Ag-Precipitated CuO Nanospheres for Enhanced Electrochemical Reduction of CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts and Electrodes
2.3. Characterization
2.4. Electrochemical Performance Testing
2.5. CO2 Electrochemical Reduction Device
2.6. Analytical Methods
3. Results
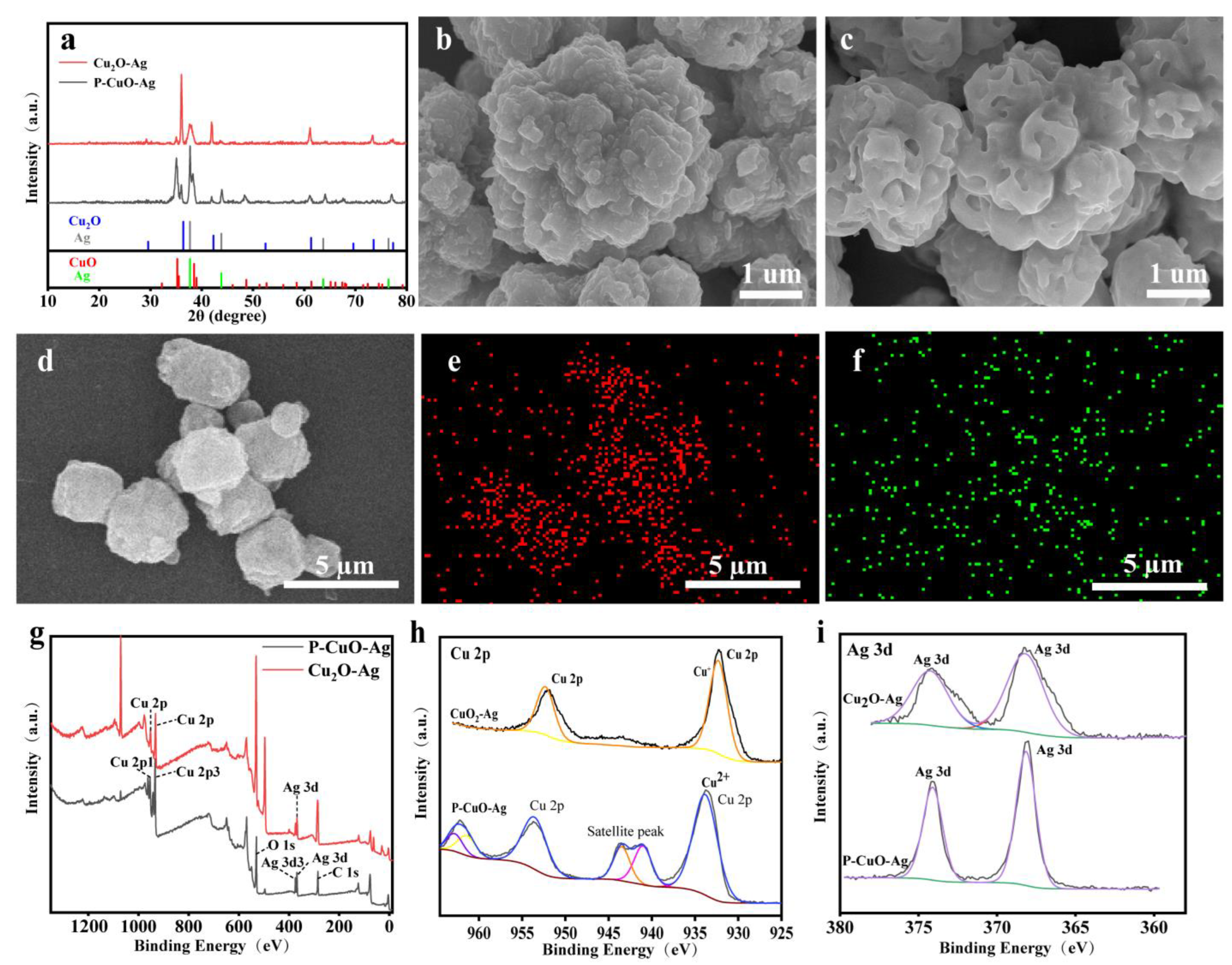
3.1. Morphological and Structural Characterization of Catalysts
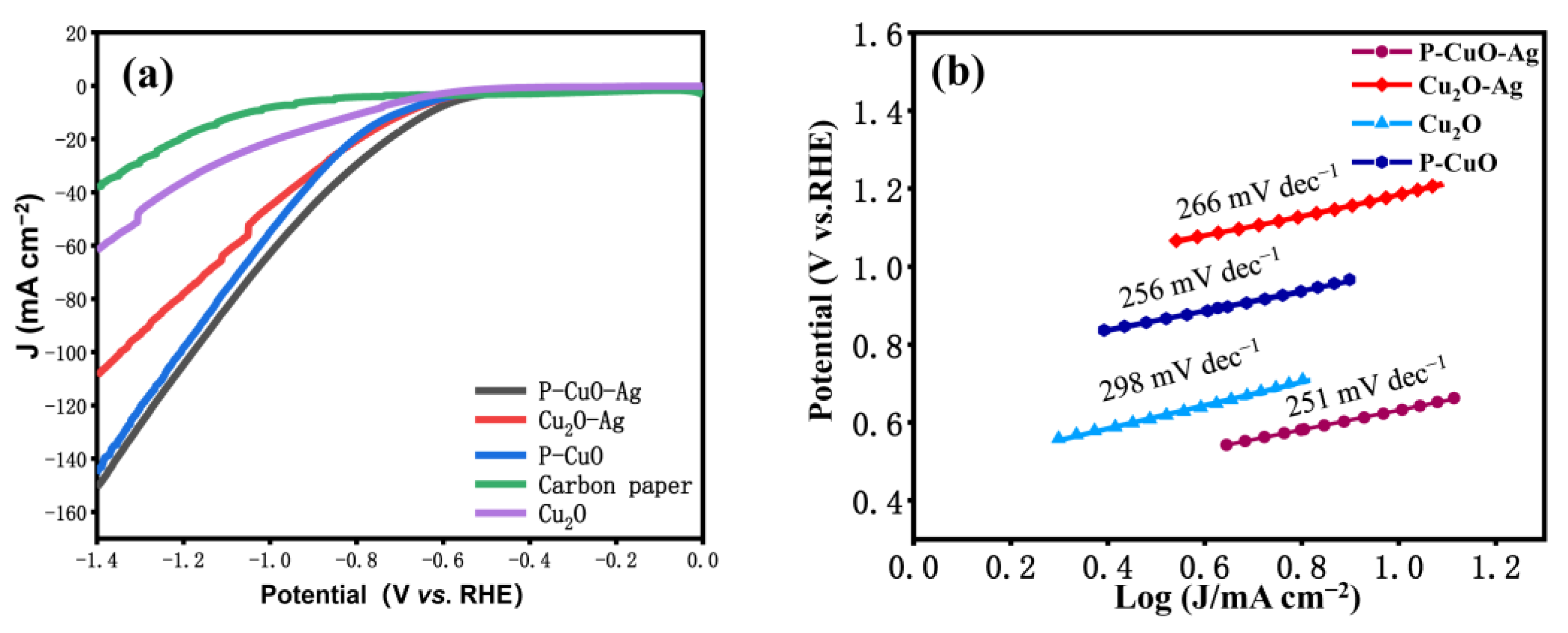
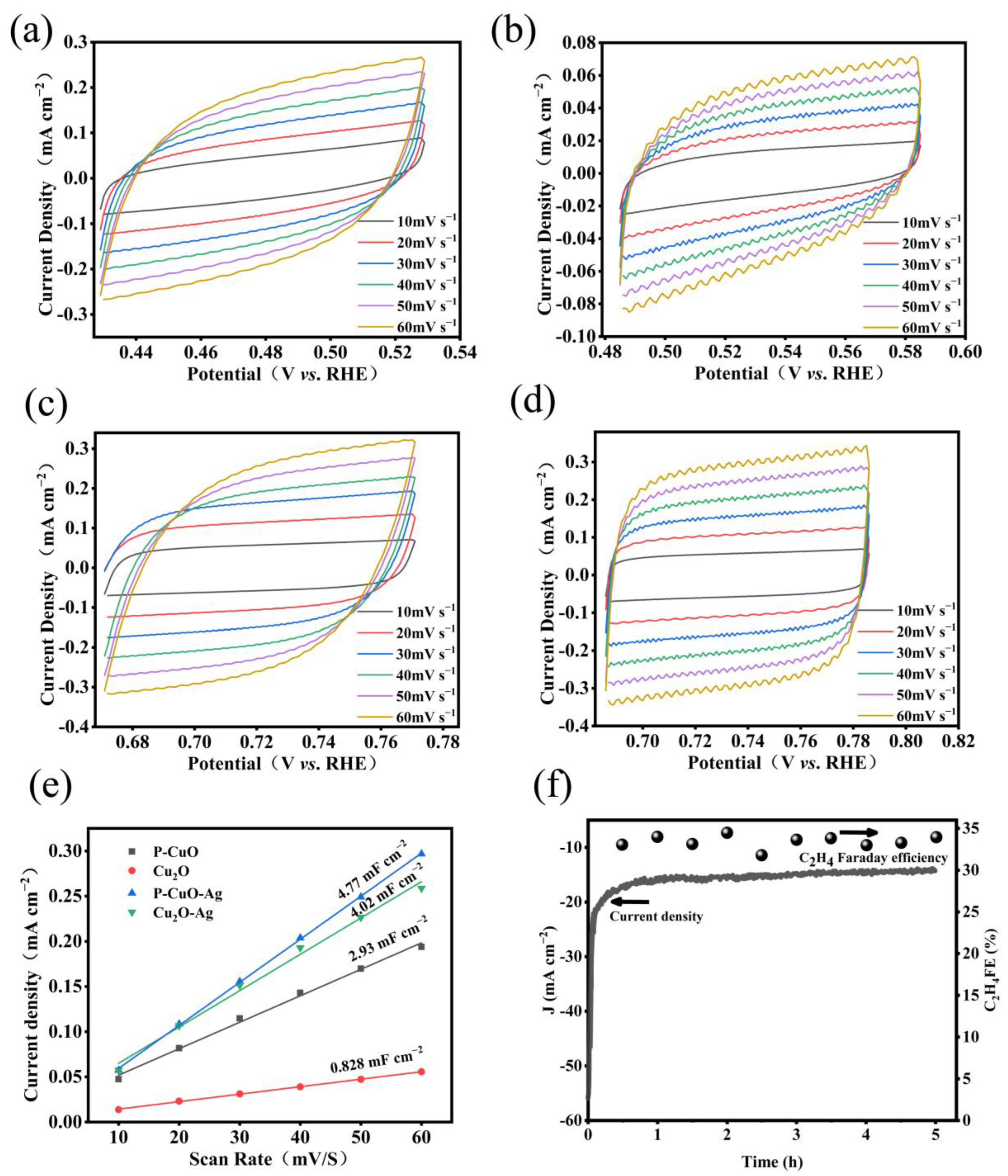
3.2. Characterization of Electrochemical Properties
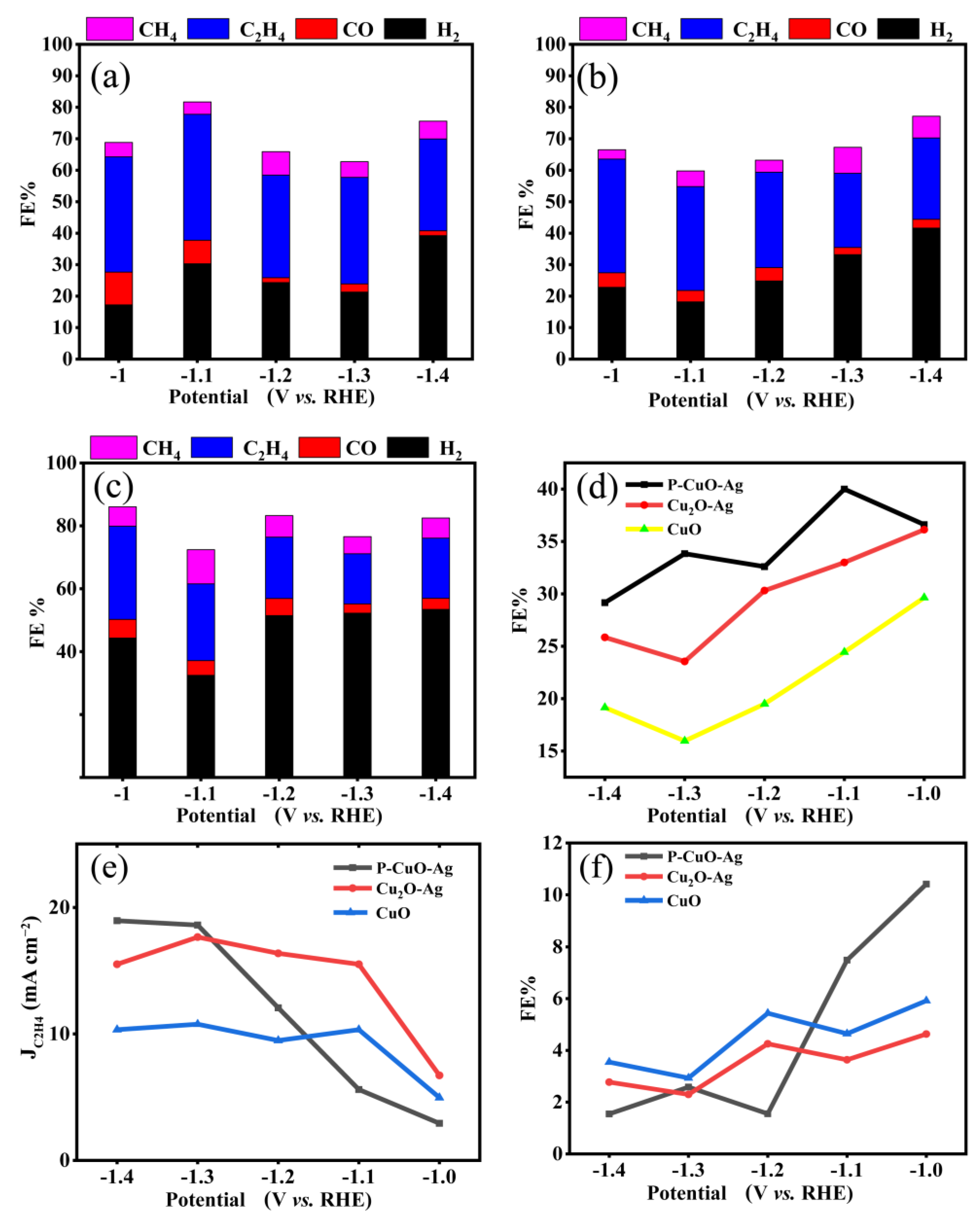
3.3. Performance Test of Electrocatalytic Reduction of CO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rong, W.F.; Zou, H.Y.; Zang, W.J.; Xi, S.B.; Wei, S.T.; Long, B.H.; Hu, J.H.; Ji, Y.F.; Duan, L.L. Size-Dependent Activity and Selectivity of Atomic-Level Copper Nanoclusters during CO/CO2 Electroreduction. Angew. Chem. Int. Ed. 2021, 60, 466–472. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Mitra, S.K.; Kozinski, J.A. The Progressive Routes for Carbon Capture and Sequestration. Energy Sci. Eng. 2016, 4, 99–122. [Google Scholar] [CrossRef]
- Zhang, H.X.; Li, S.B. Research on the Factors Influencing CO2 Emission Reduction in High-Energy-Consumption Industries under Carbon Peak. Sustainability 2023, 15, 13437. [Google Scholar] [CrossRef]
- Mercer, J.H. West Antarctic Ice Sheet and CO2 Greenhouse Effect: A Threat of Disaster. Nature 1978, 271, 321–325. [Google Scholar] [CrossRef]
- Schneider, S.H. The Greenhouse Effect: Science and Policy. Science 1989, 243, 771–781. [Google Scholar] [CrossRef][Green Version]
- Sabatino, F.; Mehta, M.; Grimm, A.; Gazzani, M.; Gallucci, F.; Kramer, G.J.; van Sint Annaland, M. Evaluation of a Direct Air Capture Process Combining Wet Scrubbing and Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2020, 59, 7007–7020. [Google Scholar] [CrossRef]
