Application of the Hydrodeoxygenation of Black Soldier Fly Larvae Lipids in Green Diesel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Meterials
2.2. Lipid Extraction
2.3. Fatty Acid Methyl Ester (FAME) Production Using Extracted BSFL Lipids
2.4. Hydrodeoxygenation (HDO) of BSFL Lipids
2.5. BSFL Lipids and Hydrodeoxygenated BSFL Oil Analysis
3. Results
3.1. Main Effects of Parameters’ Performance
3.1.1. Effect of Feeding Mixtures
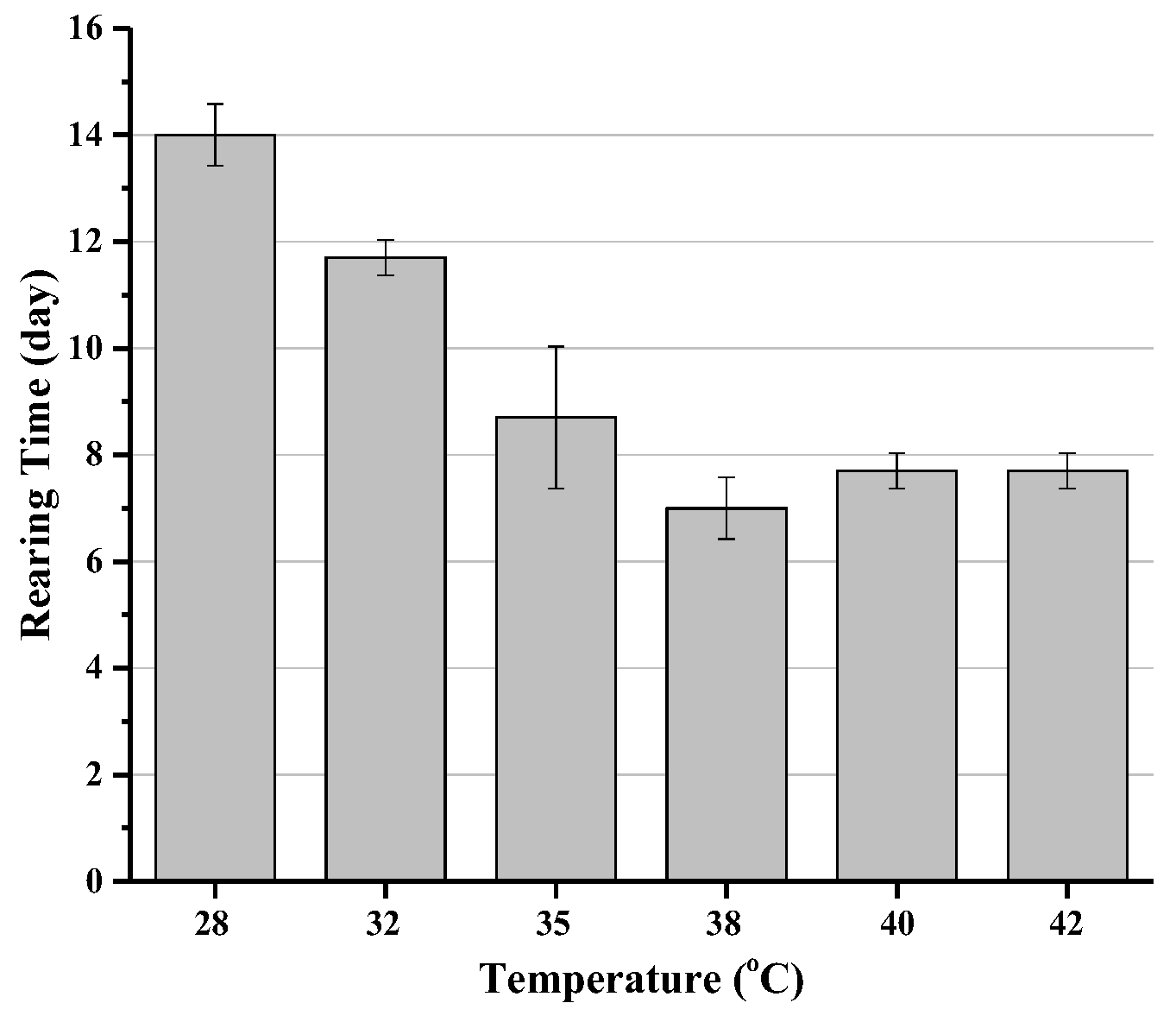
3.1.2. Effect of Growing Temperature
3.1.3. Effect of the Drying Method
3.1.4. Effect of Extraction Temperature
3.2. Characterization of BSFL Lipids and Hydrodeoxygenated BSFL Oil
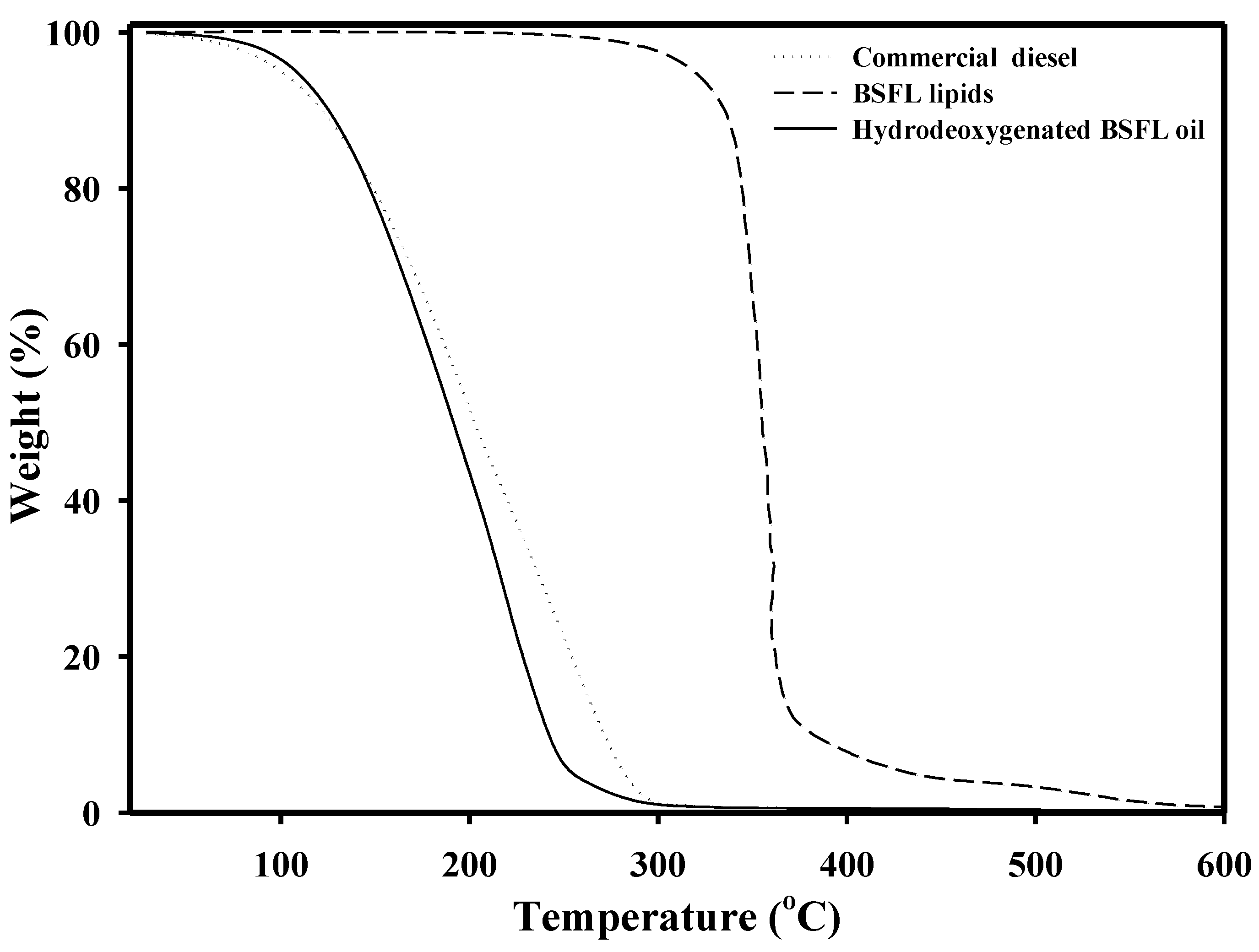
3.2.1. Hydrodeoxygenation of BSFL Lipids
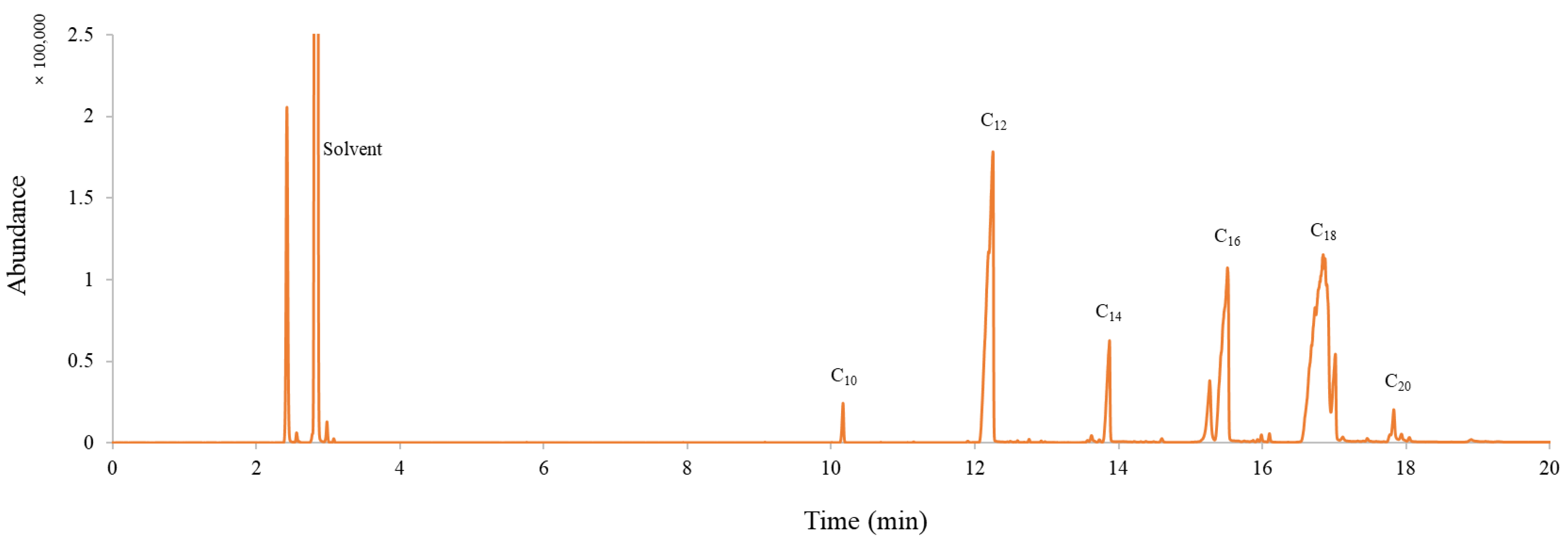
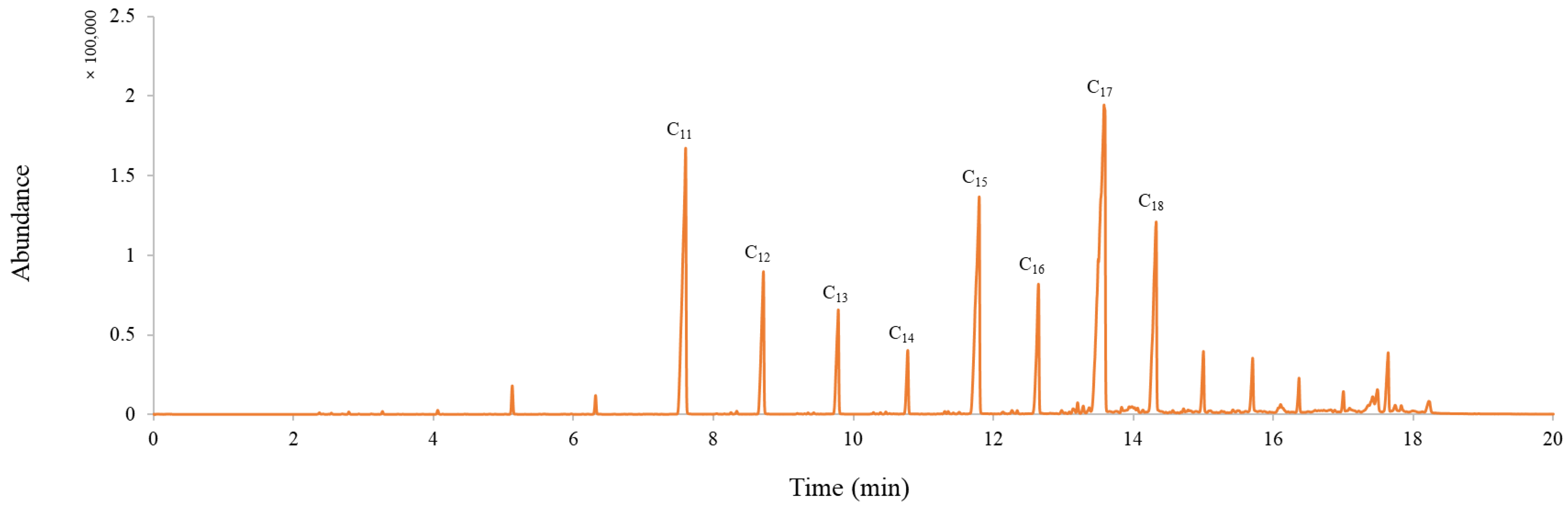
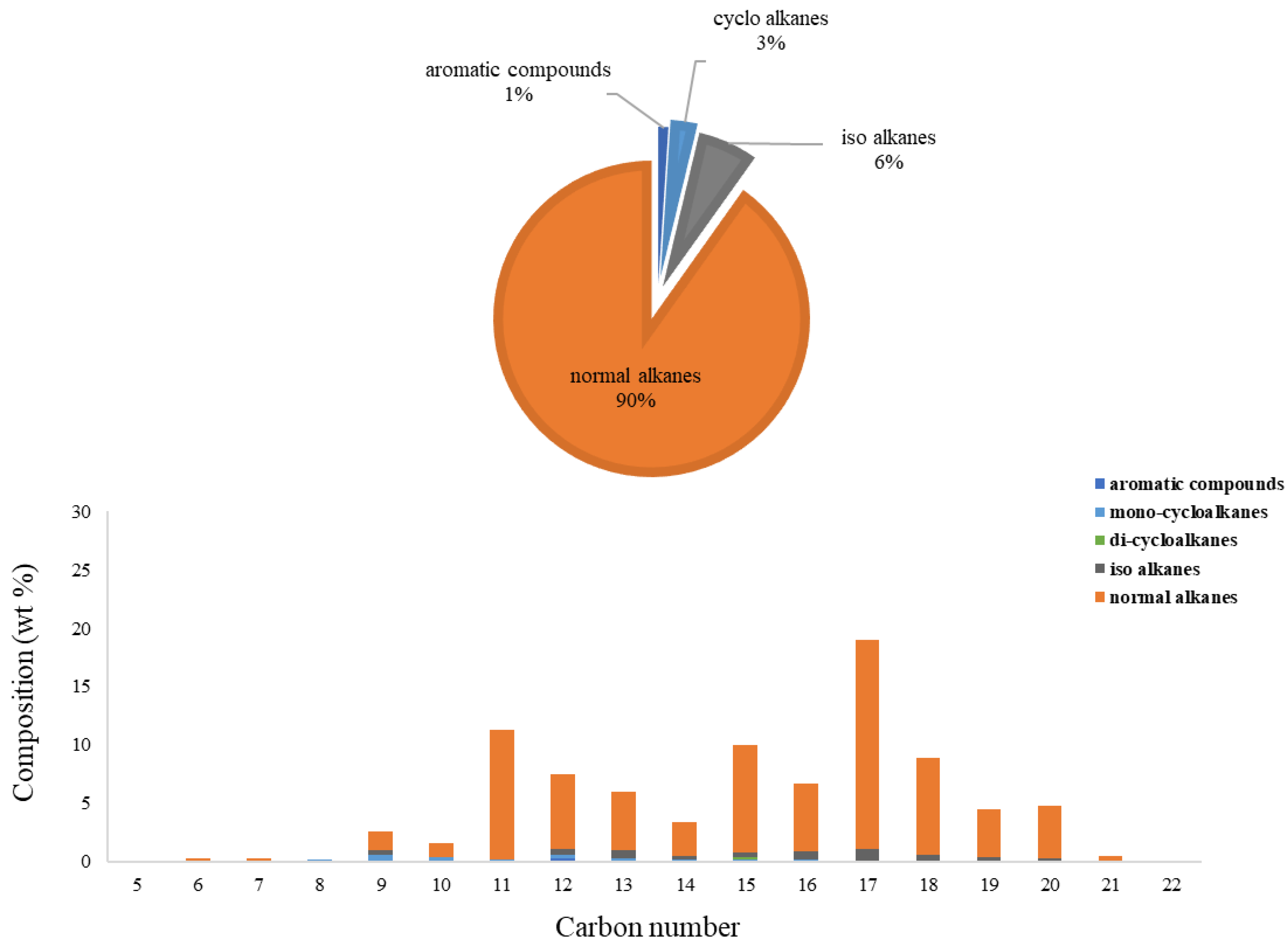
3.2.2. Composition Analysis
4. Discussion
4.1. Process Performance
4.2. Production for Green Diesel
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suresh, M.; Jawahar, C.P.; Richard, A. A Review on Biodiesel Production, Combustion, Performance, and Emission Characteristics of Non-Edible Oils in Variable Compression Ratio Diesel Engine Using Biodiesel and Its Blends. Renew. Sustain. Energy Rev. 2018, 92, 38–49. [Google Scholar] [CrossRef]
- Bazmi, A.A.; Zahedi, G.; Hashim, H. Progress and Challenges in Utilization of Palm Oil Biomass as Fuel for Decentralized Electricity Generation. Renew. Sustain. Energy Rev. 2011, 15, 574–583. [Google Scholar] [CrossRef]
