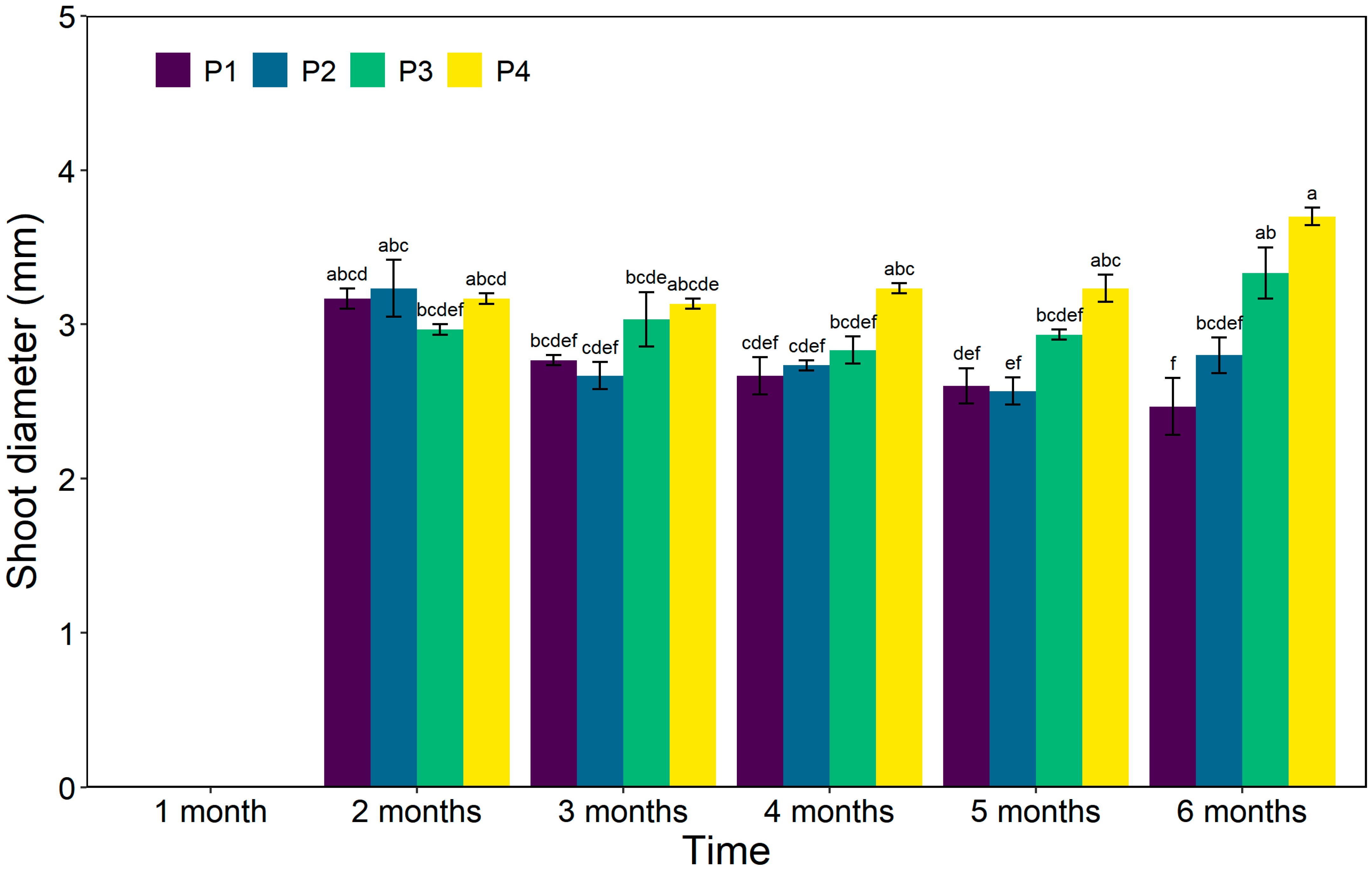
3.4. Shoot Diameter
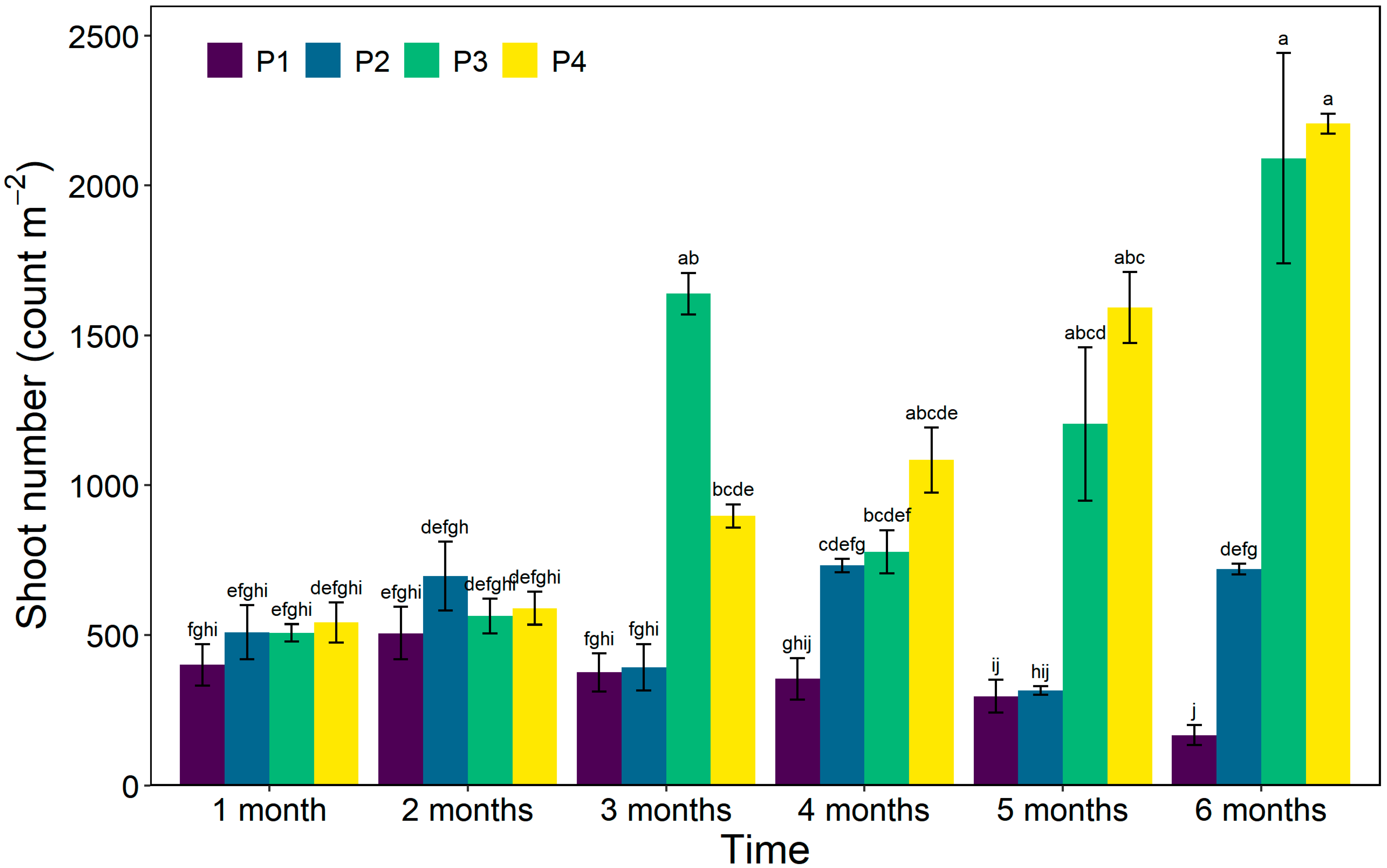
3.5. Shoot Number
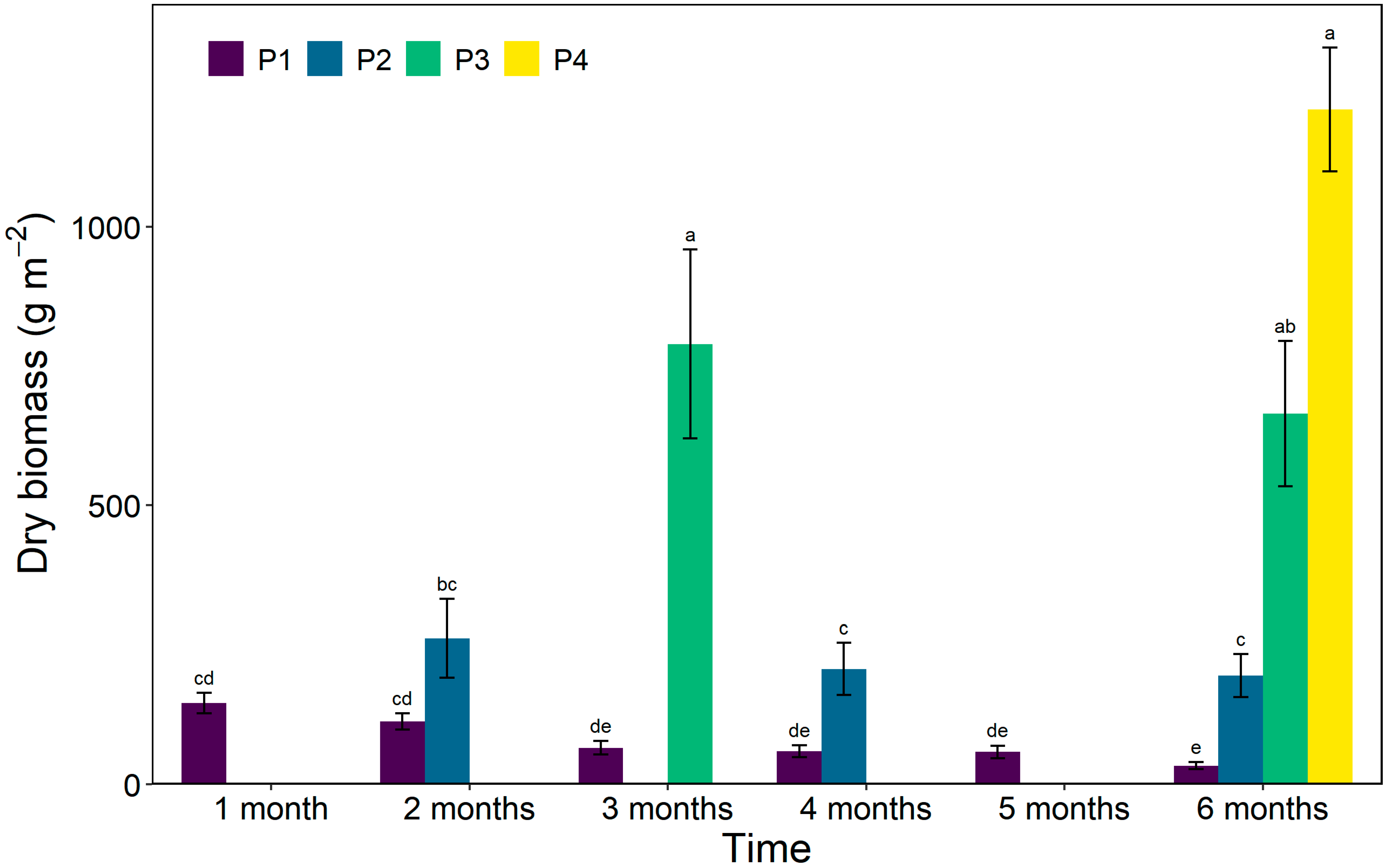
3.6. Dry Biomass
4. Discussion
4.1. Site Biomass Compared to That from Other Wetlands
4.2. Effects of Different Shoot-Cutting Intervals on L. articulata Growth and Biomass Yield
4.3. Sustainable Harvesting Period for L. articulata Biomass Yield
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dargie, G.C.; Lewis, S.L.; Lawson, I.T.; Mitchard, E.T.A.; Page, S.E.; Bocko, Y.E.; Ifo, S.A. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 2017, 542, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Englhart, S.; Jubanski, J.; Siegert, F. Quantifying dynamics in tropical peat swamp forest biomass with multi-temporal LiDAR datasets. Remote Sens. 2013, 5, 2368–2388. [Google Scholar] [CrossRef]
- Antala, M.; Juszczak, R.; van der Tol, C.; Rastogi, A. Impact of climate change-induced alterations in peatland vegetation phenology and composition on carbon balance. Sci. Total Environ. 2022, 827, 154294. [Google Scholar] [CrossRef]
- Moore, T.R.; Bubier, J.L.; Frolking, S.E.; Lafleur, P.M.; Roulet, N.T. Plant biomass and production and CO2 exchange in an ombrotrophic bog. J. Ecol. 2002, 90, 25–36. [Google Scholar] [CrossRef]
- Walker, T.N.; Ward, S.E.; Ostle, N.J.; Bardgett, R.D. Contrasting growth responses of dominant peatland plants to warming and vegetation composition. Oecologia 2015, 178, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Rupp, D.; Kane, E.S.; Dieleman, C.; Keller, J.K.; Turetsky, M. Plant functional group effects on peat carbon cycling in a boreal rich fen. Biogeochemistry 2019, 144, 305–327. [Google Scholar] [CrossRef]
- Page, S.E.; Rieley, J.O.; Shotyk, W.; Weiss, D. Interdependence of peat and vegetation in a tropical peat swamp forest. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Vitt, D.H. Peatlands. In Encyclopedia of Ecology, 2nd ed.; Elsevier: Carbondale, IL, USA, 2013; Volume 2, pp. 557–566. [Google Scholar]
- Ribeiro, K.; Pacheco, F.S.; Ferreira, J.W.; de Sousa-Neto, E.R.; Hastie, A.; Krieger Filho, G.C.K.; Alvalá, P.C.; Forti, M.C.; Ometto, J.P. Tropical peatlands and their contribution to the global carbon cycle and climate change. Glob. Chang. Biol. 2021, 27, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Sala, A.V.; Charman, D.J.; Brewer, S.; Page, S.E.; Prentice, I.C.; Friedlingstein, P.; Moreton, S.; Amesbury, M.J.; Beilman, D.W.; Björck, S.; et al. Latitudinal limits to the predicted increase of the peatland carbon sink with warming. Nat. Clim. Change 2018, 8, 907–913. [Google Scholar] [CrossRef]