- Rubiera, F.; Pevida, C. Decarbonisation of the Electricity Sector? CCUS still Imperative. Greenh. Gases Sci. Technol. 2018, 8, 396–397. [Google Scholar] [CrossRef]
- Sha, F.; Zhu, N.; Bai, Y.; Li, Q.; Guo, B.; Zhao, T.; Zhang, F.; Zhang, J. Controllable Synthesis of Various CaCO3 Morphologies Based on a CCUS Idea. ACS Sustain. Chem. Eng. 2016, 4, 3032–3044. [Google Scholar] [CrossRef]
- Wang, T.; Dong, C.D.; Lin, J.Y.; Chen, C.W.; Chang, J.S.; Kim, H.; Huang, C.P.; Hung, C.M. Recent Advances in Carbon Dioxide Conversion: A Circular Bioeconomy Perspective. Sustainability 2021, 13, 6962. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What Should We Make with CO2 and How Can We Make It? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.M.T.; Tran, D.L.; Le, H.K.; Vo, D.V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Le, Q.V. MXenes: Applications in Electrocatalytic, Photocatalytic Hydrogen Evolution Reaction and CO2 Reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Jin, Z.Y.; Guo, Y.Q.; Qiu, C.Z. Electro-Conversion of Carbon Dioxide to Valuable Chemicals in a Membrane Electrode Assembly. Sustainability 2022, 14, 5579. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y.; Chakraborty, T.; Zhou, G.; Wang, C.; Fu, X.; Wang, Y.; Zhang, J.; Li, C.; Xu, F.; et al. Asymmetrical C–C Coupling for Electroreduction of CO on Bimetallic Cu–Pd Catalysts. ACS Catal. 2022, 12, 5275–5283. [Google Scholar] [CrossRef]
- Yan, Z.F.; Hitt, J.L.; Zeng, Z.C.; Hickner, M.A.; Mallouk, T.E. Improving the Efficiency of CO2 Electrolysis by Using a Bipolar Membrane with a Weak-Acid Cation Exchange Layer. Nat. Chem. 2021, 13, 33–40. [Google Scholar] [CrossRef]
