Abstract
Recycling plastics on an industrial scale is a key approach to the circular economy. This study presents a techno-economic analysis aimed at recycling polypropylene waste, one of the main consumer plastics. Specifically, it evaluates the technical and economic feasibility of achieving a large-scale cracking process that converts polypropylene waste into an alternative fuel. Pyrolysis is considered as a promising technique to convert plastic waste into liquid oil and other value-added products, with a dual benefit of recovering resources and providing a zero-waste solution. This study concerns a fast catalytic pyrolysis in a fluidized bed reactor, with the presence of a fluid catalytic cracking catalyst of low acidity for high heat transmission, for an industrial plant with a capacity of 1 t/h of polypropylene waste provided by the Greek Petroleum Industry. From the international literature, the operational conditions were chosen pyrolysis temperature at 430 °C, pressure at 1atm, heating rate at 5 °C/min, and yields of products to 71, 14, and 15 wt.%, for liquid fuel, gas, solid product, respectively. The plant design includes a series of apparatuses, with the main one to be the pyrolyzer. The catalytic method is selected over the non-catalytic because the presence of catalyst increases the quantity and quality of the liquid product, which is the main product of the plant. The energy loops of recycling pyrolysis gas and char as a low-carbon fuel in the plant were considered. The production cost, annual revenue, for 2023, are anticipated to reach €13.7 million (115 €/t) and €15 million (15 €/t), respectively, with an estimated investment equal to €5.3 million. The Payback Time is estimated to 2.4 years to recover the cost of investment. The endeavor is rather economically sustainable. A critical parameter for large scale systems is securing feedstock with low or negligible price.
1. Introduction
Plastics are one of the most important innovations of the 20th century for two main reasons: (a) they exhibit excellent properties (low weight, low cost, and high mechanical strength) and (b) they are used in many sectors (construction, medical and engineering applications, household equipment etc.) [1]. In parallel, they consist of a serious threat to environment and health in many direct and indirect ways. Exposure to harmful chemicals can happen through packaging, or chemical manufacturing that can cancers, hormonal disruption, developmental and reproductive effects etc. In addition, this pollution chokes wildlife, damages soil and poisons groundwater, causing severe environmental damage; therefore action must be taken to recycle them in a sustainable way. The accumulation of plastic waste is very high worldwide, while their disposal in landfills reveals significant risks to the ecosystems, and human being’s health [2]. Around 70% of the world’s plastic waste is still disposed of in landfills, 8% are found in the oceans, and only 10–12% are incinerated for energy recovery. Incineration is a possible method although should be avoided since it is not environmentally friendly because it produces CO2, NOx, dioxins, and solid particles that are harmful to the environment and people’s health [3].
Other methods are proposed for the treatment of waste plastics including biological, mechanical, and thermochemical methods [4]. Primary, and secondary recycling, chemical and thermochemical conversion for energy recovery, are methods being applied in many countries [2]. Thermochemical methods such as pyrolysis, or gasification reduce the carbon footprint of plastic waste. Rotary kiln pyrolysis is simple and economical feasible. However, in the international literature, catalytic pyrolysis is cited as an environmentally friendly and efficient method with economic viability for plastics upcycling especially to liquid fuel. Catalytic pyrolysis has demonstrated high potential for direct thermochemical liquefaction of biomass for energy applications. Liquid, solid, and gaseous products can be used as biofuels [5,6]. The pyrolysis fuel can be used as a substitute or feedstock in the petrochemical industry [7,8].
The most common plastic waste to be used as pyrolysis feedstocks are polypropylene (PP), polyethylene (PE), polystyrene (PS) and polyethylene terephthalate (PET) [9]. PP is the world’s second most used plastic, after PE, usually ending up in landfills [10]. The separation of PP waste from municipal is energy intensive because they contain a mixture of plastics [3]. In more detail, PP is a thermoplastic material composed of propylene monomers. It has a very soft surface, exhibiting low friction [9]. It is a saturated polymer with a linear hydrocarbon chain with good chemical and thermal resistance. It does not react when in contact with an acid or base and it is highly resistant to electricity [8]. It has a good potential to produce liquid fuel by pyrolysis [11]. PP waste is a post-consumer waste that represents 24.3% of plastic waste, and it is the highest percentage in municipal waste [12]. It is easier to recycle PP industrial waste than mixed PP waste, because it is homogeneous [13], to up-cycle it via thermal recycling to produce liquid fuels, and chemicals, with parallel reduction of the carbon footprint [14].
Therefore, since plastics are a serious threat to environment and people’s health in many direct and indirect ways, action must be taken by the industrial sector to recycle, up-cycle and recover useful resources from PP at large-scale and in a sustainable way. The international research community has developed knowledge on plastic pyrolysis at low Technology Readiness Level (TR). Pyrolysis is considered as a promising technique to convert plastic waste into liquid oil and other value-added products, simultaneously recovering resources and providing a zero-waste solution. However, there is gap to fill in related to the deployment of large-scale PP pyrolysis. Circular economy necessitates the engineering scalability of pyrolysis of plastic material. With the implementation of large-scale industrial pyrolysis, the value of recycled materials, and the sustainability of the supply chain will be increased. Current PP pyrolysis systems are operating with relatively small or medium capacities. Additionally, modelling, that can facilitate the demonstration of large-scale PP pyrolysis process. is particularly complex due to the number of reactions involved in the process and the number of intermediate pyrolysis products [15]. Furthermore, there is a need for a techno-economic assessment (TEA) of large-scale PP pyrolysis endeavours and investments to be used for decision making. TEA is the common tool used by engineers and policymakers because it can provide the main economic indicators and determine the optimum size of industrial applications with sustainability. TEA for fuel production from PP, in the concept of circular economy and industrial symbiosis, needs investigation.
Towards this direction and filling the gap in the systemic knowledge of PP pyrolysis, this study presents a TEA, to support decision making in managing PP that is the world’s second most used plastic, after PE, usually ending up in landfills. The aim of this study is the economic sustainability and scalability of PP pyrolysis in the Greek industrial environment. Based on international literature knowledge, the following were performed: (a) design and engineering of the main apparatus of the system, (b) TEA of a PP waste catalytic pyrolysis-to-oil with a large capacity of 1 t/h PP provided by the Greek Petroleum Industry.
This study does not only contribute to the decision-making with the Greek Circular Economy strategy and environment, but it contributes to the sustainability of PP circular economy in any national environment. Pyrolysis is an eco-friendly alternative to conventional plastic disposal methods, a step towards sustainability because it addresses the SDGs by complying with sustainable waste treatment framework (Goal 12) and circular economy principles.
2. Literature Review to Define Process Design Parameters
The methodology follow in the next chapters includes (a) a literature review for the choice of the process’s operating conditions, (b) the design of the flow sheet and size determination of the equipment and (c) the economic feasibility analysis (TEA) based on economic indicators and the economic sustainability assessment.
