Remediation of Arsenic and Cadmium Co-Contaminated Soil: A Review
Abstract
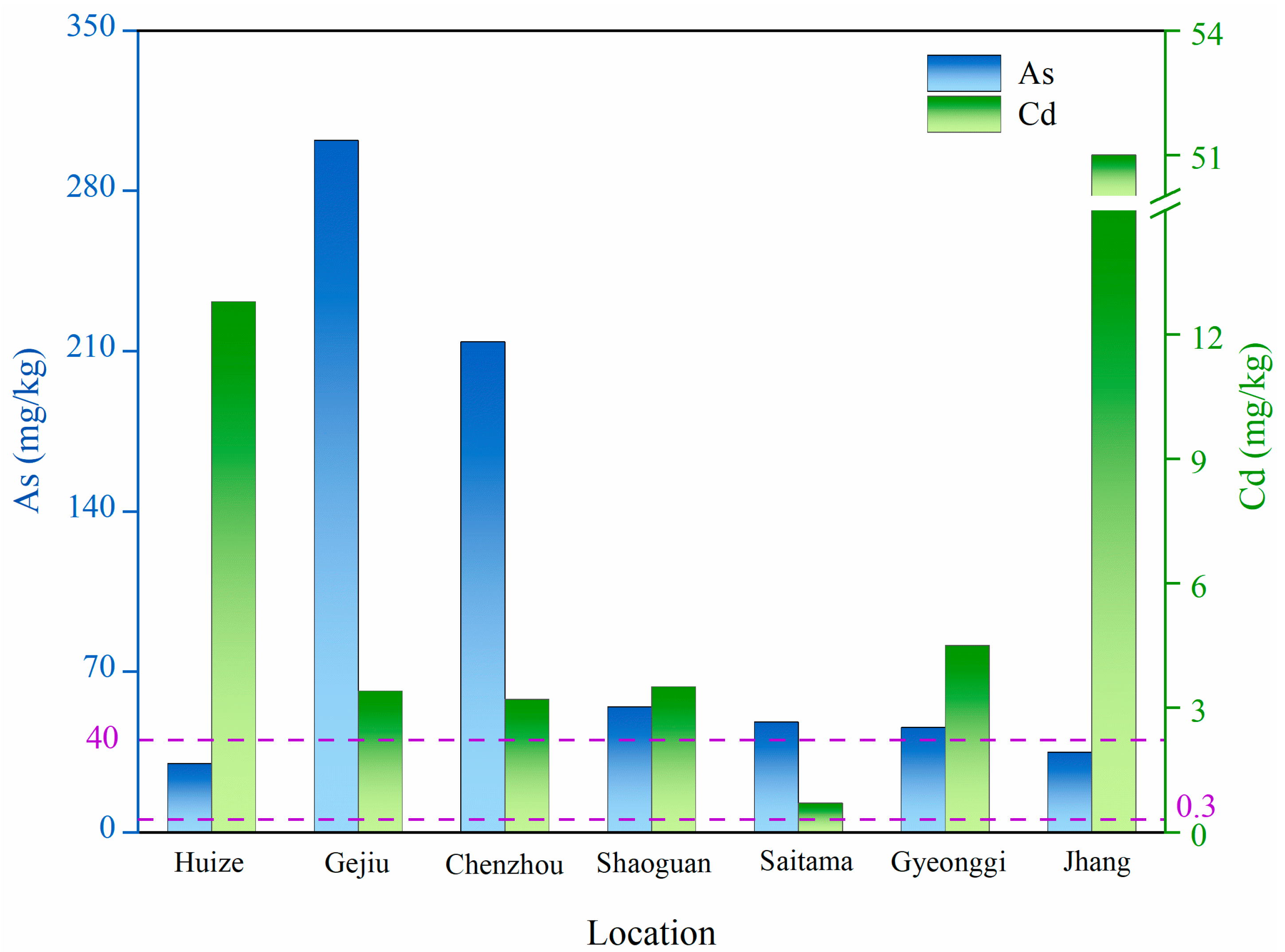
1. Introduction
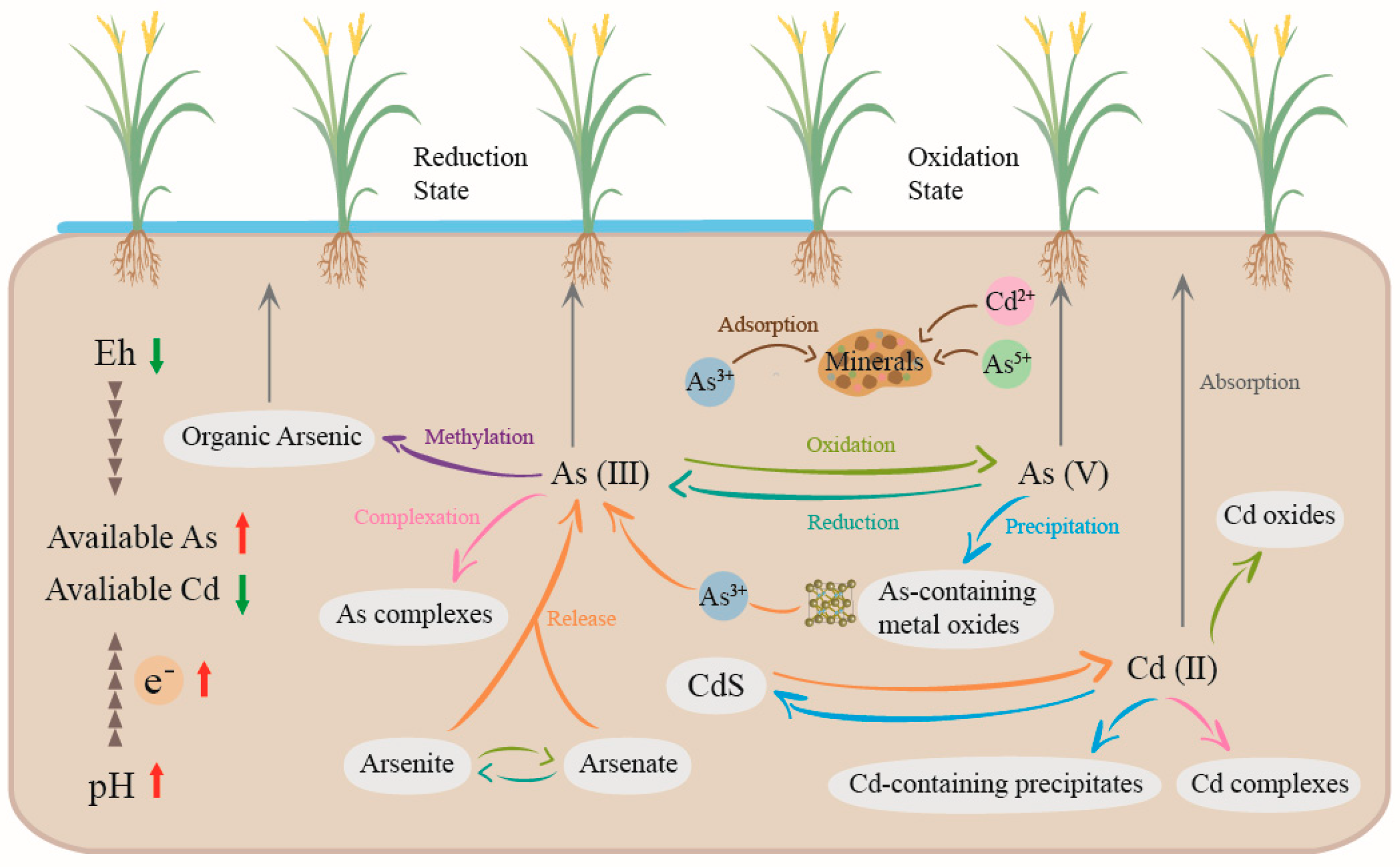
2. Forms and Transformation Mechanisms of As and Cd in Soil
2.1. Different Forms of As and Cd in Soil
2.2. Transformation Rules of As and Cd in Different pH–Eh Soil
3. Phytoremediation Techniques for As and Cd Co-Contaminated Soils
3.1. Arsenic and Cadmium Hyperaccumulators
| Plant Name | Family | Genus | Life Form | Plant Biomass | Accumulation | References |
|---|---|---|---|---|---|---|
| X. strumarium | Asteraceae | Artemisia | Annual | 470 g | As: 0.85 mg/kg Cd: 1.84 mg/kg | [48] |
| H. annuus | Asteraceae | Carthamus | Annual | 130 g | As: 1.11 mg/kg Cd: 2.31 mg/kg | [53] |
| B. juncea | Brassicaceae | Raphanus | Annual | 35.4 g | As: 576 mg/kg Cd: 5.37 mg/kg | [56,57] |
| P. vittata | Pteridaceae | Pteris | Perennial | 7.25 g | As: 8406 mg/kg Cd: 186 mg/kg | [24,49] |
3.2. Enhancement Measures for Phytoremediation
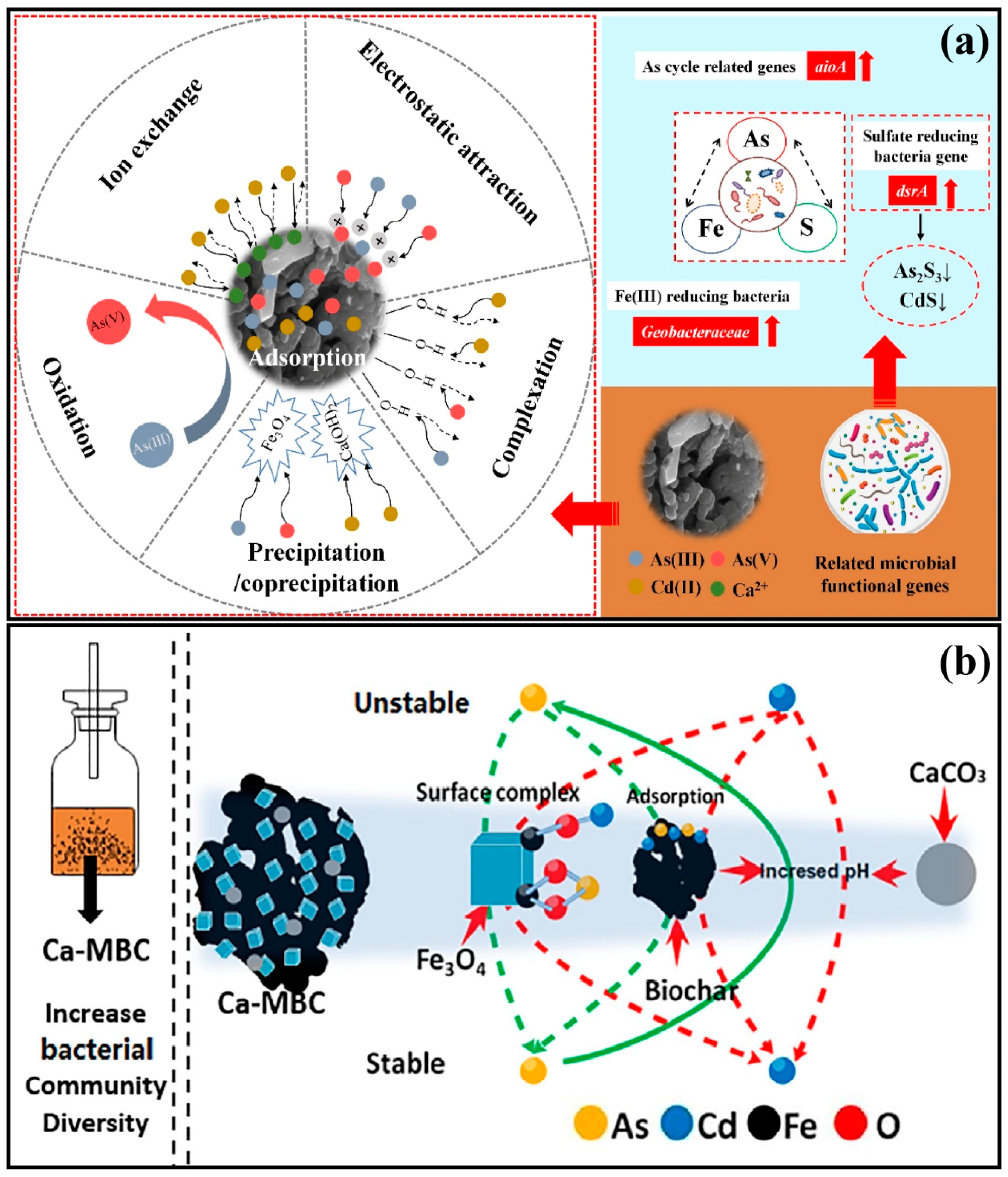
4. Chemical Immobilization Remediation for As and Cd Co-Contamination
4.1. Mechanisms of Soil Remediation through Immobilization Amendments
| Classification | Immobilization Amendments | Immobilization Mechanism | Application | Immobilization Rates | Experiment | References |
|---|---|---|---|---|---|---|
| Phosphates | Phosphates, hydroxyapatite, phosphate rock powder, phosphogypsum, and phosphate fertilizers | By forming insoluble phosphate precipitates and surface adsorption of heavy metals. | hydroxyapatite with iron | As: 69%; Cd: 44% | Pot experiment | [82,83,84,85,86] |
| Complexation of ferric sulfate and dihydrocalcium phosphate (7.2:1) | As: 69%; Cd: 41% | Pot experiment | [17] | |||
| Metals and Their Oxides | Zero-valent iron, goethite, hematite, magnetite, acicular goethite, ferrous sulfate, and red mud | Adsorption and co-precipitation effects. | Ferrous sulfate | As: 64%; Cd: 98% | Pot experiment | [68,73,87] |
| Zero-valent iron (Fe content 98%) | As: 54%; Cd: 22% | Field experiment | [88] | |||
| Steel slag (Fe content 20%) | As: 33%; Cd: 56% | Field experiment | [89] | |||
| Red mud (Fe content 21%) | As: 47%; Cd: 90% | Pot experiment | [90] | |||
| Clay Minerals | Zeolite, bentonite, loess, kaolinite, diatomaceous earth, montmorillonite, etc. | Adsorption, ion exchange, and coordination reactions. | Loess | As: 18%; Cd: 41% | Field experiment | [25,26,27,91] |
| Limestone, zeolite, and ferrous sulfate (8:4:2) | As: 55%; Cd: 51% | Pot experiment | [92] | |||
| Limestone, zeolite, and titanium dioxide (8:4:2) | As: 44%; Cd: 74% | Pot experiment | [92] | |||
| Limestone | Quicklime, hydrated lime | Increasing soil pH, altering soil CEC, and changing redox potential. | Limestone | As: 5–60%; Cd: 5–60% | Field experiment | [93,94,95] |
| Organic Fertilizers | Composting using animal manure, biomass solids, etc. | Adsorption, redox reactions, organic complexation reactions. | Fly ash mixed composting of sludge | As: 72%; Cd: 72% | Column experiment | [96,97,98] |
| Biochar | Biochar | Raising pH, adsorption, complexation, precipitation, and ion exchange. | Hydroxyapatite, zeolite, and biochar (2:1:2) | As: 55%; Cd: 33% | Pot experiment | [70,71,99] |