- Pinthong, P.; Phupaichitkun, S.; Watmanee, S.; Nganglumpoon, R.; Tungasmita, D.N.; Tungasmita, S.; Boonyongmaneerat, Y.; Promphet, N.; Rodthongkum, N.; Panpranot, J. Room Temperature Nanographene Production via CO2 Electrochemical Reduction on the Electrodeposited Bi on Sn Substrate. Nanomaterials 2022, 12, 3389. [Google Scholar] [CrossRef]
- Bell, J.G.; Underwood, T.C. Unconventional and Emerging Approaches to CO2 Reduction. Sustainability 2024, 16, 713. [Google Scholar] [CrossRef]
- Gabardo, C.M.; Seifitokaldani, A.; Edwards, J.P.; Dinh, C.-T.; Burdyny, T.; Kibria, M.G.; O’Brien, C.P.; Sargent, E.H.; Sinton, D. Combined High Alkalinity and Pressurization Enable Efficient CO2 Electroreduction to CO. Energy Environ. Sci. 2018, 11, 2531–2539. [Google Scholar] [CrossRef]
- Verma, S.; Hamasaki, Y.; Kim, C.; Huang, W.; Lu, S.; Jhong, H.-R.M.; Gewirth, A.A.; Fujigaya, T.; Nakashima, N.; Kenis, P.J.A. Insights into the Low Overpotential Electroreduction of CO2 to CO on a Supported Gold Catalyst in an Alkaline Flow Electrolyzer. ACS Energy Lett. 2017, 3, 193–198. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.C.; Masel, R.I.; Kenis, P.J.A. The Effect of Electrolyte Composition on the Electroreduction of CO2 to CO on Ag Based Gas Diffusion Electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef]
- Ma, X.J.; Liu, T.X.; Liu, E.R.; Zhang, Y.P. Preparation and Performance of Cd-MgAl-LDHs@RGO in High Efficiency Electrocatalytic Reduction of CO2 to CO. Mol. Catal. 2023, 535, 112876. [Google Scholar] [CrossRef]
- Pinthong, P.; Klongklaew, P.; Praserthdam, P.; Panpranot, J. Effect of the Nanostructured Zn/Cu Electrocatalyst Morphology on the Electrochemical Reduction of CO2 to Value-Added Chemicals. Nanomaterials 2021, 11, 1671. [Google Scholar] [CrossRef]
