Multi-Objective Optimization of Synergic Perchlorate Pollution Reduction and Energy Conservation in China’s Perchlorate Manufacturing Industry
Abstract
1. Introduction
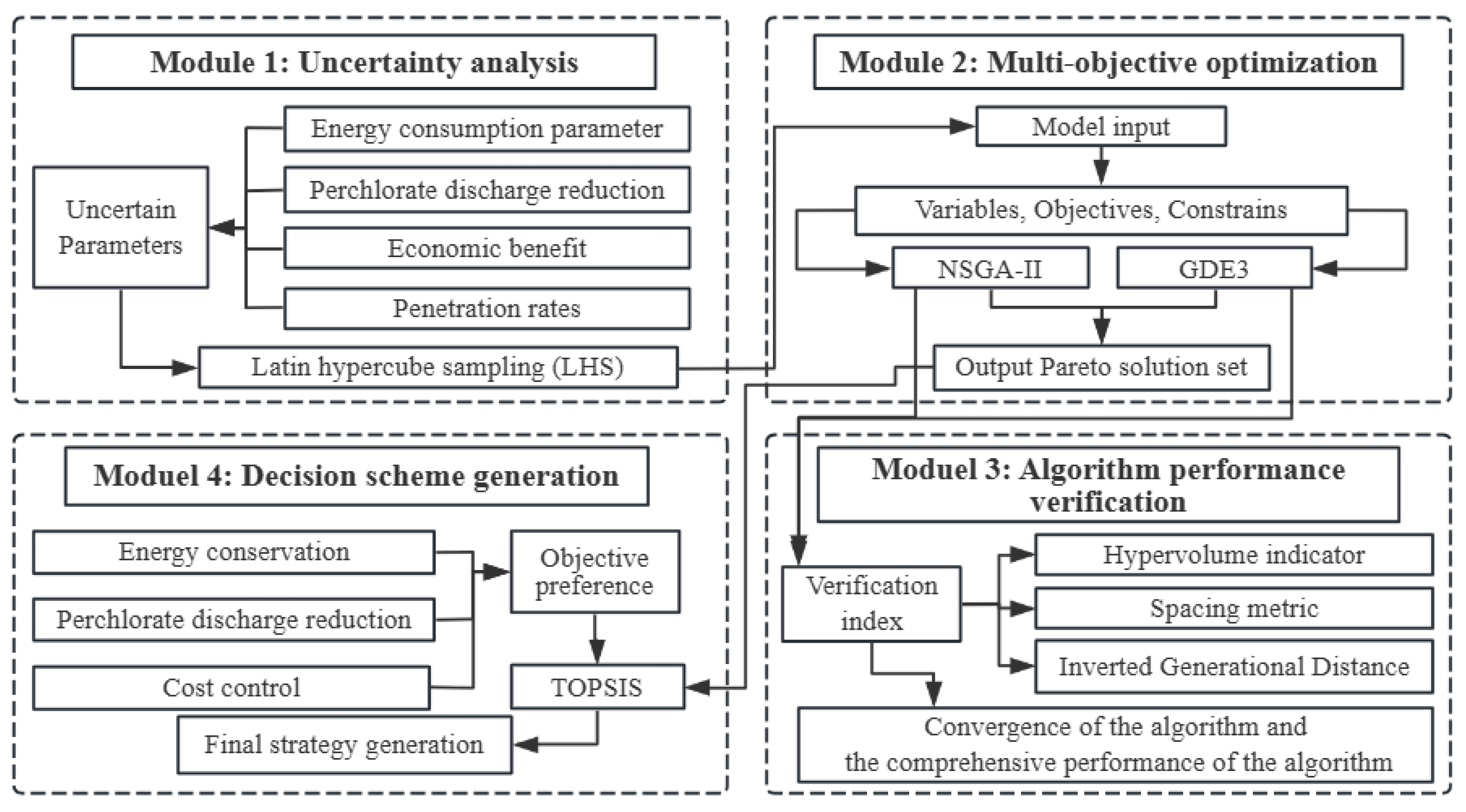
2. Methodology
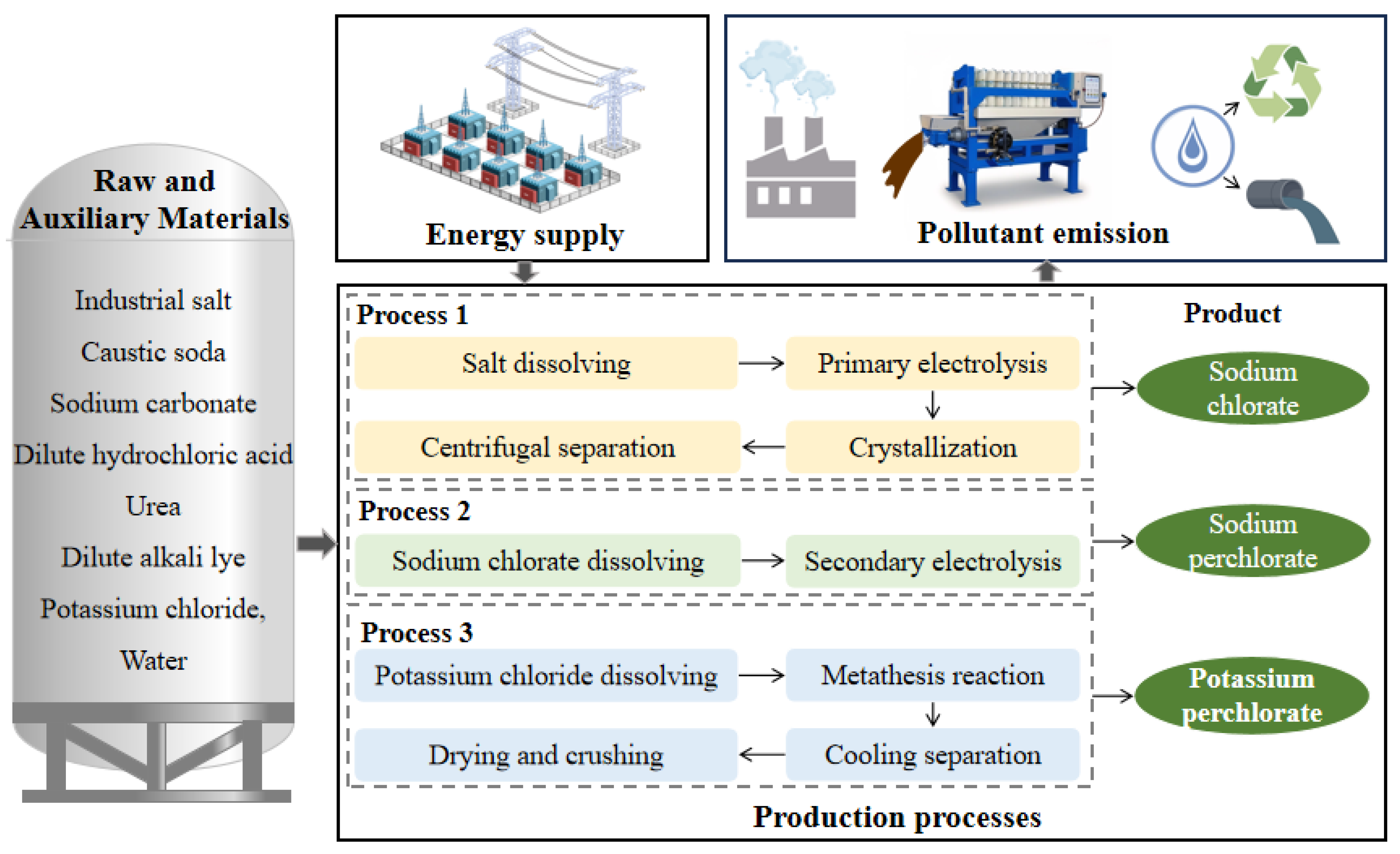
2.1. Analysis of Potassium Perchlorate Production System
2.2. The Multi-Objective Optimization Model
2.2.1. Objectives
- (1)
- Energy intensity minimization
- (2)
- Perchlorate discharge intensity minimization
- (3)
- Economic cost minimization
2.2.2. Constraints
- (1)
- Logical constraints of decision variables
- (2)
- Constraints of technology promotion
- (3)
- Mass balance of the by-products and waste constraint
2.3. Optimization Method
3. Results
3.1. Analysis of Perchlorate Production and Discharging Characteristics
- 1.
- The mother liquor from double decomposition
- 2.
- The dust from the drying shop
- 3.
- The salt sludge from pressure filters
- 4.
- The potassium perchlorate powder scattered to the floor of the packaging workshop
- 5.
- The potassium perchlorate residual on the factory road surface.
- 6.
- The potassium perchlorate carried on workers’ hands and clothing.
