Review: Microemulsions for the Sustainable Development of EOR
Abstract
1. Introduction
2. Formation Mechanisms of Microemulsions
2.1. Interface Adsorption Film Theory
2.2. Instantaneous Negative Interfacial Tension Theory
2.3. R Ratio Theory
2.4. Hydrophilic–Lipophilic Difference Theory
3. Study of the Phase Behaviors of Microemulsions
3.1. Winsor Phase Diagrams
3.2. Fish-like Diagrams
3.3. Quasi-Ternary Phase Diagrams
4. Study on the Oil Displacement Mechanisms of Middle-Phase Microemulsions
4.1. Tension
4.2. Microscopic Mechanism
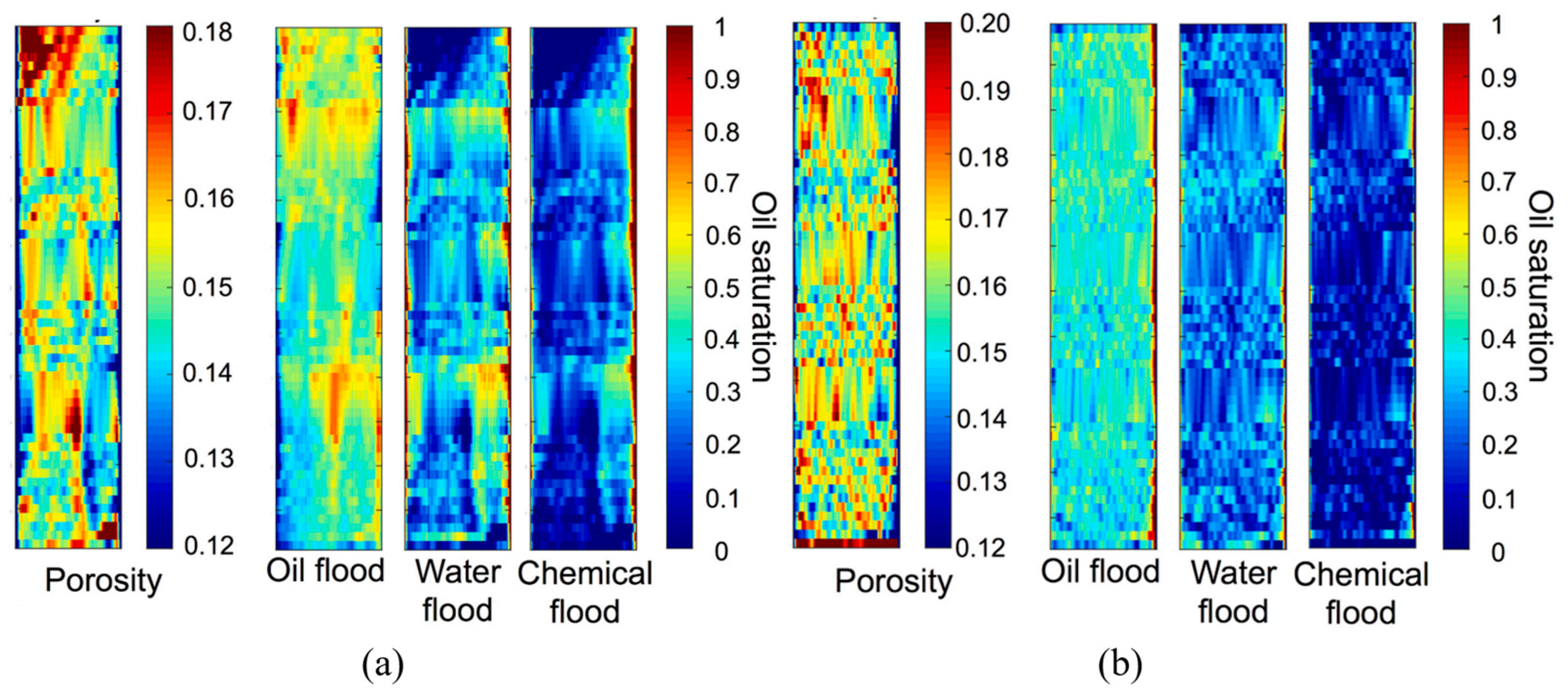
4.3. CT
5. Application of Middle-Phase Microemulsions in EOR
5.1. Development of Microemulsion EOR
5.2. Practical Application of Microemulsion EOR
6. Conclusions
- (1)
- As a low-concentration surfactant chemical flooding technique, middle-phase microemulsions offer significant advantages in terms of low dosage and high effectiveness. This is mainly reflected in three aspects of mechanism, namely, the expansion of two additional types based on the Winsor model, the quantitative modeling of the HLD equation to control different parameters for various types of surfactants, and the experimental methods linking quasi-ternary phase diagrams with the HLD equation and multiple phase diagrams. These improvements make phase diagrams more intuitive and more efficient for the screening of middle-phase microemulsion systems, which can then guide quantitative parameter-based studies on middle-phase emulsions as the next step.
- (2)
- Middle-phase microemulsion systems are environmentally friendly for enhanced oil recovery, as the use of mildly alkaline chemicals in these systems poses minimal harm to reservoirs. The application of middle-phase microemulsion flooding in practice offers enhanced safety and reliability. Microscopic mechanism studies and CT technology enable quantitative and visual representation of wettability reversal, capillary pressure reduction, and emulsification, providing strong support for the efficacy of middle-phase microemulsion flooding.
- (3)
- Middle-phase microemulsion flooding systems exhibit a wide range of applications, particularly in more complex reservoirs. In low-permeability tight reservoirs characterized by low permeability, low porosity, and poor reservoir properties, middle-phase microemulsion flooding systems can control the profile, emulsify, and reduce interfacial tension. For high-temperature, high-salinity reservoirs, middle-phase microemulsion flooding systems can enhance permeability, inhibit the formation of salt and mineral scales in the oil layer, stabilize and disperse oil droplets, and maintain stability under high-temperature, high-salinity conditions, and they can be continuously used in enhancing oil recovery.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author | Time | Block and Permeability | Agent | Effect and Conclusion | Reference |
|---|---|---|---|---|---|
| Bardhan | 2013 | None | A mixture of polyoxyethylene (20) cetyl ether (Brij-58) and a cetyl trimethyl ammonium bromide (CTAB) surfactant coupled with 1-pentanol (Pn) | Starting from molecular dynamics models, Bardhan demonstrated that the microemulsion droplet size changes with Gibbs free energy, which can be used to predict the size of nanoparticles in mixed surfactant middle-phase microemulsions. This has been proposed as a theoretical model. | [94] |
| Co | 2015 | Berea core, 300 mD | In situ SP flooding, a functionalized polymer surfactant, modified water-soluble hydrolyzable polyacrylamide (HPAM) | There is no direct correlation between reducing IFT and the recovery rate; instead, there is an optimal IFT. This system involves polymer surfactants rather than SP mixtures. | [95] |
| Nguele | 2016 | SK-1H, SK-2H, and SK-3H blocks | Cationic dimerized ammonium salt surfactant | Through adsorption experiments and infrared absorption spectroscopy, it has been proven that the hydrophilic head is preferentially adsorbed on the rock surface. Cationic dimeric ammonium surfactants can slow down the formation of by-products with acidic properties. | [96] |
| Chen | 2018 | Daqing oil field, 790 mD | In situ ASP flooding, a Na2CO3, mixed surfactant of alkyl benzene sulfonate and fatty alcohol propoxy ether sulfate | Applying a negative salinity gradient to reduce surfactant losses, with a surfactant concentration of 0.3% and a salt concentration of 2%, can result in a maximum recovery rate of up to 45.9% for the original oil in place (OOIP). This represents a 13.1% improvement in recovery compared with ASP flooding. | [97] |