- Hodgkins, S.B.; Richardson, C.J.; Dommain, R.; Wang, H.; Glaser, P.H.; Verbeke, B.; Winkler, B.R.; Cobb, A.R.; Rich, V.I.; Missilmani, M.; et al. Tropical peatland carbon storage linked to global latitudinal trends in peat recalcitrance. Nat. Commun. 2018, 9, 3640. [Google Scholar] [CrossRef]
- Giesen, W. Utilising non-timber forest products to conserve Indonesia’s peat swamp forests and reduce carbon emissions. J. Indones. Nat. Hist. 2015, 3, 17–26. [Google Scholar]
- Deshmukh, C.S.; Julius, D.; Desai, A.R.; Asyhari, A.; Page, S.E.; Nardi, N.; Susanto, A.P.; Nurholis, N.; Hendrizal, M.; Kurnianto, S.; et al. Conservation slows down emission increase from a tropical peatland in Indonesia. Nat. Geosci. 2021, 14, 484–490. [Google Scholar] [CrossRef]
- Gumbricht, T.; Roman-Cuesta, R.M.R.; Verchot, L.; Herold, M.; Wittmann, F.; Householder, E.; Herold, N.; Murdiyarso, D. An expert system model for mapping tropical wetlands and peatlands reveals South America as the largest contributor. Glob. Chang. Biol. 2017, 23, 3581–3599. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, J.; Rieley, J.O.; Mott, C.; Kimman, P.; Siegert, F. Determination of the amount of carbon stored in Indonesian peatlands. Geoderma 2008, 147, 151–158. [Google Scholar] [CrossRef]
- Baccini, A.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Change 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Warren, M.; Hergoualc’h, K.; Kauffman, J.B.; Murdiyarso, D.; Kolka, R. An appraisal of Indonesia’s immense peat carbon stock using national peatland maps: Uncertainties and potential losses from conversion. Carbon Balance Manag. 2017, 12, 12. [Google Scholar] [CrossRef]
- Page, S.; Mishra, S.; Agus, F.; Anshari, G.; Dargie, G.; Evers, S.; Jauhiainen, J.; Jaya, A.; Jovani-Sancho, A.J.; Laurén, A.; et al. Anthropogenic impacts on lowland tropical peatland biogeochemistry. Nat. Rev. Earth Environ. 2022, 3, 426–443. [Google Scholar] [CrossRef]
- Posa, M.R.C.; Wijedasa, L.S.; Corlett, R.T. Biodiversity and conservation of tropical peat swamp forests. J. Bio Sci. 2011, 61, 49–57. [Google Scholar] [CrossRef]
- Taufik, M.; Minasny, B.; McBratney, A.B.; Van Dam, J.C.; Jones, P.D.; Van Lanen, H.A.J. Human-induced changes in Indonesian peatlands increase drought severity. Environ. Res. Lett. 2020, 15, 084013. [Google Scholar] [CrossRef]
- Pudyatmoko, S.; Budiman, A.; Siregar, A.H. Habitat suitability of a peatland landscape for tiger translocation on Kampar Peninsula, Sumatra, Indonesia. Mamm. Biol. 2023, 103, 375–388. [Google Scholar] [CrossRef]