3.2. The Model Optimization Results
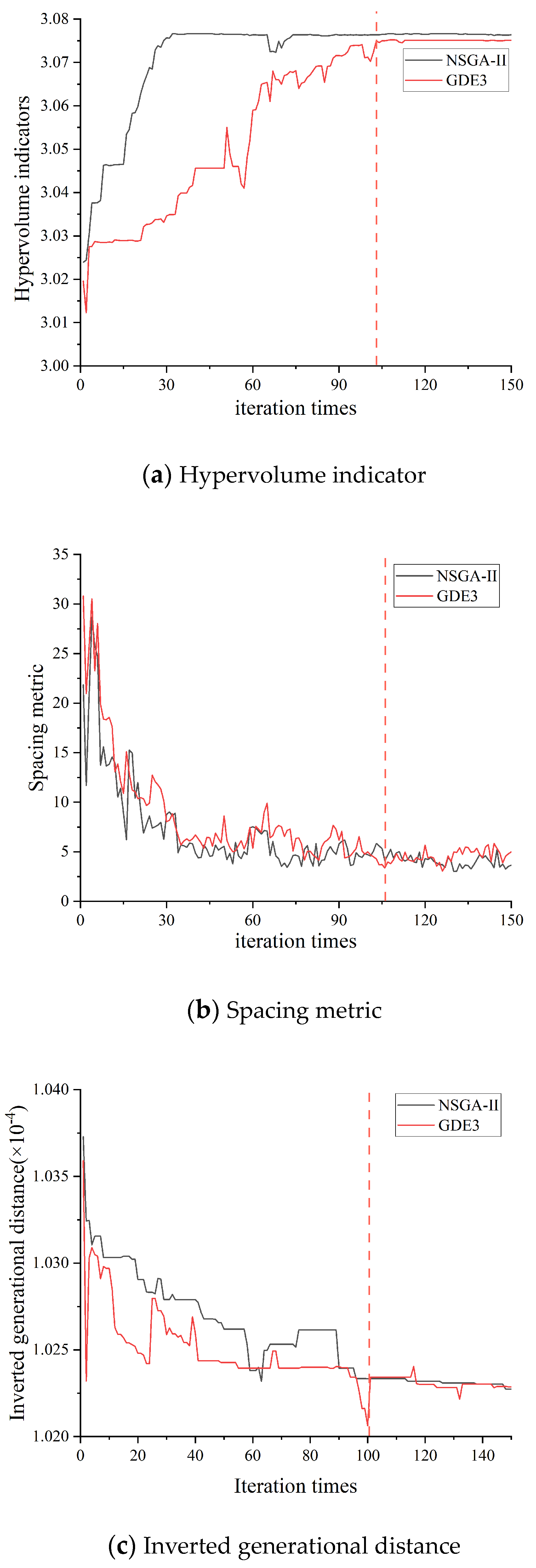
3.2.1. The Algorithm Performance Verification Results
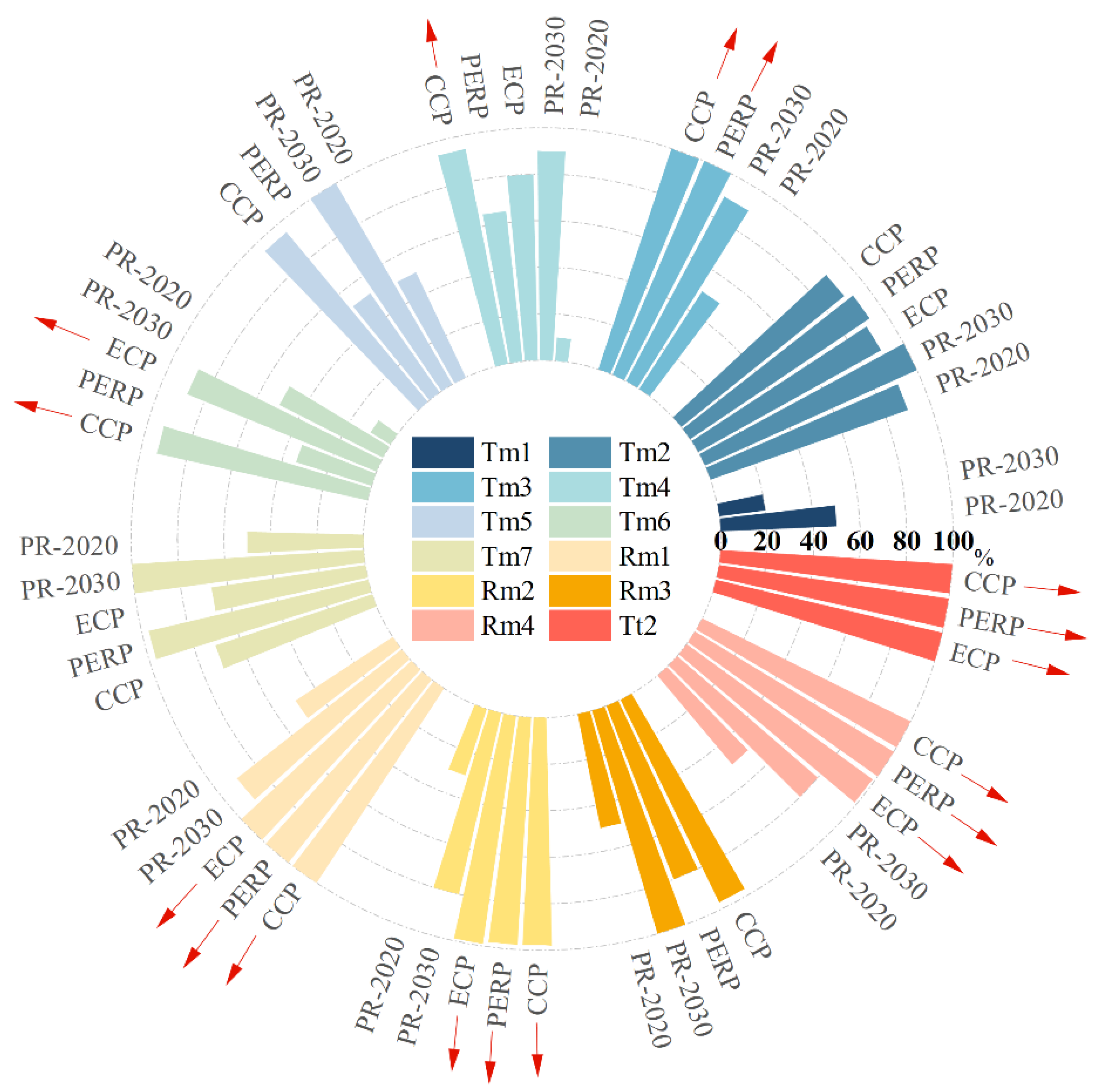
3.2.2. The Optimal Solutions
3.3. Decision Strategy
4. Discussion
5. Conclusions
- (1)
- The major perchlorate discharging nodes include the mother liquor from double decomposition, product drying dust, and refined pressed filter sludge. The comprehensive perchlorate discharge intensity for the potassium perchlorate manufacturing industry is estimated to be around 23.86 kg/t of product in China. This means 3760.34 t perchlorate per year is discharged into the environment, considering the current pollution discharging control policy lacks control over perchlorate.
- (2)
- For the optimization of the multi-objective model constructed in this study, while both the NSGA-II and GDE3 algorithms are effective in identifying the optimal trade-offs between conflicting objectives, GDE3 demonstrates superior overall performance in terms of convergence speed. It can find optimal solutions within a limited number of iterations, providing a more robust and efficient approach.
- (3)
- The optimization results have unveiled significant achievements that could be produced through ECPDR management of this industry. Implementing the optimal solution under ECP can achieve a 99.97% reduction in perchlorate discharge intensity (0.0097 kg/t) at an energy intensity of 3910.10 kWh/t. Under PERP, the optimal solution can reduce perchlorate discharge intensity to 0.0032 kg/t. Additionally, the optimal solution under CCP can yield an economic benefit of 432.53 CNY/t.
- (4)
- In the next decade of development for the potassium perchlorate manufacturing industry, hydrogen purification and recovery technology, MVR technology, bag filter dust collection technology for the drying workshop, and efficient biodegradation technology should be vigorously promoted as key technologies.