| Ayirala | 2019 | Carbonate reservoir in a stock tank reservoir | Non-ionic surfactant: ethoxylated alcohol with an average chain length of 12. Polymer: sulfonated polyacrylamide with a sulfonation degree of 25% | High salinity water: Significant shift from oil-wet to intermediate-wet or low oil-wet conditions, with noticeable effects. Low salinity water: Transition from oil-wet to low oil-wet conditions, with less pronounced effects. | [98] |
| Han | 2019 | Daqing oil field, 1018 mD | In situ ASP flooding, Na2CO3, four surfactants, including two sodium alkyl aryl sulfonates and two propoxy alcohol sulfates, with an HPAM molecular weight ranging from 12 million to 16 million | At a negative salinity gradient, the recovery rate is approximately 33.5% to 38.5% higher compared with polymer flooding and approximately 90% higher than the polymer flooding of residual oil. | [99] |
| Li | 2019 | Daqing oil field, 1800 mD | In situ ASP flooding, NaCl/Na2CO3, heavy alkyl benzene sulfonate, and polyacrylamide (HPAM) | Under the conditions of an alkali mass fraction of 1.2 wt%, a surfactant mass fraction of 0.3 wt%, and a polymer mass fraction of 2500 mg/L, as the viscosity of the flooding agent increases, the weak alkali ASP system improves the recovery rate by 22.0%. Weak alkali ASP can achieve better interfacial tension and displacement efficiency compared with strong alkali ASP. | [100] |
| Riswati | 2019 | Indonesia oil reservoir, 68.5 mD | In situ ASP flooding (0.5% linear alkyl benzene sulfonate (LAS) and 2% diethylene glycol butyl ether (DGBE), 1% Na2CO3) | A negative salinity gradient allows the front-end Winsor II microemulsion to transform into a Winsor III microemulsion, preventing surfactant failure at low salinity. The maximum recovery rate can reach 75.80%. | [101] |
| Han | 2020 | Daqing oil field, 1031.5 mD | In situ ASP flooding, two AESs with different alkyl lengths and number of POs, ABS, NaCl, and 12 million HAPM | By employing polymer SP flooding and subsequent core flooding with a negative salinity gradient, the final cumulative oil recovery reaches as high as 93.9% OOIP, with a residual oil saturation of approximately 3.4%. When compared with polymer flooding without preflushing with brine, the additional OOIP recovery from the negative salinity gradient flooding exceeds 10%. | [102] |
| Kurnia | 2020 | North Dakota shale formations, 2345 mD | In situ ASP flooding, zwitterionic and non-ionic surfactants: cocamidopropyl hydroxysulfonamide and alcohol propoxy sulfate, and 10 million HPAM | A binary surfactant system with a 0.3% surfactant concentration can displace 63–75% of the residual oil due to the ultra-low interfacial tension. | [103] |
| Dantas | 2021 | Brazil Botucatu block | Directly injected microemulsion (saponified coconut oil (SCO) is the anionic surfactant, Na2CO3 and 1-butanol are cosurfactants, and kerosene is the oil phase) | The maximum total recovery rate is 97%. | [52] |
| Liang | 2021 | Mahu tight oil reservoir Ma-131, 0.17–0.45 mD | Complex nano-fluid, 0.1 wt% DR800 and 0.1 wt% DME | The highest oil recovery rate is 31.1% higher than water flooding in the lab. The normalized cumulative oil production of Well-B2 increases by about 18.6%, from 3716.0 tons per 1000 m to 4406.4 tons per 1000 m. | [104] |
| Slamet | 2021 | None, artificial stone (synthetic core) | Sodium lignosulfonate, PEG-4000, ammonium persulfate, and acetone | The highest oil yield, 79%, is obtained by the PS1 surfactant with the highest PEG dosage in 16,000 ppm brine solution. | [105] |
| Sekerbayeva | 2022 | Oil fields in Kazakhstan | Benzenesulfonic acid, dimethyl-, mono-C11-16- alkyl deriv. and benzenesulfonic acid, C14-24-branched and linear, alkyl deriv. oxirane, and Na2CO3 | The microemulsion phase constituted nearly 40% of the total height of the oil/brine column by means of the hybrid method. The recovery factor after injecting formation water was 52%, and it increased to 61% after optimized LSW injection. After switching to the engineered brine/surfactant, the recovery factor reached 70%, which proves the effectiveness of the hybrid method. | [106] |
| Panthi | 2022 | None, 50–670 mD | In situ ASP flooding, a single surfactant with 0.5% Alfoterra, and 8 million HPAM | With the preflush of alkali and soft brine, a 0.5 PV ASP plug, and 2700 ppm polymer, the cumulative oil recovery rate from the core flooding can reach 94% to 96% of the original oil in place. | [107] |
| Mariam | 2023 | Indiana limestone outcrop cores, 111.54 mD | An anionic surfactant (Soloterra-113H), South Caspian Sea water | Four gradual negative salinity gradients design with phase behavior of Winsor Type II-III-I provided a 33.1% higher incremental of OOIP. | [108] |
