1. Introduction
In the modern world, the energy demand continues to increase, fueled by population growth, industrialization and technological advances. This growing energy consumption has significant economic and environmental impacts. Dependence on conventional energy sources, such as fossil fuels, has led to widespread pollution, resulting in climate change. It is, therefore, necessary to identify sustainable alternatives to the continued use of non-renewable resources [
1].
In this regard, solar energy is an inexhaustible source of renewable energy, capable of providing the Earth with large quantities of thermal energy on a daily basis. The use of this energy, however, is hindered by its intermittent nature, dictated by the cycle of day and night and by meteorological and seasonal fluctuations. This variability limits the reliability of solar energy, especially in applications that require constant energy production for extended periods. The adoption of an effective thermal energy storage solution is, therefore, essential to match the energy supply and demand. It is strategic to identify new technological solutions to achieve a leveling of energy load peaks and guarantee constant and prolonged energy availability [
2].
Phase change materials (PCMs) stand out as an effective solution to address these challenges, offering efficient energy storage and management capabilities. These materials, in fact, have the ability to store and release thermal energy during their phase transitions as a response to external temperature variations. They are, therefore, able to reduce the need for energy of fossil origin for heating/cooling the environments in which they are applied, guaranteeing environmental comfort [
3]. The integration of PCMs into building elements can adequately regulate the indoor climate, reducing energy consumption for heating, ventilation and air conditioning (HVAC) [
4]. Furthermore, these materials can find useful and convenient applications in very different fields, such as in automotive applications [
5], textiles [
6,
7], electronics [
8], and renewable energy systems [
9,
10,
11].
PCMs can be composed of materials with a different nature and composition, namely organic, inorganic and hybrid. Each of them is characterized by specific temperatures and enthalpy of the phase change, and they can therefore find applications in different fields. Organic phase change materials represent the most investigated and used class of PCMs thanks to their ability to store and release high quantities of thermal energy during phase transitions. The key properties that make these PCMs very attractive for a wide variety of applications are the following: (i) the high values of latent heat, which allow them to store large amounts of thermal energy per unit mass or volume; (ii) a wide range of phase change temperatures, ranging from sub-zero temperatures to over 100 °C, making them adaptable to any specific need; (iii) the good chemical and thermal stability, ensuring their reliability and long-term performance in energy storage systems [
12,
13]; and (iv) these materials can be encapsulated or shape-stabilized within various matrices (such as microcapsules [
14], polymers [
15], carbon [
16], or inert materials [
17]) to prevent losses, adapt to each application and facilitate their integration into different elements [
11,
17,
18]. Organic PCMs are widely used to maintain thermal stability in buildings, guaranteeing the internal comfort of the inhabitants and limiting the extensive use of heating/cooling devices [
18,
19]. Although they are not cheap, the use of organic PCMs can lead to long-term cost savings through reduced energy consumption and less maintenance of heating/cooling devices [
20,
21].
It is necessary to highlight that organic PCMs have some disadvantages or limitations. They may have lower heat transfer rates than traditional heat transfer fluids, affecting the speed of the thermal response, which may not be adequate for some applications. Additionally, some organic PCMs undergo degradation or phase separation after repeated thermal cycling, compromising their long-term performance and reliability [
22,
23]. Incompatibility problems can arise when integrating organic PCMs into elements of a different nature or into complex systems: careful selection of the most suitable PCM is, therefore, necessary to always guarantee adequate performance. Finally, improper encapsulation or handling of organic PCMs can cause leaks, which may pose risks to the environment and human beings and which require appropriate remedies [
3,
12]. In summary, a careful analysis of the limitations of organic PCMs is essential for the correct implementation of these materials in specific contexts.
By mixing two organic PCMs it is also possible to obtain a new phase change material that offers customized properties based on specific requirements or needs. In fact, through the physical mixing of two suitable PCMs in pre-established concentrations, it will be possible to obtain a new PCM capable of exhibiting a specific range of phase change temperatures [
24,
25]. There are numerous studies in the literature illustrating the development of binary, ternary and multicomponent organic PCMs suitable for a wide variety of applications.
Venkitaraj et al. [
26] investigated the performance of a novel organic solid–solid/low melting alloy (LMA) composite PCM for thermal energy storage applications. Although effective as solid–solid organic PCMs, polyalcohols suffer from low thermal conductivity. For this reason, the LMA was added due to its excellent thermal conductivity properties. The authors of the study analyzed the effect of adding the LMA to the composite PCM by employing different techniques, namely TGA-DTA (i.e., simultaneous thermogravimetric analysis (STA) combining thermogravimetry analysis (TGA) and differential thermal analysis (DTA)), DSC (differential scanning calorimetry), and FTIR (Fourier transform infrared spectroscopy). The effect of 100 hot–cold cycles was also evaluated. Chang et al. [
27] developed a novel PCM using poly-ethylene glycols (PEGs) of different molecular weights (i.e., PEG-200, PEG-400, PEG-600, and PEG-1000) mixed with isophorone diisocyanate in order to obtain crosslinked polyurethane. The goal of their study was to produce a PCM capable of activating at temperatures below 0 °C. To broaden the phase change temperature range of PEG-based PCMs, the authors blended PEG-4000 and PEG-20000 with polyurethane. The crosslinks present in polyurethane have been shown to limit the movement of PEG at high temperatures, thus preventing material loss. The resulting PCMs had operating temperature ranges between −27 and 73 °C, making them effective for energy storage in a wide variety of climate conditions. In another study [
28], authored by Deepak et al., a stearic acid–acetamide eutectic PCM with a melting temperature of 65.73 °C and a latent heat of 203.72 J/g was mixed with aluminum oxide nanoparticles and multi-walled carbon nanotubes (MWCNTs) to improve its thermal conductivity. This modification resulted in an increase in the melting temperature by almost 3 °C and in the latent heat by 10%, thanks to a greater molecular interaction. The eutectic mixture showed chemical and thermal stability, with an increase in the degradation temperature, even after 500 thermal cycles. Kheradmand et al. [
29] proposed incorporating a hybrid PCM into plastering mortars for façade walls to improve the energy efficiency of buildings. Three organic microencapsulated PCMs, based on paraffin wax, were inserted by mixing them directly into the mortar formulations. The thermal performance of the hybrid PCMs, in comparison to the mortar not containing any PCM, was evaluated using a prototype test cell reproducing a daily temperature profile. The results suggested that hybrid PCMs in plastering mortars are able to significantly reduce the heating and cooling energy demands to maintain indoor comfort levels. Samad Ali Taj et al. [
30] tried to improve the thermal performance of clay bricks by inserting organic PCMs as heat storage elements. Lauric acid and palmitic acid were chosen for this purpose, having melting temperature ranges suitable for the hot climate of Islamabad (Pakistan). Two test compartments were built, to simulate two adjacent rooms, to evaluate the temperature reduction and the thermal load. One contained clay bricks with embedded PCM and the other standard bricks, and both were exposed to sunlight and controlled conditions. The results of the study showed a decrease in the compartment temperature by 4–5.5 °C and a 32% reduction in the temperature fluctuation range if the PCM was included in the bricks. Aamir Khan et al. [
31] investigated the effect of different cooling methods on a 5000 mAh lithium-ion battery during its charging and discharging at different rates. The cooling systems tested were natural cooling (in air), cooling using a heat transfer fluid, cooling using a binary PCM (based on lauric acid and stearic acid), and hybrid cooling. Compared to air cooling, heat transfer fluid cooling reduced the maximum temperature by approximately 22%, mixed PCM cooling by 41%, and hybrid cooling (i.e., heat transfer fluid cooling combined with PCM) by about 46%. The effectiveness of a hybrid cooling system, i.e., supported by a PCM, was therefore demonstrated: such a system could be proposed for the cooling of lithium-ion batteries. Homlakorn et al. [
32] explored passive cooling methods using PCMs to mitigate the temperature rise in photovoltaic (PV) cells. To achieve this aim, a novel organic PCM, composed of myristic acid and stearic acid in three different proportions (60:40, 70:30, 80:20 in weight), was investigated. The test results, determined with radiation generated by a tungsten halogen bulb, showed that the PCM can effectively reduce the temperature of the photovoltaic module, improving its performance. In particular, the mixture of composition 60:40 proved to be the best performing, with a temperature reduction of over 7 °C, an increase in power of 0.454 W and an improvement in module efficiency of 4.2% compared to the reference PV module. In another study, Shoujin Chang et al. [
33] developed tailor-made PCM blends for the thermal management of electronic devices. The PCMs, obtained by mixing fatty acids and paraffin, offered adjustable melting ranges and high heat storage capacity. The developed PCMs were able to greatly delay the temperature rise of electronic devices, extending the time needed to reach the critical temperature by more than eight times. In pulsed heat flow tests, these PCMs limited the maximum temperatures to 50 °C, well below the critical thresholds.