Pyrolysis is a thermochemical conversion that allows the breaking of chemical bonds, through thermal decomposition, under non-oxidizing conditions, producing a liquid, a gas and a solid product. It is used for plastic waste treatment [16]. The liquid product formed during the pyrolysis process is the most important because it exhibits commercial fuel properties [5].
Pyrolysis can be distinguished into three categories: slow, intermediate, and fast. Slow pyrolysis can achieve cracking of plastics in the absence of oxygen, at low temperatures, with low heating rates (0.1~1 °C/s) and long residence time [17]. It can convert plastic waste into highly stable carbonaceous products (char) and non-condensable gases, which are good fuels [5]. The solid product contains a large amount of carbon (about 80 wt.%) [18]. Fast pyrolysis is the process in which plastic waste is rapidly heated to high temperatures and low residence times, producing mainly a liquid product that can be used as an alternative to commercial fuels. It is the most common method in both research and application, at the laboratory or industrial scale for plastic-to-liquid [19]. It usually requires small particle sizes and specialized equipment to collect the rapidly released gases. It can produce 37–95 wt.% of liquid products [17,18]. Slow pyrolysis has many advantages over fast pyrolysis, such as better (controlled) heat transfer, and greater control over inlet and outlet flow rates (controlled product collection), although it favours gas and biochar instead of bio-oil production [20]. Intermediate pyrolysis is a thermal cracking process with a very high heating rate, very short vapour residence times and high cracking temperatures between 450–1000 °C resulting in liquid yields of about 75 wt.% [19].
According to the environment in which pyrolysis operates, it can be further classified as oxidative pyrolysis, hydrocracking, steam pyrolysis, catalytic pyrolysis, and vacuum pyrolysis. Catalytic pyrolysis can improve selectivity and reduce energy requirements [21]. The parameters affecting pyrolysis product yields are temperature, heating rate, residence time, presence and type of catalyst, polymer/catalyst ratio, reaction gas rate, pressure, and reactor type [21,22], as are indicated in Table 1.

Table 1.
Pyrolysis type versus process parameters.
2.1. Temperature and Pressure’s Effect
The cracking of plastics is directly affected by the temperature and pressure parameters. The pressure during cracking is atmospheric. With increasing temperatures, the production of gas products is significantly higher than that of liquid ones [22]. According to literature a higher heating rate, an intermediate pyrolysis temperature, and a shorter residence time maximizes the liquid yield [23]. This could be due to heat accelerating the breaking of polymer bonds, and then undergoing a hydrogen chain transfer reaction between molecules [24].
The pyrolytic gaseous products from the PP pyrolysis primarily consist of non-condensable hydrocarbons such as methane, ethane, ethylene, propane, and propylene [1,10]. The pyrolysis oils contain mainly paraffins, while the aromatics content is around 3% [1]. Oils contain many light and heavy fractions hydrocarbon compounds with atom number from C4–C20 and its physicochemical properties closer to diesel values [22].
2.2. Reactor’s Type Effect
The reactor is considered the heart of pyrolysis. To achieve successful pyrolysis, two essential design characteristics must be addressed, where the reactor should blend the PP and catalyst effectively, deliver a large amount of heat to the feedstock, and have a short residence period. The reactor types used in the pyrolysis process include the rotary kiln, fixed-bed, batch and semi-batch, fluidized-bed, tubular, plasma, and microwave reactors. The most common reactors used are the fluidized bed and rotary kiln reactors [12].
A fluidized bed reactor is considered the best reactor to perform catalytic plastic pyrolysis since it provides better mixing of the catalyst with fluid and the catalyst can be regenerated many times without the need of discharging, especially worth considering if the catalyst is a very expensive substance. It is also the most suitable reactor for a large-scale operation in terms of the economic point of view [16,17].
Rotary kiln reactors are a common option used for pyrolyzing a variety of municipal solid waste. They provide good mixing of wastes, flexible adjustment of residence time, larger channel for the waste stream allowing feeding of heterogeneous materials, thus, preventing extensive pre-treatment of wastes, and simple maintenance. The catalytic reaction in rotary kiln reactors requires low-cost catalysts because of the difficulty of regenerating them [25].
Although they both have their advantages it is important to note that the lower cost of catalyst use that is required in the fluidized bed reactor is a significant factor for the selection of reactor, as the pyrolysis type is considered catalytic. So, if the operation of a fluidized bed reactor with a catalyst is considered lower cost opposite to rotary kiln reactor, then FBR is the optimal choice for the large-scale operation [2,16,17,25].
2.3. Inert Gas Flow Rate Effect
Carrier gas plays a role in the process by reducing secondary reactions and by diluting the reactor atmosphere and transferring the pyrolysis products. The pyrolysis carrier gas is a stable and non-reactive gas which is causing the degradation of material without oxidation. Many carrier gases such as helium, nitrogen, hydrogen, steam, and also pyrolytic gas have been used in tire pyrolysis. Helium is generally used for the pyrolysis of polymers. N2 is the most common used carrier gas because it can help control reaction conditions, improve product quality, ensure process safety, and optimize the efficiency of the pyrolysis reactions. When plastic is pyrolyzed with high-temperature water vapor as a heat carrier fluid, the water vapor will participate in the pyrolysis reaction of organic matter, to change the release characteristics of oil and gas products. The importance of inert gas flow is to help the transfer of evaporated products to the condenser and to prevent the organic material from oxidation without participation in the pyrolysis process. The molecular size of the carrier gas helps in the transport of the products and is temperature-dependent [21]. In the pyrolysis industry, the most used gases are H2, N2, He, and Ar. Nitrogen is the predominant because it does not interfere with the cracking reaction and enhances the movement of volatiles in the condenser. Usually, the nitrogen flow rate is maintained at about 10 mL/s for most plastic pyrolysis.
Regarding the heating rate, the results are that liquid yield increased as the heating rate increased; however, higher rates decreased its yield. The optimum temperature found under these conditions was 650 °C and a heating rate of 15 °C min−1, obtaining 94.37% of liquid hydrocarbon. The composition of the liquid hydrocarbon corresponded to a concentration of 84.74% of styrene [1,2,3,8].
2.4. Heating Rate and Residence Time Effect
Two main categories set the conditions of heating rate and residence time. Slow pyrolysis consists of low temperature, slow heating rate and long residence time whereas fast pyrolysis consists of higher temperature, higher heating rate and low residence time. The heating rate is crucial for the yields obtained from pyrolysis processes as it directly affects heat transfer. The higher the heating rate, the faster the volatiles are formed and the lower the heating rate, the longer the residence time for the volatiles. Low temperatures and low heating rates favour the production of charcoal, but it cannot be used for the efficient production of good-quality oil [25]. Generally, fast pyrolysis is employed to maximize the liquid product yield, while slow pyrolysis is employed to maximize the solid product yield [26,27]. Although, slow pyrolysis has better (controlled) heat transfer, greater control over inlet and outlet flow rates (controlled product collection) over fast pyrolysis [1,2,3,8,20].