- Giannini, V.; Oehmke, C.; Silvestri, N.; Wichtmann, W.; Dragoni, F.; Bonari, E. Combustibility of biomass from perennial crops cultivated on a rewetted Mediterranean peatland. Ecol. Eng. 2016, 97, 157–169. [Google Scholar] [CrossRef]
- Maimunah, S.; Rahman, S.A.; Samsudin, Y.B.; Artati, Y.; Simamora, T.I.; Andini, S.; Lee, S.M.; Baral, H. Assessment of suitability of tree species for bioenergy production on burned and degraded peatlands in Central Kalimantan, Indonesia. Land 2018, 7, 115. [Google Scholar] [CrossRef]
- Leksono, B.; Windyarini, E.; Hasnah, T.M.; Rahman, S.A.; Baral, H. Calophyllum inophyllum—A viable prospect for green energy and landscape restoration? In Bioenergy for Landscape Restoration and Livelihoods: Re-creating Energy-Smart Ecosystems on Degraded Landscapes; Baral, H., Leksono, B., Seol, M., Eds.; Center for International Forestry Research: Bogor, Indonesia, 2022; pp. 178–191. [Google Scholar]
- Maulidya, A.; Suwignyo, R.A.; Priadi, D.P.; Baral, H.; Choi, E.; Adriansyah, F.; Yang, H. Survival and growth performance of Calophyllum inophyllum L. seedlings in peat soil and at different levels of groundwater. Land 2024, 13, 879. [Google Scholar] [CrossRef]
- Premono, B.T.; Wakhid, N.; Handayani, D.; Nurzakiah, S.; Tata, H.L. Home garden mixed cropping practice by communities living on peatland in household’s income resilience and climate adaptation. IOP Conf. Ser. Earth Environ. Sci. 2024, 1315, 012003. [Google Scholar] [CrossRef]
- Dohong, A.; Aziz, A.A.; Dargusch, P. A review of the drivers of tropical peatland degradation in South-East Asia. Land Use Policy 2017, 69, 349–360. [Google Scholar] [CrossRef]
- Budiman, I.; Bastoni, S.; Sari, E.N.; Hadi, E.E.; Asmaliyah; Siahaan, H.; Januar, R.; Hapsari, R.D. Progress of paludiculture projects in supporting peatland ecosystem restoration in Indonesia. Glob. Ecol. Conserv. 2020, 23, e01084. [Google Scholar] [CrossRef]
- Tan, Z.D.; Lupascu, M.; Wijedasa, L.S. Paludiculture as a sustainable land use alternative for tropical peatlands: A review. Sci. Total Environ. 2021, 753, 142111. [Google Scholar] [CrossRef]
- Pusvita, E.; Mulyana, A.; Adriani, D.; Antoni, M. Farmer’s interest and economics model of agrosilvofishery restoration on degraded peatland in OKI Regency south Sumatra, Indonesia. JOSAET 2023, 1, 99–111. [Google Scholar]
- Wunbua, J.; Nakhapakorn, K.; Jirakajohnhool, S. Change detection and identification of land potential for planting Krajood (Lepironia articulata) in Thale Noi, Southern Thailand. Sist. 2012, 34, 329–336. [Google Scholar]