In the present study, different organic PCMs with different characteristics and origins were analyzed. Two of them were by-products of the lost wax casting industry. To the best of our knowledge, little is known about the waste materials from the casting industry process, and only a few studies have analyzed them as PCMs [
34,
35].
The lost wax casting industry generates several types of waste, among which the main wastes are waxy materials used in the initial production stage to fabricate sacrificial parts. During production, these waxy materials melt and are removed from inside the mold formed by multiple layers of ceramic paste, which constitutes the cavity for pouring the molten metal. Due to the significant expansion of this industry, increasing quantities of these waxy materials are left in landfills. Therefore, the possibility of using, or even valorizing, this waste is essential to guarantee the sustainability of production, offering environmental and economic benefits. The objective of this study was, in fact, to verify whether these waxy by-products could be reused as effective and low-cost PCMs. The research aimed to analyze different types of waxy by-products, comparing their performance with commercial (microencapsulated and non-encapsulated) PCMs. Two commercial PCMs were used as a basis for comparison: a commercial paraffin and a microencapsulated PCM. The possibility of combining this waxy waste with a commercial PCM to improve its properties has also been explored. The selected/developed PCMs were analyzed to determine their chemical composition, thermal characteristics, thermal stability as well as reliability after being subjected to 100 cycles of melting and crystallization processes.
2. Materials
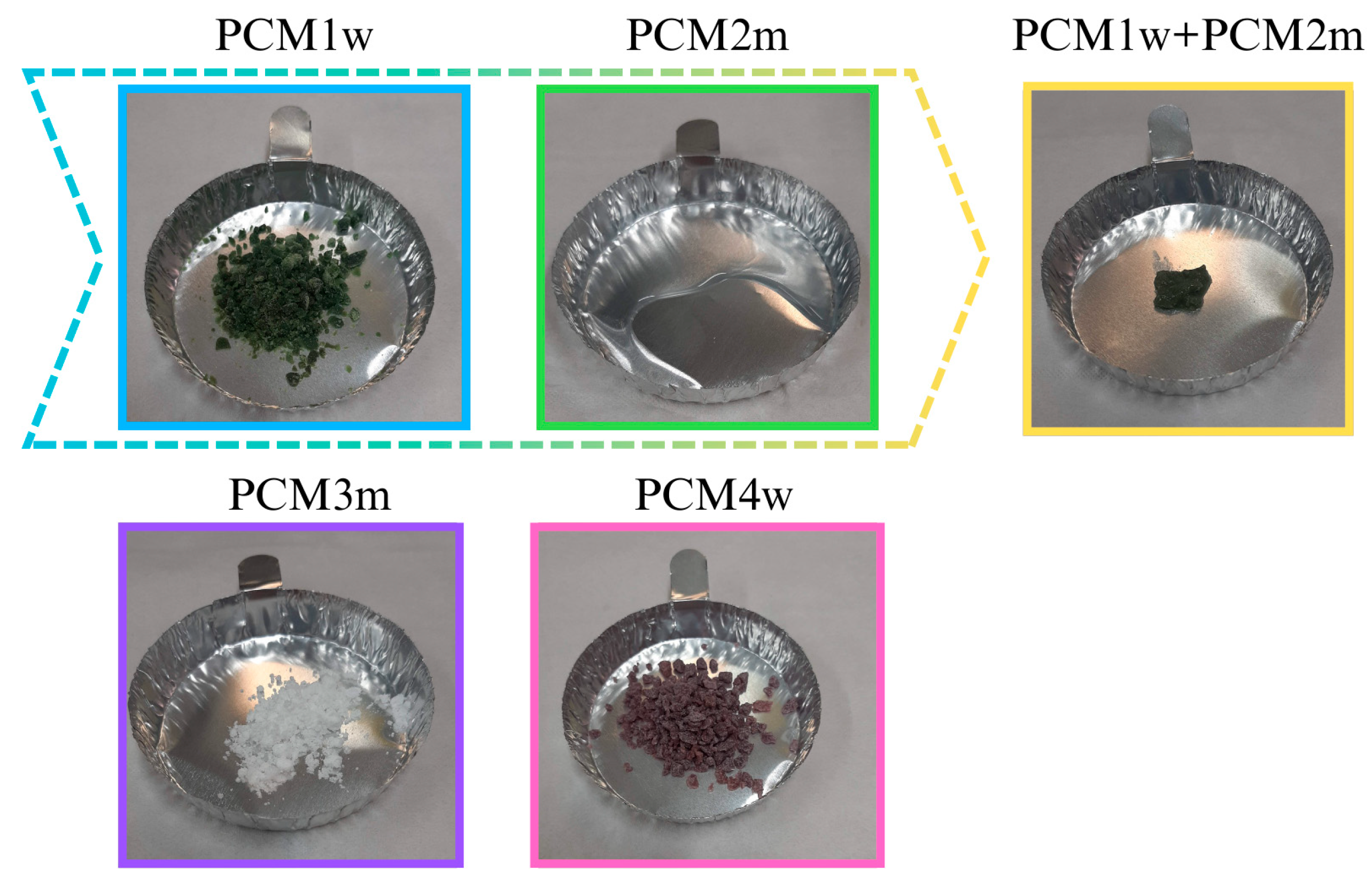
Five different organic PCMs (shown in
Figure 1) were analyzed in this study, one of which was developed by the authors. For more immediate identification, the PMCs under study were indexed based on their origin: commercial PCMs are referred to as PCMm, where “m” stands for “market”; PCMs obtained as by-products of the lost wax casting industry are referred to as PCMw, where “w” stands for “wax”.
PCM1w is a by-product of the lost wax casting industry. It was supplied by a Portuguese company (Ferespe, Vila Nova de Famalicão, Portugal). It was originally produced by Paramelt (Heerhugowaard, The Netherlands) under the trade name Paracast® FW 14632, but not as a phase change material. PCM1w is mainly composed of a mixture of refined hydrocarbon waxes, resins and polymers. It was supplied in boards (20 × 30 × 10 cm3) with a green hue; after a grinding process, it was reduced to granular form. It has a density of 1013 kg/m3.