- Ayompe, L.M.; Schaafsma, M.; Egoh, B.N. Towards Sustainable Palm Oil Production: The Positive and Negative Impacts on Ecosystem Services and Human Wellbeing. J. Clean. Prod. 2021, 278, 123914. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second Generation Bioethanol Production: A Critical Review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, Properties and Challenges of Algae Biomass for Biofuel Application: An Overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; de Boer, I.J.M.; Ripoll-Bosch, R. The Battle for Biomass: A Systematic Review of Food-Feed-Fuel Competition. Glob. Food Secur. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Humpenöder, F.; Popp, A.; Bodirsky, B.L.; Weindl, I.; Biewald, A.; Lotze-Campen, H.; Dietrich, J.P.; Klein, D.; Kreidenweis, U.; Müller, C.; et al. Large-Scale Bioenergy Production: How to Resolve Sustainability Trade-Offs? Environ. Res. Lett. 2018, 13, 024011. [Google Scholar] [CrossRef]
- Embrandiri, A.; Singh, R.P.; Ibrahim, H.M.; Ramli, A.A. Land Application of Biomass Residue Generated from Palm Oil Processing: Its Potential Benefits and Threats. Environmentalist 2012, 32, 111–117. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Microalgae Biofuels: A Critical Review of Issues, Problems and the Way Forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef]
- Putro, L.H.S. Emissions of CH4 and CO2 from Wastewater of Palm Oil Mills: A Real Contribution to Increase the Greenhouse Gas and Its Potential as Renewable Energy Sources. Environ. Nat. Resour. J. 2022, 20, 61–72. [Google Scholar] [CrossRef]
- Azhar, B.; Saadun, N.; Prideaux, M.; Lindenmayer, D.B. The Global Palm Oil Sector Must Change to Save Biodiversity and Improve Food Security in the Tropics. J. Environ. Manag. 2017, 203, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Shay, E.G. Diesel fuel from vegetable oils: Status and opportunities. Biomass Bioenergy 1993, 4, 227–242. [Google Scholar] [CrossRef]