- Sinaga, N.I.; Lense, O.N.; Hendry; Tukayo, R. Association among Lepironia articulata Cyperaceae, peat soil and people in Wanggate. IOP Conf. Ser. Earth Environ. Sci. 2022, 1025, 012003. [Google Scholar] [CrossRef]
- Rasaminirina, F.; Larridon, I. The genera of Cyperaceae of Madagascar. Plant Ecol. Evol. 2023, 156, 276–310. [Google Scholar] [CrossRef]
- Jacobs, S.W.L. New South Walse Flora Online: Lepironia articulate. Royal Botanic Gardens & Domain Trust, Sydney Australia. Available online: https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Lepironia~articulata (accessed on 9 July 2024).
- Oishi, R.; Nioh, I.; Hayatsu, M.; Nioh, T.; Limtong, P. Isolation and characterization of heterotrophic chemoorganotrophic bacteria from the roots of Lepironia articulata (reedgrass) grown on strongly acidic sulfate soil in South Thailand. Microb. Environ. 1999, 14, 69–75. [Google Scholar] [CrossRef][Green Version]
- Said, N.S.M.; Kurniawan, S.B.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N.I. Competence of Lepironia articulata in eradicating chemical oxygen demand and ammoniacal nitrogen in coffee processing mill effluent and its potential as green straw. Sci. Total Environ. 2021, 799, 149315. [Google Scholar] [CrossRef]
- Sim, C.H.; Yusoff, M.K.; Shutes, B.; Ho, S.C.; Mansor, M. Nutrient removal in a pilot and full scale constructed wetland, Putrajaya City Malaysia. J. Environ. Manag. 2008, 88, 307–317. [Google Scholar] [CrossRef]
- Bensink, A.H.A.; Burton, H.; Stradbroke Island, N. A place for freshwater invertebrates. Proc. R. Soc. Qld. 1975, 86, 29–45. [Google Scholar]
- McFarland, D. The composition, microhabitat use and response to fire of the avifauna of subtropical heathlands in Cooloola National Park, Queensland. Emu—Austral Ornithol. 1988, 88, 249–257. [Google Scholar] [CrossRef]
- Marshall, J.C.; McGregor, G.B. The influence of water depth on the distribution of the emergent sedge Lepironia articulalta (Cyperaceae) in two dune lakes of Southern Queensland Wallum wetlands. Proc. R. Soc. Qld. 2011, 117, 193–199. [Google Scholar]
- Domyos, P.; Te-Chato, S. In vitro propagation of Lepironia articulate in Kuan Kreng peatlands, Nakhon Si Thammrat. J. Agric. Technol. 2013, 9, 1993–2004. [Google Scholar]
- Salim, J.M.; Lee, G.E.; Salam, M.R.; Shahimi, S.; Pesiu, E.; Jani, J.M.; Horsali, N.A.I.; Shahrudin, R.; Nor, S.M.M.; Chong, J.L.; et al. A checklist of vascular plants and uses of some species for livelihood-making in Setiu Wetlands, Terengganu, Malaysia. PhytoKeys 2020, 160, 7–43. [Google Scholar] [CrossRef]