PCM2m is a paraffin, which appears as a colorless liquid; it is produced and sold as a PCM by Rubitherm (Berlin, Germany). PCM2m has densities in the solid and liquid states of 760 kg/m3 and 700 kg/m3, respectively.
By mixing PCM1w with PCM2m (in weight composition 1:1), the organic–organic PCM1w + PCM2m phase change material was obtained. The mixture was prepared by simply mixing the two components at a temperature of 30 °C for 2 min. The obtained PCM1w + PCM2m has a green, pasty consistency.
PCM3m was supplied in the form of microcapsules composed of a melamine–formaldehyde shell and a paraffin core. It is commercialized as a PCM by Devan Chemicals (Ronse, Belgium), under the trade name Mikathermic D24. The microcapsules have a particle size distribution between 5.8 and 339 μm, with 80% of the particle size between 10.4 and 55.2 μm and an average particle size of 43.91 μm. The density of PCM3m is 880 kg/m3.
Finally, PCM4w is again a by-product of the lost wax casting industry, supplied by Ferespe company (Vila Nova de Famalicão, Portugal). It was originally produced by Paramelt (Muskegon, MI, USA) under the trade name Cerita® Casting Wax F28-46M, not for PCM purposes. PCM4w has a red hue. It was supplied as boards (20 × 30 × 10 cm3) and then ground to obtain small granules. A density of 1013 kg/m3 is reported for PCM 4. It is composed of a mixture of waxes, resins, polymers, cross-linked polystyrene, terephthalic acid and oil soluble dye.
3. Methods
The thermal energy storage (TES) characteristics are the main properties to be determined in relation to a PCM. They must be determined to evaluate the behavior of a PCM during the phase transition process and the temperature range at which it absorbs and releases heat. For this purpose, the PCMs under analysis were subjected to calorimetric analysis using differential scanning calorimetry (DSC, Stare System, Mettler Toledo, Columbus, OH, USA). With this method, it was possible to measure for each PCM the range of phase transition temperatures, with the relative enthalpies (ΔH) of melting or crystallization. The latter is proportional to the area under the melting or crystallization peaks, and it represents the heat absorbed (melting process) or released (crystallization process) during the transitions of the PCMs. Each sample was subjected to a single thermal cycle, from 0 °C to 100 °C, with a heating rate of 10 °C/min, and from 100 °C to 0 °C, with a cooling rate of 10 °C/min. Although lower test rates, such as 0.5 or 1 °C/min, may provide more accurate data on phase transitions and thermal stability [
36,
37], the used speed (i.e., 10 °C/min) is the one most reported in scientific papers and employed in industrial applications. In fact, this heating–cooling rate was also chosen to facilitate a direct comparison with existing results in the literature. The calorimetric tests were carried out in an inert atmosphere (i.e., nitrogen gas, flow rate equal to 60 mL/min). The samples, with an average weight of 10 to 20 mg, were sealed in aluminum crucibles and then analyzed in a DSC module. Three measurements were performed, and the results were averaged.
Fourier transform infrared spectroscopy (FTIR, Jasco 6300 spectrometer, JASCO Corporation, Tokyo, Japan) was employed to assess the chemical structure (i.e., composition and chemical bonds) of the phase change materials under study. This technique also identified possible chemical interactions between PCM1w and PCM2m when mixed to obtain the PCM1w + PCM2m system. The infrared spectra were recorded in the wavelength range from 500 to 4000 cm−1, using 64 scans and a resolution of 4 cm−1. This test was repeated three times on each PCM system to ensure the repeatability of the results.
Thermogravimetric analysis (TGA, NETZSCH STA 409, thermal analyzer, Selb, Germany) was used to evaluate the mass variation of the PCMs due to exposure up to very high temperatures. This evaluation is important to assess the thermal resistance of the PCMs, even under extreme temperature conditions. The TGA analysis was performed in an inert atmosphere (i.e., nitrogen), varying the test temperature between 20 °C and 1000 °C at a heating rate of 10 °C/min. The test was repeated three times on each PCM, and the results were averaged.
Finally, the tested PCMs were subjected to 100 melting and solidification cycles to evaluate their thermal stability and in-service reliability. After thermal cycling, the PCMs were analyzed again with DSC and FTIR, using the same test parameters already reported. In this way, it was possible to verify whether their phase change properties (using DSC analysis) and chemical structure (by FTIR spectroscopy) were modified as a result of thermal cycling.
Figure 2 summarizes the tests performed in this study.
4. Results and Discussion
4.1. Chemical Analysis
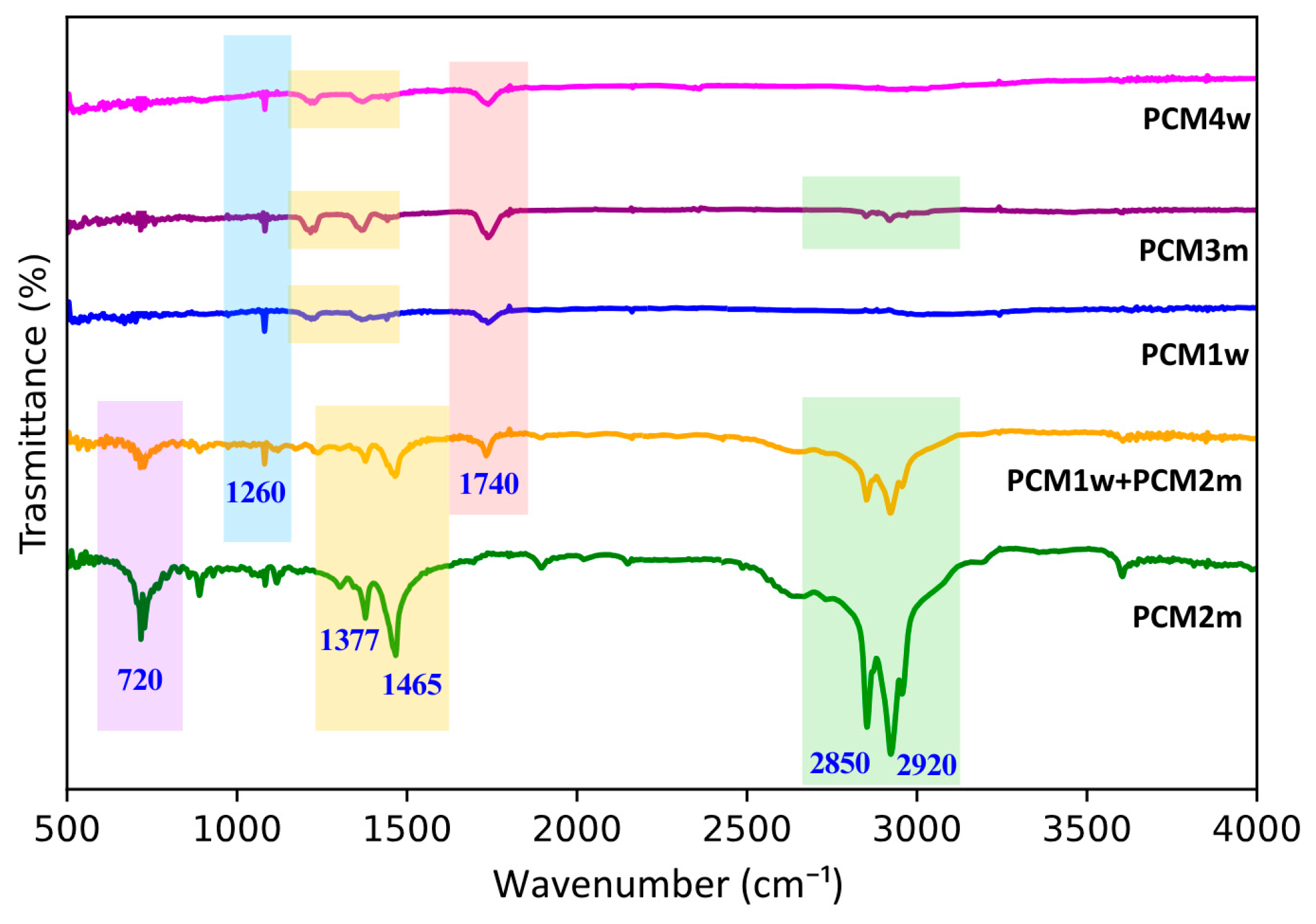
The spectra obtained from the FTIR analysis performed on the PCMs under study are reported in
Figure 3.