- Hosseindoust, A.; Ha, S.H.; Mun, J.Y.; Kim, J.S. Quality Characteristics of Black Soldier Flies Produced by Different Substrates. Insects 2023, 14, 500. [Google Scholar] [CrossRef]
- Naser El Deen, S.; van Rozen, K.; Elissen, H.; van Wikselaar, P.; Fodor, I.; van der Weide, R.; Hoek-van den Hil, E.F.; Rezaei Far, A.; Veldkamp, T. Bioconversion of Different Waste Streams of Animal and Vegetal Origin and Manure by Black Soldier Fly Larvae Hermetia Illucens L. (Diptera: Stratiomyidae). Insects 2023, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.G.d.S. An Overview of Lipids from Insects. Biocatal. Agric. Biotechnol. 2021, 33, 101967. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Dias, C.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Could Consumption of Insects, Cultured Meat or Imitation Meat Reduce Global Agricultural Land Use? Glob. Food Secur. 2017, 15, 22–32. [Google Scholar] [CrossRef]
- Yakti, W.; Schulz, S.; Marten, V.; Mewis, I.; Padmanabha, M.; Hempel, A.J.; Kobelski, A.; Streif, S.; Ulrichs, C. The Effect of Rearing Scale and Density on the Growth and Nutrient Composition of Hermetia Illucens (L.) (Diptera: Stratiomyidae) Larvae. Sustainability 2022, 14, 1772. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. The Nutritive Value of Black Soldier Fly Larvae Reared on Common Organic Waste Streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of Rearing Substrate on Growth Performance, Waste Reduction Efficiency and Chemical Composition of Black Soldier Fly (Hermetia Illucens) Larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty Acid Composition of Black Soldier Fly Larvae (Hermetia Illucens)—Possibilities and Limitations for Modification through Diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Martínez, A.E.; Magallanes, L.M.; Tarditto, L.V.; Pramparo, M.C.; Gayol, M.F. Fatty Acids Methyl Esters from Soybean Oil for Biobased Surfactants Industry: Obtention C16/C18 Concentrate for Use as Feedstock. Ind. Crops Prod. 2022, 190, 115892. [Google Scholar] [CrossRef]
