Unlocking the Potential of Plant Growth-Promoting Rhizobacteria to Enhance Drought Tolerance in Egyptian Wheat (Triticum aestivum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Screening of PGPR
2.2. Evaluation of Plant Growth-Promoting (PGP) Characteristics
2.2.1. Production of Indole Acetic Acid (IAA)
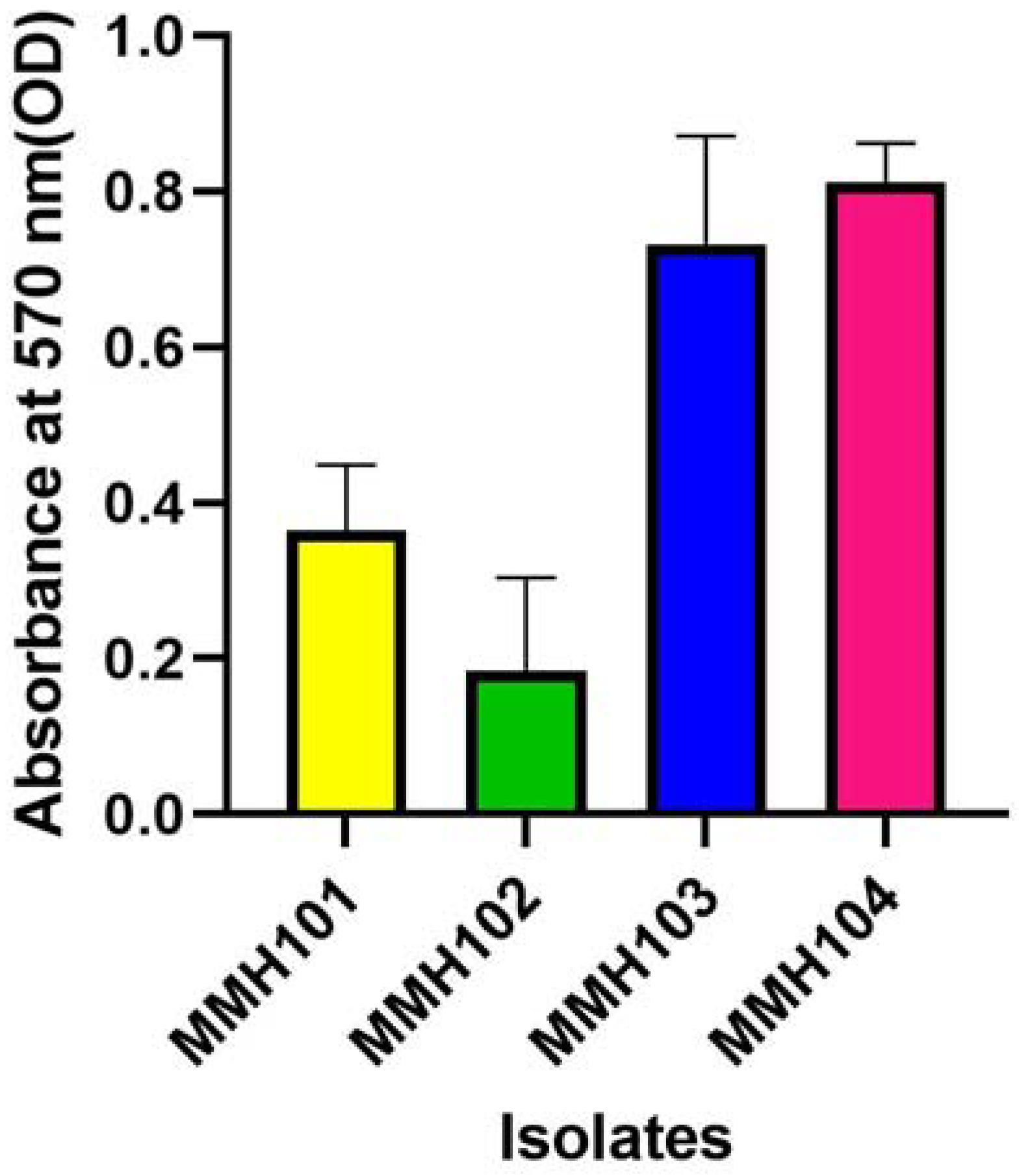
2.2.2. Biofilm Production
2.2.3. Production of Siderophores
2.2.4. ACC Deaminase Activity
2.2.5. Phosphate Solubilization
2.2.6. Nitrogen Fixation
2.2.7. Antagonism Assay
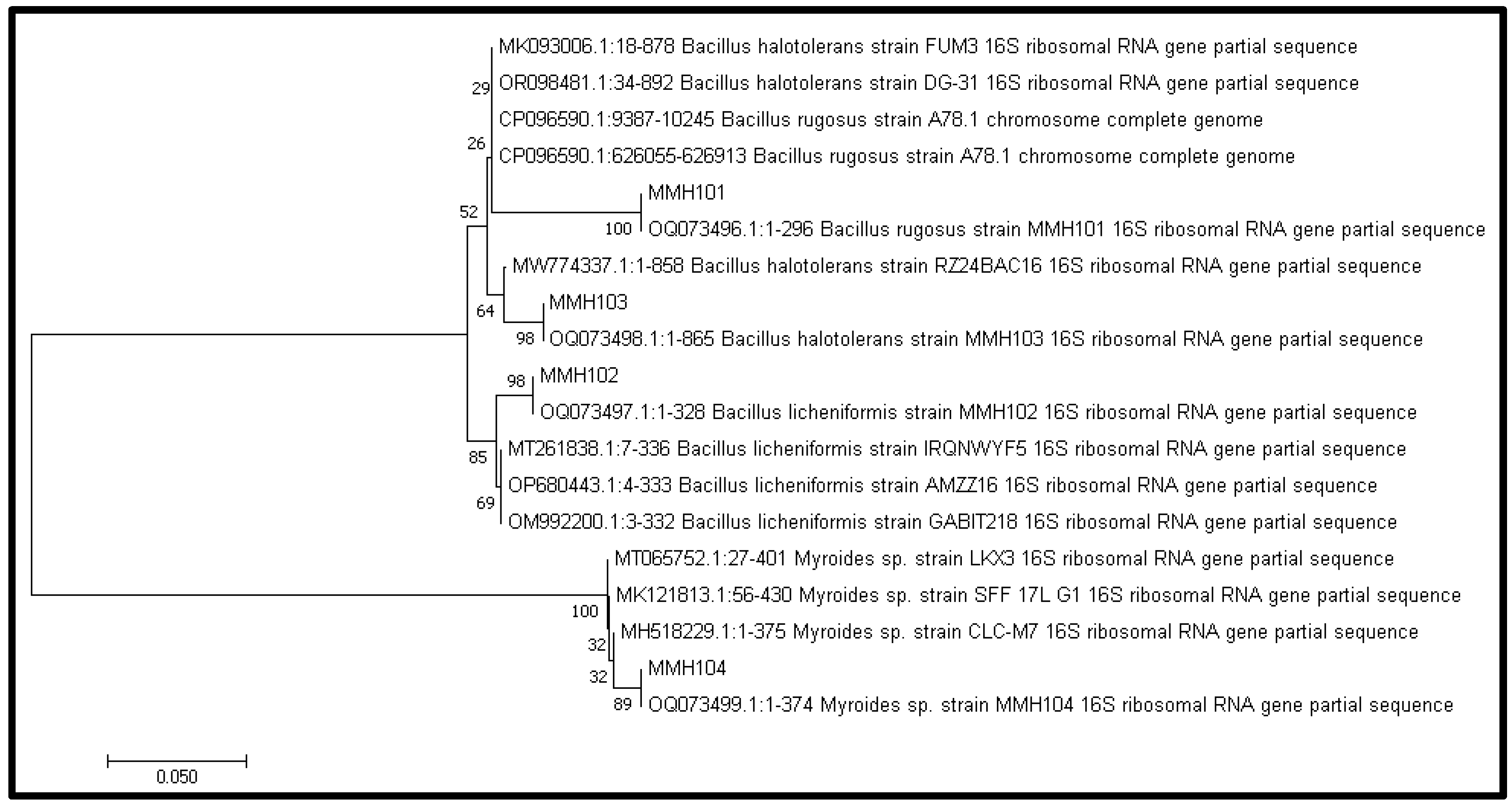
2.3. 16S rDNA Gene Analysis of Bacterial Isolates
2.4. Pot Experiment and Drought Treatment
2.5. Evaluating the Impact of PGPR on Wheat Drought Tolerance
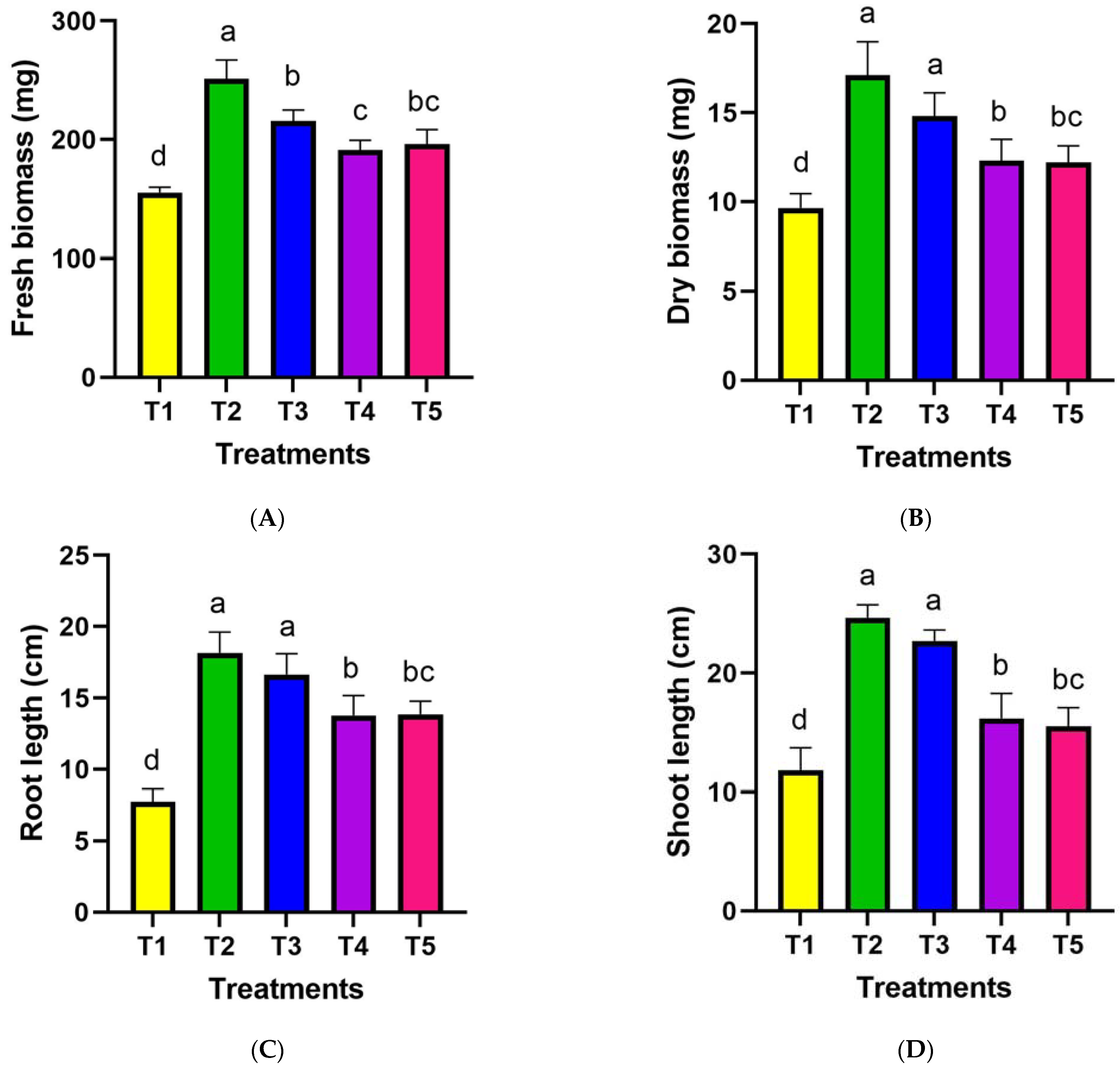
2.5.1. Morphological Traits Measurement
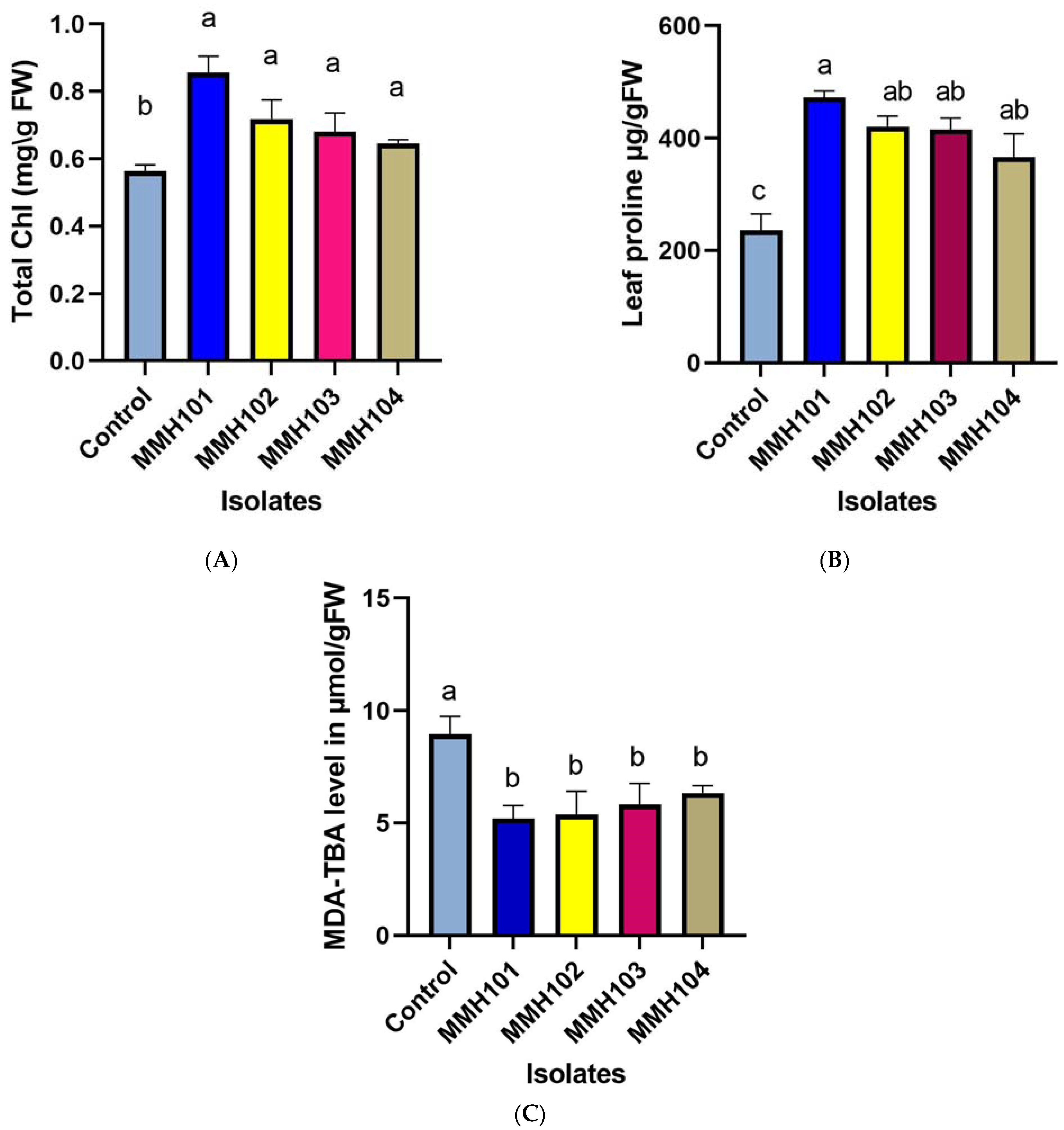
2.5.2. Chlorophyll Content
- Chlorophyll a (mg/g FW) = [{(12.7 × OD663) − (2.69 × OD645)} × V × 1000 × W]
- Chlorophyll b (mg/g FW) = [{(22.9 × OD645) − (4.68 × OD663)} × V × 1000 × W]
2.5.3. Proline
2.5.4. Malondialdehyde (MDA) Content
2.6. Statistical Analysis
3. Results
3.1. Isolation and Identification of Bacteria
3.2. Qualitative Evaluation of Plant Growth-Regulating Traits