The FTIR analysis of PCM2m revealed distinct peaks at 2920 cm
−1 and 2850 cm
−1: they are relative to alkane C–H stretching vibrations (asymmetric and symmetric), typical of long-chain hydrocarbons such as paraffins. Additionally, peaks at 1465 cm
−1 and 1377 cm
−1 were recorded, corresponding to C–H bending vibrations characteristic of CH
2 and CH
3 groups in alkanes. The presence of paraffin is further confirmed by the peak at 720 cm
−1, corresponding to the C–H rocking vibration peak [
38].
PCM1w, i.e., the waxy material consisting of different components, displayed a clear peak at 1740 cm
−1, relative to C=O stretching, probably related to the ester groups present in its polymeric phase. The peak at 1260 cm
−1 suggests the presence of C–O stretching, which is also consistent with the ester groups. Two weak peaks like those recorded for the PCM2m sample were also observed, probably associated with the bending vibrations of CH
2 and CH
3. They appear to be shifted to lower wavenumbers, suggesting potential changes in the molecular structure or possible interactions with other components in the mixture. It should be noted that this can also occur in cross-linked polymers: shifts in the peaks are generally attributed to changes in the polymer network or different crosslink density [
39].
The FTIR spectrum obtained for the PCM1w + PCM2m system presents a combination of the peaks recorded for PCM1w and PCM2m: this proves that a physical mixture has formed between the two starting PCMs, without the formation of new chemical compounds. In fact, no new peaks are observed in the PCM1w + PCM2m FTIR spectrum.
The characteristic peaks observed in the spectra of PCM3m and PCM4w are very similar to those recorded for PCM1w. However, PCM3m showed weaker peaks at both 2920 cm−1 and 2850 cm−1, suggesting a smaller amount of paraffin in this system.
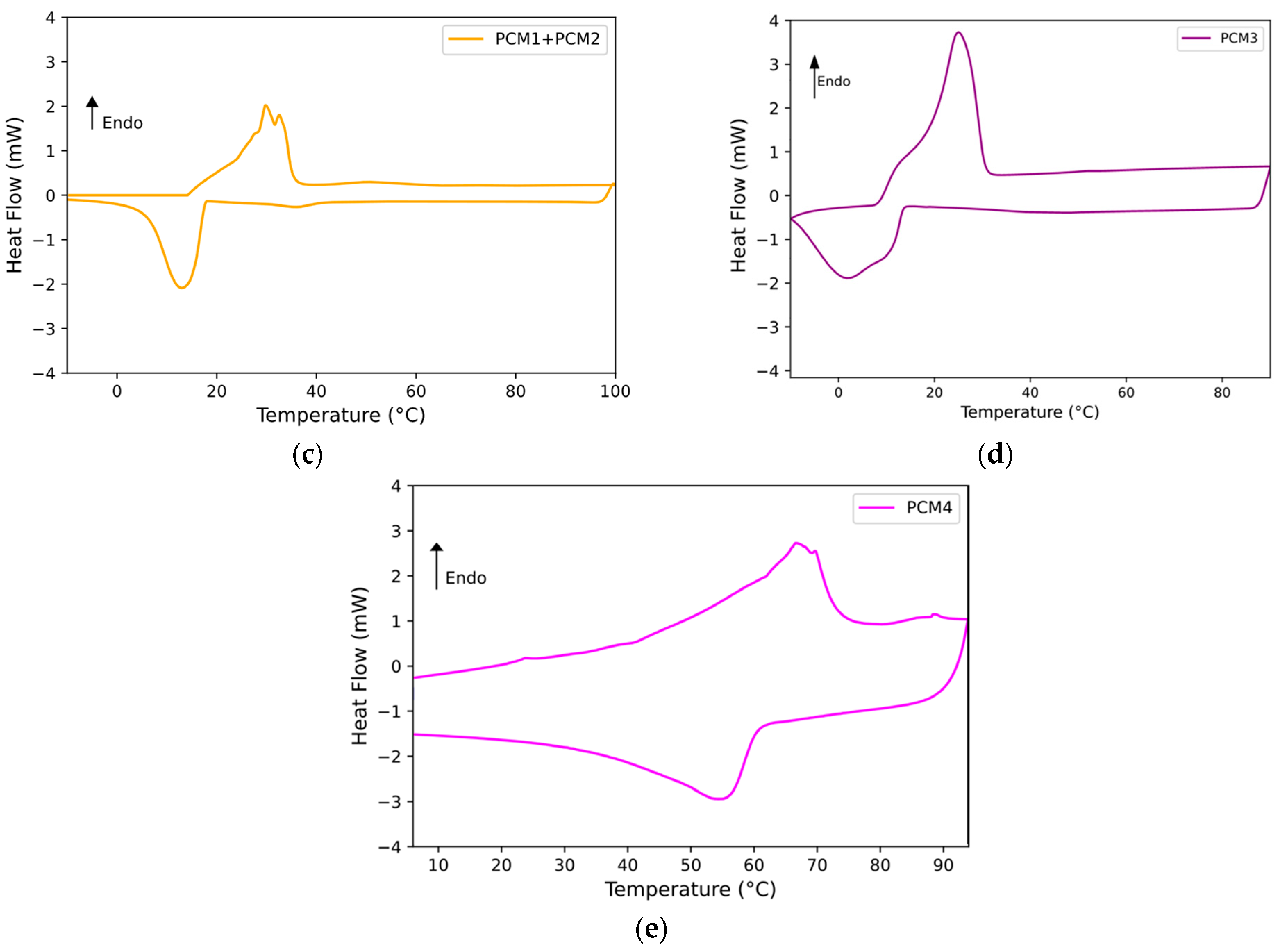
4.2. Characterization of Phase Change Behavior
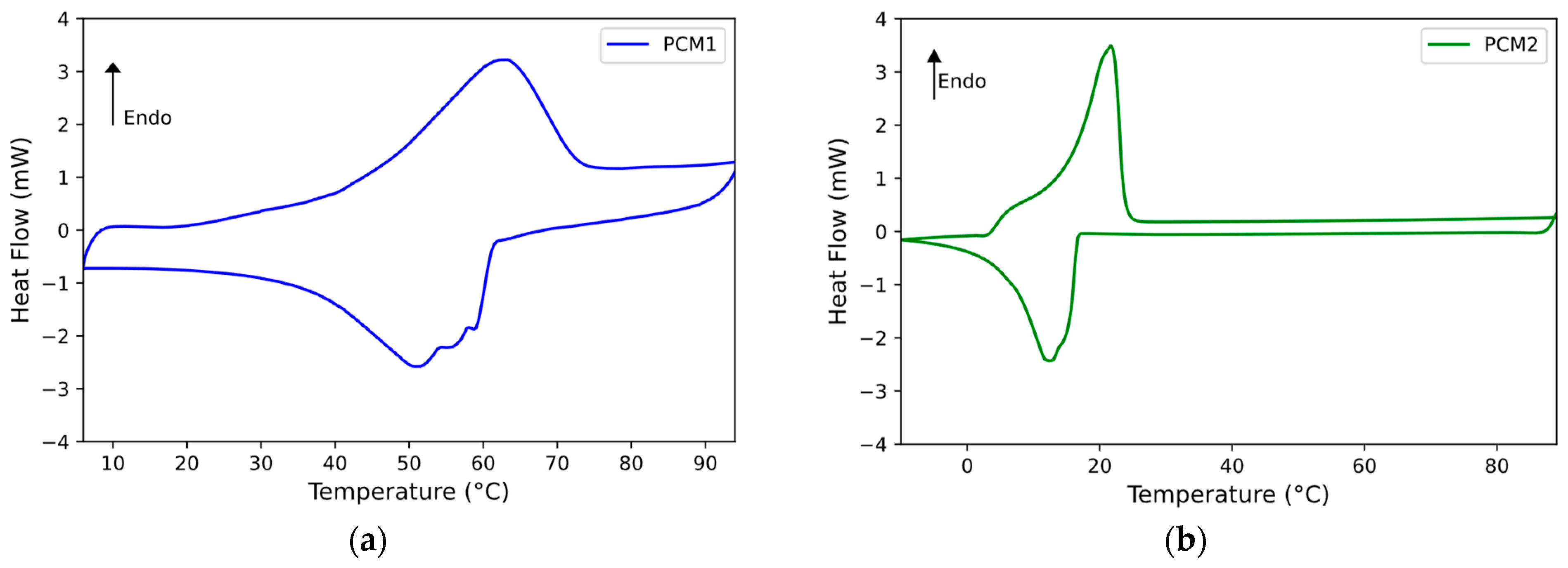
The measurement of the melting and crystallization temperature ranges and the related enthalpies of the PCMs under study were performed by DSC analysis. These, in fact, are the main TES characteristics that effective PCMs must possess. The DSC curves recorded for each PCM are reported in
Figure 4, and the corresponding results are presented in
Table 1.
All the PCMs analyzed, except for the PCM1w + PCM2m mixture, show a single endothermic peak (i.e., related to the melting process). On the other hand, the PCM1w + PCM2m mixture presents two close endothermic peaks (as visible in
Figure 4c): this is due to the fact that the blend is composed of two organic phases characterized by different melting points, where PCM1w has a melting peak at around 63 °C (