- (5)
- Considering both perchlorate discharge reduction and economic cost control, production processes without primary electrolysis should be gradually phased out. Instead, primary electrolysis processes equipped with MVR technology should be added.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Process 1. Preparation of sodium chlorate by primary electrolysis
- Process 2. Preparation of sodium perchlorate by secondary electrolysis
- Process 3. Preparation of potassium perchlorate by double decomposition reaction
Appendix B
| NO. | Energy Conservation (kWh/t) | Perchlorate Reduction (kg ClO4-/t) | Fixed Investment (CNY/t) | Operational Cost (CNY/t) | Benefits (CNY/t) | Penetration Rate-2020 (%) | Penetration Rate-2030 (%) |
|---|---|---|---|---|---|---|---|
| Tm1 | −200 | 0 | 10 | 150 | 50 | 50 | 20 |
| Tm2 | 1000 | 0 | 120 | 1700 | 10 | 90 | 100 |
| Tm3 | −1 | 0 | 0.4 | 1 | 50 | 50 | 90 |
| Tm4 | −5 | 0.05 | 0.2 | 1 | 1 | 10 | 90 |
| Tm5 | −0.1 | 0.005 | 0.2 | 1 | 1 | 50 | 100 |
| Tm6 | −1 | 0.005 | 0.1 | 1 | 1 | 10 | 50 |
| Tm7 | −1 | 0.05 | 0.5 | 1 | 2 | 50 | 100 |
| Tm8 | −4570.4 | 29.70 | 177.12 | 3500.99 | 4620.05 | 30 | 80 |
| Tm9 | −9000 | 29.16 | 300 | 7000 | 9464.6 | 30 | 10 |
| Rm1 | −130.95 | 0 | 8.47 | 129.4 | 680 | 50 | 90 |
| Rm2 | −70.4 | 0.835 | 27.12 | 0.99 | 155.25 | 30 | 80 |
| Rm3 | 0 | 0.1 | 0 | 0 | 1 | 50 | 100 |
| Rm4 | 0 | 2 | 1 | 0 | 20 | 50 | 80 |
| Tt1 | −1.2 | (99%) * | 111.12 | 17.28 | 0 | 0 | / |
| Tt2 | −1.0 | (99.92%) * | 10 | 17.04 | 0 | 0 | / |
| Tt3 | −280 | (99%) * | 106.66 | 160 | 0 | 0 | / |
| Categories | Uncertainty Parameter | Range |
|---|---|---|
| Energy performance | Energy consumption intensity in the base year | ±10% |
| Energy-saving effect of advanced technology | ±20% | |
| Energy intensity of by-product and waste recovery technology | ±20% | |
| Parameter of perchlorate discharge | Perchlorate discharge intensity in base year | ±10% |
| Direct perchlorate discharge reduction amount | ±20% | |
| Economic cost | Electricity price | ±10% |
| Potassium perchlorate price | ±20% | |
| Present situation of application | Penetration rate | ±20% |
| NO. | Baseline Year Penetration Rate (%) | Predicted Penetration Rate-2030 (%) | ECP (%) | PERP (%) | CCP (%) |
|---|---|---|---|---|---|
| Tm1 | 50 | 20 | / | 0 | 0 |
| Tm2 | 90 | 100 | 90 | 93 | 90 |
| Tm3 | 50 | 90 | / | 100 | 100 |
| Tm4 | 10 | 90 | 80 | 65 | 94 |
| Tm5 | 50 | 100 | / | 52 | 95 |
| Tm6 | 10 | 50 | 88 | 34 | 93 |
| Tm7 | 50 | 100 | 67 | 97 | 71 |
| Tm8 | 30 | 80 | 0 | / | / |
| Tm9 | 30 | 10 | 0 | / | / |
| Rm1 | 50 | 90 | 100 | 100 | 100 |
| Rm2 | 30 | 80 | 99 | 98 | 98 |
| Rm3 | 50 | 100 | / | 80 | 97 |
| Rm4 | 50 | 80 | 99 | 100 | 100 |
| Tt1 | 0 | / | 0 | 0 | 0 |
| Tt2 | 0 | / | 100 | 100 | 100 |
| Tt3 | 0 | / | 0 | 0 | 0 |
| Parameter | Population Size | Iteration Times | Mutation Probability | Crossover Probability |
|---|---|---|---|---|
| Value | 100 | 150 | 0.04 | 0.9 |
References
- Kumarathilaka, P.; Oze, C.; Indraratne, S.P.; Vithanage, M. Perchlorate as an emerging contaminant in soil, water and food. Chemosphere 2016, 150, 667–677. [Google Scholar] [CrossRef]
- Qu, D.; Zhang, J.; Wan, D.; Niu, Z. Perchlorate removal by a combined heterotrophic and bio-electrochemical hydrogen autotrophic system. Sci. Total Environ. 2022, 851, 158178. [Google Scholar] [CrossRef] [PubMed]
- Vinson, V. The double punch of perchlorate. Science 2020, 368, 1444–1445. [Google Scholar]
- Kumar, K.S.; Kavitha, S.; Parameswari, K.; Sakunthala, A.; Sathishkumar, P. Environmental occurrence, toxicity and remediation of perchlorate—A review. Chemosphere 2023, 311, 137017. [Google Scholar] [CrossRef]
- Han, Y.; Li, D.; Zou, C.; Li, Y.; Zhao, F. Effects of perchlorate, nitrate, and thiocyanate exposures on serum total testosterone in children and adolescents. Sci. Total Environ. 2023, 861, 160566. [Google Scholar] [CrossRef]