| Co-application of steel slag and biomass charcoal (4:1) | As: 20%; Cd: 42% | Field experiment | [100] | |||
| Modified biochar, acid-modified zeolite, and acid-modified vermiculite (27:23:50) | As: 99%; Cd: 81% | Pot experiment | [101] |
4.2. Arsenic and Cadmium Co-Immobilization Amendments
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Zarei, A.; Esmaeilzadeh, M.; Taghavi, M.; Yousefi, M.; Yousefi, Z.; Sedighi, F.; Javan, S. Assessment of heavy metal pollution and human health risks assessment in soils around an industrial zone in Neyshabur, Iran. Biol. Trace Elem. Res. 2020, 195, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-Z.; Qin, M.-L.; Lin, X.-Y.; Zhu, Z.-W.; Chen, M.-X. Sulfur supply reduces cadmium uptake and translocation in rice grains (Oryza sativa L.) by enhancing iron plaque formation, cadmium chelation and vacuolar sequestration. Environ. Pollut. 2018, 238, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Dixit, G.; Singh, A.P.; Kumar, A.; Singh, P.K.; Kumar, S.; Dwivedi, S.; Trivedi, P.K.; Pandey, V.; Norton, G.J.; Dhankher, O.P.; et al. Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J. Hazard. Mater. 2015, 298, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Delang, C.O. Heavy metal contamination of soils in China: Standards, geographic distribution, and food safety considerations. A review. DIE ERDE J. Geogr. Soc. Berl. 2018, 149, 261–268. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, X.; Shohag, J.I.; Gu, M. Bioaccessibility and human exposure assessment of cadmium and arsenic in pakchoi genotypes grown in co-contaminated soils. Int. J. Environ. Res. Public Health 2017, 14, 977. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Rahaman, S.; Rahman, M.; Mise, N.; Sikder, T.; Ichihara, G.; Uddin, K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Liu, J.; Chen, Z.; Jiang, W.; Huang, X.; Xue, Q. Mechanical properties evolution and microscopic mechanisms of arsenic and cadmium co-contaminated clayey soils. Bull. Eng. Geol. Environ. 2023, 82, 229. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Zia, Z.; Fahad, S.; Abbas, S.; Hammad, H.M.; Shahzad, A.N.; Abbas, F.; Alharby, H.; Shahid, M. Arsenic uptake, accumulation and toxicity in rice plants: Possible remedies for its detoxification: A review. Environ. Sci. Pollut. Res. 2017, 24, 9142–9158. [Google Scholar] [CrossRef] [PubMed]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Chen, Y.; Weng, L.; Ma, J.; Khan, Z.H.; Liao, Z.; Magid, A.S.I.A.; Li, Y. Watering techniques and zero-valent iron biochar pH effects on As and Cd concentrations in rice rhizosphere soils, tissues and yield. J. Environ. Sci. 2021, 100, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.D.; Feng, C.; Li, J.S.; Wang, K.K. Soil-water characteristic and microscopic mechanism of arsenic and cadmium composite heavy metal contaminated soil. Rock Soil Mech. 2022, 43, 2841–2851. [Google Scholar] [CrossRef]
- Bolan, N.; Mahimairaja, S.; Kunhikrishnan, A.; Naidu, R. Sorption–bioavailability nexus of arsenic and cadmium in variable-charge soils. J. Hazard. Mater. 2013, 261, 725–732. [Google Scholar] [CrossRef]