2.5. Catalyst and Catalyst/Plastic Ratio Effect
In general, the addition of catalysts to the cracking process can improve the quality of condensate oil and gas products by increasing the yield and quality (calorific value) of the liquid (hydrocarbon-rich) products [28], compared to non-catalytic pyrolysis [1].
The acidity of a catalyst is a significant parameter in the liquid product yield of PP pyrolysis. Higher liquid yields are obtained from low acidity catalysts, instead of high acidity ones, with liquid yields that can reach up to 75% [29]. The structure and pore distribution of the catalyst can affect also the selectivity of the reaction [26].
Typical catalysts are FCC (Fluid catalytic cracking catalyst) alumina, Y-zeolite, HZSM, MCM and ZSM-5 [5]. The three main catalysts widely used in plastic pyrolysis are zeolites, FCC and silica-alumina catalysts [12]. The reactivity of the three types of catalysts mentioned above is due to metal or acid sites contained on the surface.
The use of zeolites in pyrolysis gives a higher calorific value fuel [28]. The most researched zeolites are HZSM-5 due to their good selectivity and high catalytic activity. However, the cost of zeolites is significantly high [26]. Typical zeolites commonly used for fuel production are categorized into two groups, (a) microporous (HZSM-5, HY, HMOR, HUSY, etc.), and (b) mesoporous catalysts (MCM-41, SBA-15, etc.) [1].
The silica-alumina catalyst is an amorphous acid catalyst containing Bronsted acid sites with a hydrogen atom capable of being ionized and a Lewis acid site, electron accepting sites. The acid concentration of the silica-alumina catalyst is determined by the molar ratio SiO2/Al2O3. Unlike zeolite, the acid strength of silica-alumina is determined oppositely, i.e., the high SiO2/Al2O3 ratio indicates the high acid strength. For example, SA-1 (SiO2/Al2O3 = 4.99) has higher acidity than SA-2 (SiO2/Al2O3 = 0.27) where both are the commercial silica-alumina available in the market [12].
The FCC catalyst has certain advantages such as increasing the efficiency of the pyrolysis process and reducing energy demand. It results in high liquid yield due to smaller microporous area, formation of branched hydrocarbons in the liquid product (due to carbon chain isomerism), reduced concentration of heavy aliphatic compounds (C20+), increased concentration of light hydrocarbons (C13), increased C2 and C4 and reduced methane production [4]. FCC catalysts are widely used in the petrochemical industry. The addition of FCC in the PP pyrolysis can enhance the production of liquid products while the addition of ZSM-5 decreases the liquid yield and increases the yield in gaseous products [30].
Several studies have reported that the polymer-to-catalyst ratio has a significant effect on both the yield and composition of plastic pyrolysis products. However, by increasing the amount of catalyst, no direct proportionality is achieved in terms of increased conversion or overall efficiency. An increase in the amount of catalyst increases conversion up to a certain limit, but a further increase in the percentage of catalyst gives no appreciable increase in conversion rate [1,2,3,14,21].
The ratio between catalyst and polymer usually varies from 5 to 20% by weight of catalyst [31]. The maximum yield in the condensate is obtained with a 10% FCC catalyst. However, catalyst-free cracking showed maximum condensate yield consisting of waxes (>90 wt.%) that were not suitable for fuel. In general, high surface area catalysts can change the nature of the pyrolysis and even affect the results at low content. Results show that the non-condensable product and coke yields increase with catalyst content [14,21].
The effect of catalyst type and catalyst/polymer ratio on pyrolysis liquid product yields is shown in Table 2 and Table 3, respectively.

Table 2.
Effect of catalyst on specific temperature for pyrolysis product yields.

Table 3.
Effect of catalyst/PP ratio on pyrolysis liquid/gas/solid yields [21].
Taken in consideration all the above, a fast catalytic pyrolysis, carried out in a fluidized bed reactor (FBR) with an FCC catalyst of low acidity, selected due to high heat transmission, was considered for the case study of an industrial plant with capacity of 1 t/h of PP, provided by the Greek Petroleum Industry.
3. Engineering Framework for the Eco-Technological Design of PP Pyrolysis
The present study concerns an industrial pyrolysis plant with a capacity of 1 t/h of industrial PP waste, in Northern Greece, that is the partnership of two leading industries in the Balkans: (1) CaO-Hellas, a lime manufacturing industry, the pyrolysis system developer and owner and (2) Greek Petroleum company (ELPE), the PP provider, in a collaboration within the circular economy and industrial symbiosis models. Cao-Hellas is interested in the application of technologies that will reduce its energy footprint while simultaneously promoting the circular economy to reduce the environmental footprint and the production of materials of added value utilizing materials that would otherwise be discarded in the environment at the end of their life cycle. The conceptual representation of PP pyrolysis facility in Greece within the concepts of resource recovery, zero-waste, and sustainability as studied here is depicted schematically in Figure 1.
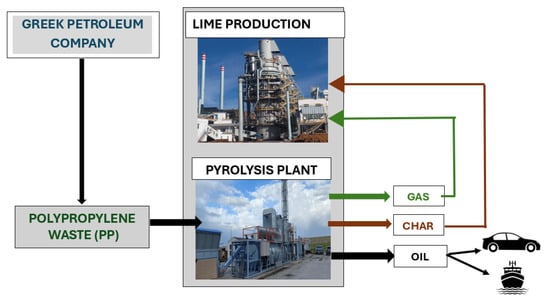
Figure 1.
Conceptual representation of PP pyrolysis facility in Greece within the concepts of resource recovery, zero-waste, and sustainability.
3.1. Feedstock Characteristics
The PP waste is provided by the Greek Petroleum Company (ELPE) in the form of granules of irregular shape, with 5–10 mm diameter and density of 550 kg/m3. The characteristics of the supplied PP waste are depicted in Table 4.

Table 4.
PP waste feedstock characteristics.
3.2. Choices of Operating Conditions and Hypotheses
The pyrolysis operating conditions choices were based on the literature review. The choices made for, and their justification are depicted in Table 5.

Table 5.
Choices and hypotheses made in this study.
Therefore, for the modelling and simulation purposes of PP waste pyrolysis, we considered as pyrolysis products the following substances: methane, ethane, ethylene, cyclopropane, cyclopropane, n-butane, 1-decahexene, 1-C28=, 2,4-dimethyl-1-heptene, n-pentane, 2-methyl-1-pentene.