- Van Stempvoort, D.R.; MacKay, D.R.; Brown, S.J.; Collins, P. Environmental fluxes of perchlorate in rural catchments, Ontario, Canada. Sci. Total Environ. 2020, 720, 137426. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Praamsma, M.L.; Oldi, J.F.; Kunisue, T.; Sinha, R.K. Occurrence of perchlorate in drinking water, groundwater, surface water and human saliva from India. Chemosphere 2009, 76, 22–26. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks to public health related to the presence of perchlorate in food, in particular fruits and vegetables. EFSA J. 2014, 12, 10. [Google Scholar]
- Muñoz-Arango, D.; Torres-Rojas, F.; Tapia, N.; Vega, M.; Alvear, C.; Pizarro, G.; Pastén, P.; Cortés, S.; Vega, A.S.; Calderón, R.; et al. Perchlorate and chlorate assessment in drinking water in northern Chilean cities. Environ. Res. 2023, 233, 116450. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Stroble, S.T.; Anderson, R.M.; Moore, Q.; Catling, D.C.; Douglas, S.; McKay, C.P.; Ming, D.W.; Smith, P.H.; Tamppari, L.K.; et al. Discovery of Natural Perchlorate in the Antarctic Dry Valleys and Its Global Implications. Environ. Sci. Technol. 2010, 44, 2360–2364. [Google Scholar] [CrossRef]
- Cao, F.; Jaunat, J.; Sturchio, N.; Cancès, B.; Morvan, X.; Devos, A.; Barbin, V.; Ollivier, P. Worldwide occurrence and origin of perchlorate ion in waters: A review. Sci. Total Environ. 2019, 661, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.W.; Brady, B.B. Perchlorate Leaching from Solid Rocket Motor Propellant in Water. J. Propuls. Power 2005, 21, 937–941. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Jayasena, H.C. Perchlorate Contamination in Groundwater and Associated Health Risks from Fireworks Manufacturing Area (Sivakasi region) of South India. Expo. Health 2022, 14, 359–373. [Google Scholar] [CrossRef]
- Chen, W.; He, N.; Shi, Y.; An, W.; Yang, M. Analysis of exposure routes and contribution rate of perchlorate in China. Chin. Sci. Bull. 2020, 65, 1387–1394. (In Chinese) [Google Scholar] [CrossRef]
- GB 5749-2022; Standards for Drinking Water Quality. State Administration for Market Regulation and National Administration of Standardization: Beijing, China, 2022.
- Shi, G. A new process for the combined production of potassium perchlorate. Chem. Eng. Equip. 2014, 3, 54–58. (In Chinese) [Google Scholar]
- Wang, Y.; Wen, Z.; Yao, J.; Dinga, C.D. Multi-objective optimization of synergic energy conservation and CO2 emission reduction in China’s iron and steel industry under uncertainty. Renew. Sustain. Energy Rev. 2020, 134, 110128. [Google Scholar] [CrossRef]
- Ma, H.; Fei, M.; Jiang, Z.; Li, L.; Zhou, H.; Crookes, D. A Multipopulation-Based Multiobjective Evolutionary Algorithm. IEEE Trans. Cybern. 2020, 50, 689–702. [Google Scholar] [CrossRef]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T.A.M.T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Li, L. Survey on Multi-Objective Evolutionary Algorithms. J. Phys. Conf. Ser. 2019, 1288, 012057. [Google Scholar] [CrossRef]
- Chen, P. Effects of the entropy weight on TOPSIS. Expert Syst. Appl. 2021, 168, 114186. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yoon, K. Methods for multiple attribute decision making. In Multiple Attribute Decision Making: Methods and Applications a State-of-the-Art Survey; Springer: Berlin/Heidelberg, Germany, 1981; pp. 58–191. [Google Scholar]
- Opricovic, S.; Tzeng, G.H. Compromise solution by MCDM methods: A comparative analysis of VIKOR and TOPSIS. Eur. J. Oper. Res. 2004, 156, 445–455. [Google Scholar] [CrossRef]
- China’s Development and Reform Commission. Notice on the Issuance of the Plan for Improving the Double Control Degree of Energy Consumption Intensity and Total Amount. Available online: https://www.gov.cn/zhengce/zhengceku/2021-09/17/content_5637960.htm (accessed on 11 September 2021).
