Comparative Analysis of Japanese Soils: Exploring Power Generation Capability in Relation to Bacterial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Analysis
2.2. Microbial Fuel Cell Assembly
2.3. DNA Extraction and Amplicon Analysis
2.4. Nucleotide Sequence Accession Numbers
2.5. Statistical Analyses
3. Results
3.1. Soil Properties before and after MFC Operation
3.2. Power Generation of Soils in MFCs
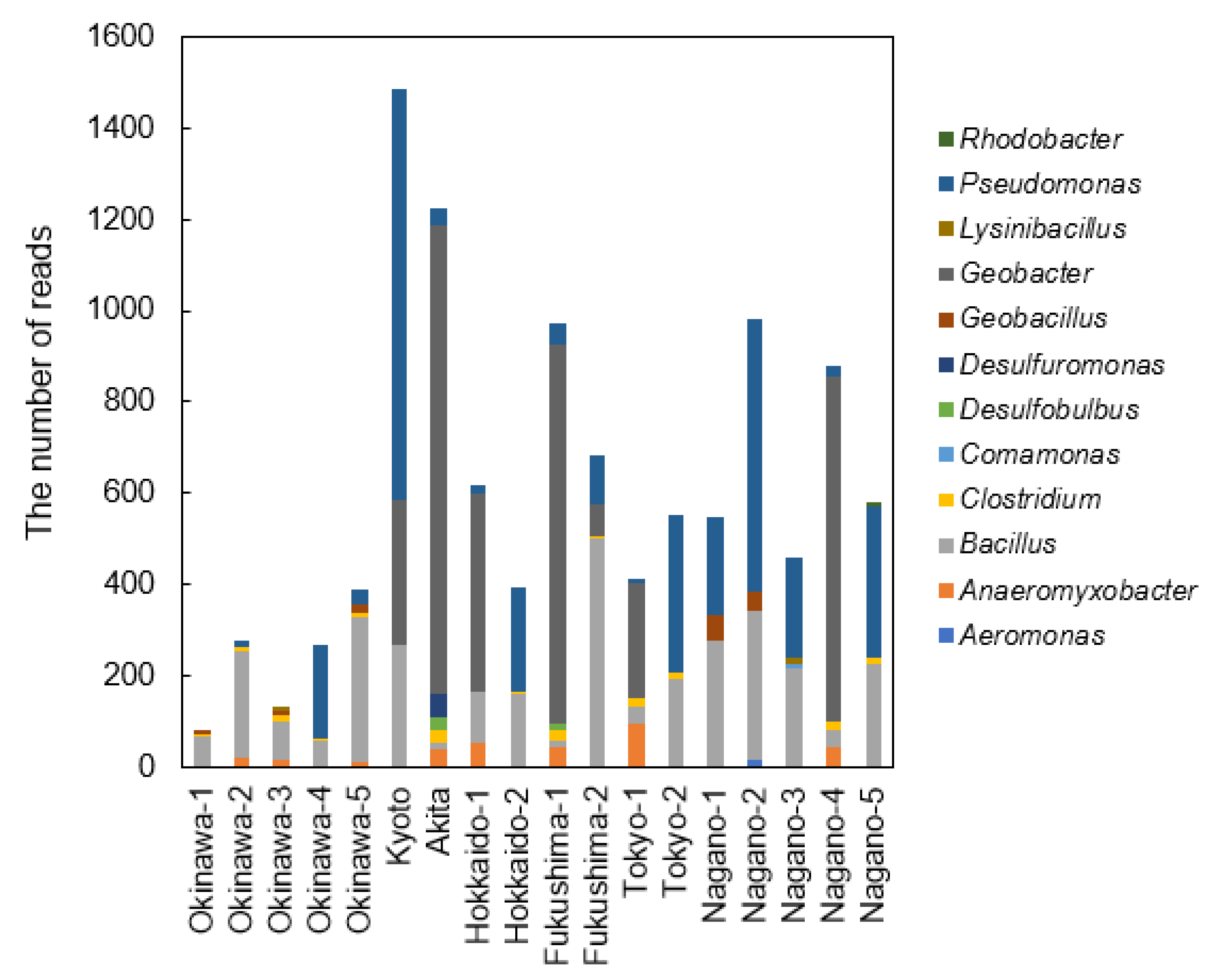
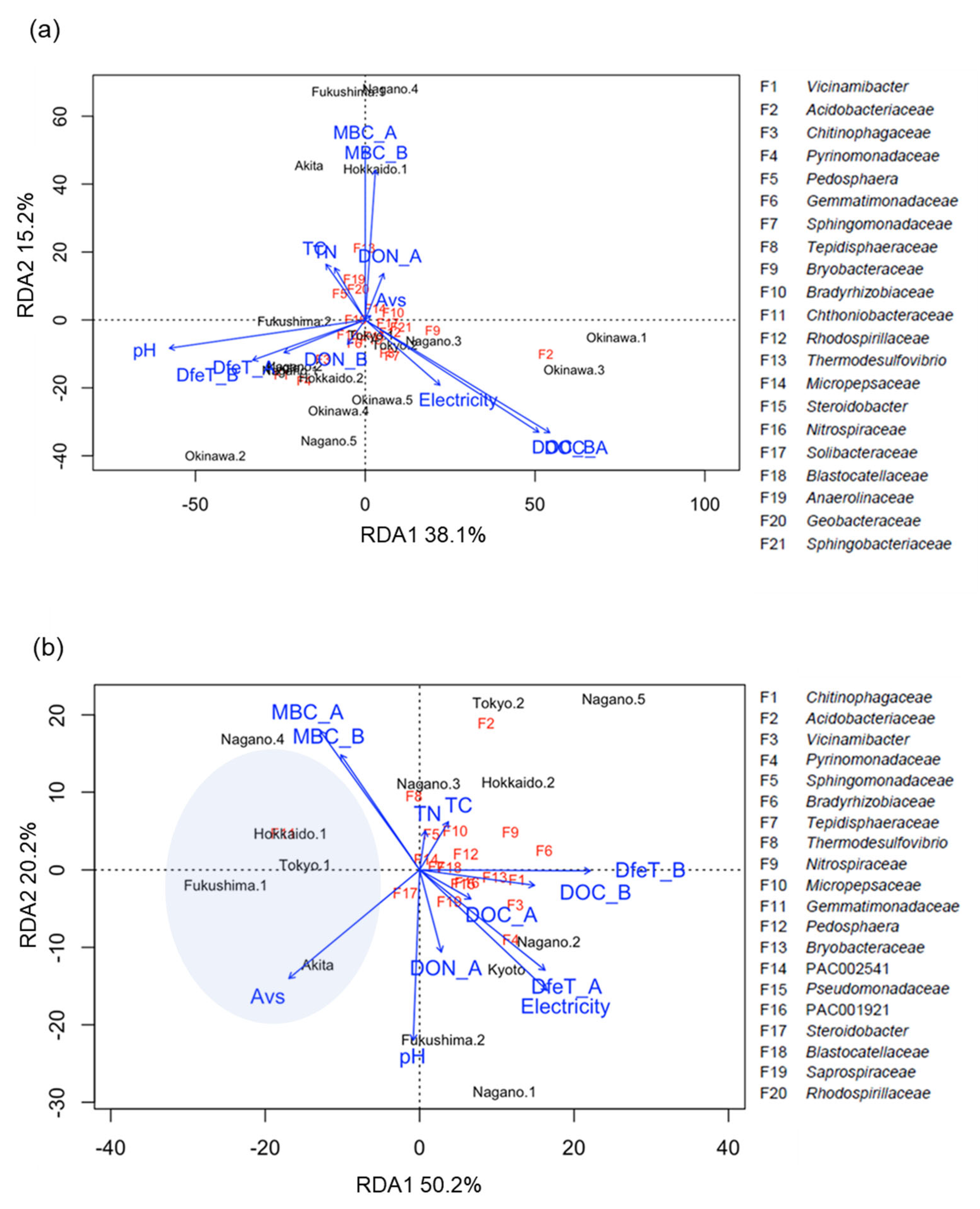
3.3. Analyses of Bacterial Community Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Ma, F.; Pan, S.; Lin, M.; Zhang, G.; Xiong, B.; Wang, Y.; Liang, Y.; Yang, Z. Earth energy evolution, human development and carbon neutral strategy. Pet. Explor. Dev. 2022, 49, 468–488. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sust. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, A.; Raghavan, V.; Tartakovsky, B. Carbon source and energy harvesting optimization in solid anolyte microbial fuel cells. J. Power Sources 2017, 356, 324–330. [Google Scholar] [CrossRef]
- Logan, B.E. Essential data and techniques for conducting microbial fuel cell and other types of bioelectrochemical system experiments. ChemSusChem 2012, 5, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.A.; Pham, N.; Pham, H.T. Wastewater treatment performance and microbial community of anode electrodes of membrane and membrane-less MFCs under effect of sunlight. J. Water Process. Eng. 2021, 42, 102159. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Yang, W.; Logan, B.E. Regenerable nickel-functionalized activated carbon cathodes enhanced by metal adsorption to improve hydrogen production in microbial electrolysis cells. Environ. Sci. Technol. 2018, 52, 7131–7137. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S.P. Biofuel cells—Recent advances and applications. Biosens. Bioelectron. 2007, 22, 1224–1235. [Google Scholar] [CrossRef]
- Michie, I.S.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Electrogenic biofilm development determines charge accumulation and resistance to ph perturbation. Energies 2020, 13, 3521. [Google Scholar] [CrossRef]