| Kurnia | 2023 | Bangko oil field, Berea cores, 406.87 mD | Anionic (S11) and amphoteric (S20) surfactants, NaCl, trisodium citrate dihydrate Na3C6H5O7·2H2O, and Fe3O4 | Core flooding experiment showed an improvement in the recovery factor when using surfactant-Fe3O4 nanofluid, namely 5.09% of OOIP or 17.80% of ROIP with total oil recovery of 76.50% of OOIP. | [109] |
References
- Nelson, R.C.; Pope, G.A. Phase Relationships in Chemical Flooding. Soc. Pet. Eng. J. 1978, 18, 325–338. [Google Scholar] [CrossRef]
- Wasan, D.T.; McNamara, J.J.; Shah, S.M.; Sampath, K.; Aderangi, N. The Role of Coalescence Phenomena and Interfacial Rheological Properties in Enhanced Oil Recovery: An Overview. J. Rheol. 1979, 23, 181–207. [Google Scholar] [CrossRef]
- Doscher, T.M.; Wise, F.A. Enhanced Crude Oil Recovery Potential-An Estimate. J. Pet. Technol. 1976, 28, 575–585. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Block Copolymer Stabilized Nonaqueous Biocompatible Sub-Micron Emulsions for Topical Applications. Int. J. Pharm. 2013, 448, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Gogarty, W.B. Oil Recovery with Surfactants: History and a Current Appraisal, in Improved Oil Recovery by Surfactant and Polymer Flooding. Acad. Press 1977, 37, 27–54. [Google Scholar]
- Pokhriyal, N.K.; Devi, S. Effect of Water Solubility of Monomer on Reaction Kinetics Oil-Water Microemulsion Copolymerisation. Eur. Polym. J. 2000, 36, 333–343. [Google Scholar] [CrossRef]
- Shinoda, K.; Friberc, S. Microemulsions: Colloidal aspects. Adv. Colloid Interface Sci. 1975, 4, 281–300. [Google Scholar] [CrossRef]
- Giang, H.; Shlomovitz, R.; Schick, M. Microemulsions, Modulated Phases and Macroscopic Phase Separation: A Unified Picture of Rafts. Essays Biochem. 2015, 57, 21–32. [Google Scholar] [CrossRef][Green Version]
- Pei, H.; Zhang, G.; Ge, J.; Jin, L. The Effect of Oil Viscosity, Permeability, and Residual Oil Saturation on the Performance of Alkaline Flooding in the Recovery of Heavy Oil. Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 702–710. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Y.; Zhang, Y.; Wang, Z. The Key to Surfactant-Free Microemulsion Demulsification: CO2 Promotes the Transfer of Amphiphilic Solvent to Aqueous Phase. J. Mol. Liq. 2022, 345, 117000. [Google Scholar] [CrossRef]
- Sottmann, T.; Strey, R. Ultralow Interfacial Tensions in Water–n-Alkane–Surfactant Systems. J. Chem. Phys. 1997, 106, 8606–8615. [Google Scholar] [CrossRef]
- Roger, K.; Cabane, B.; Olsson, U. Formation of 10−100 Nm Size-Controlled Emulsions through a Sub-PIT Cycle. Langmuir 2010, 26, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Elraies, K.A. Microemulsion in Enhanced Oil Recovery. In Science and Technology Behind Nanoemulsions; Karakuş, S., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-570-8. [Google Scholar]
- Liu, D.; Xu, J.; Zhao, H.; Zhang, X.; Zhou, H.; Wu, D.; Liu, Y.; Yu, P.; Xu, Z.; Kang, W.; et al. Nanoemulsions Stabilized by Anionic and Non-Ionic Surfactants for Enhanced Oil Recovery in Ultra-Low Permeability Reservoirs: Performance Evaluation and Mechanism Study. Colloids Surf. Physicochem. Eng. Asp. 2022, 637, 128235. [Google Scholar] [CrossRef]
- Wang, Z.; Pang, R.; Le, X.; Peng, Z.; Hu, Z.; Wang, X. Survey on Injection–Production Status and Optimized Surface Process of ASP Flooding in Industrial Pilot Area. J. Pet. Sci. Eng. 2013, 111, 178–183. [Google Scholar] [CrossRef]
- Bourrel, M.; Schechter, R.S. Microemulsions and Related Systems: Formulation, Solvency, and Physical Properties; Surfactant Science Series; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 1988. [Google Scholar]
- Rousseau, D.; Le Gallo, C.; Wartenberg, N.; Courtaud, T. Mobility of Microemulsions: A New Method to Improve Understanding and Performances of Surfactant EOR. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 18 April 2022; p. D021S013R001. [Google Scholar]
- Roger, K.; Cabane, B.; Olsson, U. Emulsification through Surfactant Hydration: The PIC Process Revisited. Langmuir 2011, 27, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Winsor, P. Solubilisation with Amphiphilic Compounds. Chem. Ind. 1960, 23, 632–644. [Google Scholar]
- Jaffar, N.A.; Luan, N.; Arisandi, E. Novel Microemulsion Breaker System Remove Drill-in-Fluids Filter Cake and Remediate Near Wellbore Damage to Enhance Productivity of Horizontal Wells of Offshore Sabah, Malaysia. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 18 March 2022; p. D031S021R005. [Google Scholar]