- Wen, Z.; Wang, Y.; Zhang, C.; Zhang, X. Uncertainty analysis of industrial energy conservation management in China’s iron and steel industry. J. Environ. Manag. 2018, 225, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Sandberg, G.; Dahlblom, O. On Latin hypercube sampling for structural reliability analysis. Struct. Saf. 2003, 25, 47–68. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, Y.; Sun, S.; Liu, T.; Shan, Y. A comprehensive survey on NSGA-II for multi-objective optimization and applications. Artif. Intell. Rev. 2023, 56, 15217–15270. [Google Scholar] [CrossRef]
- Huang, D.; Dinga, C.D.; Wen, Z.; Razmadze, D. Industrial-environmental management in China’s iron and steel industry under multiple objectives and uncertainties. J. Environ. Manag. 2022, 310, 114785. [Google Scholar] [CrossRef]
- Menzel, G.; Och, S.H.; Mariani, V.C.; Moura, L.M.; Domingues, E. Multi-objective optimization of the volumetric and thermal efficiencies applied to a multi-cylinder internal combustion engine. Energy Convers. Manag. 2020, 216, 112930. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Tao, Y.; Wen, Z.; Chen, B.; Zhang, H. A many-objective optimization of industrial environmental management using NSGA-III: A case of China’s iron and steel industry. Appl. Energy 2019, 242, 46–56. [Google Scholar] [CrossRef]
- Sandoval, C.; Cuate, O.; González, L.C.; Trujillo, L.; Schütze, O. Towards fast approximations for the hypervolume indicator for multi-objective optimization problems by Genetic Programming. Appl. Soft Comput. 2022, 125, 109103. [Google Scholar] [CrossRef]
- Guerreiro, A.P.; Fonseca, C.M.; Paquete, L. The Hypervolume Indicator. ACM Comput. Surv. 2021, 54, 1–42. [Google Scholar] [CrossRef]
- Yang, K.; Emmerich, M.; Deutz, A.; Bäck, T. Multi-Objective Bayesian Global Optimization using expected hypervolume improvement gradient. Swarm Evol. Comput. 2019, 44, 945–956. [Google Scholar] [CrossRef]
- Shang, K.; Ishibuchi, H.; He, L.; Pang, L.M. A Survey on the Hypervolume Indicator in Evolutionary Multiobjective Optimization. IEEE Trans. Evol. Comput. 2021, 25, 1–20. [Google Scholar] [CrossRef]
- Li, F.; Shang, Z.; Shen, H.; Liu, Y.; Huang, P.Q. Combining modified inverted generational distance indicator with reference-vector-guided selection for many-objective optimization. Appl. Intell. 2023, 53, 12149–12162. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, J.J.; Lin, J.H.; Chiang, M.C. Domestic open-end equity mutual fund performance evaluation using extended TOPSIS method with different distance approaches. Expert Syst. Appl. 2010, 37, 4642–4649. [Google Scholar] [CrossRef]
- GB8978-1996; Integrated Wastewater Discharge Standard. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 1996.
- GB 31573−2015; Emission Standards of Pollutants for the Inorganic Chemical Industry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2015.

| NO. | Methods | Method A | Method B |
|---|---|---|---|
| Tm1 | Improved coating formulation technology for single-pole gas stripping external circulation electrolyzers; it could be used in primary electrolysis, could enhance current density and reduce equipment footprint, but significantly increase energy consumption intensity. | √ | |
| Tm2 | Continuous cycle electrolysis, which could be used in secondary electrolysis, could improve the current efficiency compared with the traditional deep electrolysis technology. | √ | √ |
| Tm3 | Filtering electrolyzed brine with a precise membrane filtration system could improve brine quality and lower electrolysis energy consumption. | √ | |
| Tm4 | Treatment and reuse of workshop floor and plant flushing water could reduce the perchlorate escaping from the production process into the environment. | √ | √ |
| Tm5 | Separate storage and management of hazardous waste and general solid waste could save some unnecessary cost input, and the management is more reasonable. | √ | |
| Tm6 | Vacuum dust collection system: it could be employed for dust cleaning on workers’ hands and workwear surfaces and could prevent perchlorate materials from being discharged into the environment through workers’ hands and clothing. | √ | √ |