3.3. Quantitative Evaluation of Plant Growth-Regulating Traits
3.4. Molecular Identification of PGPR Isolates
3.5. Assessment of Inoculated Wheat Morphological Traits under Drought Stress
3.6. Assessment of Inoculated Wheat Physiological Traits under Drought Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byregowda, R.; Prasad, S.R.; Oelmüller, R.; Nataraja, K.N.; Prasanna Kumar, M.K. Is endophytic colonization of host plants a method of alleviating drought stress? Conceptualizing the hidden world of endophytes. Int. J. Mol. Sci. 2022, 23, 9194. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef]
- Omar, S.A.; Fetyan, N.A.; Eldenary, M.E.; Abdelfattah, M.H.; Abd-Elhalim, H.M.; Wrobel, J.; Kalaji, H.M. Alteration in expression level of some growth and stress-related genes after rhizobacteria inoculation to alleviate drought tolerance in sensitive rice genotype. Chem. Biol. Technol. Agric. 2021, 8, 41. [Google Scholar] [CrossRef]
- Bueno-Ramos, N.; González-Hernández, A.I.; Marcos-Barbero, E.L.; Miranda-Apodaca, J.; Bendou, O.; Gutiérrez-Fernández, I.; Arellano, J.B.; Morcuende, R. Impact of Water Deficit on Primary Metabolism at the Whole Plant Level in Bread Wheat Grown under Elevated CO2 and High Temperature at Different Developmental Stages. Chem. Proc. 2022, 10, 6. [Google Scholar]