- De Mot, B.; Hereijgers, J.; Daems, N.; Breugelmans, T. Insight in the Behavior of Bipolar Membrane Equipped Carbon Dioxide Electrolyzers at Low Electrolyte Flowrates. Chem. Eng. J. 2022, 428, 131170. [Google Scholar] [CrossRef]
- Ali, S.; Yasin, G.; Iqbal, R.; Huang, X.; Su, J.; Ibraheem, S.; Zhang, Z.; Wu, X.Q.; Wahid, F.; Ismail, P.M.; et al. Porous Aza-Doped Graphene-Analogous 2D Material a Unique Catalyst for CO2 Conversion to Formic-Acid by Hydrogenation and Electroreduction Approaches. Mol. Catal. 2022, 524, 112285. [Google Scholar] [CrossRef]
- Kumar, N.; Seriani, N.; Gebauer, R. DFT Insights into Electrocatalytic CO2 Reduction to Methanol on α-Fe2O3(0001) Surfaces. Phys. Chem. Chem. Phys. 2020, 22, 10819–10827. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2020, 11, 28. [Google Scholar] [CrossRef]
- O’Brien, C.P.; Miao, R.K.; Liu, S.; Xu, Y.; Lee, G.; Robb, A.; Huang, J.E.; Xie, K.; Bertens, K.; Gabardo, C.M.; et al. Single Pass CO2 Conversion Exceeding 85% in the Electrosynthesis of Multicarbon Products via Local CO2 Regeneration. ACS Energy Lett. 2021, 6, 2952–2959. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, J.-J.; Yang, M.-P.; Meng, W.-J.; Wang, H.; Lu, J.-X. CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol. Catalysts 2018, 8, 171. [Google Scholar] [CrossRef]
- Genovese, C.; Ampelli, C.; Perathoner, S.; Centi, G. Mechanism of C–C Bond Formation in the Eectrocatalytic Reduction of CO2 to Acetic Acid. A Challenging Reaction to Use Renewable Energy with Chemistry. Green Chem. 2017, 19, 2406–2415. [Google Scholar] [CrossRef]