| Tm7 | Constructing rainwater collection ponds could prevent the dispersion of perchlorate from the factory road during rainy days. | √ | √ |
| Tm8 | Adding the primary electrolysis process (accompanied by Mechanical vapor recompression (MVR)) could realize the reuse of the mother liquor from double decomposition, and the potassium perchlorate and sodium chloride separated by MVR can bring certain economic benefits. | √ | |
| Tm9 | Adding the primary electrolysis process (without MVR) could realize the reuse of the mother liquor from double decomposition; some excess sodium chlorate will be sold to the market, but this part of sodium chlorate contains a small amount of perchlorate. | √ | |
| Rm1 | Hydrogen purification and recovery technology could recover hydrogen from an electrolysis tank. | √ | √ |
| Rm2 | MVR could efficiently recover NaCl and potassium perchlorate from the double decomposition mother liquor. | √ | √ |
| Rm3 | Extracting and reusing perchlorate from filter-pressed sludge. | √ | |
| Rm4 | Recovering dust from the potassium perchlorate drying workshop using a bag filter. | √ | √ |
| Tt1 | In ion exchange, the modified resin material has a selective adsorption capacity for perchlorate ions in wastewater, and the adsorption capacity of the resin material is greatly improved. | √ | √ |
| Tt2 | Efficient biodegradation technology. The efficient and harmless transformation of perchlorate in wastewater was achieved by adding efficient reducing bacteria, controlling the REDOX potential of the reactor, and regulating hydraulic conditions of the UASB unit to promote sludge flocculation. | √ | √ |
| Tt3 | The catalytic reduction technique includes pretreatment and the “HJ-PERCl” REDOX system, which can decompose perchlorate into chloride under specific conditions. | √ | √ |
| NO. | Nodes | Intensities (kg/t KClO4) | Method A | Method B |
|---|---|---|---|---|
| ① | The mother liquor from double decomposition | 36.15 | 6.45 | √ |
| ② | The dust from the drying shop | 1.90 | √ | √ |
| ③ | The salt sludge from pressure filters | 1.20 | √ | |
| ④ | The potassium perchlorate powder scattered to the floor of the packaging workshop | 0.051 | √ | √ |
| ⑤ | The potassium perchlorate residual on the factory road surface | 0.001 | √ | √ |
| ⑥ | The potassium perchlorate carried on workers’ hands and clothing | 0.0056 | √ | √ |
| Total (kg/t KClO4) | 9.61 | 38.11 | ||
| Method A | Method B | |||||
|---|---|---|---|---|---|---|
| Value in 2020 | Certainty | Uncertainty | Value in 2020 | Certainty | Uncertainty | |
| Energy intensity (kW·h/t) | 7820 | 7622.35 | 7620.11 | 3790 | 3692.77 | 3693.52 |
| Perchlorate discharging intensity (kg/t) | 9.61 | 3.17 × 10−3 | 3.17 × 10−3 | 38.11 | 2.4 × 10−7 | 3 × 10−6 |
| Economic cost (CNY/t) | / | −457.03 * | −456.31 * | / | −514.65 * | −531.16 * |
| Objective | ECP | PERP | CCP |
|---|---|---|---|
| Energy intensity (kW·h/t) | 3910.10 | 7831.42 | 7840.82 |
| Perchlorate discharge intensity (kg/t) | 0.0097 | 0.0032 | 0.0032 |
| Economic cost (CNY/t) | −310.95 | −417.90 | −442.09 |
| Preference | Key Measures |
|---|---|
| ECP | Tm6, Rm1, Rm2, Rm4, Tt2 |
| PERP | Tm3, Rm1, Rm2, Rm4, Tt2 |
| CCP | Tm3, Tm4, Tm6, Rm1, Rm2, Rm4, Tt2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, H.; Zhu, G. Multi-Objective Optimization of Synergic Perchlorate Pollution Reduction and Energy Conservation in China’s Perchlorate Manufacturing Industry. Sustainability 2024, 16, 6924. https://doi.org/10.3390/su16166924
Li Y, Wang H, Zhu G. Multi-Objective Optimization of Synergic Perchlorate Pollution Reduction and Energy Conservation in China’s Perchlorate Manufacturing Industry. Sustainability. 2024; 16(16):6924. https://doi.org/10.3390/su16166924
Chicago/Turabian StyleLi, Ying, Hongyang Wang, and Guangcan Zhu. 2024. "Multi-Objective Optimization of Synergic Perchlorate Pollution Reduction and Energy Conservation in China’s Perchlorate Manufacturing Industry" Sustainability 16, no. 16: 6924. https://doi.org/10.3390/su16166924
APA StyleLi, Y., Wang, H., & Zhu, G. (2024). Multi-Objective Optimization of Synergic Perchlorate Pollution Reduction and Energy Conservation in China’s Perchlorate Manufacturing Industry. Sustainability, 16(16), 6924. https://doi.org/10.3390/su16166924