- Shen, B.; Wang, X.; Zhang, Y.; Zhang, M.; Wang, K.; Xie, P.; Ji, H. The optimum pH and Eh for simultaneously minimizing bioavailable cadmium and arsenic contents in soils under the organic fertilizer application. Sci. Total Environ. 2020, 711, 135229. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.L.; Yang, Z.H.; Cai, L.Y.; Liu, L.; Liao, Y.P. Remediation effect of phosphorus and ferric amendments on the soil complexly contaminated by Pb, Cd and Ad and the process optimization. J. Saf. Environ. 2015, 15, 314–319. [Google Scholar] [CrossRef]
- Komarek, M.; Vanek, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef]
- Marques, A.P.; Rangel, A.O.; Castro, P.M.L. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Lin, H.; Pan, H.L.; He, Y.H.; Dong, Y.B.; Li, B. Research advances of remedying arsenic-contaminated farmland soil by chemical techniques. Environ. Pollut. Control 2020, 42, 780–787. [Google Scholar] [CrossRef]
- Bosso, L.; Scelza, R.; Testa, A.; Cristinzio, G.; Rao, M.A. Depletion of pentachlorophenol contamination in an gricultural soil treated with Byssochlamys nivea, Scopulariopsis brumptii and urban waste compost: A laboratory microcosm study. Water Air Soil Pollut. 2015, 226, 183. [Google Scholar] [CrossRef]
- Tomasini, A.; León-Santiesteban, H. The role of the filamentous fungi in bioremediation. Fungal Bioremediat. Fundam. Appl. 2019, 1, 3–21. [Google Scholar]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a potential strategy for remediation of contaminated mining soils: Mechanisms, applications, and future perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.B.; Fan, Z.L.; Lei, M.; Wei, C.Y.; Huang, Z.C. Arsenic super-enriched plant Centipede grass and its enrichment characteristics for arsenic. Chin. Sci. Bull. 2002, 47, 207–210. [Google Scholar]
- Wang, H.Y.; Wen, S.L.; Chen, P.; Zhang, L.; Cen, K.; Sun, G.X. Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environ. Sci. Pollut. Res. 2016, 23, 3781–3788. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Wang, Y.; Ling, X.; Chen, Z.; Tang, Y.; Qiu, H.; Ying, R.; Qiu, R. Effects of an iron-silicon material, a synthetic zeolite and an alkaline clay on vegetable uptake of As and Cd from a polluted agricultural soil and proposed remediation mechanisms. Environ. Geochem. Health 2017, 39, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- GB15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Li, P.; Lin, C.; Cheng, H.; Duan, X.; Lei, K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol. Environ. Saf. 2015, 113, 391–399. [Google Scholar] [CrossRef]
- Ran, J.W.; Ning, P.; Sun, X.; Liang, D.L. Heavy metal pollution characteristics and potential risks of soil and crops in gejiu, yunnan. Environ. Monit. China 2019, 35, 62–68. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, M.; Wang, G.J.; Zhou, H.; Yang, W.T.; Gu, J.F.; Liao, B.H. Remediation of paddy soil complexly polluted with cadmium and arsenic using 2 combined amendments. Acta Sci. Circumstantiae 2018, 38, 2008–2013. [Google Scholar] [CrossRef]
- Yun, S.-W.; Park, C.-G.; Jeon, J.-H.; Darnault, C.J.; Baveye, P.C.; Yu, C. Dissolution behavior of As and Cd in submerged paddy soil after treatment with stabilizing agents. Geoderma 2016, 270, 10–20. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, K.; Ashraf, M.; Parveen, R.; Mustafa, I.; Khan, A.; Bibi, Z.; Akram, N. Bioaccumulation of heavy metals and metalloids in luffa (Luffa cylindrica L.) Irrigated with domestic wastewater in jhang, pakistan: A prospect for human nutrition. Pak. J. Bot. 2015, 47, 217–224. [Google Scholar]