- Wu, S.Q.; Sun, T.T.; Cai, Z.Z.; Shen, J.; Yang, W.Z.; Zhao, Z.M.; Yang, D.P. Biolubricant Base Stock with Improved Low Temperature Performance: Ester Complex Production Using Housefly (Musca Domestica L.) Larval Lipid. Renew. Energy 2020, 162, 1940–1951. [Google Scholar] [CrossRef]
- Hobbi, P.; Okoro, O.V.; Hajiabbas, M.; Hamidi, M.; Nie, L.; Megalizzi, V.; Musonge, P.; Dodi, G.; Shavandi, A. Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application. Molecules 2023, 28, 675. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jung, S.; Na, Y.G.; Jeon, C.H.; Cheon, H.Y.; Yun, E.Y.; Lee, S.H.; Kwon, E.E.; Kim, J.K. Biodiesel Production from the Black Soldier Fly Larvae Grown on Food Waste and Its Fuel Property Characterization as a Potential Transportation Fuel. Environ. Eng. Res. 2022, 27, 200704. [Google Scholar] [CrossRef]
- Han, G.B.; Jang, J.H.; Ahn, M.H.; Suh, Y.W.; Choi, M.; Park, N.K.; Lee, M.E.; Kim, J.K.; Jeong, B. Operation of Bio-Aviation Fuel Manufacturing Facility via Hydroprocessed Esters and Fatty Acids Process and Optimization of Fuel Property for Turbine Engine Test. Korean J. Chem. Eng. 2021, 38, 1205–1223. [Google Scholar] [CrossRef]
- Aprianto, M.A.; Muhlisin; Kurniawati, A.; Hanim, C.; Ariyadi, B.; Al Anas, M. Effect Supplementation of Black Soldier Fly Larvae Oil (Hermetia Illucens L.) Calcium Salt on Performance, Blood Biochemical Profile, Carcass Characteristic, Meat Quality, and Gene Expression in Fat Metabolism Broilers. Poult. Sci. 2023, 102, 102984. [Google Scholar] [CrossRef] [PubMed]