3.3. Plant Design
The plant design (the flow sheet made by using Visio, https://www.microsoft.com/el-gr/microsoft-365/visio/flowchart-software, accessed on 20 February 2023) is given in Figure 2. The procedure is the following: PP and water are introduced into a washing device in which the PP is cleaned of any impurities. Then the clean PP is introduced into a dryer which removes any amount of water from the polymer, with the aid of nitrogen gas, which is directly introduced into the fluidized bed reactor for pyrolysis. At the same time, the resulting gas stream (206) from the dryer contains steam and nitrogen and is fed to the CN-4 condenser for separation, to recycle the nitrogen and return it as an input to the dryer. Then pyrolysis takes place. The gaseous products are directed to two successive heat exchangers to reduce their temperature. The stream resulting from the second exchanger is directed to a hydrocyclone for the separation of water and polypropylene containing water, and to two successive condensers. It is then separated into a volatile substances mixture and a concentrated liquid product stream (oil), that can be utilized in a variety of ways in the fuel industry. The solid residue from the reactor is fed to a burner for energy production. Water collected from the four condensers in the process (of high temperature) is cooled down to 25 °C and it comes back in a closed loop, to the condensers. The description of each device is given below.
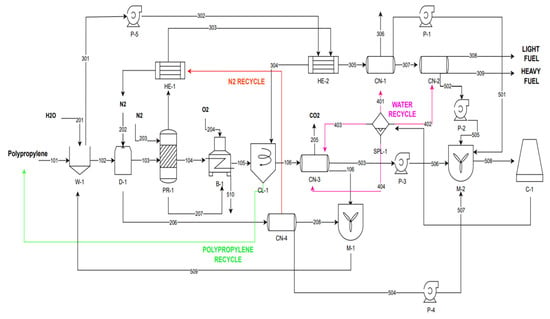
Figure 2.
Plant design of the proposed pyrolysis facility for converting PP plastic waste to oil. The numbers indicate streams of the process (see Section below).
Description of the Plant
Washing device/rotary drum washer W-1
The raw material is transported by trucks to the production plant where it is stored in a suitable warehouse. From there it is conveyed by a conveyor belt to the washing device, to which a certain amount of water is added. The device aims to clean the polypropylene particles so that there are no significant impurities such as organic waste, stones, and metals [36]. The washing device is a rotating screw which through a water supply cleans the polypropylene feed from impurities and finally separates the water from the polymer. The washing efficiency is at 99% with the purest polypropylene upon exiting the device having retained 1% wash water as the correspondingly same percentage of polypropylene exits along with the used wash water [37].
Spray dryer D-1
Drying controls, the moisture content of the polypropylene and is a very basic device of the process. Drying is the evaporation and removal of water from a solid-liquid mixture, in this case polypropylene water, to form a dry solid. Spray heavy fuel, and the other is light fuel. The heavy fuel is the final desired liquid product [9].
Catalytic cracking reactor PR-1
The catalytic cracking process takes place in a fluidized bed reactor. This is a type of reactor in which the catalyst is placed on a distribution plate through which a fluidization gas passes, with the feed particles passing through the reactor in a liquid phase. The liquefaction gas is an inert gas (also known as carrier gas) which is only involved in the transport of evaporated products without participating in the pyrolysis. Therefore, the catalyst is mixed with the fluid, which increases the contact surface for reaction. This type of reactor was chosen as it provides almost constant temperature, with high mass and heat transfer, giving shorter residence time in the reactor and consequently more uniform product range, compared to other types of reactors [12].
Burner B-1
The solid product resulting from catalytic cracking can be applied as a feedstock in the production of activated carbon and as a fuel in boilers/burners [32]. To utilize all by-products, the solid resulting from cracking will be used as a fuel for energy production in a burner. The burner operates at a temperature of 500 °C [1] and produces clean coal and a mixture of gaseous by-products.
Condensers CN-1, CN-2
The gaseous products resulting from catalytic cracking enter a condenser where the light and heavy fractions are separated. Then the output stream with the heavy fractions enters a second evaporator to further condense the less volatile components [2]. Therefore, two different streams result, one of which is heavy fuel, and the other is light fuel. The heavy fuel is the final desired liquid product [9].
Condensers CN-3, CN-4
For the separation of gas mixtures of streams 106 and 206, the condensation method is again used. This is because both streams have steam which is easily converted to water by dropping the temperature below its boiling point and separated from the other gas. These streams must separate their components as the carbon dioxide contained in stream 106 must be removed for environmental pollution reasons and the steam and nitrogen contained in stream 206 must be separated as the nitrogen has to be recycled.
Hydrocyclone CL-1
The hydrocyclone is a device which is used to separate solid products from liquid fractions [9]. In this case, streams 105 and 304 are introduced into the cyclone where the objective is to separate the solid content in polypropylene from the total liquid fluid. The hydrocyclone achieves the separation through centrifugal force.
Tube-shell heat exchangers HE-1, HE-2
The heat exchangers of the processes are tube-shell heat exchangers and operate in a uniform mode. Specifically, heat is transferred from a hot stream to a cold stream as it flows in parallel through the heat exchanger.
Mixing devices M-1, M-2
M-1 is a vessel into which the streams 208,106 and M-2 is a vessel into which the streams 501,505,506 and 507 end up to mix.
Cooling tower C-1
The counterflow cooling tower is a cooling device designed to remove heat from the process fluid by cooling it. With coil exchangers mounted in the tower, the entire cooling process is accomplished through heat exchange between the air, spray water and water circulating through the coil exchanger inside. In the closed-circuit counter-flow cooling tower, the air is introduced at the bottom of the tower and flows from the bottom to the top, in the opposite direction of the spray water, therefore, it is called a counter-flow cooling tower. It is widely used in industrial refrigeration. So, the current is introduced into the cooling system to reduce its temperature from 90 °C to 25 °C.
3.4. Sizing of the Plant
The process’s devices are sized according to standard methods of the chemical engineering [38,39,40,41,42] and are presented in Table 6.

Table 6.
Apparatus sizing (diameter, height).
4. Techno-Economic Analysis and Economic Sustainability Assessment (TEA)
The assessment of economic viability is an evaluation of the various economic parameters of a plant or system, helping decision-makers. It is a financial analysis, and the main tool is the cost-benefit analysis (CBA), expressing costs and benefits in monetary terms. The techno-economic assessment performed in this study breaks down costs into capital expenses (CapEx), operational expenses (OpEx), and raw material costs. Market prices are collected to show the possible profit margin. The main parameters estimated are a) the investment capital (IC), b) production cost (PC), raw materials cost (CRM), cost of auxiliary provisions (CUT), and waste treatment costs (CWT).
4.1. Investment Capital (IC)
Fixed costs, indirect costs or overheads are business expenses that are not dependent on the level of goods or services produced by the business, i.e., not reliant on output. Considering the change in the time value of money, the Chemical Engineering Plant Cost Index (CEPCI or CE) was used to extrapolate the costs to 2022 [39]. For 2022 and beyond, values for the index are not yet available, so they will be found by extrapolating the trend line shown by the previous ones. Below in Figure 3, CEPCI values for the period 1995–2021 are given.