- Xu, F.; Ouyang, D.-l.; Rene, E.R.; Ng, H.Y.; Guo, L.-l.; Zhu, Y.-j.; Zhou, L.-l.; Yuan, Q.; Miao, M.-s.; Wang, Q. Electricity production enhancement in a constructed wetland-microbial fuel cell system for treating saline wastewater. Bioresour. Technol. 2019, 288, 121462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Zheng, J.; Zhang, L.; Fu, Q.; Zhu, X.; Liao, Q. Response of anodic biofilm and the performance of microbial fuel cells to different discharging current densities. Bioresour. Technol. 2017, 233, 1–6. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Naha, S.; Goswami, P. Bacterial community structure of electrogenic biofilm developed on modified graphite anode in microbial fuel cell. Sci. Rep. 2023, 13, 1255. [Google Scholar] [CrossRef]
- Khater, D.Z.; Amin, R.; Zhran, M.; Abd El-Aziz, Z.K.; Mahmoud, M.; Hassan, H.M.; El-Khatib, K. The enhancement of microbial fuel cell performance by anodic bacterial community adaptation and cathodic mixed nickel–copper oxides on a graphene electrocatalyst. J. Genet. Eng. Biotechnol. 2022, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Stratford, J.P.; Beecroft, N.J.; Slade, R.C.; Grüning, A.; Avignone-Rossa, C. Anodic microbial community diversity as a predictor of the power output of microbial fuel cells. Bioresour. Technol. 2014, 156, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Grüning, A.; Beecroft, N.J.; Avignone-Rossa, C. Low-potential respirators support electricity production in microbial fuel cells. Microb. Ecol. 2015, 70, 266–273. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A. Microbial community structure and its functional implications. Nature 2009, 459, 193–199. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Levy, R.; Borenstein, E. Emergent biosynthetic capacity in simple microbial communities. PLoS Comput. Biol. 2014, 10, e1003695. [Google Scholar] [CrossRef]
- Mahmoud, R.H.; Gomaa, O.M.; Hassan, R.Y. Bio-electrochemical frameworks governing microbial fuel cell performance: Technical bottlenecks and proposed solutions. RSC Adv. 2022, 12, 5749–5764. [Google Scholar] [CrossRef] [PubMed]
- Kaku, N.; Yonezawa, N.; Kodama, Y.; Watanabe, K. Plant/microbe cooperation for electricity generation in a rice paddy field. Appl. Microbiol. Biotechnol. 2008, 79, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Okita, N.; Iwama, E.; Naoi, K. Recent advances in supercapacitors: Ultrafast materials make innovations. Electrochemistry 2020, 88, 83–87. [Google Scholar] [CrossRef]
- Takanezawa, K.; Nishio, K.; Kato, S.; Hashimoto, K.; Watanabe, K. Factors affecting electric output from rice-paddy microbial fuel cells. Biosci. Biotechnol. Biochem. 2010, 74, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Jiang, Y.; Zhou, Y.; Shen, K.; Zhong, W. Using electrical signals of microbial fuel cells to detect copper stress on soil microorganisms. Eur. J. Soil Sci. 2015, 66, 369–377. [Google Scholar] [CrossRef]