- Kissinger, K.; Yamani, A.; Pitri, R.M.N.; Nasrulloh, A.V. Impacts of Forest Farmers Management on Lepironia articulata Retz.: Conservation Based on Utilization of Peat Ecosystem Biodiversity in South Kalimantan. Pol. J. Environ. Stud. 2024, 33, 1203–1213. [Google Scholar] [CrossRef]
- Ullah, A.; Kaewsichan, L.; Tohdee, K. Evaluation of hydrothermally carbonized alternative biomass (Lepironia articulate) as a source of alternative energy and adsorbent. JPIChE 2019, 47, 1–13. [Google Scholar] [CrossRef]
- Al-Ajalin, F.A.H.; Idris, M.; Abdullah, S.R.S.; Kurniawan, S.B.; Imron, M.F. Effect of wastewater depth to the performance of short-term batching-experiments horizontal flow constructed wetland system in treating domestic wastewater. Environ. Technol. Innov. 2020, 20, 101106. [Google Scholar] [CrossRef]
- Binti Hairolnizam, N.F.A.; Nasarudin, M.A.S.; Mohamad Termizi, A.Z.-A.; Amalina, F.; Abd Razak, A.S.; Sulaiman, S. The potential of biodegradable compostable eco-straw from Lepironia Articulata sp. (Purun/Kercut). Mater. Today Proc. 2024; in press. [Google Scholar] [CrossRef]
- Homkhiew, C.; Srivabut, C.; Boonchouytan, W.; Rawangwong, S. Sandwich composites from recycled plastic reinforced with krajood (Lepironia articulata) fiber for building applications. Iran. Polym. J. 2024, 33, 839–853. [Google Scholar] [CrossRef]
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability study of active chitosan biopolymer films enriched with Quercus polyphenol extract in different soil types. Environ. Technol. Innov. 2021, 21, 101318. [Google Scholar] [CrossRef]
- Oberlintner, A.; Likozar, B.; Novak, U. Hydrophobic functionalization reactions of structured cellulose nanomaterials: Mechanism, kinetics and in silico multi-scale models. Carbohydr. Polym. 2021, 1, 117742. [Google Scholar] [CrossRef] [PubMed]
- Bjelić, A.; Hočevar, B.; Grilc, M.; Novak, U.; Likozar, B. A review of sustainable lignocellulose biorefining applying (natural) deep eutectic solvents (DESs) for separations, catalysis and enzymatic biotransformation processes. Rev. Chem. Eng. 2022, 38, 243–272. [Google Scholar] [CrossRef]
- Chucheep, K.; Suwanpayak, N.; Phanchindawan, N. A study of the feasibility of using grey sedge residue to facilitate zero waste production. Recycling 2022, 7, 34. [Google Scholar] [CrossRef]
- Monti, A.; Bezzi, G.; Pritoni, G.; Venturi, G. Long-term productivity of lowland and upland switchgrass cytotypes as affected by cutting frequency. Bioresour. Technol. 2008, 99, 7425–7432. [Google Scholar] [CrossRef]