Figure 4a) and PCM2m at about 22 °C. The mixture of the two PCMs presents two melting peaks (i.e., about 29 °C and 33 °C, respectively) that are closer to the melting peak of commercial PCM2m, i.e., that characterized by the greater melting enthalpy. Passing to analyze the cooling stage, the crystallization peak of PCM1w is rather broad and less regular than that presented by PCM2m. This could be expected because PCM1w is not a commercial product and is composed of several organic components. The PCM1w + PCM2m mixture presents a crystallization peak at around 16 °C, once again close to the crystallization peak of the commercial product, i.e., PCM2m. Two very small melting and crystallization peaks can be identified in the DSC of the blend, at around 50 °C on heating and 37 °C on cooling: they are probably due to part of the PCM1w phase, i.e., the one that presents the highest phase change temperatures, which fails to blend entirely into the mixture.
In terms of the melting enthalpy, PCM1w has an enthalpy of 49 J/g and PCM2m of 151 J/g. The PCM1w + PCM2m mixture shows a heat of melting of approximately 98 J/g. Bearing in mind that the blend is composed of 50% by weight of PCM1w and 50% of PCM2m, the melting enthalpy of PCM1w + PCM2m precisely respects the mixture rule. Approximately the same is observed for the crystallization enthalpy of the blend with respect to the heat of solidification of the individual PCMs. Therefore, by adding an inexpensive by-product (i.e., PCM1w) to a commercial PCM, it is possible to obtain a good phase change material, reusing a waste product and saving on the cost of the commercial product. As reported in
Figure 4d and in
Table 1, microencapsulated PCM3m displays single melting and crystallization peaks at around 25 °C and 2 °C, respectively. The commercial product again shows quite high phase change enthalpies, about 133 J/g on heating and 121 J/g in the cooling stage, respectively. Finally, PCM 4, i.e., a by-product of the lost wax casting industry, shows not very regular melting and crystallization peaks at around 69 °C and 57 °C, respectively. This can be again explained by its non-uniform composition. The enthalpies of melting and crystallization are lower than those measured for commercial PCMs, approximately 33 J/g and 30 J/g, respectively.
From the results of the calorimetric tests, it can be concluded that all the systems analyzed, both the commercial and experimental ones, offer thermal characteristics that allow them to behave like a PCM in various applications. It is also possible to reuse waste products from other production processes, alone or in mixtures with commercial PCMs, to reduce the costs of the final PCM.
4.3. Thermal Stability
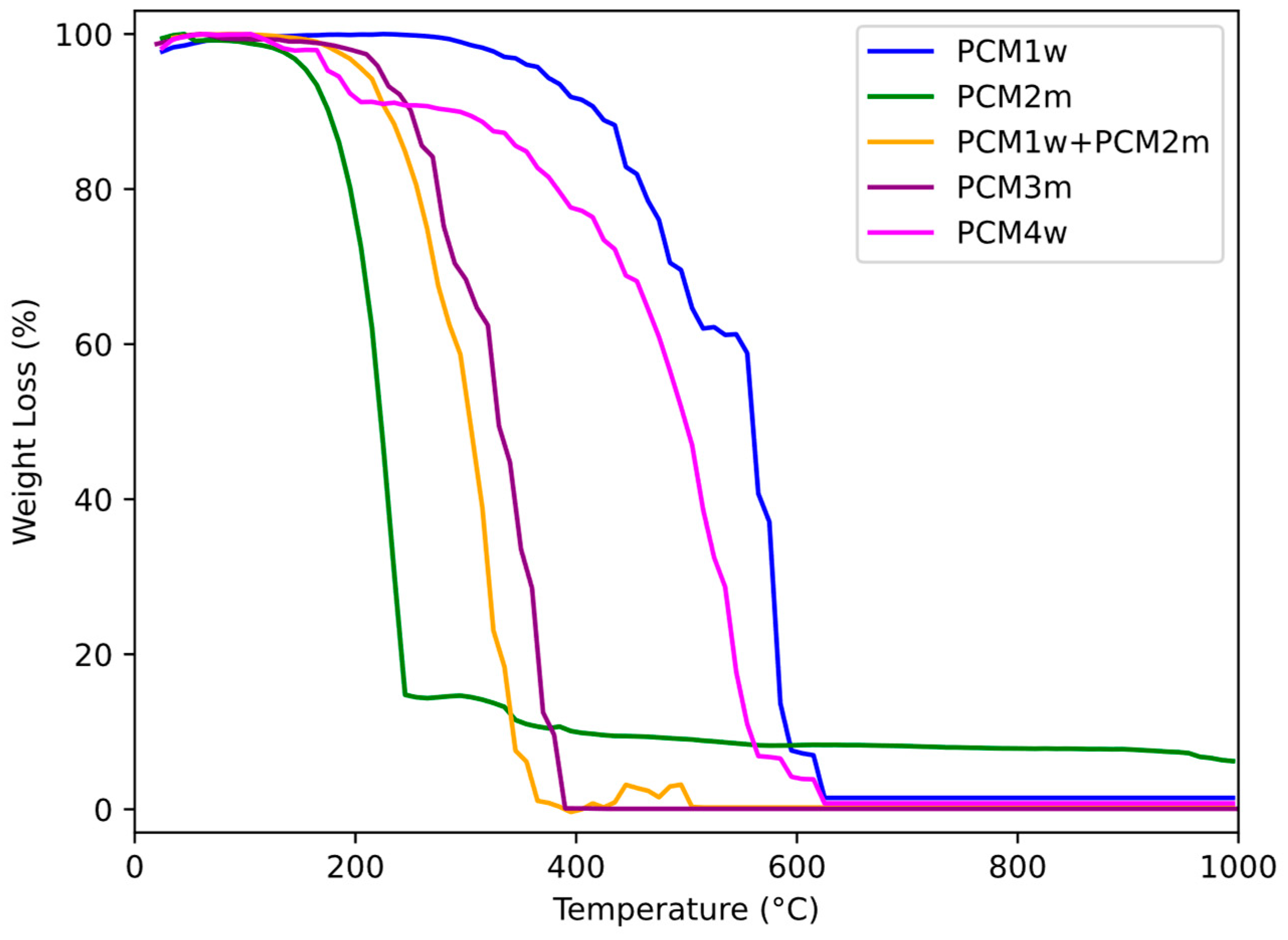
The thermal degradation of the PCMs was analyzed by monitoring their weight loss with an increasing temperature using thermogravimetric (TGA) analysis. The obtained TGA curves for the analyzed PCMs are reported in
Figure 5, while the corresponding results are presented in
Table 2.