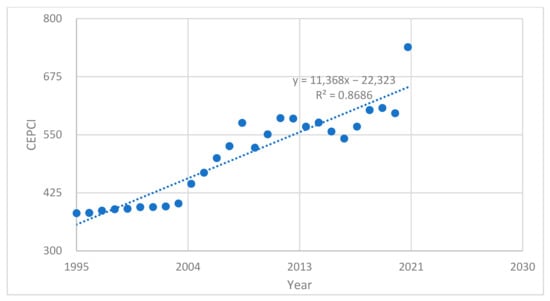
Figure 3.
Estimated CECPI index by year for the period 1995–2025.
CEPCI index for 2022 will be, then:
The total cost of installed equipment for 2022, which relates to the supply and installation of equipment, is calculated. Guthrie Method [39] is mostly used among others [40,41]. Sizing methods are presented in Table 7.

Table 7.
Estimated cost of installed equipment.
Cost of Fixed Capital (FCI) consists of Direct Cost (DC) and Indirect Cost (ID). The data presented above in Table 7 are used to estimate the total cost of installed equipment Cp, which is part of FCI. According to [40] fixed capital costs can be broken down into individual costs, where each of the direct or indirect costs can be expressed as a percentage of CP. FCI can also be expressed as a function of Cp. Thus, in the case of new facilities, it is assumed that the cost for offsite facilities is about 35% of FCI and the indirect cost is about 15% of FCI. The FCI is equal to [39]:
However, the result of these calculations leads to a significant underestimation of the FCI. For this reason, the equation used by the National Renewable Energy Laboratory (NREL) of the United States was used to estimate the FCI:
The cost of offsite facilities can be estimated as 45% of the cost of onsite facilities, therefore:
The total FCI is calculated according to [40] and is equal to 5,020,000€.
As mentioned above, the Cost of Working Capital (CWC) is expressed as a percentage of FCI, as 5% of FCI [39]. Thus, it can be easily calculated and is equal to 251,000,000€. In summary, the total investment capital is estimated as the sum of FCI and WCC, based on all the above and is equal to approximately 5,300,000€.
4.2. Production Cost (PC)
The viability of an investment project involving the construction of a production plant depends on the total cost of production. These costs can be broken down into the following categories: Direct Costs (DC), Fixed Costs (FC) and General Expenses (GE). The TPC and its costs can be expressed as [40]:
where:
TPC = DC + FC + GE = 1.22(CRM + CUT + CWT) + 2.66COL + 0.295FCI
CRM: Raw materials, CUT: Ancillary Benefits, COL: Direct Work, CWT: Waste treatment.
The construction time of the plant is assumed to be 2 years; therefore, the operating costs of the plant should be reported in 2024 and beyond. The 2024 CEPCI, according to Figure 2, will be:
4.3. Cost of Raw Materials (CRM)
The raw materials used throughout the process are PP, FCC and N2. PP is recycled in a small percentage for this reason and its purchase until permanent status is achieved is for a partially larger quantity. The values for each and the respective time to which they refer are 54€/tn for PP [43], 987.55€/tn for FCC [43], and 740€/tn for N2. Figure 4 shows various PP prices in the USA, Asia and the United Kingdom for pure (dark blue) and mixed (light blue) polypropylene. As can be expected, the price of PP plastic is cheaper in Asia and the UK and quite expensive in America. It is also understandable that the purer the polypropylene the more expensive it is anywhere.
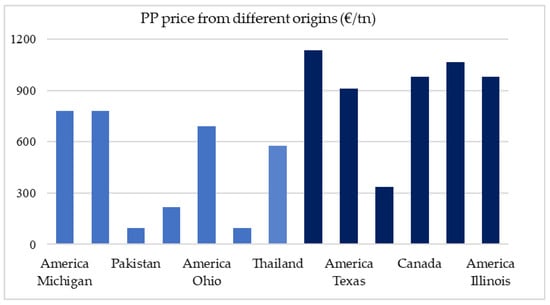
Figure 4.
PP price from different origins and in different countries (€/tn). Pure → dark blue and Mix (PP and other impurities) → light blue.
In this study, the price of waste PP provided by Hellenic Petroleum (HELPE) was assumed ~54€/tn [43] considering transportation costs to be incorporated in this price (Figure 4).
The projected cost of raw materials (PP, FCC Catalyst, N2) for 2024, for specific flow rates, was calculated with the help of Microsoft Excel software 365 and is equal to CRM = 6,784,732.28€.
4.4. Cost of Auxiliary Provisions (CUT)
The auxiliary supplies for this system are water, air, and electricity. Since the burner can fully meet the electricity needs by using char as fuel, the cost of the auxiliary supplies consists mainly of the cost of the purchase of water and air.
According to the Greek Water Company (EYATH), the cost of water in 2022 is 0.98 €/m3 It was assumed that the amount of water to be purchased is that needed for the first 3 days of operation, (9.19 m3/h × 24 h × 3 = 661.68 m3). After the first 3 days, the condensate recycling process will start, so the water will be recycled. As for air, the cost is equal to €0.356100 std/m3 [39], and the amount of capacity required is equal to 1,168,992 tn/yr. The total cost of the water provision for 2022 is 279,493.61€.
4.5. Direct Labor Costs (COL)
The number of employees in the plant is estimated to the Ulrich method, in which each employee works a total of 40 h per week, and factors such as normal leave and sick leave are included. Thus, approximately 16 employees are needed to fully cover the needs [39].
The number of devices needed are: 1 Washing device W-1, 1 Reactor PR-1, 1 Spray dryer D-1, 1 Burner B-1, 4 Compressors CN-1/4, 1 Hydrocyclone CL-1, 2 Heat exchangers HΕ-1/2, 2 Mixing Devices M-1/2, 1 Cooling Towel and 4 Storage Containers V-1/4.
The average hourly rate for 2019 in the EU countries was 30.3€/h according to Eurostat [44] and is equal to 31.3€/h for the year 2024. The total cost for the 16 employees who work 40 h/week is calculated with the help of Microsoft Excel. For 16 employees and worker price 31.3€/h (2024) the total cost is 1,322,112€.
4.6. Pyrolysis Waste Treatment Costs (CWT)
Waste in the process is the CO2 gas stream produced during the condensation process in the CN-3 device (Stream 205), and the solid carbon stream resulting from the combustion process (Stream 510). The treatment cost in 2013 was $36/t, for non-toxic waste [39]. Microsoft Excel is used to calculate the cost.
4.7. Depreciation
The fixed assets of the plant are subject to depreciation over the life of the plant due to operational and time-related wear and tear, as well as depreciation resulting from legal commitments. This reduction is called depreciation [39]. The calculation is done using MS Excel:
where, CFo is the direct DC cost, S is the residual value and N is the payback period. The depreciation period is 15 years, a practice that many entrepreneurs follow when considsering a commercial plant. The residual value was considered as neglisable. The results of the order can be estimated by using the MACRS method (Modified Accelerated Cost Recovery System).
The total production cost (TPC) is calculated with the help of each individual cost per year, according to following equation [39]:
Therefore, the total cost of production per year is estimated at approximately 13,715,000€.