- Moyo, H.; Scholes, M.C.; Twine, W. The effects of repeated cutting on coppice response of Terminalia sericea. Trees 2015, 29, 161–169. [Google Scholar] [CrossRef]
- Applegate, G.; Freeman, B.; Tular, B.; Sitadevi, L.; Jessup, T.C. Application of agroforestry business models to tropical peatland restoration. Ambio 2022, 51, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Jeong, J.; Artati, Y.; Yang, H.; Adriani, D.; Yang, A.-R. Local perspectives on agrosilvofishery in peatlands: A case study of Perigi Village, South Sumatra, Indonesia. Land 2024, 13, 539. [Google Scholar] [CrossRef]
- Omar, M.S.; Ifandi, E.; Sukri, R.S.; Kalaitzidis, S.; Christanis, K.; Lai, D.T.C.; Bashir, S.; Tsikouras, B. Peatlands in Southeast Asia: A comprehensive geological review. Earth Sci. Rev. 2022, 232, 104149. [Google Scholar] [CrossRef]
- Cahya, M.; Suwignyo, R.A.; Sodikin, E.; Baral, H. Increasing rice productivity in degraded peatlands using improved planting methods and rice varieties. Biol. Res. J. 2022, 8, 69–82. [Google Scholar] [CrossRef]
- Anda, M.; Ritung, S.; Suryani, E.; Sukarman; Hikmat, M.; Yatno, E.; Mulyani, A.; Subandiono, R.E.; Suratman; Husnain. Revisiting tropical peatlands in Indonesia: Semi-detailed mapping, extent and depth distribution assessment. Geoderma 2021, 402, 115235. [Google Scholar] [CrossRef]
- Rinaldi, P.S.; ’Akromah, Z.N.; Ramadhan, H.; Husna, S.; Syamsudin, D.L.; Panggabean, P.B.; Murdianti, R.A.; Fatahillah, M.H.; Perala, I.; Rizqia, E.K.; et al. Physical and Chemical Analysis of Land in Forest Peat Swamp in Resort Pondok soar, Tanjung Puting National Park, Central Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2019, 394, 012037. [Google Scholar] [CrossRef]
- Hikmatullah, S. Physical and chemical properties of cultivated peat soils in four trial sites of ICCTF in Kalimantan and Sumatra, Indonesia. J. Trop. Soils 2014, 19, 131–141. [Google Scholar] [CrossRef]
- Klimatologi Kelas I Sumatera Selatan (1994–2023), Badan Meteorologi Klimatologi Dan Geofisika Stasiun, Palembang, Indonesia.
- Rengsirikul, K.; Ishii, Y.; Kangvansaichol, K.; Pripanapong, P.; Sripichitt, P.; Punsuvon, V.; Vaithanomsat, P.; Nakamanee, G.; Tudsri, S. Effects of inter-cutting interval on biomass yield, growth components and chemical composition of napiergrass (Pennisetum purpureum Schumach) cultivars as bioenergy crops in Thailand. Grassl. Sci. 2011, 57, 135–141. [Google Scholar] [CrossRef]
- Kulik, M.; Sender, J.; Bochniak, A.; Jaźwa, M.; Ciesielski, D. The influence of mowing frequency on the growth and development of Phragmites australis. J. Nat. Conserv. 2023, 76, 126492. [Google Scholar] [CrossRef]