- Wang, X.Q.; Liu, C.P.; Du, Y.H.; Liu, X.W.; Tan, J.; Liu, D.H.; Li, F.B. Effects of stabilizing remediation of Cd and As in paddy rice by applying combined zero-valent iron and humus. Ecol. Environ. Sci. 2018, 27, 2329–2336. [Google Scholar] [CrossRef]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jing, C. A review of arsenic interfacial geochemistry in groundwater and the role of organic matter. Ecotoxicol. Environ. Saf. 2019, 183, 109550. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M. Plant responses to arsenic: Uptake, metabolism and resistance to arsenic toxicity—A review. Zemdirb. Agric. 2017, 104, 369–376. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Rao, P. Toxicity, bioavailability and metal speciation. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1993, 106, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Huang, J.; Li, H.; Peng, X.; Xia, L.; Zhou, L.; Zhang, T.; Liu, Z.; He, Q.; Luo, F.; et al. Biogeochemical behavior and pollution control of arsenic in mining areas: A review. Front. Microbiol. 2023, 14, 1043024. [Google Scholar] [CrossRef]
- Panthri, M.; Gupta, M. An insight into the act of iron to impede arsenic toxicity in paddy agro-system. J. Environ. Manag. 2022, 316, 115289. [Google Scholar] [CrossRef]
- Porter, S.K.; Scheckel, K.G.; Impellitteri, C.A.; Ryan, J.A. Toxic metals in the environment: Thermodynamic considerations for possible immobilization strategies for Pb, Cd, As, and Hg. Crit. Crit. Rev. Environ. Sci. Technol. 2004, 34, 495–604. [Google Scholar] [CrossRef]
- Suda, A.; Makino, T. Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: A review. Geoderma 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Bandara, T.; Franks, A.; Xu, J.; Bolan, N.; Wang, H.; Tang, C. Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit. Rev. Environ. Sci. Technol. 2020, 50, 903–978. [Google Scholar] [CrossRef]
- Bolan, N.S.; Makino, T.; Kunhikrishnan, A.; Kim, P.-J.; Ishikawa, S.; Murakami, M.; Naidu, R.; Kirkham, M.B. Cadmium contamination and its risk management in rice ecosystems. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands; Volume 119, pp. 183–273. [CrossRef]
- Naidu, R.; Bolan, N.; Kookana, R.S.; Tiller, K. Ionic-strength and pH effects on the sorption of cadmium and the surface charge of soils. Eur. J. Soil Sci. 1994, 45, 419–429. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, F.B.; Liu, C.S.; Huang, W.; Liu, T.X.; Yu, W.M. Iron redox cycling coupled to transformation and immobilization of heavy metals: Implications for paddy rice safety in the red soil of South China. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 279–317. [Google Scholar] [CrossRef]