- Ferioun, M.; Srhiouar, N.; Tirry, N.; Belahcen, D.; Siang, T.C.; Louahlia, S.; El Ghachtouli, N. Optimized drought tolerance in barley (Hordeum vulgare L.) using plant growth-promoting rhizobacteria (PGPR). Biocatal. Agric. Biotechnol. 2023, 50, 102691. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) with biofilm-forming ability: A multifaceted agent for sustainable agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Wang, S.; Ouyang, L.; Ju, X.; Zhang, L.; Zhang, Q.; Li, Y. Survey of plant drought-resistance promoting bacteria from Populus euphratica tree living in arid area. Indian J. Microbiol. 2014, 54, 419–426. [Google Scholar] [CrossRef]
- Khan, M.R.; Ahmad, F.; Shah, A.A.; Ali, S.M. Role of plant growth-promoting rhizobacteria (PGPR) in the biocontrol of plant diseases. In Plant Growth-Promoting Rhizobacteria (PGPR) for Sustainable Agriculture; Springer: Cham, Switzerland, 2014; pp. 195–221. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Mokhtar, M.M.; Abd-Elhalim, H.M.; El Allali, A. A large-scale assessment of the quality of plant genome assemblies using the LTR assembly index. AoB Plants 2023, 15, plad015. [Google Scholar] [CrossRef]