- Jiang, Y.-B.; Deng, H.; Sun, D.-M.; Zhong, W.-H. Electrical signals generated by soil microorganisms in microbial fuel cells respond linearly to soil Cd2+ pollution. Geoderma 2015, 255, 35–41. [Google Scholar] [CrossRef]
- Deng, H.; Wu, Y.-C.; Zhang, F.; Huang, Z.-C.; Chen, Z.; Xu, H.-J.; Zhao, F. Factors affecting the performance of single-chamber soil microbial fuel cells for power generation. Pedosphere 2014, 24, 330–338. [Google Scholar] [CrossRef]
- Huang, D.-Y.; Zhou, S.-G.; Chen, Q.; Zhao, B.; Yuan, Y.; Zhuang, L. Enhanced anaerobic degradation of organic pollutants in a soil microbial fuel cell. Chem. Eng. J. 2011, 172, 647–653. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.; Zhou, Q.; Zhang, Z.; Chen, C. Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnol. Bioeng. 2012, 109, 426–433. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Eds.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Gee, G.W.; Or, D. Particle-size analysis in Methods of Soil Analysis: Part 4 Physical Methods; Dane, J.H., Topp, G.C., Eds.; Number 5 in the Soil Science Society of America Book Series; Soil Science Society of America, Inc.: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Hardy, F. The maximum water-retaining capacity of colloidal soils; the interpretation of this and of certain other soil moisture constants. J. Agric. Sci. 1923, 13, 340–351. [Google Scholar] [CrossRef]
- Tsuji, T. Studies on causes and measures for the growth disorders of early planting rice in the Northern District of Shiga Prefecture (Part 3). Transformation of sulfur compounds in paddy soils causing growth disorders in rice and their present status in soils. J. Jpn. Soc. Soil Sci. Plant Nutr. 2000, 71, 472–479. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- McKeague, J.; Day, J. Dithionite-and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci. 1966, 46, 13–22. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Wang, J.; Deng, H.; Wu, S.-S.; Deng, Y.-C.; Liu, L.; Han, C.; Jiang, Y.-B.; Zhong, W.-H. Assessment of abundance and diversity of exoelectrogenic bacteria in soil under different land use types. Catena 2019, 172, 572–580. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kaku, N.; Watanabe, K. Microbial electricity generation in rice paddy fields: Recent advances and perspectives in rhizosphere microbial fuel cells. Appl. Microbiol. Biotechnol. 2014, 98, 9521–9526. [Google Scholar] [CrossRef]