- Ma, M.; Djanashvili, K.; Smith, W.A. Controllable Hydrocarbon Formation from the Electrochemical Reduction of CO2 over Cu Nanowire Arrays. Angew. Chem. Int. Ed. 2016, 55, 6680–6684. [Google Scholar] [CrossRef]
- Ma, D.; Jin, T.; Xie, K.; Huang, H. An Overview of Flow Cell Architecture Design and Optimization for Electrochemical CO2 Reduction. J. Mater. Chem. A 2021, 9, 20897–20918. [Google Scholar] [CrossRef]
- Usman, M.; Humayun, M.; Garba, M.D.; Ullah, L.; Zeb, Z.; Helal, A.; Suliman, M.H.; Alfaifi, B.Y.; Iqbal, N.; Abdinejad, M.; et al. Electrochemical Reduction of CO2: A Review of Cobalt Based Catalysts for Carbon Dioxide Conversion to Fuels. Nanomaterials 2021, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.Y.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]
- Christophe, J.; Doneux, T.; Buess-Herman, C. Electroreduction of Carbon Dioxide on Copper-Based Electrodes: Activity of Copper Single Crystals and Copper–Gold Alloys. Electrocatalysis 2012, 3, 139–146. [Google Scholar] [CrossRef]
- Peterson, A.A.; Nørskov, J.K. Activity Descriptors for CO2 Electroreduction to Methane on Transition-Metal Catalysts. J. Phys. Chem. Lett. 2012, 3, 251–258. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P.D. Synergistic Geometric and Electronic Effects for Electrochemical Reduction of Carbon Dioxide Using Gold-Copper Bimetallic Nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.S.; Choe, S.; Kim, M.J.; Lim, T.; Kim, S.-K.; Kim, J.J. Electrodeposited Ag Catalysts for the Electrochemical Reduction of CO2 to CO. Appl. Catal. B Environ. 2017, 208, 35–43. [Google Scholar] [CrossRef]