- Irmawati, I. (Sriwijaya University, Palembang, Indonesia). Personal communication, 2024. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Ikusima, I. Primary production and population ecology of the aquatic sedge Lepironia articulata in a tropical swamp, Tasek Bera, Malaysia. Aquat. Bot. 1978, 4, 269–280. [Google Scholar] [CrossRef]
- Southern, W.; Ash, J.; Brodie, J.; Ryan, P. The flora, fauna and water chemistry of Tagimaucia crater, a tropical highland lake and swamp in Fiji. Freshw. Biol. 1986, 16, 509–520. [Google Scholar] [CrossRef]
- De Ryck, S.; Reheul, D.; De Cauwer, B. Impact of regular mowing, mowing height, and grass competition on tuber number and tuber size of yellow nutsedge clonal populations (Cyperus esculentus L.). Weed Res. 2023, 63, 371–381. [Google Scholar] [CrossRef]
- Li, B.; Shibuya, T.; Yogo, Y.; Hara, T. Effects of ramet clipping and nutrient availability on growth and biomass allocation of yellow nutsedge. Ecol. Res. 2004, 19, 603–612. [Google Scholar] [CrossRef]
- Bar, K.D.; Verschoren, V.; Sara, J.R.; Merie, P.; Schoelynck, J. Consequences of Different Cutting Regimes on Regrowth and Nutrient Stoichiometry of Sparganium erectum L. and Potamogeton natans L. River Res. Appl. 2017, 33, 1420–1427. [Google Scholar] [CrossRef]
- Terer, T.; Triest, L.; Muthama Muasya, A.M. Effects of harvesting Cyperus papyrus in undisturbed wetland, Lake Naivasha, Kenya. Hydrobiologia 2012, 680, 135–148. [Google Scholar] [CrossRef]
- Fike, J.H.; Parrish, D.J.; Wolf, D.D.; Balasko, J.A.; Green, J.T., Jr.; Rasnake, M.; Reynolds, J.H. Switchgrass production for the upper southeastern USA: Influence of cultivar and cutting frequency on biomass yields. Biomass Bioenergy 2006, 30, 207–213. [Google Scholar] [CrossRef]
- Liu, X.-J.A.; Fike, J.H.; Galbraith, J.M.; Fike, W.B. Switchgrass response to cutting frequency and biosolids amendment: Biomass yield, feedstock quality, and theoretical ethanol yield. BioEnergy Res. 2014, 7, 1191–1200. [Google Scholar] [CrossRef]
- Li, B.; Shibuya, T.; Yogo, Y.; Hara, T. Effects of temperature on bud-sprouting and early growth of Cyperus esculentus in the dark. J. Plant Res. 2000, 113, 19–27. [Google Scholar] [CrossRef]
| Cutting Interval | Time | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | ||||||||
| P1 | P | 1st | 2nd | 3rd | 4th | 5th | 6th | ||||||
| P2 | 1st | 2nd | 3rd | ||||||||||
| P3 | 1st | 2nd | |||||||||||
| P4 | 1st | ||||||||||||
| Parameter | Green Shoot | 1/3 Dry Shoot | Dry Shoot |
|---|---|---|---|
| Shoot number (m−2) | 2113 ± 304 | 523 ± 44 | 1713 ± 354 |