- Wang, Z.; Lin, X.; Zhang, L.; Zhong, H.; Fan, M.; Yu, T. Opportunities for Fluorocarbons Interior Coating Technology in ASP Flooding EOR: Evaluation of Corrosion Protection and Drag Reduction. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 12 November 2016; p. D011S010R005. [Google Scholar]
- Shen, P.; Wang, J.; Yuan, S.; Zhong, T.; Jia, X. Study of Enhanced-Oil-Recovery Mechanism of Alkali/Surfactant/Polymer Flooding in Porous Media From Experiments. SPE J. 2009, 14, 237–244. [Google Scholar] [CrossRef]
- Choi, K.-O.; Choi, S.J.; Lee, S. Characterization of Phase and Diffusion Behaviors of Oil, Surfactant, and Co-Surfactant Ternary Systems for Lipid-Based Delivery Carriers. Food Chem. 2021, 359, 129875. [Google Scholar] [CrossRef]
- Hohl, L.; Kraume, M. The Formation of Complex Droplets in Liquid Three Phase Systems and Their Effect on Dispersion and Phase Separation. Chem. Eng. Res. Des. 2018, 129, 89–101. [Google Scholar] [CrossRef]
- Feng, J.; Rodríguez-Abreu, C.; Esquena, J.; Solans, C. A Concise Review on Nano-emulsion Formation by the Phase Inversion Composition (PIC) Method. J. Surfactants Deterg. 2020, 23, 677–685. [Google Scholar] [CrossRef]
- Herrera, D.; Chevalier, T.; Frot, D.; Barré, L.; Drelich, A.; Pezron, I.; Dalmazzone, C. Monitoring the Formation Kinetics of a Bicontinuous Microemulsion. J. Colloid Interface Sci. 2022, 609, 200–211. [Google Scholar] [CrossRef]
- Zhao, X.; Zhan, F.; Liao, G.; Liu, W.; Su, X.; Feng, Y. In Situ Micro-Emulsification during Surfactant Enhanced Oil Recovery: A Microfluidic Study. J. Colloid Interface Sci. 2022, 620, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of Formation and Structure of Micro Emulsions by Electron Microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Robbins, M. Microemulsions—Theory and Practice. Abstr. Pap. Am. Chem. Soc. 1976, 172, 48. [Google Scholar]
- Ostrovsky, M.; Good, R. Mass-Transfer And Dynamic Liquid-Liquid Interfacial-Tension. 3. Theory of Nonequilibrium Pressure Coefficient of Interfacial-Tension. J. Colloid Interface Sci. 1985, 106, 140–145. [Google Scholar] [CrossRef]
- Bier, M. Nonequilibrium Interfacial Tension during Relaxation-All Databases. Phys. Rev. E 2015, 92, 042128. [Google Scholar] [CrossRef] [PubMed]
- Surfactant Science Series Vol. 39. Interfacial Phenomena in Biological Systems—All Databases. Available online: https://www.webofscience.com/wos/alldb/full-record/BIOSIS:PREV199243035081 (accessed on 18 September 2023).
- Lin, T.; Kurihara, H.; Ohta, H. Effects of Phase Inversion and Surfactant Location on Formation of O-W Emulsions. J. Soc. Cosmet. Chem. 1975, 26, 121–139. [Google Scholar]
- Salager, J.L.; Morgan, J.C.; Schechter, R.S.; Wade, W.H.; Vasquez, E. Optimum Formulation of Surfactant/Water/Oil Systems for Minimum Interfacial Tension or Phase Behavior. Soc. Pet. Eng. J. 1979, 19, 107–115. [Google Scholar] [CrossRef]
- Wu, Z.; Mu, G.; Liu, H.; Ding, C. Study on the Influence of Different Types of Inorganic Salts on Microemulsion System Using Fishlike Phase Diagram-All Databases. Chem. Res. Appl. 2015, 27, 1564–1568. [Google Scholar]
- Arpornpong, N.; Charoensaeng, A.; Khaodhiar, S.; Sabatini, D.A. Formulation of Microemulsion-Based Washing Agent for Oil Recovery from Spent Bleaching Earth-Hydrophilic Lipophilic Deviation Concept. Colloids Surf. Physicochem. Eng. Asp. 2018, 541, 87–96. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Sheng, L.; Wang, M.; Liu, H. The Phase Behavior and Solubilization Ability of Nonionic Surfactant-Distillate Fraction of Crude Oil Microemulsion System. Colloids Surf. Physicochem. Eng. Asp. 2020, 603, 125181. [Google Scholar] [CrossRef]
- Park, J.; Mohanty, K. Design of Surrogate Oils for Surfactant-Brine-Oil Phase Behavior. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 18 April 2022; p. D011S008R003. [Google Scholar]
- Jin, L.; Jamili, A.; Li, Z.; Lu, J.; Luo, H.; Ben Shiau, B.J.; Delshad, M.; Harwell, J.H. Physics Based HLD–NAC Phase Behavior Model for Surfactant/Crude Oil/Brine Systems. J. Pet. Sci. Eng. 2015, 136, 68–77. [Google Scholar] [CrossRef]
- Jin, L.; Budhathoki, M.; Jamili, A.; Li, Z.; Luo, H.; Ben Shiau, B.J.; Delshad, M.; Harwell, J.H. Predicting Microemulsion Phase Behavior Using Physics Based HLD-NAC Equation of State for Surfactant Flooding. J. Pet. Sci. Eng. 2017, 151, 213–223. [Google Scholar] [CrossRef]
- Yao, T.; Li, J. An Experiments Study on Chenmical Flood for Heavy Oil Reservoirs-All Databases. Oilfield Chem. 2010, 27, 84–87+42. [Google Scholar]
- Yin, D.; Yang, K.; Huang, K. Effect of Salt and Alcohol on the Displacement Performance of Dodecyl Dimethyl Betaine Microemulsion System Using Winsor Phase Diagram Method-All Databases. Oilfield Chem. 2018, 35, 119–124+130. [Google Scholar]