- Matsumoto, A.; Nagoya, M.; Tsuchiya, M.; Suga, K.; Inohana, Y.; Hirose, A.; Yamada, S.; Hirano, S.; Ito, Y.; Tanaka, S. Enhanced electricity generation in rice paddy-field microbial fuel cells supplemented with iron powders. Bioelectrochemistry 2020, 136, 107625. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory fe (iii) and mn (iv) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [PubMed]
- Barbato, R.A.; Foley, K.L.; Toro-Zapata, J.A.; Jones, R.M.; Reynolds, C.M. The power of soil microbes: Sustained power production in terrestrial microbial fuel cells under various temperature regimes. Agric. Ecosyst. Environ. Appl. Soil Ecol. 2017, 109, 14–22. [Google Scholar] [CrossRef]
- Schiessl, K.T.; Hu, F.; Jo, J.; Nazia, S.Z.; Wang, B.; Price-Whelan, A.; Min, W.; Dietrich, L.E. Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun. 2019, 10, 762. [Google Scholar] [CrossRef]
- Shrestha, N. Detecting Multicollinearity in Regression Analysis. Am. J. Appl. Math. Stat. 2020, 8, 39–42. [Google Scholar] [CrossRef]
- Fedorovich, V.; Knighton, M.C.; Pagaling, E.; Ward, F.B.; Free, A.; Goryanin, I. Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2009, 75, 7326–7334. [Google Scholar] [CrossRef] [PubMed]
- Damo, J.L.C.; Shimizu, T.; Sugiura, H.; Yamamoto, S.; Agake, S.-i.; Anarna, J.; Tanaka, H.; Sugihara, S.; Okazaki, S.; Yokoyama, T. The Application of Sulfur Influences Microbiome of Soybean Rhizosphere and Nutrient-Mobilizing Bacteria in Andosol. Microorganisms 2023, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Agyekum, D.V.; Kobayashi, T.; Dastogeer, K.M.; Yasuda, M.; Sarkodee-Addo, E.; Ratu, S.T.; Okazaki, S. Diversity and function of soybean rhizosphere microbiome under nature farming. Front. Microbiol. 2023, 14, 1130969. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Narsing Rao, M.P.; Shi, Y.; Lian, Z.-H.; Jin, P.-J.; Wang, W.; Li, Y.-M.; Wang, K.-K.; Banerjee, A. The shift of soil microbial community induced by cropping sequence affect soil properties and crop yield. Front. Microbiol. 2023, 14, 1095688. [Google Scholar] [CrossRef]
- Jiang, Y.-B.; Deng, H. Characterization of electricity generated by soil in microbial fuel cells and the isolation of soil source exoelectrogenic bacteria. Front. Microbiol. 2016, 7, 223047. [Google Scholar] [CrossRef]



| Soil | Crop | Fertilization of NPK | Location | Sand | Silt | Clay | Soil Moisture | MWHC |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | ||||
| Okinawa-1 | Sugarcane | Chemical | 25°57′7″ N, 31°17′42″ E | 0.7 | 19.7 | 79.6 | 22.0 | 88.4 |