- Taufek, N.M.; Mohamad Zulkifli, N.F.N.; Hamizah, A.N. Upcycling of Food Waste Generated from the Fresh Market by Utilising Black Soldier Fly Larvae: Influence on Growth, Bioconversion, and Nutritional Composition. J. Environ. Manag. 2024, 349, 119467. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.S.; Mong, G.R.; Lim, J.W.; Raksasat, R.; Rawindran, H.; Hassan, M.A.; Lam, M.K.; Khoo, K.S.; Zango, Z.U. Low-Temperature Thermal Pre-Treated Sewage Sludge for Feeding of Black Soldier Fly (Hermetia Illucens) Larvae: Protein, Lipid and Biodiesel Profile and Characterization. Renew. Sustain. Energy Rev. 2023, 178, 113241. [Google Scholar] [CrossRef]
- Salam, M.; Shahzadi, A.; Zheng, H.; Alam, F.; Nabi, G.; Dezhi, S.; Ullah, W.; Ammara, S.; Ali, N.; Bilal, M. Effect of Different Environmental Conditions on the Growth and Development of Black Soldier Fly Larvae and Its Utilization in Solid Waste Management and Pollution Mitigation. Environ. Technol. Innov. 2022, 28, 102649. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Liu, J.; Guo, Y.; Chen, A.; Chen, B.; Hu, P.; Zhu, X.; Li, W.; Zhao, W.; et al. Optimization of Extraction Factor and Nutritional Characterization of Black Soldier Fly Larvae Oil via Subcritical Butane Extraction. LWT 2023, 186, 115221. [Google Scholar] [CrossRef]
- No, S.Y. Application of Hydrotreated Vegetable Oil from Triglyceride Based Biomass to CI Engines—A Review. Fuel 2014, 115, 88–96. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic Hydrodeoxygenation of Triglycerides: An Approach to Clean Diesel Fuel Production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Okoro, O.V.; Preat, V.; Karimi, K.; Nie, L.; Debaste, F.; Shavandi, A. Optimizing the Subcritical Water Valorization of Insect (Hermetia Illucens l.) Farming Waste for Biodiesel Production. Chem. Eng. Res. Des. 2023, 196, 413–426. [Google Scholar] [CrossRef]