- Luo, M.; Si, X.; Yang, Z.; Gong, J. Preparation and Performance of Microemulsion for Corrosion Inhibition of Coiled Tubing-All Databases. Fine Chem. 2018, 35, 1758–1764. [Google Scholar]
- Luo, M.; Liu, J.; Wen, Q.; Liu, H.; Jia, Z. Fracturing Cleanup Effectiveness Improved by Environment-Friendly MES Middle Phase Microemulsion-All Databases. Acta Pet. Sin. Pet. Process. Sect. 2011, 27, 454–460. [Google Scholar]
- Liu, H.; Zhang, X.; Ding, C.; Chen, S.; Qi, X. Phase Behavior of Sodium Dodecyl Sulfate-n-Butanol-Kerosene-Water Microemulsion System. Chin. J. Chem. Eng. 2014, 22, 699–705. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Shi, C.; Chai, J. Middle-Phase Microemulsions Formed by n -Dodecyl Polyglucoside and Lauric-N-Methylglucamide. J. Dispers. Sci. Technol. 2013, 34, 147–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.Y.; Chai, J.L.; Cui, X.C.; Pan, J.; Song, J.W.; Sun, B.; Lu, J.J. The Phase Behavior and Solubilization of Isopropyl Myristate in Microemulsions Containing Hexadecyl Trimethyl Ammonium Bromide and Sodium Dodecyl Sulfate. J. Mol. Liq. 2017, 244, 262–268. [Google Scholar] [CrossRef]
- Hou, N.; Chai, J.-L.; Zhang, J.-Q.; Song, J.-W.; Zhang, Y.; Lu, J.-J. Application of ε-β Fishlike Phase Diagrams on the Microemulsion Solubilizations of Dense Nonaqueous Phase Liquids. Fluid Phase Equilibria 2016, 412, 211–217. [Google Scholar] [CrossRef]
- Javanbakht, G.; Arshadi, M.; Qin, T.; Goual, L. Micro-Scale Displacement of NAPL by Surfactant and Microemulsion in Heterogeneous Porous Media. Adv. Water Resour. 2017, 105, 173–187. [Google Scholar] [CrossRef]
- Naik, P.K.; Kundu, D.; Bairagya, P.; Banerjee, T. Phase Behavior of Water-Menthol Based Deep Eutectic Solvent-Dodecane System. Chem. Thermodyn. Therm. Anal. 2021, 3–4, 100011. [Google Scholar] [CrossRef]
- Furlanetto, S.; Cirri, M.; Piepel, G.; Mennini, N.; Mura, P. Mixture Experiment Methods in the Development and Optimization of Microemulsion Formulations. J. Pharm. Biomed. Anal. 2011, 55, 610–617. [Google Scholar] [CrossRef]
- Neuma De Castro Dantas, T.; Viana, F.F.; Thaise Costa De Souza, T.; Dantas Neto, A.A.; Aum, P.T.P. Study of Single-Phase Polymer-Alkaline-Microemulsion Flooding for Enhancing Oil Recovery in Sandstone Reservoirs. Fuel 2021, 302, 121176. [Google Scholar] [CrossRef]
- Acosta, E.J.; Kiran, S.K.; Hammond, C.E. The HLD-NAC Model for Extended Surfactant Microemulsions. J. Surfactants Deterg. 2012, 15, 495–504. [Google Scholar] [CrossRef]
- Kanan, K.; Al-Jabari, M.; Kayali, I. Phase Behavioral Changes in SDS Association Structures Induced by Cationic Hydrotropes. Arab. J. Chem. 2017, 10, S314–S320. [Google Scholar] [CrossRef]
- Porada, J.H.; Mansueto, M.; Laschat, S.; Stubenrauch, C. Microemulsions with Hydrophobic Ionic Liquids: Influence of the Structure of the Anion. J. Mol. Liq. 2017, 227, 202–209. [Google Scholar] [CrossRef]
- Salager, J.-L.; Antón, R.E.; Sabatini, D.A.; Harwell, J.H.; Acosta, E.J.; Tolosa, L.I. Enhancing Solubilization in Microemulsions—State of the Art and Current Trends. J. Surfactants Deterg. 2005, 8, 3–21. [Google Scholar] [CrossRef]
- Kasaka, Y.; Bibouche, B.; Volovych, I.; Schwarze, M.; Schomäcker, R. Investigation of Phase Behaviour of Selected Chemical Reaction Mixtures in Microemulsions for Technical Applications. Colloids Surf. Physicochem. Eng. Asp. 2016, 494, 49–58. [Google Scholar] [CrossRef]
- Al-Sahhaf, T.; Ahmed, A.S.; Elkamel, A. Producing Ultralow Interfacial Tension at the Oil/Water Interface. Pet. Sci. Technol. 2002, 20, 773–788. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.-H.; Zhang, Q.; Zhang, F.; Ma, G.-Y.; Zhang, L.; Zhang, L. Effect of Electrolyte on Synergism for Reducing Interfacial Tension between Betaine and Petroleum Sulfonate. Energy Fuels 2020, 34, 3188–3198. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (Alkaline Surfactant Polymer Enhanced Oil Recovery) Technology in the Petroleum Industry: Prospects and Challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Baek, K.H.; Liu, M.; Argüelles-Vivas, F.J.; Abeykoon, G.A.; Okuno, R. The Effect of Surfactant Partition Coefficient and Interfacial Tension on Oil Displacement in Low-Tension Polymer Flooding. J. Pet. Sci. Eng. 2022, 214, 110487. [Google Scholar] [CrossRef]
- He, W.; Ge, J.; Zhang, G.; Jiang, P.; Jin, L. Effects of Extended Surfactant Structure on the Interfacial Tension and Optimal Salinity of Dilute Solutions. ACS Omega 2019, 4, 12410–12417. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery Theory and Practice Preface; Gulf Publishing Company: Houston, TX, USA, 2011; p. XIII-+. ISBN 978-0-08-096163-7. [Google Scholar]