- Rahaman, M.; Dutta, A.; Zanetti, A.; Broekmann, P. Electrochemical Reduction of CO2 into Multicarbon Alcohols on Activated Cu Mesh Catalysts: An Identical Location (IL) Study. ACS Catal. 2017, 7, 7946–7956. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Verma, S.; Ma, S.C.; Fister, T.T.; Timoshenko, J.; Frenkel, A.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous Copper Silver Alloys by Additive-Controlled Electrodeposition for the Selective Electroreduction of CO2 to Ethylene and Ethanol. J. Am. Chem. Soc. 2018, 140, 5791–5797. [Google Scholar] [CrossRef]
- Niu, D.; Wei, C.; Lu, Z.; Fang, Y.; Liu, B.; Sun, D.; Hao, X.; Pan, H.; Wang, G. Cu2O-Ag Tandem Catalysts for Selective Electrochemical Reduction of CO2 to C2 Products. Molecules 2021, 26, 2175. [Google Scholar] [CrossRef]
- Zhu, W.L.; Michalsky, R.; Metin, Ö.; Lv, H.F.; Guo, S.J.; Wright, C.J.; Sun, X.L.; Peterson, A.A.; Sun, S.H. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, M.J.; Ahn, S.H.; Choi, I.; Jang, J.H.; Ham, Y.S.; Kim, J.J.; Kim, S.-K. Electrochemical CO2 Reduction to CO on Dendritic Ag–Cu Electrocatalysts Prepared by Electrodeposition. Chem. Eng. J. 2016, 299, 37–44. [Google Scholar] [CrossRef]
- Jiang, J.; Park, S.; Piao, L. One-Pot Synthesis of Monodisperse Cu2O Nanoparticle Aggregates Through an in Situ Seed Generation Process. CrystEngComm 2020, 22, 18–23. [Google Scholar] [CrossRef]
- Yang, Z.X.; Guo, X.X.; Chen, Y.; Gao, L.J.; Wei, R.P.; Xiao, G.M. Modulating Surface Microenvironment Based on Ag-adorned CuO Flower-liked Nanospheres for Strengthening C-C Coupling during CO2RR. Surf. Interfaces 2024, 48, 104267. [Google Scholar] [CrossRef]
- Wang, W.S.; Dahl, M.; Yin, Y.D. Hollow Nanocrystals through the Nanoscale Kirkendall Effect. Chem. Mater. 2013, 25, 1179–1189. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vesaghi, M.A.