- Tassi, E.; Pouget, J.; Petruzzelli, G.; Barbafieri, M. The effects of exogenous plant growth regulators in the phytoextraction of heavy metals. Chemosphere 2008, 71, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Huang, Y.-Z.; Bao, Q.-L.; Wei, X.-D.; Tie, B.-Q.; Zhang, S.-N.; Han, N.; Huang, Y.-C. Effect of chelating agents and organic acids on remediation of cadmium and arsenic complex contaminated soil using xanthium sibiricum. Environ. Sci. 2022, 43, 4292–4300. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Yang, A.J.; Hu, X.; Deng, Q.J.; Fan, J.Y. Study on enrichment effect and the vivo existing form in vivo of As, Sb and Cd in Pteris vittata L. Guangdong Agric. Sci. 2019, 46, 68–78. [Google Scholar] [CrossRef]
- Chen, C.D.; Zhang, A.N.; La, M.; Qi, G.; Zhao, G.Q.; Chu, C.J. Soil heavy metal contamination and enrichment of dominant plants in coal waste piles in Pingdingshan area. Ecol. Environ. Sci. 2019, 28, 1216–1223. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Li, H.; Liao, B.; Zeng, Q. Study of the potential of two agricultural cropping patterns for remediating heavy metals from soils. Acta Ecol. Sin. 2016, 36, 688–695. [Google Scholar] [CrossRef]
- Luo, Q.; He, L.Q.; Yang, W.M. Research progress of oil sunflower repairing heavy metals contaminated farmland. Mod. Agric. Sci. Technol. 2016, 1, 225–226+232. [Google Scholar]
- Han, N. Effect of chelating agent on phytoremediation of cadmium and arsenic compound contaminated farmland. Chin. Acad. Agric. Sci. 2020. [Google Scholar] [CrossRef]
- Chen, H.; Cutright, T. EDTA and HEDTA effects on Cd, Cr, and Ni uptake by Helianthus annuus. Chemosphere 2001, 45, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.X.; Chai, T.Y. Research progress on tolerance of Indian mustard (Brassica juncea L.) to heavy metal. Chin. J. Eco-Agric. 2011, 19, 226–234. [Google Scholar] [CrossRef]
- Yang, W.; Luo, L.; Bostick, B.C.; Wiita, E.; Cheng, Y.; Shen, Y. Effect of combined arsenic and lead exposure on their uptake and translocation in Indian mustard. Environ. Pollut. 2021, 274, 116549. [Google Scholar] [CrossRef] [PubMed]
- Gurajala, H.K.; Cao, X.; Tang, L.; Ramesh, T.M.; Lu, M.; Yang, X. Comparative assessment of Indian mustard (Brassica juncea L.) genotypes for phytoremediation of Cd and Pb contaminated soils. Environ. Pollut. 2019, 254, 113085. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, A.; Iqbal, M. Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicol. Environ. Saf. 2009, 72, 626–634. [Google Scholar] [CrossRef]
- Kang, H.; Yang, Z.; Wang, H.L.; Li, B.W. Study on the absorption accumulation characteristics of Indian mustard to heavy metals Pb, Cd and Zn in soil. J. Anhui Agric. Sci. 2010, 38, 19378–19381. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 411, 438. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, T.; An, Z.; Lei, M.; Huang, Z.; Liao, X.; Liu, Y. Potential of Pteris vittata L. for phytoremediation of sites co-contaminated with cadmium and arsenic: The tolerance and accumulation. J. Environ. Sci. 2008, 20, 62–67. [Google Scholar] [CrossRef]
- El Aafi, N.; Brhada, F.; Dary, M.; Maltouf, A.F.; Pajuelo, E. Rhizostabilization of metals in soils using Lupinus luteus inoculated with the metal resistant rhizobacterium Serratia sp. Msmc541. Int. J. Phytorem. 2012, 14, 261–274. [Google Scholar] [CrossRef]