- Afolabi, F.; Mahmood, S.M.; Sharifigaliuk, H.; Bin Kamarozaman, M.I.H.; Mansor, F.N.N.B.M. Investigations on the Enhanced Oil Recovery Capacity of Novel Bio-Based Polymeric Surfactants. J. Mol. Liq. 2022, 368, 120813. [Google Scholar] [CrossRef]
- Latief, F.W.; Setiati, R.; Mardiana, D.A. The Effect of Alkali Type on IFT Value for Surfactant-Alkali Injection. In Proceedings of the 5th Annual Applied Science and Engineering Conference (AASEC 2020), Online, 21–22 April 2020; IoP Publishing Ltd.: Bristol, UK, 2021; Volume 1098, p. 062032. [Google Scholar]
- Liu, M.; Fang, H.; Jin, Z.; Xu, Z.; Zhang, L.; Zhang, L.; Zhao, S. Interfacial Tensions of Ethoxylated Fatty Acid Methyl Ester Solutions Against Crude Oil. J. Surfactants Deterg. 2017, 20, 961–967. [Google Scholar] [CrossRef]
- Hematpur, H.; Abdollahi, R.; Safari-Beidokhti, M.; Esfandyari, H. Experimental Microemulsion Flooding Study to Increase Low Viscosity Oil Recovery Using Glass Micromodel. Math. Probl. Eng. 2021, 2021, 5021868. [Google Scholar] [CrossRef]
- Bao, B.; Shi, J.; Feng, J. Research Progress of Surfactant Enhanced Oil Recovery Based on Microfluidics Technology. Acta Pet. Sin. 2022, 43, 432–442+452. [Google Scholar]
- Lapteva, M.; Kalia, Y.N. Microstructured Bicontinuous Phase Formulations: Their Characterization and Application in Dermal and Transdermal Drug Delivery. Expert Opin. Drug Deliv. 2013, 10, 1043–1059. [Google Scholar] [CrossRef]
- Bao, B.; Zhao, S. A Review of Experimental Nanofluidic Studies on Shale Fluid Phase and Transport Behaviors. J. Nat. Gas Sci. Eng. 2021, 86, 103745. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, D.; Wang, D.; Zhang, C.; Yang, Z. Experiment Investigation of Microemulsion Enhanced Oil Recovery in Low Permeability Reservoir. J. Mater. Res. Technol. 2020, 9, 8306–8313. [Google Scholar] [CrossRef]
- Yu, F.; Gao, Z.; Zhu, W. Experimental Research on Imbibition Mechanisms of Fractured Reservoirs by Microfluidic Chips. Pet. Explor. Dev. 2021, 48, 1004–1013. [Google Scholar] [CrossRef]
- Yang, W.; Fu, C.; Du, Y.; Xu, K.; Baihoff, M.T.; Weston, J.; Lu, J. Dynamic Contact Angle Reformulates Pore-Scale Fluid-Fluid Displacement at Ultralow Interfacial Tension. SPE J. 2021, 26, 1278–1289. [Google Scholar] [CrossRef]
- Yu, F.; Gao, Z.; Zhu, W.; Wang, C.; Liu, F.; Xu, F.; Jiang, H.; Li, J. Experiments on Imbibition Mechanisms of Fractured Reservoirs by Microfluidic Chips. Pet. Explor. Dev. 2021, 48, 1162–1172. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.; Ma, M. Study of the Variation of Pore-Scale Residual Oil Flow Based on a Microfluidic Model. Pet. Sci. Bull. 2020, 5, 376–391. [Google Scholar]
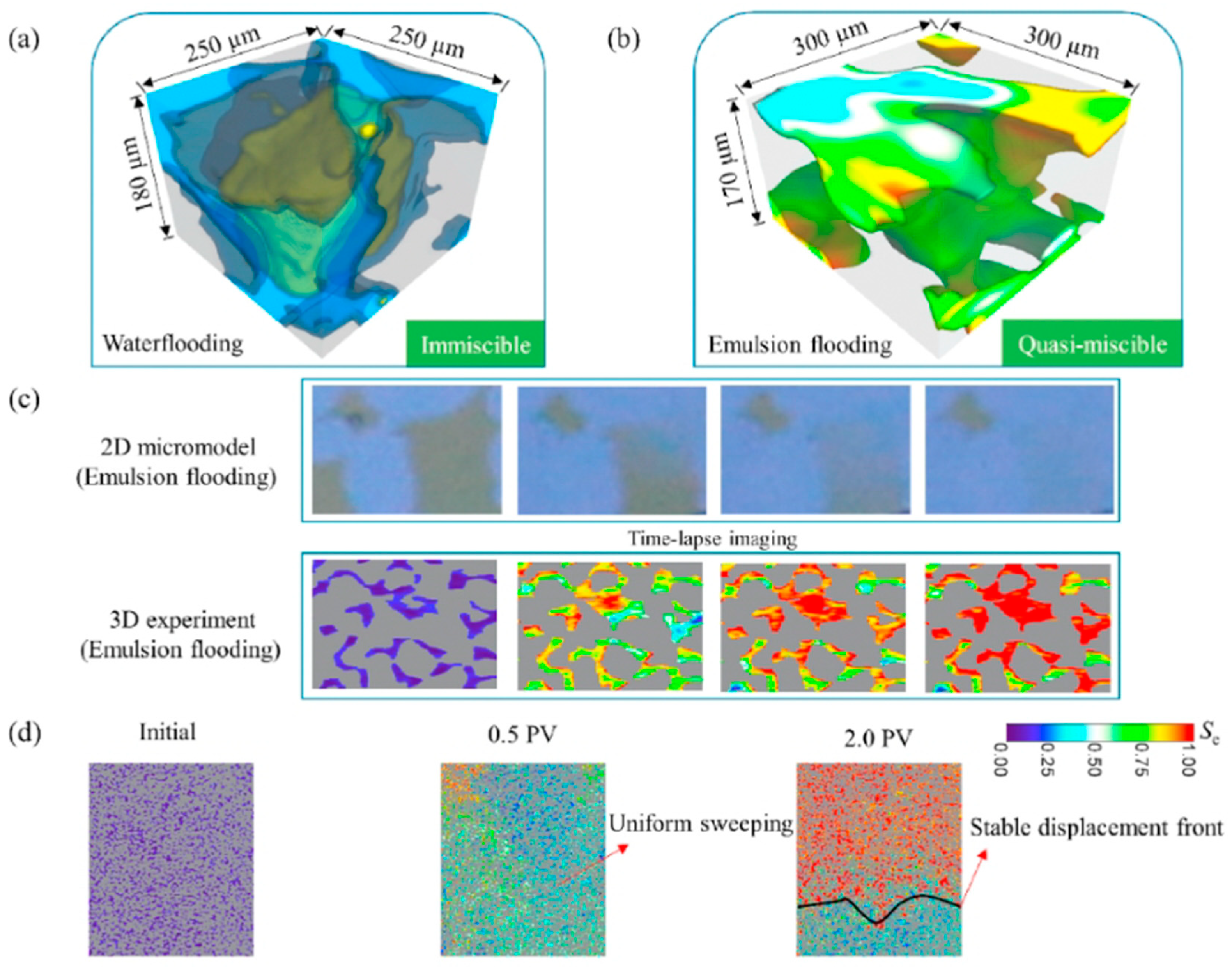
- She, Y.; Wang, W.; Hu, Y.; Mahardika, M.A.; Nasir, M.; Zhang, C.; Patmonoaji, A.; Matsushita, S.; Suekane, T. Pore-Scale Investigation on Microemulsion-Based Quasi-Miscible Flooding for EOR in Water-Wet/Oil-Wet Reservoirs: A 3D Study by X-Ray Microtomography. J. Pet. Sci. Eng. 2022, 216, 110788. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.; Fang, Y.; Jin, G.; Xia, Y. What Dominates the Interfacial Properties of Extended Surfactants: Amphipathicity or Surfactant Shape? J. Colloid Interface Sci. 2019, 547, 190–198. [Google Scholar] [CrossRef]
- Taber, J. Dynamic and Static Forces Required to Remove a Discontinuous Oil Phase from Porous Media Containing Both Oil and Water. Soc. Pet. Eng. J. 1969, 9, 3–12. [Google Scholar] [CrossRef]
- Holm, L.W. Soluble Oils for Improved Oil Recovery, in Improved Oil Recovery by Surfactant and Polymer Flooding; Academic Press: Cambridge, MA, USA, 1977; pp. 453–485. [Google Scholar]
- Hirasaki, G.J. Application of the Theory of Multicomponent, Multiphase Displacement to Three-Component, Two-Phase Surfactant Flooding. Soc. Pet. Eng. J. 1981, 21, 191–204. [Google Scholar] [CrossRef]
- Dreher, K.D.; Shoppman, T.D. Separation of Oil and Water Produced by Micellar-Solution/ Polymer Flooding. J. Pet. Technol. 1985, 37, 1459–1465. [Google Scholar] [CrossRef]
- Corsano, A. Field Test of an Aqueous Surfactant System For Oil Recovery, Benton Field, Illinois. J. Pet. Technol. 1973, 25, 195–204. [Google Scholar]
- Glover, C.J.; Puerto, M.C.; Maerker, J.M.; Sandvik, E.L. Surfactant Phase Behavior and Retention in Porous Media. Soc. Pet. Eng. J. 1979, 19, 183–193. [Google Scholar] [CrossRef]
- Shi, P.; Luo, H.; Tan, X.; Lu, Y.; Zhang, H.; Yang, X. Molecular Dynamics Simulation Study of Adsorption of Anionic-Nonionic Surfactants at Oil/Water Interfaces. RSC Adv. 2022, 12, 27330–27343. [Google Scholar] [CrossRef]