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS Study of the Cu@Cu2O Core-Shell Nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Sun, D.H.; Li, P.Y.; Yang, B.; Xu, Y.; Huang, J.L.; Li, Q.B. Monodisperse AgPd Alloy Nanoparticles as a Highly Active Catalyst towards the Methanolysis of Ammonia Borane for Hydrogen Generation. RSC Adv. 2016, 6, 105940–105947. [Google Scholar] [CrossRef]
- Yang, F.; Ma, X.Y.; Cai, W.B.; Song, P.; Xu, W.L. Nature of Oxygen-Containing Groups on Carbon for High-Efficiency Electrocatalytic CO2 Reduction Reaction. J. Am. Chem. Soc. 2019, 141, 20451–20459. [Google Scholar] [CrossRef]
- Zahid, A.; Shah, A.; Shah, I. Oxide Derived Copper for Electrochemical Reduction of CO2 to C2+ Products. Nanomaterials 2022, 12, 1380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, M.; Zhao, L.; Zhong, G.; Zhang, Y.; Zhang, Z.; Sun, Y.; Hu, X.; Peng, Z.; Wang, Y.; et al. Ag-Precipitated CuO Nanospheres for Enhanced Electrochemical Reduction of CO2. Sustainability 2024, 16, 5888. https://doi.org/10.3390/su16145888
Xu J, Li M, Zhao L, Zhong G, Zhang Y, Zhang Z, Sun Y, Hu X, Peng Z, Wang Y, et al. Ag-Precipitated CuO Nanospheres for Enhanced Electrochemical Reduction of CO2. Sustainability. 2024; 16(14):5888. https://doi.org/10.3390/su16145888
Chicago/Turabian StyleXu, Jinyun, Ming Li, Liping Zhao, Guoqiang Zhong, Yu Zhang, Ziqi Zhang, Yu Sun, Xudong Hu, Zhe Peng, Yicong Wang, and et al. 2024. "Ag-Precipitated CuO Nanospheres for Enhanced Electrochemical Reduction of CO2" Sustainability 16, no. 14: 5888. https://doi.org/10.3390/su16145888
APA StyleXu, J., Li, M., Zhao, L., Zhong, G., Zhang, Y., Zhang, Z., Sun, Y., Hu, X., Peng, Z., Wang, Y., Zheng, C., & Sun, X. (2024). Ag-Precipitated CuO Nanospheres for Enhanced Electrochemical Reduction of CO2. Sustainability, 16(14), 5888. https://doi.org/10.3390/su16145888