- Abd El-Halim, H.M.; Ismail, I.M.; Al Aboud, N.M.; Elghareeb, D.; Metry, E.A.; Hossien, A.F.; Fahmy, E.M. Evaluation of two promoters for generating transgenic potato plants as salicylic acid biosensors. Biol. Plant. 2020, 64, 535–540. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Synergistic effects of biofilm-producing PGPR strains on wheat plant colonization, growth and soil resilience under drought stress. Saudi J. Biol. Sci. 2023, 30, 103664. [Google Scholar] [CrossRef]
- Furlan, F.; Saatkamp, K.; Volpiano, C.G.; Franco, F.D.A.; dos Santos, M.F.; Vendruscolo, E.C.G.; Guimarães, V.F.; da Costa, A.C.T. Plant growth-promoting bacteria effect in withstanding drought in wheat cultivars. Sci. Agrar. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Ramasamy, K.P.; Mahawar, L. Coping with salt stress-interaction of halotolerant bacteria in crop plants: A mini review. Front. Microbiol. 2023, 14, 1077561. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Samant, M. Plant-beneficial Bacillus, Pseudomonas, and Staphylococcus spp. from Kumaon Himalayas and their drought tolerance response. Front. Sustain. Food Syst. 2023, 7, 1085223. [Google Scholar] [CrossRef]