The first commercial PCM under investigation, i.e., PCM2m, shows a first large degradation step starting at approximately 175 °C. At higher temperatures, i.e., around 300 °C, it presents limited weight loss. At the end of the TGA test, a residue of approximately 5% was recorded. Comparing its thermogram with those obtained for the other PCMs, it was concluded that PCM2m shows the lowest degradation onset temperature, which means a lower thermal resistance. Similar degradation temperatures for paraffin-based PCMs are reported in other studies, such as in the works by Bharathiraja et al. [
40] and Manoj Kumar et al. [
41].
Commercial microencapsulated PCM3m shows a very small degradation step, from 180 °C to 220 °C, probably attributable to the decomposition of the paraffin core. A second degradation step is recorded from 280 °C to 390 °C. Significant weight loss occurs in this temperature range. This suggests that the primary component of this PCM is melamine formaldehyde, a compound that degrades in this temperature range, as reported by Xu et al. [
42] and by Huang et al. [
43]. Thermal degradation of PCM3m is essentially complete above 390 °C (residual weight nearly 0%).
The thermograms shown in
Figure 5 for PCM1w and PCM 4w, i.e., the products composed of several organic components, are very similar to each other. In them, it is possible to identify different degradation phases: one begins at around 190–195 °C, a very broad one begins at around 350–360 °C, and a final one at around 590 °C. The behavior of these PCMs, characterized by a degradation profile with multiple weight loss stages, can be explained by their complex composition. As regards the residual weight, while PCM4w degrades almost completely, PCM1w has a residual weight greater than 10% up to 1000 °C. Evidently, in the complex composition of PCM1w, there is a component with high thermal resistance. Ultimately, the thermal resistance of the PCMs obtained as a by-product of the lost wax casting industry is generally higher than that exhibited by the commercial PCMs. This result confirms the possibility of using these waste materials as PCMs for different applications.
The PCM1w + PCM2m system shows an essentially single-step degradation process, starting at around 220 °C, with no residual weight. Being a blend of PCM1w and PCM2m, as expected, this system shows an intermediate thermal resistance, i.e., greater than the commercial product PCM2m and very similar to that recorded for commercial PCM3m. Even the PCM mix, therefore, could be proposed as a phase change material for different purposes.
Taking into account the results of the TGA analysis of the different PCMs, some applications can be imagined based on their thermal degradation characteristics. PCM1w and PCM4w, for example, could be suitable for industrial processes where materials need to be stable up to high temperatures. PCM2m, PCM3m and the PCM1w + PCM2m mixture could be proposed for applications where the PCM is exposed to moderate temperatures, i.e., in building elements, for passive solar heating devices, in automotive components, in electronic devices to prevent overheating or in smart packaging for temperature-sensitive products.
4.4. Thermal and Chemical Stability after Thermal Cycling
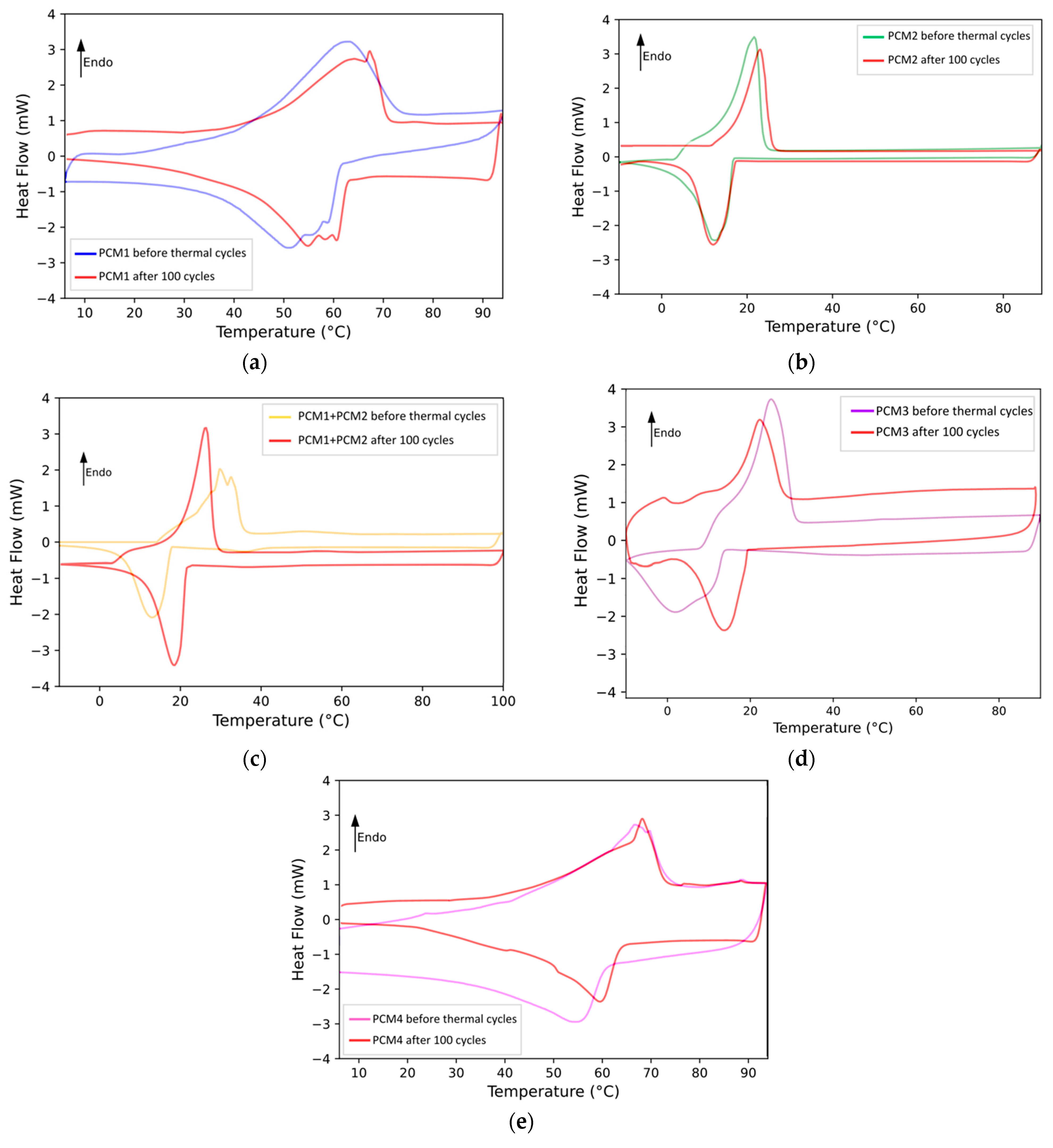
To be reliable as thermal energy storage systems, PCMs must maintain constant thermal properties and chemical characteristics, even after numerous cycles of thermal energy absorption and release. To assess their stability in service, therefore, the PCMs under analysis were subjected to 100 heating–cooling cycles. Then, the thermal properties (via DSC testing) and chemical characteristics (through FTIR analysis) of the PCMs were examined before and after the thermal cycles to verify their durability. The results of the DSC analysis are presented in
Figure 6 and summarized in
Table 3. They can be compared with those reported in
Table 1 to assess any modification in the peak temperatures of melting and crystallization and/or in the enthalpies associated with the phase transitions due to the thermal cycles.