- Vozka, P.; Šimáček, P.; Kilaz, G. Impact of HEFA Feedstocks on Fuel Composition and Properties in Blends with Jet A. Energy Fuels 2018, 32, 11595–11606. [Google Scholar] [CrossRef]
- Pérez, W.; Marín, J.; del Río, J.; Peña, J.; Rios, L. Upgrading of Palm Oil Renewable Diesel through Hydroisomerization and Formulation of an Optimal Blend. Fuel 2017, 209, 442–448. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Assabumrungrat, S. Roles of Monometallic Catalysts in Hydrodeoxygenation of Palm Oil to Green Diesel. Chem. Eng. J. 2015, 278, 249–258. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Zhou, G.; Shen, S.; Rong, L. Hydrotreatment of Jatropha Oil over NiMoLa/Al2O3 Catalyst. Green Chem. 2012, 14, 2499–2505. [Google Scholar] [CrossRef]
- Li, Y.; Mu, C.; Chu, G.; Wang, Y.; Xu, J.; Guo, X.; Zhao, Y.; Wang, S.; Ma, X. Maximizing Jet Fuel Production from N-Hexadecane Hydrocracking over Pt-Supported Y Catalysts: Importance of Ideal Hydrocracking Characteristics. Fuel 2023, 346, 128286. [Google Scholar] [CrossRef]
| Feeding Mixtures | Ground Corn (wt %) | Food Waste (wt %) | Meat By-Product (wt %) |
|---|---|---|---|
| A | 100 | 0 | 0 |
| B | 70 | 30 | 0 |
| C | 50 | 50 | 0 |
| D | 30 | 70 | 0 |
| E | 70 | 0 | 30 |
| F | 50 | 0 | 50 |
| G | 30 | 0 | 70 |
| H | 0 | 100 | 0 |
| I | 0 | 70 | 30 |
| J | 0 | 50 | 50 |
| K | 0 | 30 | 70 |
| L | 33 | 33 | 33 |
| Crude Protein (wt %) | Crude Lipid (wt %) | Crude Ash (wt %) | Crude Fiber (wt %) | Moisture (wt %) | Calcium (wt %) | Phosphorus (wt %) | |
|---|---|---|---|---|---|---|---|
| Ground corn | 16.0 | 4.0 | 6.0 | 8.0 | 12.0 | 0.70 | 1.20 |