| Okinawa-2 | Sugarcane | Chemical | 25°56′56″ N, 131°17′17″ E | 19.3 | 16.5 | 64.2 | 25.0 | 107.2 |
| Okinawa-3 | Sugarcane | Chemical | 25°56′40″ N, 131°17′21″ E | 2.9 | 15.6 | 81.5 | 23.7 | 85.9 |
| Okinawa-4 | Sugarcane | Chemical | 25°56′8″ N, 131°17′46″ E | 2.3 | 17.5 | 80.2 | 20.8 | 96.0 |
| Okinawa-5 | Sugarcane | Chemical | 25°57′6″ N, 131°19′27″ E | 2.5 | 17.2 | 80.3 | 23.2 | 96.7 |
| Kyoto | Soybean (Kurodaizu) | Compost and chemical | 35°1′3″ N, 135°34′14″ E | 50.2 | 32.8 | 17.0 | 14.4 | 79.0 |
| Akita | Rice (paddy) | Chemical | 40°1′4″ N, 139°57′35″ E | 43.8 | 26.7 | 29.5 | 41.2 | 184.5 |
| Hokkaido-1 | Rice (paddy) | Compost only | 43°00′48″ N, 141°23′28″ E | 42.0 | 38.4 | 19.5 | 38.5 | 155.5 |
| Hokkaido-2 | Rye | Compost and chemical | 43°5′27″ N, 141°20′27″ E | 52.4 | 34.1 | 13.5 | 20.9 | 81.4 |
| Fukushima-1 | Rice (paddy) | Compost only | 37°36′18″ N, 140°34′48″ E | 60.3 | 22.9 | 16.8 | 35.0 | 149.5 |
| Fukushima-2 | Vegetable | Compost only | 37°45′1″ N, 40°23′25″ E | 45.3 | 33.0 | 21.7 | 35.6 | 100.3 |
| Tokyo-1 | Rice (paddy) | Compost and chemical | 35°39′57″ N, 139°28′5″ E | 31.5 | 39.5 | 29.1 | 32.0 | 117.9 |
| Tokyo-2 | Vegetable | Compost and chemical | 35°39′57″ N, 139°28′35″ E | 26.4 | 42.2 | 31.4 | 38.2 | 154.1 |
| Nagano-1 | Vegetable | Natural Farming without chemical fertilizer nor chemical herbicide | 36°11′43″ N, 137°51′3″ E | 53.6 | 29.7 | 16.7 | 20.6 | 90.9 |
| Nagano-2 | Peach (orchard) | Chemical | 36°45′11″ N, 138°23′10″ E | 45.7 | 34.7 | 19.6 | 21.0 | 86.3 |
| Nagano-3 | Apple (orchard) | Chemical | 36°45′8″ N, 138°22′59″ E | 41.7 | 34.4 | 23.9 | 23.8 | 94.8 |
| Nagano-4 | Rice (paddy) | Chemical | 36°45′08.4” N 138°22′58” E | 43.1 | 37.4 | 19.6 | 19.0 | 94.5 |
| Nagano-5 | Rice (paddy) | Natural Farming without chemical fertilizer nor chemical herbicide | 36°11′43″ N, 137°51′3″ E | 42.9 | 38.1 | 19.0 | 31.6 | 119.9 |
| Soil | pH Before | EC (mS/m) Before | TC (g/kg) Before | TN (g/kg) Before | AvS (mg/kg) Before | DFeT (g/kg) Before | DFeT (g/kg) After | DOC (mg/kg) Before | DOC (mg/kg) After | DON (mg/kg) Before | DON (mg/kg) After | MBC (mg/kg) Before | MBC (mg/kg) After |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Okinawa-1 | 5.2 | 8.9 | 13 | 2 | 39 | 1 | 2 | 306 | 226 | 55 | 67 | 93 | 100 |
| Okinawa-2 | 7.7 | 13.8 | 14 | 2 | 17 | 3 | 3 | 117 | 89 | 34 | 26 | 95 | 66 |
| Okinawa-3 | 5.7 | 13.8 | 18 | 2 | 49 | 2 | 3 | 202 | 165 | 45 | 102 | 158 | 7 |
| Okinawa-4 | 6.3 | 31.1 | 11 | 2 | 78 | 1 | 2 | 156 | 129 | 33 | 27 | 23 | 8 |