Analyzing the effect of thermal cycling on PCM1w, the peak temperatures for the melting and crystallization processes increase by a few degrees compared to those found for the unaged material (compare the data in
Table 3 with those reported in
Table 1). The shape of the melting and crystallization peaks recorded for this PCM is not very regular: this feature can be explained by the fact that PCM1w is a mix of many different compounds. Anyhow, the enthalpies of melting and crystallization (data reported in
Table 3) remain practically unchanged compared to those measured on unaged PCM1w (data reported in
Table 1), showing small differences within the experimental errors.
As regards the commercial PCM2m, a small increase (about 3 °C) is only observed for the Tm, following the thermal cycles. The peak of the crystallization process, however, is practically unchanged when taking into account the range of variation of the results. Both ΔH values decrease after the thermal cycles, by almost 5% for melting and by almost 6% for crystallization. This observation, together with the results obtained from the thermogravimetric tests, confirms that PCM2m is to some extent vulnerable to temperature.
The PCM1w + PCM2m mixture appears to be influenced to some extent by thermal cycling. In fact, the melting temperature decreases by about 5–8 °C while the Tc seems to increase slightly (by about 3–4 °C). Both enthalpies increase by about 5% following thermal cycling. Furthermore, after repeated thermal cycles, the blend presents a more regular thermal curve: in fact, the double peak observable during the melting of the unaged material disappears.
As regards PCM3m, the second commercial product, the peak melting temperature decreases slightly, by approximately 3 °C, as a consequence of the thermal cycles. Furthermore, the shape of the melting peak appears not very regular. The T
c increases significantly (about 14 °C) after repeated thermal cycles. This behavior is generally attributed to molecular rearrangements and alterations in the crystal structure during thermal cycling, leading to increased crystallinity and purification effects, as reported in several studies [
44,
45,
46]. Both enthalpies recorded during the phase changes decrease after the cycles, about 10% for melting and almost 13% for crystallization.
The melting behavior of PCM4w remains practically unchanged after the thermal cycles, both in terms of the shape and temperature of the peak and the value of the enthalpy. Even the changes during the crystallization process are minimal: the peak crystallization temperature increases by about 3–4 °C while the ΔH of the same process decreases by approximately 7%.
It can be concluded that the PCMs obtained as by-products of the lost wax casting industry are able to resist thermal cycling reasonably well, where their behavior is certainly not worse than that of the commercial PCMs. They maintain the melting and crystallization enthalpies almost unchanged, guaranteeing the reliability of the PCMs, even for long periods of service. Finally, the PCM mixture is greatly influenced by the behavior of the commercial component, i.e., PCM2m, which is the material least resistant to thermal aging.
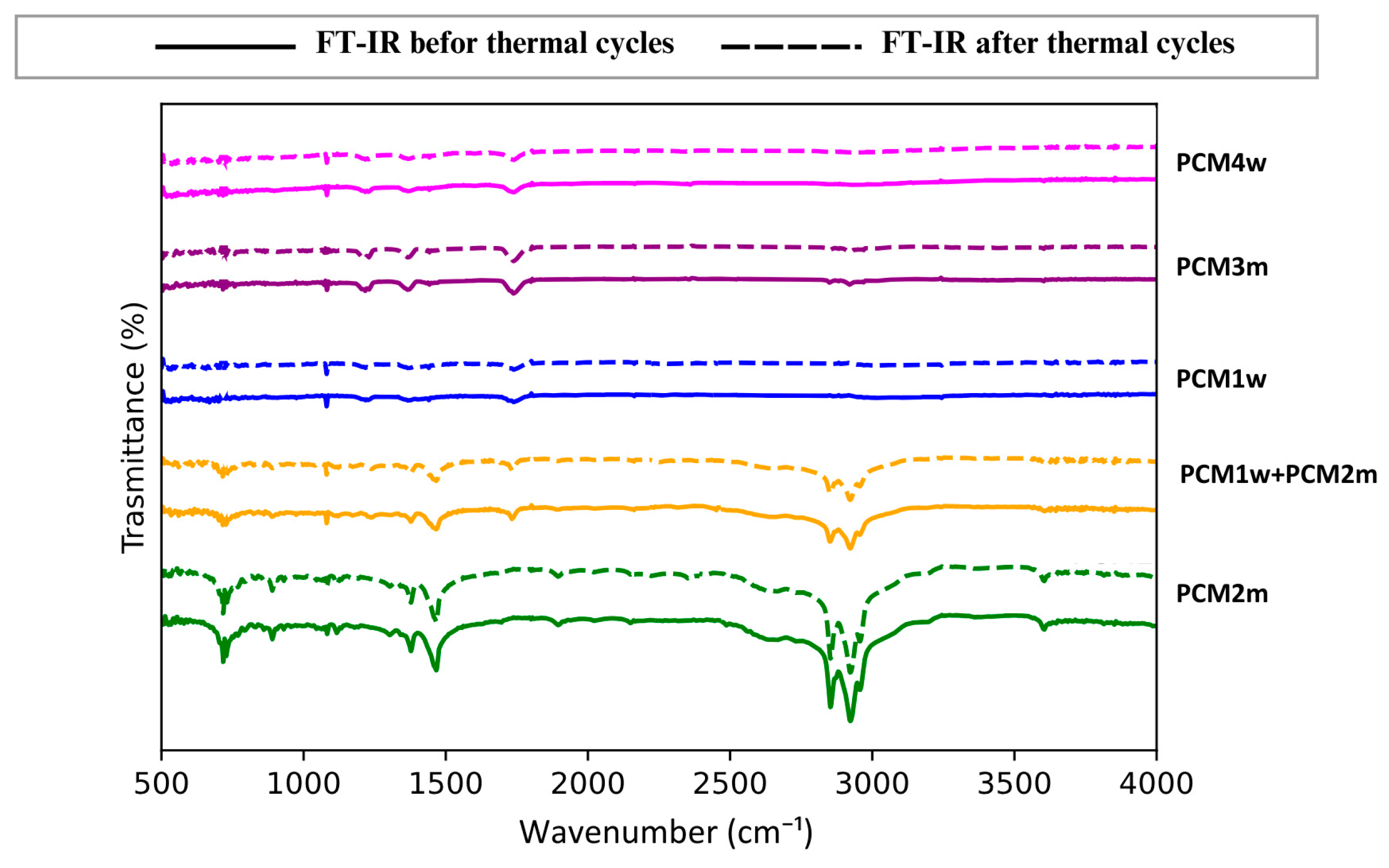
FTIR analysis was used to evaluate the stability of the PCMs after thermal cycling, verifying any changes in the chemical bonds of the phase change materials under analysis. The FTIR spectra of all the analyzed PCMs, both before and after 100 thermal cycles, are shown in
Figure 7.