- Witthayapanyanon, A.; Phan, T.T.; Heitmann, T.C.; Harwell, J.H.; Sabatini, D.A. Interfacial Properties of Extended-Surfactant-Based Microemulsions and Related Macroemulsions. J. Surfactants Deterg. 2010, 13, 127–134. [Google Scholar] [CrossRef]
- Sheng, J.J. A Comprehensive Review of Alkaline-Surfactant-Polymer (ASP) Flooding: A Comprehensive Review of Asp Flooding. Asia-Pac. J. Chem. Eng. 2014, 9, 471–489. [Google Scholar] [CrossRef]
- Stoll, W.M. Alkaline/Surfactant/Polymer Flood: From the Laboratory to the Field. SPE Res. Eval. Eng. 2011, 14, 702–712. [Google Scholar] [CrossRef]
- Zhu, Y. Current Developments and Remaining Challenges of Chemical Flooding EOR Techniques in China. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11 August 2015; p. D021S012R003. [Google Scholar]
- James-Smith, M.A.; Shekhawat, D.; Cheung, S.; Moudgil, B.M.; Shah, D.O. Effect of Chain Length on Binding of Fatty Acids to Pluronics in Microemulsions. Colloids Surf. B-Biointerfaces 2008, 62, 5–10. [Google Scholar] [CrossRef]
- Lettow, J.S.; Lancaster, T.M.; Glinka, C.J.; Ying, J.Y. Small-Angle Neutron Scattering and Theoretical Investigation of Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) Stabilized Oil-in-Water Microemulsions. Langmuir 2005, 21, 5738–5746. [Google Scholar] [CrossRef]
- Liang, X.-D.; Liu, Y.-F.; Zhou, D.; Yu, W.; Yin, J.-Z. Critical Microemulsion Concentration and Molar Ratio of Water-to-Surfactant of Supercritical CO2 Microemulsions with Commercial Nonionic Surfactants: Experiment and Molecular Dynamics Simulation. J. Chem. Eng. Data 2016, 61, 3135–3143. [Google Scholar] [CrossRef]
- Su, H.; Zhou, F.; Wang, Q.; Yu, F.; Dong, R.; Xiong, C.; Li, J.; Liang, T. Flow Physics of Polymer Nanospheres and Diluted Microemulsion in Fractured Carbonate Reservoirs: An Investigation into Enhanced Oil Recovery Mechanisms. SPE J. 2021, 26, 2231–2244. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, B.; Qi, D.; Lv, D.; He, J.; Zhou, S.; Wang, Z.; Han, R. Intensive Fracture Cluster Completion Strategy Using Microemulsion Flowback Technology in a Daqing Tight Oil Field Application: Case Study. In Proceedings of the SPE Russian Petroleum Technology Conference, Virtual, 26 October 2020; p. D033S010R004. [Google Scholar]
- Bardhan, S.; Kundu, K.; Saha, S.K.; Paul, B.K. Physicochemical Investigation of Mixed Surfactant Microemulsions: Water Solubilization, Thermodynamic Properties, Microstructure, and Dynamics. J. Colloid Interface Sci. 2013, 411, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Co, L.; Zhang, Z.; Ma, Q.; Watts, G.; Zhao, L.; Shuler, P.J.; Tang, Y. Evaluation of Functionalized Polymeric Surfactants for EOR Applications in the Illinois Basin. J. Pet. Sci. Eng. 2015, 134, 167–175. [Google Scholar] [CrossRef]
- Nguele, R.; Sasaki, K.; Salim, H.S.-A.; Sugai, Y.; Widiatmojo, A.; Nakano, M. Microemulsion and Phase Behavior Properties of (Dimeric Ammonium Surfactant Salt—Heavy Crude Oil—Connate Water) System. J. Unconv. Oil Gas Resour. 2016, 14, 62–71. [Google Scholar] [CrossRef]
- Chen, Z.; Han, X.; Kurnia, I.; Yu, J.; Zhang, G.; Li, L. Adoption of Phase Behavior Tests and Negative Salinity Gradient Concept to Optimize Daqing Oilfield Alkaline-Surfactant-Polymer Flooding. Fuel 2018, 232, 71–80. [Google Scholar] [CrossRef]
- Ayirala, S.C.; Boqmi, A.; Alghamdi, A.; AlSofi, A. Dilute Surfactants for Wettability Alteration and Enhanced Oil Recovery in Carbonates. J. Mol. Liq. 2019, 285, 707–715. [Google Scholar] [CrossRef]
- Han, X.; Kurnia, I.; Chen, Z.; Yu, J.; Zhang, G. Effect of Oil Reactivity on Salinity Profile Design during Alkaline-Surfactant-Polymer Flooding. Fuel 2019, 254, 115738. [Google Scholar] [CrossRef]
- Li, J.; Niu, L.; Lu, X. Performance of ASP Compound Systems and Effects on Flooding Efficiency. J. Pet. Sci. Eng. 2019, 178, 1178–1193. [Google Scholar] [CrossRef]
- Riswati, S.S.; Bae, W.; Park, C.; Permadi, A.K.; Efriza, I.; Min, B. Experimental Analysis to Design Optimum Phase Type and Salinity Gradient of Alkaline Surfactant Polymer Flooding at Low Saline Reservoir. J. Pet. Sci. Eng. 2019, 173, 1005–1019. [Google Scholar] [CrossRef]
- Han, X.; Chen, Z.; Zhang, G.; Yu, J. Surfactant-Polymer Flooding Formulated with Commercial Surfactants and Enhanced by Negative Salinity Gradient. Fuel 2020, 274, 117874. [Google Scholar] [CrossRef]
- Kurnia, I.; Zhang, G.; Han, X.; Yu, J. Zwitterionic-Anionic Surfactant Mixture for Chemical Enhanced Oil Recovery without Alkali. Fuel 2020, 259, 116236. [Google Scholar] [CrossRef]
- Tianbo, L.; Xurong, Z.; Shuai, Y.; Jiawei, Z. Surfactant-EOR in Tight Oil Reservoirs: Current Status and a Systematic Surfactant Screening Method with Field Experiments-All Databases. J. Pet. Sci. Eng. 2021, 196, 108097. [Google Scholar] [CrossRef]