| Okinawa-5 | 6.3 | 63.5 | 16 | 2 | 356 | 1 | 2 | 168 | 151 | 319 | 104 | 54 | 42 |
| Kyoto | 6.3 | 11.3 | 15 | 1 | 14 | 2 | 2 | 60 | 56 | 53 | 63 | 106 | 64 |
| Akita | 7.3 | 21.6 | 24 | 3 | 88 | 3 | 3 | 56 | 61 | 41 | 75 | 51 | 133 |
| Hokkaido-1 | 5.9 | 33.9 | 68 | 6 | 101 | 4 | 4 | 106 | 103 | 161 | 143 | 316 | 358 |
| Hokkaido-2 | 6.7 | 5.2 | 26 | 2 | 14 | 5 | 5 | 64 | 70 | 19 | 51 | 46 | 44 |
| Fukushima-1 | 6.2 | 27.9 | 24 | 2 | 91 | 2 | 2 | 75 | 67 | 104 | 65 | 300 | 232 |
| Fukushima-2 | 6.3 | 54.3 | 42 | 5 | 138 | 3 | 4 | 201 | 154 | 237 | 92 | 241 | 145 |
| Tokyo-1 | 5.9 | 31.0 | 38 | 4 | 92 | 4 | 3 | 87 | 134 | 140 | 112 | 257 | 236 |
| Tokyo-2 | 6.1 | 25.2 | 41 | 4 | 89 | 4 | 3 | 152 | 88 | 126 | 115 | 681 | 463 |
| Nagano-1 | 6.8 | 19.4 | 47 | 4 | 74 | 5 | 5 | 112 | 109 | 69 | 168 | 412 | 190 |
| Nagano-2 | 6.0 | 16.0 | 36 | 3 | 17 | 5 | 5 | 110 | 82 | 72 | 184 | 252 | 175 |
| Nagano-3 | 5.6 | 11.1 | 46 | 4 | 20 | 4 | 4 | 104 | 119 | 55 | 117 | 232 | 41 |
| Nagano-4 | 5.8 | 14.6 | 28 | 3 | 26 | 2 | 3 | 66 | 65 | 43 | 102 | 1085 | 1264 |
| Nagano-5 | 5.6 | 31.7 | 52 | 5 | 18 | 4 | 2 | 141 | 122 | 188 | 62 | 292 | 442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Z.; Yuan, K.; Seki, M.; Agake, S.-I.; Matsumura, K.; Okita, N.; Naoi, W.; Naoi, K.; Toyota, K.; Tanaka, H.; et al. Comparative Analysis of Japanese Soils: Exploring Power Generation Capability in Relation to Bacterial Communities. Sustainability 2024, 16, 4625. https://doi.org/10.3390/su16114625
Yue Z, Yuan K, Seki M, Agake S-I, Matsumura K, Okita N, Naoi W, Naoi K, Toyota K, Tanaka H, et al. Comparative Analysis of Japanese Soils: Exploring Power Generation Capability in Relation to Bacterial Communities. Sustainability. 2024; 16(11):4625. https://doi.org/10.3390/su16114625
Chicago/Turabian StyleYue, Zihan, Kun Yuan, Mayuko Seki, Shin-Ichiro Agake, Keisuke Matsumura, Naohisa Okita, Wako Naoi, Katsuhiko Naoi, Koki Toyota, Haruo Tanaka, and et al. 2024. "Comparative Analysis of Japanese Soils: Exploring Power Generation Capability in Relation to Bacterial Communities" Sustainability 16, no. 11: 4625. https://doi.org/10.3390/su16114625
APA StyleYue, Z., Yuan, K., Seki, M., Agake, S.-I., Matsumura, K., Okita, N., Naoi, W., Naoi, K., Toyota, K., Tanaka, H., Sugihara, S., Yasuda, M., & Ohkama-Ohtsu, N. (2024). Comparative Analysis of Japanese Soils: Exploring Power Generation Capability in Relation to Bacterial Communities. Sustainability, 16(11), 4625. https://doi.org/10.3390/su16114625