- Han, N.; Huang, Y.Z.; Wei, X.D.; Tie, B.Q.; Zhang, S.N.; Wang, B.S.; Bao, Q.L.; Huang, Y.C. Effect of chelating agents on remediation of cadmium and arsenic complex contaminated soil using oil sunflower. J. Agro-Environ. Sci. 2019, 38, 1891–1900. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Feng, W.; Xin, L.; Xu, Z. Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci. Total Environ. 2019, 650, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, X.; Chi, Y.; Huang, L.; Li, W.C.; Ye, Z. Rhizosphere bacterial community composition affects cadmium and arsenic accumulation in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2021, 222, 112474. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, C.; Xiao, X.; Guo, Z.; Peng, C.; Wang, X. Phytoextraction potential of arsenic and cadmium and response of rhizosphere microbial community by intercropping with two types of hyperaccumulators. Environ. Sci. Pollut. Res. 2022, 29, 91356–91367. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, B.S.; Huang, Y.Y.; Yao, Y.W.; Lin, J.M.; Liu, K.H.; Yu, F.M. Mechanism study on the phytoremediation of cadmium- and arsenic-contaminated soil by polygonaceae plants with Enterobacter sp. J. Agro-Environ. Sci. 2020, 39, 304–312. [Google Scholar] [CrossRef]
- Jiang, K.; Zhou, K. Chemical immobilization of lead, cadmium, and arsenic in a smelter-contaminated soil using 2,4,6-trimercaptotriazine, trisodium salt, nonahydrate and ferric sulfate. J. Soils Sediments 2018, 18, 1060–1065. [Google Scholar] [CrossRef]
- Cui, H.; Fan, Y.; Xu, L.; Zhou, D.; Mao, J.; Fang, G.; Cang, L.; Zhu, Z. Sustainability of in situ remediation of Cu- and Cd-contaminated soils with one-time application of amendments in Guixi, China. J. Soils Sediments 2016, 16, 1498–1508. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Harvey, O.R.; Herbert, B.E.; Rhue, R.D.; Kuo, L.-J. Metal Interactions at the Biochar-Water Interface: Energetics and Structure-Sorption Relationships Elucidated by Flow Adsorption Microcalorimetry. Environ. Sci. Technol. 2011, 45, 5550–5556. [Google Scholar] [CrossRef]
- Zhang, H.M.; Xu, M.G.; Lv, J.L.; Li, J.M. A review of studies on effects of pH on cadmium sportion and desorption in soil. J. Agro-Environ. Sci. 2005, 24, 320–324. [Google Scholar]
- Qiao, J.-T.; Liu, T.-X.; Wang, X.-Q.; Li, F.-B.; Lv, Y.-H.; Cui, J.-H.; Zeng, X.-D.; Yuan, Y.-Z.; Liu, C.-P. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Lin, C.-F.; Lo, S.-S.; Lin, H.-Y.; Lee, Y. Stabilization of cadmium contaminated soils using synthesized zeolite. J. Hazard. Mater. 1998, 60, 217–226. [Google Scholar] [CrossRef]
- Xiong, S.J.; Huang, X.C. Research progress of zeolite in cadmium-contaminated soil remediation. Contemp. Hortic. 2017, 1, 8–9+56. [Google Scholar] [CrossRef]
- Liu, C.H.; Yi, X.; Zhou, J.; Xu, L. Application of passive materials in remediation of heavy metal contaminated soils. Anhui Agric. Sci. Bull. 2017, 23, 74–77+85. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Review on remediation technologies for arsenic-contaminated soil. Front. Environ. Sci. Eng. 2019, 14, 24. [Google Scholar] [CrossRef]
- Chen, S.; Xu, M.; Ma, Y.; Yang, J. Evaluation of different phosphate amendments on availability of metals in contaminated soil. Ecotoxicol. Environ. Saf. 2007, 67, 278–285. [Google Scholar] [CrossRef]