| Food waste | 23.05 | 9.37 | 16.60 | 16.20 | 3.77 | 4.81 | 1.06 |
| Meat by-product | 28.34 | 3.43 | 2.50 | 0.19 | 65.03 | 0.018 | 0.13 |
| Feeding Mixtures | Mean ± SE | ||||
|---|---|---|---|---|---|
| Moisture (%) | Crude Lipid (%) | Period (Day) | Production Rate (kg/Clutch) | Feeding Mixtures Price (USD/kg) | |
| A | 3.53 ± 0.3 | 26.92 ± 2.0 | 8.33 ± 0.3 | 3.39 ± 0.4 | 0.51 |
| B | 5.06 ± 0.4 | 26.69 ± 2.0 | 8.00 ± 0.5 | 4.54 ± 0.2 | 0.36 |
| C | 7.03 ± 0.4 | 26.27 ± 0 | 6.17 ± 0.3 | 3.57 ± 0.2 | 0.26 |
| D | 4.54 ± 0.1 | 27.19 ± 1.2 | 7.17 ± 0.3 | 3.24 ± 0.3 | 0.16 |
| E | 6.88 ± 0.1 | 27.22 ± 0.3 | 6.00 ± 0.5 | 5.23 ± 0.3 | 0.36 |
| F | 4.92 ± 0.2 | 29.01 ± 0.8 | 7.17 ± 0.3 | 3.29 ± 0.2 | 0.26 |
| G | 5.27 ± 0.1 | 30.55 ± 0.3 | 8.00 ± 0.5 | 3.63 ± 0.2 | 0.16 |
| H | 7.63 ± 0.2 | 21.28 ± 0.7 | 10.33 ± 0.6 | 3.68 ± 0.1 | 0.01 |
| I | 9.42 ± 0.1 | 19.77 ± 0.6 | 8.23 ± 0.9 | 5.40 ± 0.4 | 0.01 |
| J | 5.10 ± 0.2 | 18.24 ± 0.8 | 10.17 ± 0.3 | 4.55 ± 0.2 | 0.01 |
| K | 10.48 ± 0.3 | 15.89 ± 1.4 | 10.33 ± 0.3 | 4.63 ± 0.1 | 0.01 |
| L | 6.48 ± 0.1 | 25.81 ± 1.2 | 6.00 ± 0.5 | 4.56 ± 0.2 | 0.18 |
| Mean ± SE | |||
|---|---|---|---|
| Hot Air Dryer | Rotary Dryer | Microwave | |
| Crude lipids (g) | 123.73 ± 6.82 | 176.86 ± 3.40 | 206.96 ± 3.46 |
| Dry matter (g) | 183.59 ± 8.79 | 130.46 ± 5.60 | 100.36 ± 5.69 |
| Lipid Yield (wt %) | 40.83 ± 2.83 | 57.95 ± 1.80 | 67.66 ± 1.83 |
| Mean ± SE | |||
|---|---|---|---|
| 25–30 °C | 35–45 °C | 65–75 °C | |
| Crude lipids (g) | 120.31 ± 5.58 | 160.56 ± 5.68 | 203.86 ± 6.52 |
| Dry matter (g) | 187.01 ± 3.39 | 143.76 ± 6.49 | 100.46 ± 5.67 |
| Lipid Yield (wt %) | 39.73 ± 1.09 | 53.6 ± 2.09 | 67.6 ± 1.83 |
| Content (wt %) | |||||
|---|---|---|---|---|---|
| N | C | H | S | O | |