5. Results
Plastic waste pollution is a serious world problem while recycling rates are relatively low. This necessitates the implementation of eco-technologies to transform plastic waste into energy and materials. With the depletion of fossil fuels, it has become increasingly important to develop eco-technologies for alternative sources of energy to fuels. Plastic pyrolysis is regarded as the most environmentally friendly technology in terms of both its Global Warming Potential (GWP) and Total Energy Use (TEU) [41]. For a sustainable energy transition and a circular economy, research and development in pyrolysis-to-oil production is important, with specific advantages of reduction in carbon footprint and recovery of energy and resources. As a strategy for improving plastic recycling, research into the economic and technical feasibility (TEA) of plastic waste is recommended. An engineering design and economic viability assessment of an industrial PP waste catalytic pyrolysis system was developed in this study. Based on the TEA performed, the pyrolysis was determined to be scalable and sustainable.
To produce oil, gas, and char products, the crushed PP is fed into the pyrolysis reaction chamber in ambient nitrogen (N2) gas with a flow rate of 20 L/min < 500 °C for almost 1 h. The heavy oil fraction is collected at the bottom, whereas the formulated hot gases were swiftly passed through the cyclone to separate the tar fraction. In the interim, the char remained in the reaction chamber and can exit after cooling it. The oil produced from industrial PP waste, via thermal and catalytic de-polymerization addresses the twin problem of waste dispose and depletion of fossil fuel reserves.
The annual income is calculated by using the equation:
where, i = each product.
Each year, the expense of the services and materials transported undergoes a fluctuation by the overall economic state of a nation. Variation is expressed through inflation. The average inflation rate in Greece between 2011 and 2021 is 1.41%, and the prices of raw materials and utilities can be estimated for the next 30 years based on this rate. The selling prices of manufactured goods are also affected by inflation. Therefore, the value of annual income fluctuates over time and can be quantified. With the aid of these calculations, it is feasible to evaluate the selected business plan and determine its viability.
The Net Present Value (NPV) was calculated for 30 years of operation and is equal to 55.754.143,300. Furthermore, due to calculations, the equivalent annual value (EAV) is estimated at 4.952.497,45 €. The return on investment (ROI) was calculated at 33.86% > 10%. The Payback Time (POT) was estimated to be 2.4 years. Please note that year 2 is the year in which the investment acquires capital and construction of the plant begins. Therefore, years 2 and 1 refer to the first and second years of construction, and year 0 refers to the first year of operation.
6. Discussion
Catalytic pyrolysis-to-oil is an appealing technological option for the up-cycling of PP plastic waste. The estimated values of economic indicators show that PP catalytic pyrolysis at an industrial scale is viable and profitable, particularly in the context of the circular economy. Pyrolysis is an effective eco-method that brings many benefits compared to incineration and disposal in landfills. The cost of this service is usually indicated as a negative value, often referred to as a “gate fee” in landfills or waste incineration plants. Because the provided PP waste is homogenous, no limitations were faced when collecting and separating the PP plastic.
Care was taken for the pyrolysis waste’s recycling and further energy recovery using solid and gas pyrolysis products, as energy sources to fulfil the energy needs of the plant, in a closed loop.
6.1. Assumptions Made
For the TEA, we made some assumptions, which are summarised below:
- It was assumed that it takes 2–3 years to build an industrial plant. In the present investigation, it was determined that the duration required for the complete construction of the plant was 2 years.
- Regarding the source of funding for the investment project, it was assumed that 40% of the funding would be derived from the investor’s funds, while the remaining 60% would be obtained from bank loans, with an interest rate of 8%.
- This loan will be repaid over 20 years in equal instalments.
- The minimum acceptable rate of return (MARR) was set at 8%.
- The price of light fuel and heavy fuel is considered equal to the current price of gasoline and diesel, respectively (2€/L and 1.9€/L).
- The burner generates additional electricity which is available for sale.
6.2. Critical Issues
For advancing pyrolysis technology to industrial applications the most relevant critical issues are:
- (1)
- Design for meeting technical requirements.
- (2)
- Technical requirements process for converting PP to high yields of pyrolysis-oil.
- (3)
- Reactor designs capable of meeting technical requirements.
- (4)
- Pyrolysis oil stability issues and recent findings that address the problem.
- (5)
- Product specifications and standards that need to be established.
- (6)
- Environmental, safety, and health issues.
These health issues are important to both the producer and consumer of bio-oils, regarding the toxicity of pyrolysis-oil, affecting the plant operating personnel, transportation, and consumers safety.
Other critical parameters for large-scale systems are
- Securing feedstock availability.
- Decreasing environmental impact from the logistical challenges of gathering and transporting the PP waste.
In this study, the cost of transporting the PP waste was considered zero, because the Greek Petroleum Company (HELPE) will provide PP waste to the Greek lime manufacturing company (CAO Hellas) without any cost, towards enhancing the collaboration and the industrial symbiosis concepts.
6.3. Limitations of the Study
The current study has several limitations, many due to the lack of laboratory data as well as the conceptual nature of the analysis itself. From the previous critical issues described in Section 6.2, only the three first issues (design for meeting technical requirements, technical requirements process for converting PP to high yields of pyrolysis-oil, reactor designs capable of meeting technical requirements) were dealt in this study. The other 3 issues (pyrolysis oil stability issues and recent findings that address the problem, product specifications and standards that need to be established, environmental, safety, and health) will be the objective of another study including experimental work and measurements.
6.4. Comparison with Pyrolysis in Rotary Kiln Reactor
Several reactor designs can convert PP waste to oil. The most common are:
- (a)
- Fluidized beds, both bubbling and circulating.
- (b)
- Rotary kiln.
- (c)
- Ablative.
Of these designs, the fluidized and transported beds appear to have good acceptance because they can produce pyrolysis-oil in high yields. This was the reason we made the choice of FBR in this study. Rotary kiln is the most common reactor used in waste pyrolysis because it presents several advantages, such as simple construction, low purchase costs, simple operation, and feedstock flexibility.
A comparison of the TEA of this study with the results of our previous work related to the economic assessment of PP waste pyrolysis in a smaller scale industrial plant with a rotary kiln reactor [45], was attempted. For the rotary kiln pyrolysis plant with a capacity of 200,000 t/year of PP waste, the economic indicators were, [46,47]:
- Annual profits ~37.3 M€.
- ROI ~81%.
- POT ~1.16 years.
- Profit per t of PP wate treated ~186.5 €/t.
Comparing the economic indicators of the previous work [47] with those of the present study, it can be assumed that the catalytic pyrolysis at large scale is potentially less profitable than that of non-catalytic in rotary kiln, in terms of economic sustainability only.
6.5. Benefits of PP Waste Pyrolysis
The benefits of PP waste pyrolysis are diverse:
- Mitigating plastic pollution.
- Diverting plastic from landfills.