- Slamet, P.; Ronny Windu, S.; Suherman, S. High-Performance Polymeric Surfactant of Sodium Lignosulfonate-Polyethylene Glycol 4000 (SLS-PEG) for Enhanced Oil Recovery (EOR) Process-All Databases. Period. Polytech. Chem. Eng. 2021, 66, 114–124. [Google Scholar] [CrossRef]
- Sekerbayeva, A.; Pourafshary, P.; Hashmet, M.R. Application of Anionic Surfactant\engineered Water Hybrid EOR in Carbonate Formations: An Experimental Analysis-All Databases. Petroleum 2022, 8, 466–475. [Google Scholar] [CrossRef]
- Panthi, K.; Mohanty, K.K. Chemical Flood with a Single Surfactant. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 18 April 2022; p. D021S013R002. [Google Scholar]
- Mariam, S.; Aida, S.; Peyman, P.; Muhammad, R.H. Optimization of Low Salinity Water/Surfactant Flooding Design for Oil-Wet Carbonate Reservoirs by Introducing a Negative Salinity Gradient-All Databases. Energies 2023, 15, 9400. [Google Scholar] [CrossRef]
- Sumadi, P.; Yoga, R.; Ivan, K.; Oki, M. Synergy of Surfactant Mixtures and Fe3O4 Nanoparticles for Enhanced Oil Recovery (EOR)-All Databases. Inorg. Chem. Commun. 2023, 155, 111125. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, Z.; Yang, Z.; Luyu, W. Experimental Study on Characteristics and Mechanisms of Matrix Pressure Transmission near the Fracture Surface during Post-Fracturing Shut-in in Tight Oil Reservoirs-All Databases. J. Pet. Sci. Eng. 2022, 219, 111133. [Google Scholar] [CrossRef]
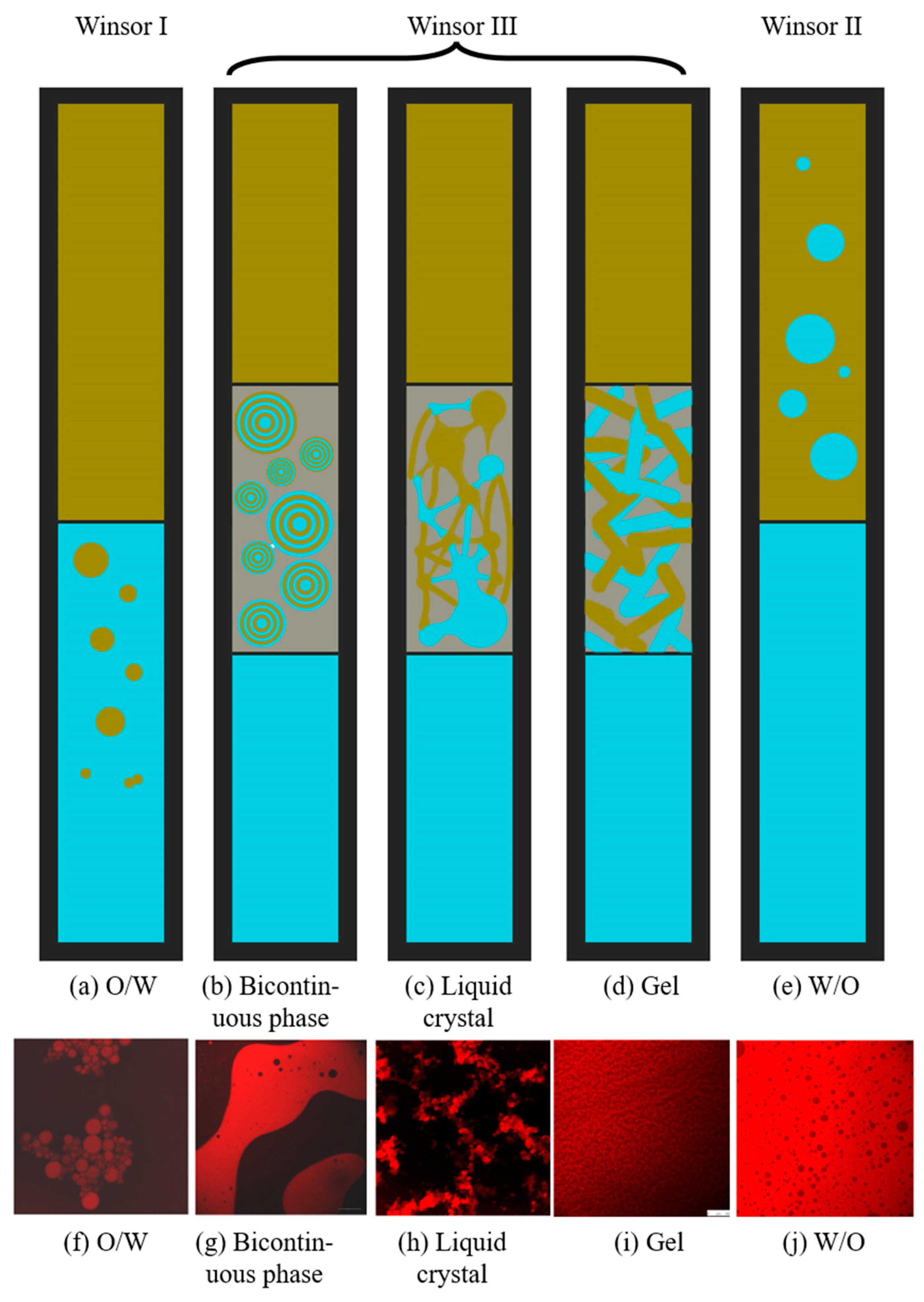

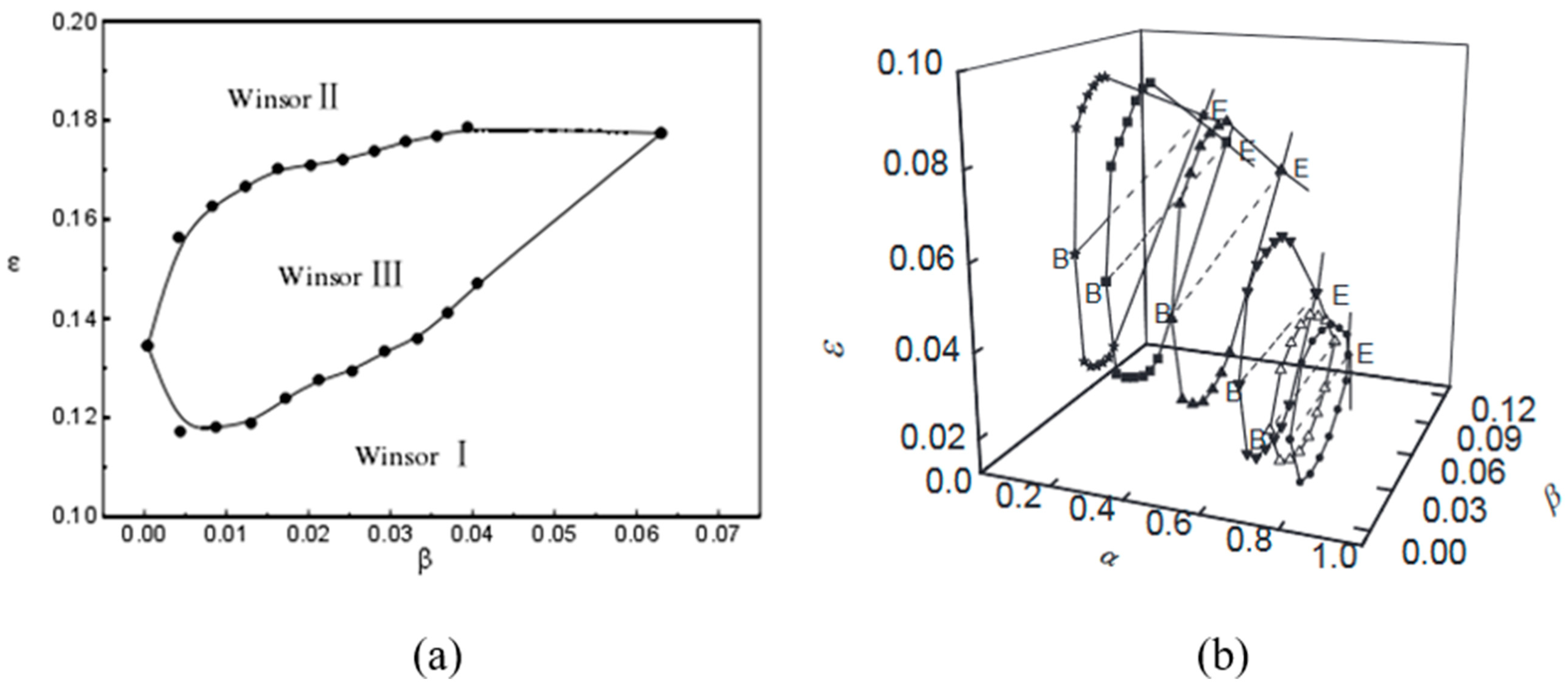



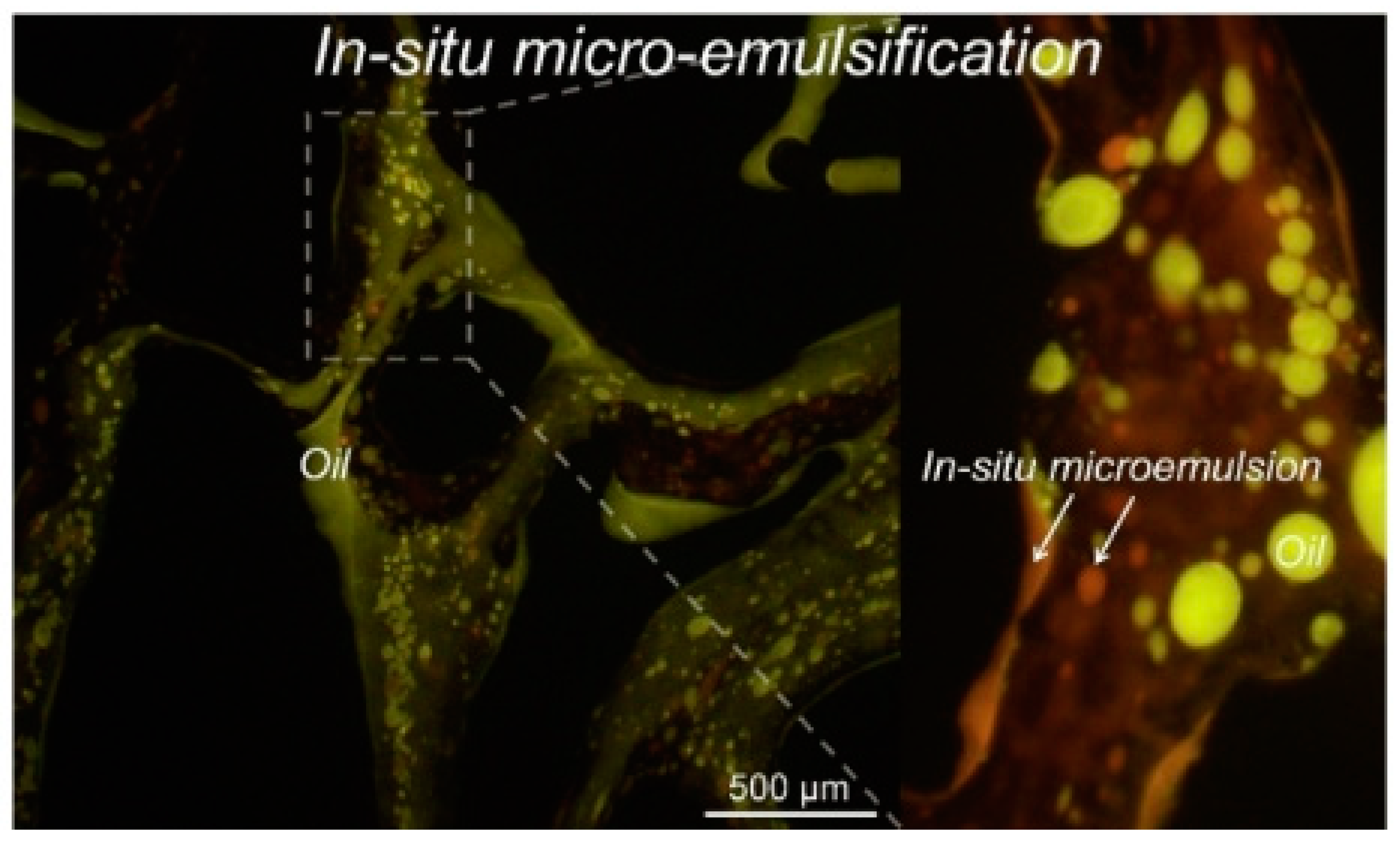

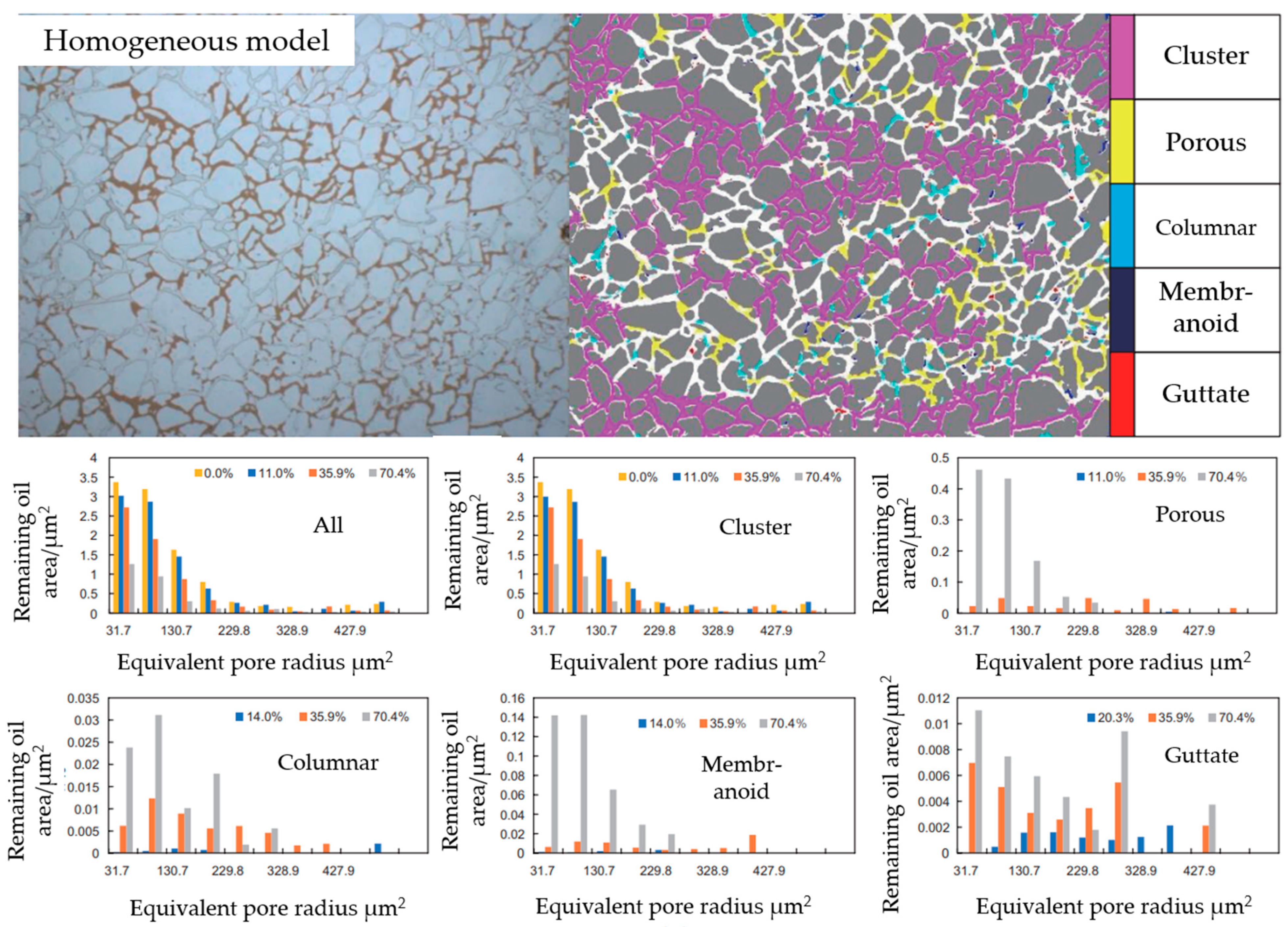
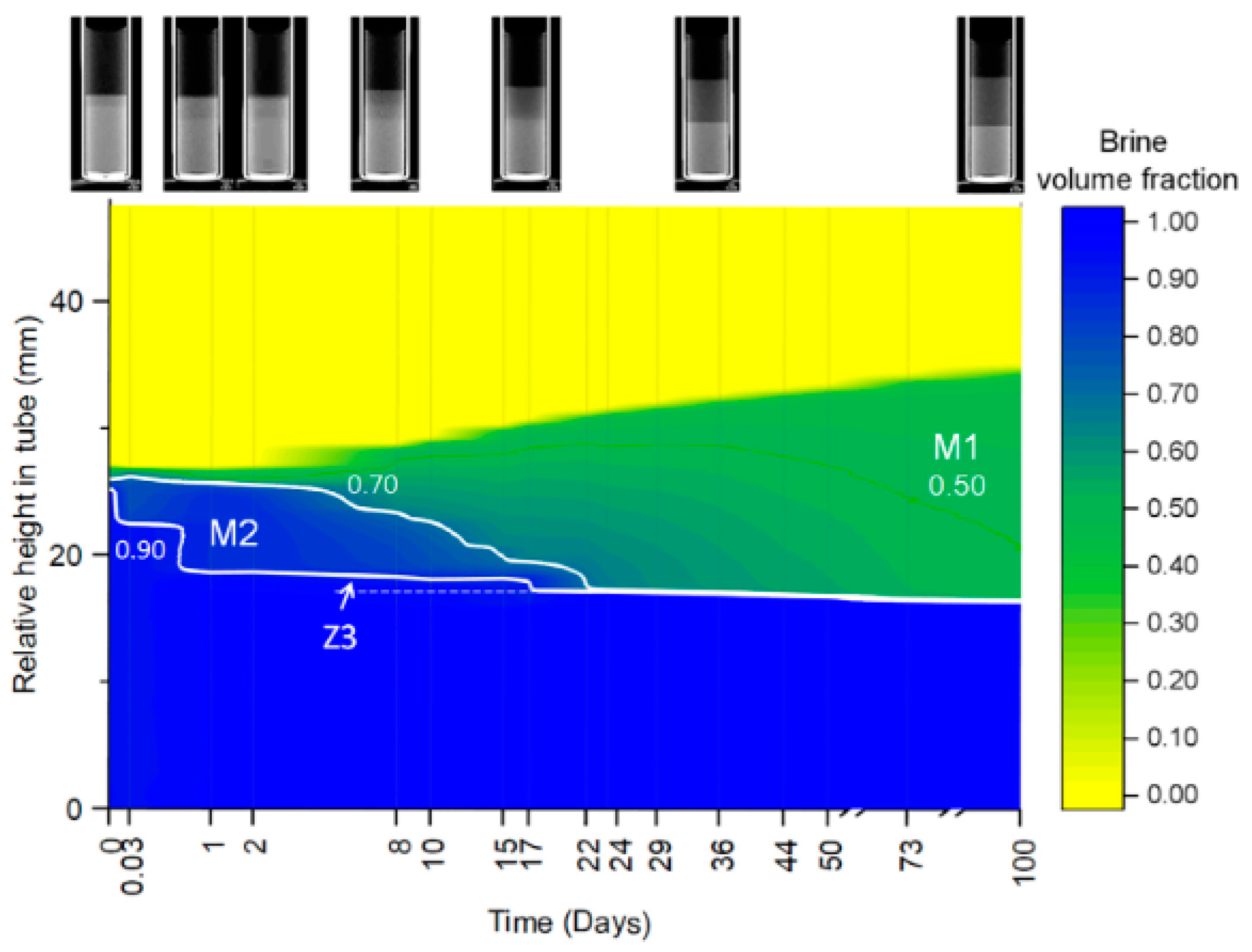


| R Ratio | Microemulsion Structure Types |
|---|---|
| 1 | Middle-phase microemulsion |
| >1 | O/W microemulsion |
| <1 | W/O microemulsion |
| Author | Year | Control Parameters | Research Content | Reference |
|---|---|---|---|---|
| Wu | 2016 | EACN, K, CC | Effect of alcohol on EACN, obtaining surfactant parameters with different K values and Cc values | [35] |
| Arpornpong | 2018 | Calculate optimal salinity from the slope intercept | [36] | |
| Liu | 2020 | EACN, T | Effect of temperature on wax content and equivalent alkane carbon number | [37] |
| Jaebum | 2022 | EACN | Comparison of the difference in EACN number between dead oil and live oil | [38] |
| Author | Year | Research Content | Reference |
|---|---|---|---|
| Roger | 2011 | Roger studied the phase diagram positions when the boundary elimination (CB) of different oil-to-water ratios occurs. The CB front edge represents a single-phase state (reverse micelle, lamellar, cubic, or sponge) or a two-phase state resulting from phase transitions between single-phase states, aligning with the middle-phase transformation process | [18] |
| Acosta | 2012 | Acosta investigated the corresponding positions in the three-phase diagram when HLD = 0 or HLD approaches 0, indicating the formation of a bicontinuous phase | [53] |
| Kanan | 2017 | Kanan demonstrated different phase diagram positions for middle-phase microemulsions by using cationic surfactants to prepare microemulsions | [54] |
| Javanbakht | 2017 | Javanbakht proved the dynamic transition of Winsor III-type non-aqueous phase liquids (NAPLs) from an emulsion to a transparent phase | [49] |
| Porada | 2017 | Porada investigated the impact of anionic polarity on the size of the three-phase region | [55] |
| Choi | 2021 | The surfactant-to-oil ratio (SOR) is an important factor in determining the type of microemulsion. Bicontinuous microemulsions form when the SOR is greater than or equal to 2. On the other hand, when the SOR is less than 2, it favors the formation of W/O microemulsions | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Zhang, Q.; Tian, M.; Li, Y.; Han, X.; Guo, R. Review: Microemulsions for the Sustainable Development of EOR. Sustainability 2024, 16, 629. https://doi.org/10.3390/su16020629
Hu H, Zhang Q, Tian M, Li Y, Han X, Guo R. Review: Microemulsions for the Sustainable Development of EOR. Sustainability. 2024; 16(2):629. https://doi.org/10.3390/su16020629
Chicago/Turabian StyleHu, Haibin, Qun Zhang, Maozhang Tian, Yuan Li, Xu Han, and Rui Guo. 2024. "Review: Microemulsions for the Sustainable Development of EOR" Sustainability 16, no. 2: 629. https://doi.org/10.3390/su16020629
APA StyleHu, H., Zhang, Q., Tian, M., Li, Y., Han, X., & Guo, R. (2024). Review: Microemulsions for the Sustainable Development of EOR. Sustainability, 16(2), 629. https://doi.org/10.3390/su16020629