- Karlsson, T.; Elgh-Dalgren, K.; Björn, E.; Skyllberg, U. Complexation of cadmium to sulfur and oxygen functional groups in an organic soil. Geochim. Cosmochim. Acta 2007, 71, 604–614. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Zeng, G.-M.; Cheng, M.; Gong, X.-M.; Wan, J.; Luo, H. Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour. Technol. 2018, 261, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.Y.; Jiang, L.L.; Zhang, C.L.; Zhou, Y.X.; Xie, T.; Liao, C.J. Passivation effects of silkwo202rm organic fertilizer and iron base compound material on heavy metals in contaminated soil by cadmium, arsenic and zinc. J. South. Agric. 2019, 50, 2436–2442. [Google Scholar] [CrossRef]
- Fu, D.; He, Z.; Su, S.; Xu, B.; Liu, Y.; Zhao, Y. Fabrication of α-FeOOH decorated graphene oxide-carbon nanotubes aerogel and its application in adsorption of arsenic species. J. Colloid Interface Sci. 2017, 505, 105–114. [Google Scholar] [CrossRef]
- Warren, G.P.; Alloway, B.J. Reduction of arsenic uptake by lettuce with ferrous sulfate applied to contaminated soil. J. Environ. Qual. 2003, 32, 767–772. [Google Scholar] [CrossRef]
- Liang, Y.; Li, F.Y.; Yang, F.; Shi, W.L. Immobilization and its mechanisms of heavy metal contaminated soils by phosphate-containing amendment and biochar. J. Agro-Environ. Sci. 2013, 32, 2377–2383. [Google Scholar] [CrossRef]
- Lei, M.; Zeng, M.; Liao, B.-H.; Hu, L.-Q.; Zhou, H.; Long, S.-B. Effects of phosphorus-containing substances on arsenic uptake by rice. Environ. Sci. 2014, 35, 3149–3154. [Google Scholar] [CrossRef]
- Yuan, Y.; Chai, L.; Yang, Z.; Yang, W. Simultaneous immobilization of lead, cadmium, and arsenic in combined contaminated soil with iron hydroxyl phosphate. J. Soils Sediments 2017, 17, 432–439. [Google Scholar] [CrossRef]
- Casiot, C.; Egal, M.; Elbaz-Poulichet, F.; Bruneel, O.; Bancon-Montigny, C.; Cordier, M.-A.; Gomez, E.; Aliaume, C. Hydrological and geochemical control of metals and arsenic in a Mediterranean river contaminated by acid mine drainage (the Amous River, France); preliminary assessment of impacts on fish (Leuciscus cephalus). Appl. Geochem. 2009, 24, 787–799. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.-R.; Tang, J.-F.; Cotner, J.B.; Xu, Y.-Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Nakamura, K.; Katou, H.; Ishikawa, S.; Ito, M.; Honma, T.; Miyazaki, N.; Takehisa, K.; Sano, S.; Matsumoto, S.; et al. Simultaneous decrease of arsenic and cadmium in rice (Oryza sativa L.) plants cultivated under submerged field conditions by the application of iron-bearing materials. Soil Sci. Plant Nutr. 2016, 62, 340–348. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Q.; Zhou, X.; Ren, H.; Zhang, H.; Xu, R.; Wang, X.; Peters, M.; Zhu, G.; Wei, R.; et al. Red mud (RM)-Induced enhancement of iron plaque formation reduces arsenic and metal accumulation in two wetland plant species. Int. J. Phytoremediation 2015, 18, 269–277. [Google Scholar] [CrossRef]
- Zotiadis, V.; Argyraki, A.; Theologou, E. Pilot-scale application of attapulgitic clay for stabilization of toxic elements in contaminated soil. J. Geotech. Geoenviron. Eng. 2012, 138, 633–637. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zou, J.-L.; Yang, W.-T.; Zhou, H.; Liao, B.-H. Synergetic control of bioavailability of Pb, Cd and As in the rice paddy system by combined amendments. Environ. Sci. 2016, 37, 4004–4010. [Google Scholar] [CrossRef]