- Recovering valuable resources.
- Pyrolysis promotes resource recovery by converting plastic waste into fuel.
- Contributing to a greener and more sustainable plastic waste management.
- Reducing carbon footprint.
- Pyrolysis is an energy-efficient process with lower emissions.
- Promising a sustainable avenue for profitability and market development.
- Stimulating job creation and sustainable development by creating job opportunities in plant operations and recycling sectors.
- Contributing to SDGs, ensuring sustainable consumption and production patterns (Goal 12).
6.6. The Need for Regulatory Support
There is a need for regulatory support that includes:
- Government initiatives
For industrial implementation of plastic waste pyrolysis at large scale and in the circular economy, governments and regulatory bodies worldwide must offer incentives, subsidies, and favorable policies to promote the adoption of this environmentally friendly technology.
- Aligning with circular economy principles
PP pyrolysis is in line with the circular economy concept because via pyrolysis PP waste is minimized, energy resources are conserved, we can have economic benefits. We can use the pyrolysis gas and solid product to fulfill the energy requirements of the facility, in the closed-loop principle, reducing the need for fossil fuels.
6.7. Challenges-Barriers
To achieve a successful implementation of pyrolysis-to-oil, some challenges must be overcome. These are:
- Collection, separation, sorting, and cleaning operations of plastic waste must be considered, and their cost must be estimated.
- As pyrolysis oil from plastic waste is a secondary product, there is still a debate regarding its classification as a product suitable for industrial applications.
- There is a need for specifications and standardization of pyrolysis oil, which characteristics may vary according to type of plastic and pyrolysis reactor, temperature, reaction time, heating rate, etc.
- Pyrolysis oil from PP waste has very high potential to substitute commercial diesel fuel through further research and by improvement of properties by different post-treatment procedures [46].
- To improve the properties of crude pyrolysis oil, post treatment procedures such as distillation and hydrotreatment are needed [47].
There are several barriers that must be overcome to advance the technology at large scale. These are:
- Specifications or standards for pyrolysis oil must be created.
- Pyrolysis oil quality and reproducibility which is important especially with respect to stability in storage.
- Environmental, safety and health issues.
- Long term supplies of the plastic resource.
7. Conclusions
PP waste and in general plastic waste that originally are produced by crude oil, can be recycled as feedstocks to produce synthetic oil and gas through the thermochemical process of pyrolysis, mitigating waste environmental impacts and increasing resource recovery.
This study provides an engineering scalability solution for PP waste valorization, that it has interest in the process industry and recycling sectors. PP plastic waste pyrolysis-to-oil (fuel) was considered in the TEA performed. The plant characteristics are: FCC pyrolysis in FBR, temperature ~430 °C, pressure ~1 atm, N2 as heating gas, and heating rate at 5 °C/min. Under those conditions, the yields of the liquid, gas, and solid pyrolysis products can reach 71, 14, and 15 wt.%, respectively.
The plant includes a washing device, spray dryer, fluidized bed reactor, burner, condensers, hydro cyclone, heat exchangers, cooling tower, pumps, and mixing vessels. The sizing of the equipment of the plant was performed, using proper engineering formulae.
The TEA breakdowns costs into capital expenses (CapEx), operational expenses (OpEx), and raw material costs (RM). Market prices are collected to show the possible profit margin. Assumptions were made mainly regarding the lifetime (30-years), time in building it (2-years), MARR rate (8%) and depreciation time (20-year). The economic indicators estimated as: capital investment (fixed and working capital) ~€5.300.000,00, annual revenue ~€15.000.000,00 for the year 2023, POT to 2.5 years after the start of construction, and NPV to ~€56,000,000.00. These indicators show that the plant is economically viable.
Critical parameters for the sustainability of PP waste pyrolysis are the type of pyrolysis reactor, securing feedstock availability, decreasing environmental impact from the logistical challenges of gathering and transporting this feedstock. Industrial symbiosis can play a prominent role in circular economy and sustainability of industrial networks, by reducing the costs of raw material, as well as decreasing the environmental impact of businesses.
PP waste pyrolysis contributes to achieve sustainable development goals (SDGs) and circular economy. For accelerating the industrial large-scale application of pyrolysis for plastic management, incentives, subsidies, and favorable policies to promote the adoption of this environmentally friendly technology are needed, by governments and regulatory bodies.
Author Contributions
A.Z.: Conceptualization; methodology; project administration; funding acquisition; review and editing; supervision. A.C.: formal analysis; investigation; resources; data curation; writing—original draft preparation; visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ESPA 2021–2027, the Region of Central Macedonia, Greece, grant number KMP6-0283869.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the Region of Central Macedonia, Greece, and the support of EC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Hasan, M.M.; Hazrat, M.A. Transport fuel from waste plastics pyrolysis—A review on technologies, challenges, and opportunities. Energy Convers. Manag. 2022, 258, 115451. [Google Scholar] [CrossRef]
- Dwivedi, P.; Mishra, P.K.; Mondal, M.K.; Srivastava, N. Non-biodegradable polymeric waste pyrolysis for energy recovery. Heliyon 2019, 5, e02198. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraj, S.; Ashokkumar, V.; Pandiyan, R.; Halimatul Munawaroh, H.S.; Chew, K.W.; Chen, W.H.; Ngamcharussrivichai, C. Pyrolysis: An effective technique for degradation of COVID-19 medical wastes. Chemosphere 2021, 275, 130092. [Google Scholar] [CrossRef]
- Frank, J.R.; Brown, T.R.; Malmsheimer, R.W.; Volk, T.A.; Ha, H. The financial trade-off between the production of biochar and biofuel via pyrolysis under uncertainty. Biofuels Bioprod. Biorefining 2020, 14, 594–604. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2022, 803, 149911. [Google Scholar] [CrossRef]