| Dry biomass (g m−2) | 1911.3 ± 912.7 | 755.2 ± 162.8 | 1962.5 ± 375.6 |
| Parameters | d.f. | F | p |
|---|---|---|---|
| Shoot height | |||
| Time | 3 | 70.14 | <0.001 ** |
| Shoot-cutting interval | 5 | 14.04 | <0.001 ** |
| Time × Shoot-cutting interval | 15 | 14.90 | <0.001 ** |
| Shoot diameter | |||
| Time | 3 | 22.42 | <0.001 ** |
| Shoot-cutting interval | 5 | 9.55 | <0.001 ** |
| Time × Shoot-cutting interval | 13 | 4.09 | <0.001 ** |
| Shoot number | |||
| Time | 3 | 85.31 | <0.001 ** |
| Shoot-cutting interval | 5 | 6.92 | <0.001 ** |
| Time × Shoot-cutting interval | 15 | 12.13 | <0.001 ** |
| Dry biomass | |||
| Time | 3 | 113.63 | <0.001 ** |
| Shoot-cutting interval | 5 | 6.55 | <0.001 ** |
| Time × Shoot-cutting interval | 3 | 2.20 | 0.11 ns |
| Cumulative shoot number | |||
| Shoot-cutting interval | 3 | 7.51 | <0.05 * |
| Cumulative dry biomass | |||
| Shoot-cutting interval | 3 | 6.54 | <0.05 * |
| Treatments | Cumulative Shoot Number (m−2) |
|---|---|
| P1 | 2102 ± 630 b |
| P2 | 2148 ± 156 b |
| P3 | 3729 ± 677 a |
| P4 | 2207 ± 58 b |
| Treatments | Cumulative Dry Biomass (g m−2) |
|---|---|
| P1 | 470.1 ± 123.8 b |
| P2 | 661.0 ± 259.1 ab |
| P3 | 1453.5 ± 518.4 a |
| P4 | 1210.5 ± 193.0 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodikin, E.; Irmawati, I.; Suwignyo, R.A.; Halimi, E.S.; Tampubolon, M.; Yang, A.-R.; Yang, H.; Baral, H. Growth and Biomass Yield of Grey Sedge (Lepironia articulata Retz. Domin) under Different Shoot-Cutting Intervals in a Tropical Peatland. Sustainability 2024, 16, 8896. https://doi.org/10.3390/su16208896
Sodikin E, Irmawati I, Suwignyo RA, Halimi ES, Tampubolon M, Yang A-R, Yang H, Baral H. Growth and Biomass Yield of Grey Sedge (Lepironia articulata Retz. Domin) under Different Shoot-Cutting Intervals in a Tropical Peatland. Sustainability. 2024; 16(20):8896. https://doi.org/10.3390/su16208896
Chicago/Turabian StyleSodikin, Erizal, Irmawati Irmawati, Rujito A. Suwignyo, Entis S. Halimi, Marudut Tampubolon, A-Ram Yang, Hyunyoung Yang, and Himlal Baral. 2024. "Growth and Biomass Yield of Grey Sedge (Lepironia articulata Retz. Domin) under Different Shoot-Cutting Intervals in a Tropical Peatland" Sustainability 16, no. 20: 8896. https://doi.org/10.3390/su16208896
APA StyleSodikin, E., Irmawati, I., Suwignyo, R. A., Halimi, E. S., Tampubolon, M., Yang, A.-R., Yang, H., & Baral, H. (2024). Growth and Biomass Yield of Grey Sedge (Lepironia articulata Retz. Domin) under Different Shoot-Cutting Intervals in a Tropical Peatland. Sustainability, 16(20), 8896. https://doi.org/10.3390/su16208896