| BSFL lipids | 0.48 | 74.67 | 12.43 | 0.04 | 12.42 |
| Hydrodeoxygenated BSFL oil | 0.56 | 83.93 | 15.21 | Trace | 0.30 |
| Peak No. | Retention Time (min) | Area (%) | FAME Species | Compound Name |
|---|---|---|---|---|
| 1 | 10.16 | 0.970 | 10:0 | Decanoic acid, methyl ester |
| 2 | 12.25 | 25.723 | 12:0 | Dodecanoic acid, methyl ester |
| 3 | 13.87 | 4.691 | 14:0 | Tetradecanoic acid, methyl ester |
| 4 | 15.27 | 3.459 | 16:1 | 9-Hexadecenoic acid, methyl ester |
| 5 | 15.51 | 16.946 | 16:0 | Hexadecanoic acid, methyl ester |
| 6 | 16.85 | 39.764 | 18:2 | 8,11-Octadecadienoic acid, methyl ester |
| 7 | 17.01 | 4.326 | 18:0 | Octadecanoic acid, methyl ester |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.E.; Jang, H.S.; Yun, Y.J.; Han, G.B.; Park, Y.K.; Yang, Y.C.; Jang, J.H. Application of the Hydrodeoxygenation of Black Soldier Fly Larvae Lipids in Green Diesel Production. Sustainability 2024, 16, 584. https://doi.org/10.3390/su16020584
Lee JE, Jang HS, Yun YJ, Han GB, Park YK, Yang YC, Jang JH. Application of the Hydrodeoxygenation of Black Soldier Fly Larvae Lipids in Green Diesel Production. Sustainability. 2024; 16(2):584. https://doi.org/10.3390/su16020584
Chicago/Turabian StyleLee, Ji Eun, Hyun Sung Jang, Yeo Jin Yun, Gi Bo Han, Young Kyu Park, Young Cheol Yang, and Jung Hee Jang. 2024. "Application of the Hydrodeoxygenation of Black Soldier Fly Larvae Lipids in Green Diesel Production" Sustainability 16, no. 2: 584. https://doi.org/10.3390/su16020584
APA StyleLee, J. E., Jang, H. S., Yun, Y. J., Han, G. B., Park, Y. K., Yang, Y. C., & Jang, J. H. (2024). Application of the Hydrodeoxygenation of Black Soldier Fly Larvae Lipids in Green Diesel Production. Sustainability, 16(2), 584. https://doi.org/10.3390/su16020584