- Fivga, A.; Dimitriou, I. Pyrolysis of plastic waste for production of heavy fuel substitute: A techno-economic assessment. Energy 2018, 149, 865–874. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; El-Fatah Abomohra, A.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Parku, G.K.; Collard, F.X.; Görgens, J.F. Pyrolysis of waste polypropylene plastics for energy recovery: Influence of heating rate and vacuum conditions on composition of fuel product. Fuel Process. Technol. 2020, 209, 106522. [Google Scholar] [CrossRef]
- Riesco-Avila, J.M.; Vera-Rozo, J.R.; Rodríguez-Valderrama, D.A.; Pardo-Cely, D.M.; Ramón-Valencia, B. Effects of Heating Rate and Temperature on the Yield of Thermal Pyrolysis of a Random Waste Plastic Mixture. Sustainability 2022, 14, 9026. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Taghavi, N.; Udugama, I.A.; Zhuang, W.Q.; Baroutian, S. Challenges in biodegradation of non-degradable thermoplastic waste: From environmental impact to operational readiness. Biotechnol. Adv. 2021, 49, 107731. [Google Scholar] [CrossRef]
- Huang, J.; Veksha, A.; Chan, W.P.; Giannis, A.; Lisak, G. Chemical recycling of plastic waste for sustainable material management: A prospective review on catalysts and processes. Renew. Sustain. Energy Rev. 2022, 154, 111866. [Google Scholar] [CrossRef]
- Kruse, T.M.; Wong, H.W.; Broadbelt, L.J. Mechanistic modeling of polymer pyrolysis: Polypropylene. Macromolecules 2003, 36, 9594–9607. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Erdogan, S. Recycling of Waste Plastics into Pyrolytic Fuels and Their Use in IC Engines. In Sustainable Mobility; Llamas, B., Ortega Romero, M.F., Sillero, E., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Hoseini, S.M.F.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 2013, 487676. [Google Scholar] [CrossRef]
- Abdallah, R.; Juaidi, A.; Assad, M.; Salameh, T.; Manzano-Agugliaro, F. Energy Recovery from Waste Tires Using Pyrolysis: Palestine as Case of Study. Energies 2020, 13, 1817. [Google Scholar] [CrossRef]
- Pratama, N.N.; Saptoadi, H. Characteristics of waste plastics Pyrolytic Oil and Its Applications AS alternative Fuel on Four Cylinder Diesel Engines. Int. J. Renew. Energy Dev. 2014, 3, 13–20. [Google Scholar] [CrossRef]
- Basu, P. Chapter 5—Pyrolysis. In Biomass Gasification, Pyrolysis and Torrefaction, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 155–187. ISBN 9780128129920. [Google Scholar]
- Thahir, R.; Irwan, M.; Alwathan, A.; Ramli, R. Effect of temperature on the pyrolysis of plastic waste using zeolite ZSM-5 using a refinery distillation bubble cap plate column. Results Eng. 2021, 11, 100231. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Daligaux, V.; Richard, R.; Manero, M.H. Deactivation and regeneration of zeolite catalysts used in pyrolysis of plastic wastes-a process and analytical review. Catalysts 2021, 11, 770. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Constantinescu, M.; Bucura, F.; Ionete, E.I.; Ion-Ebrasu, D.; Sandru, C.; Zaharioiu, A.; Marin, F.; Miricioiu, M.G.; Niculescu, V.C.; Oancea, S.; et al. From Plastic to Fuel-New Challenges. Mater. Plast. 2019, 56, 721–729. [Google Scholar] [CrossRef]
- Du, Y.; Solène, B.; Glarborg, P.; Wu, H. Plastics as a reburning fuel: Pyrolysis at reburning temperatures and NO-reburning by simulated pyrolysis gas. Fuel 2024, 361, 130664. [Google Scholar] [CrossRef]
- Miskolczi, N.; Angyal ALaszlo, B.; Valkai, I. Fuels by pyrolysis of waste plastics from agricultural and packaging sectors in a pilot scale reactor. Fuel Process. Technol. 2009, 90, 1032–1040. [Google Scholar] [CrossRef]
- Quesada, L.; Calero de Hoces, M.; Martín-Lara, M.A.; Luzón, G.; Blázquez, G. Performance of Different Catalysts for the In Situ Cracking of the Oil-Waxes Obtained by the Pyrolysis of Polyethylene Film Waste. Sustainability 2020, 12, 5482. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail IM, I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.S. Catalytic pyrolysis of plastic waste: Moving towards pyrolysis based biorefineries. Front. Energy Res. 2019, 7, 437000. [Google Scholar] [CrossRef]
- Sakata, Y.; Uddin, M.A.; Muto, A. Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. J. Anal. Appl. Pyrolysis 1999, 51, 135–155. [Google Scholar] [CrossRef]
- Cahyono, M.S.; Fenti, U.I. Influence on heating rate and temperature on the yield and properties of pyrolysis oil obtained from waste plastic bag. Conserve: J. Energy Environ. Stud. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low-density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical, and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Sahu, J.N.; Mahalik, K.K.; Nam, H.K.; Ling, T.Y.; Woon, T.S.; bin Abdul Rahman, M.S.; Mohanty, Y.K.; Jayakumar, N.S.; Jamuar, S.S. Feasibility study for catalytic cracking of waste plastic to produce fuel oil with reference to Malaysia and simulation using ASPEN Plus. Environ. Prog. Sustain. Energy 2014, 33, 298–307. [Google Scholar] [CrossRef]
- APTSOL Limited. 2020. Available online: https://www.healthtekpak.com/events/aptsol-showcases-spray-dryer-pharmintech-2022/ (accessed on 20 January 2022).
- Koukos, I. Introduction to Chemical Plant Design, 2nd ed.; Tziola Publications: Thessaloniki, Greece, 2019. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 2020. [Google Scholar]
- Motin, A.; Petty, C.; Tarabara, V.; Benard, A. Hydrocyclone separation principles for cleaning produced water. In Proceedings of the Produced Water Society 2014 Seminar, Houston, TX, USA, 14–16 January 2017. [Google Scholar] [CrossRef]
- Walas, S.M. Chemical Process Equipment-Selection and Design; Brenner, H., Acrivos, A., Bailey, J.E., Morari, M., Nauman, E.B., Prud’homme, R.K., Eds.; Butterworth-Heinemann Series in Chemical Engineering; Institute of Technology: Cambridge, MA, USA, 1990. [Google Scholar]
- Cai, N.; Xia, S.; Li, X.; Sun, L.; Bartocci, P.; Fantozzi, F.; Zhang, H.; Chen, H.; Williams, P.T.; Yang, H. Influence of the ratio of Fe/Al2O3 on waste polypropylene pyrolysis for high value-added products. J. Clean. Prod. 2021, 315, 128240. [Google Scholar] [CrossRef]
- Eurostat. ‘Archive: Wages and Labour Costs’. 2022. Available online: https://ec.europa.eu/eurostat/eurostatistics-explained/index.php?title=Archive:%CE%9C%CE%B9%CF%83%CE%B8%CE%BF%CE%AF_%CE%BA%CE%B1%CE%B9_%CE%BA%CF%8C%CF%83%CF%84%CE%BF%CF%82_%CE%B5%CF%81%CE%B3%CE%B1%CF%83%CE%AF%CE%B1%CF%82 (accessed on 20 March 2022).
- Zabaniotou, A.; Vaskalis, I. Economic Assessment of Polypropylene Waste (PP) Pyrolysis in Circular Economy and Industrial Symbiosis. Energies 2023, 16, 593. [Google Scholar] [CrossRef]
- Faisal, F.; Rasul, M.G.; Jahirul, M.I.; Chowdhury, A.A. Waste plastics pyrolytic oil is a source of diesel fuel: A recent review on diesel engine performance, emissions, and combustion characteristics. Sci. Total Environ. 2023, 886, 163756. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A.; Dimitriadis, A. Catalyst evaluation for waste cooking oil hydroprocessing. Fuel 2012, 93, 638–641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).