- Wilson, S.C.; Leech, C.D.; Butler, L.; Lisle, L.; Ashley, P.M.; Lockwood, P.V. Effects of nutrient and lime additions in mine site rehabilitation strategies on the accumulation of antimony and arsenic by native Australian plants. J. Hazard. Mater. 2013, 261, 801–807. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Zhu, G.X.; Wu, T.L.; Liang, Q.; Wang, X.X. Effects of lime on chemical forms of heavy metals under combined pollution of Cu, Cd, Pb and Zn in soils. Environ. Eng. 2019, 37, 158–164. [Google Scholar] [CrossRef]
- Sun, L.J.; Qin, Q.; Song, K.; Qiao, H.X.; Xue, Y. The remediation and safety utilization techniques for Cd contaminated farmland soil: A review. Ecol. Environ. Sci. 2018, 27, 1377–1386. [Google Scholar] [CrossRef]
- Silver, S.; Phung, L.T. A bacterial view of the periodic table: Genes and proteins for toxic inorganic ions. J. Ind. Microbiol. Biotechnol. 2005, 32, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Luo, L.Q. Research progress on the application and interaction mechanism between specific microorganisms and heavy metals in soil. Rock Miner. Anal. 2017, 36, 209–221. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, T.; Chen, Z.; Pan, W. Solidification /stabilization treatment of gold mining waste residue. Chin. J. Environ. Eng. 2013, 7, 4951–4957. [Google Scholar]
- Gu, J.-F.; Zhou, H.; Yang, W.-T.; Peng, P.-Q.; Zhang, P.; Zeng, M.; Liao, B.-H. Effects of an additive (hydroxyapatite–biochar–zeolite) on the chemical speciation of Cd and As in paddy soils and their accumulation and translocation in rice plants. Environ. Sci. Pollut. Res. 2018, 25, 8608–8619. [Google Scholar] [CrossRef]
- Cao, J.; Chen, Z.; Wu, Q.; Wu, Z.H.; Dong, H.Y.; Qiu, R.L.; Wang, S.Z.; He, E.K.; Tang, Y.T. Mitigation of cadmium and arsenic in rice plant by soil application of steel slag and/or biochar with water management. J. Agro-Environ. Sci. 2018, 37, 1475–1483. [Google Scholar] [CrossRef]
- Xiong, J.; Guo, L.L.; Li, S.P.; Lin, Q.M.; Chen, Y.J. Optimizing the formulation and stabilization effects of an amendment for cadmium and arsenic contaminated soil. J. Agro-Environ. Sci. 2019, 38, 1909–1918. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Y.; Sun, Y.; Chen, X.; Yuan, L. Coal gangue-based magnetic porous material for simultaneous remediation of arsenic and cadmium in contaminated soils: Performance and mechanisms. Chemosphere 2023, 338, 139380. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Ma, S.; Liu, J.; Jiang, X.; Dai, D. Remediation of Arsenic and Cadmium Co-Contaminated Soil: A Review. Sustainability 2024, 16, 687. https://doi.org/10.3390/su16020687
Lin M, Ma S, Liu J, Jiang X, Dai D. Remediation of Arsenic and Cadmium Co-Contaminated Soil: A Review. Sustainability. 2024; 16(2):687. https://doi.org/10.3390/su16020687
Chicago/Turabian StyleLin, Mengting, Sairu Ma, Jie Liu, Xusheng Jiang, and Demin Dai. 2024. "Remediation of Arsenic and Cadmium Co-Contaminated Soil: A Review" Sustainability 16, no. 2: 687. https://doi.org/10.3390/su16020687
APA StyleLin, M., Ma, S., Liu, J., Jiang, X., & Dai, D. (2024). Remediation of Arsenic and Cadmium Co-Contaminated Soil: A Review. Sustainability, 16(2), 687. https://doi.org/10.3390/su16020687