Comparing the FTIR spectra recorded for each PCM, before and after the cyclic treatment, no significant changes are observed and no new peaks appear. These results demonstrate that the PCMs, even the non-commercial ones, maintain their chemical structure substantially unchanged after being subjected to repeated thermal cycling. Therefore, even the experimental PCMs obtained as by-products of the lost wax casting industry can be proposed as energy storage materials.
The encouraging results of the conducted research are expected to have significant practical applications. However, as the work is still in the experimental phase, it is not yet possible to identify precise fields of application. Anyhow, the experimentation conducted was fundamental to understand that these waxy by-products of the lost wax casting industry can be used effectively as PCMs. For example, DSC analysis has shown that these materials have appropriate melting and crystallization temperatures for various applications, from construction to photovoltaic panels and electronic devices. The same characterization identified a limitation of these waxy by-products in terms of their enthalpy, which is lower than commercial PCMs. The latent heat capacity of a PCM depends directly on its enthalpy. Therefore, the solution of mixing the waxy PCM with a commercial PCM with a higher enthalpy achieved an increase in the enthalpy of the mixed PCM, potentially solving this problem. This promising attempt does not exclude the possibility of identifying other new ways to increase the latent heat capacity of these waxy materials. Furthermore, the TGA analysis provided important information on the thermal stability: the waxy PCMs were found, in fact, to be more resistant to high temperatures than the reference commercial PCMs. Furthermore, the thermal cycling used to evaluate the thermal reliability of the PCMs demonstrated that these materials do not degrade significantly under repeated melting and crystallization cycles, i.e., their thermal properties remain stable over time in service. The FTIR analysis conducted before and after the thermal cycles further confirmed that PCMs do not undergo chemical changes.
In conclusion, the results obtained from this investigation all confirm the possibility of using waxy by-products as PCMs. This opens up possibilities for their application in various fields.
5. Expected Economic and Environmental Impacts
The lost wax casting industry currently generates significant quantities of paraffin wax waste, a form of waste that cannot be fed back into manufacturing processes. Therefore, it is generally transported and left in landfill, resulting in disposal costs and negative impacts on the environment. This study aimed to explore the possibility of reusing these industrial by-products as PCMs as an economical and sustainable alternative to commercial PCMs.
First, reusing waxy by-products as PCMs can offer economic benefits. Commercial PCMs cost between EUR 9 and EUR 16 per kg, not including the costs of their transportation to the application site. On the contrary, the cost of acquiring waxy by-products can be considered zero, allowing companies to save on the costs of transporting and disposing of this waste in landfill. Although the transformation of these waste materials, which is necessary to meet PCM specifications, may require additional costs, the savings resulting from reduced use of raw materials and management of wax waste could offset these expenses. The authors are not able to quantify these costs since they are not publicly accessible and are not made available by the companies. Because of this, pilot projects could be developed in regions with a developed investment casting industry to demonstrate the validity of the proposed solution and the economic benefits. The success of these experiences could encourage wider adoption of such production processes.
The potential environmental benefit of reusing waxy by-products as PCMs is also significant. The proposed approach could, in fact, reduce the volume of industrial waste from these production processes disposed of in landfill while at the same time preserving precious resources. By reducing the areas allocated to landfills, and by decreasing greenhouse gas emissions associated with the transport and decomposition of waste, this solution would be in line with sustainable development policies.
Despite the many potential benefits, some limits remain for the industrial scalability of these materials, such as the variability of their availability, which depends on the location and production volume of companies. In areas where the lost wax casting industry is not sufficiently developed, collecting and transporting these by-products can be difficult and expensive. However, it is possible to consider adapting the approach tested in this study to local conditions. For example, ad hoc logistics solutions and local partnerships can be trialed to overcome the challenges associated with the collection and transport of materials.
In summary, the use of waxy by-products from the lost wax casting industry as PCMs offers a sustainable and economically viable alternative to commercial products, with potential applications in several fields. Offering positive economic and environmental benefits, this approach can contribute to the development of low-cost and environmentally friendly materials, enabling the sustainable conservation of resources.
6. Conclusions
This study presents an original contribution to the development of sustainable PCMs produced from waste by-products of industrial production. Waxy by-products from the lost wax casting industry were, in fact, investigated as potential effective and low-cost PCMs. Given the significant expansion of this sector, and the consequent growing quantity of waxy waste deposited in landfill, our research aimed to identify sustainable alternatives for the profitable reuse of these waste materials. The proposed solution for the reuse of waxy materials is expected to bring environmental and economic benefits.
In the present study, the chemical and thermal characteristics and resistance to thermal loads of five organic PCMs with different origins were analyzed. Two of them, i.e., paraffin PCM2m and microencapsulated PCM3m, are true commercial phase change products. Another couple of PCMs, i.e., PCM1w and PCM4w, are composed of by-products of the lost wax casting industry. A fifth experimental PCM, indicated as PCM1w + PCM2m, was produced by blending commercial and non-commercial products to explore if it is possible to achieve improved performance in reusing, at least partly, a by-product of a different industry. These waste products may represent an attractive alternative if they are shown to offer suitable TES properties.
The investigation carried out led to the following results:
- -
Both the commercial and experimental PCMs, as analyzed by calorimetric analysis, showed adequate TES capabilities. The latent heat of the phase change of the commercial products, however, was always found to be appreciably higher than that measured for the waxy by-products. However, by blending a waxy by-product (i.e., PCM1w) with a commercial product (i.e., PCM2m), it is possible to improve the enthalpy in both the melting and crystallization processes of the final PCM blend.
- -
The analysis of the thermal degradation, as carried out by the TGA technique, showed that the commercial product PCM2m is the most sensitive to high temperatures, starting the degradation process at relatively low temperatures (i.e., around 175 °C). On the other hand, the experimental PCM1w exhibited the greatest thermal resistance. As a direct consequence, the resistance to thermal degradation of the PCM blend, i.e., the PCM1w + PCM2m system, was higher than that of PCM2m alone. Therefore, it is confirmed that the production of a blend allows for maintaining an adequate phase change enthalpy, together with good thermal resistance.
- -
The reliability of the PCMs was evaluated by comparing the thermal properties, employing the DSC technique, and the chemical characteristics, using FTIR analysis, of the different systems before and after 100 consecutive thermal cycles of melting and crystallization. All the analyzed materials retained good thermal and chemical characteristics, even after the thermal cycles, guaranteeing adequate thermal energy storage capabilities over time.