- Gusmiaty Restu, M.; Bachtiar, B.; Larekeng, S.H. Gibberellin and IAA production by rhizobacteria from various private forest. IOP Conf. Ser. Earth Environ. Sci. 2019, 270, 012018. [Google Scholar] [CrossRef]
- Melo, L.D.; Ferreira, R.; Costa, A.R.; Oliveira, H.; Azeredo, J. Efficacy and safety assessment of two enterococci phages in an in vitro biofilm wound model. Sci. Rep. 2019, 9, 6643. [Google Scholar] [CrossRef]
- Sultana, S.; Alam, S.; Karim, M.M. Screening of siderophore-producing salt-tolerant rhizobacteria suitable for supporting plant growth in saline soils with iron limitation. J. Agric. Food Res. 2021, 4, 100150. [Google Scholar] [CrossRef]
- Dworkin, M.; Foster, J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef]
- Penrose, D.M.; Moffatt, B.A.; Glick, B.R. Determination of 1-aminocycopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can. J. Microbiol. 2001, 47, 77–80. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologiya 1948, 17, 362–370. [Google Scholar]
- Khoury, C.K.; Heider, B.; Castañeda-Álvarez, N.P.; Achicanoy, H.A.; Sosa, C.C.; Miller, R.E.; Scotland, R.W.; Wood, J.R.; Rossel, G.; Eserman, L.A.; et al. Distributions, ex situ conservation priorities, and genetic resource potential of crop wild relatives of sweetpotato [Ipomoea batatas (L.) Lam., I. series Batatas]. Front. Plant Sci. 2015, 6, 251. [Google Scholar] [CrossRef]
- Calvo, P.; Ormeño-Orrillo, E.; Martínez-Romero, E.; Zúñiga, D. Characterization of Bacillus isolates of potato rhizosphere from andean soils of Peru and their potential PGPR characteristics. Braz. J. Microbiol. 2010, 41, 899–906. [Google Scholar] [CrossRef]
- Abdelatty, A.; Mandouh, M.; Mohamed, S.; Busato, S.; Badr, O.; Bionaz, M.; Elolimy, A.; Moustafa, M.; Farid, O.; Al-Mokaddem, A. Azolla leaf meal at 5% of the diet improves growth performance, intestinal morphology and p70S6K1 activation, and affects cecal microbiota in broiler chicken. Animal 2021, 15, 100362. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Khan, M.Y.; Nadeem, S.M.; Sohaib, M.; Waqas, M.R.; Alotaibi, F.; Ali, L.; Zahir, Z.A.; Al-Barakah, F.N.I. Potential of plant growth promoting bacterial consortium for improving the growth and yield of wheat under saline conditions. Front. Microbiol. 2022, 13, 958522. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Plant-Microbe Interactions; Springer: New York, NY, USA, 2021. [Google Scholar]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; de-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum sp. B515CD-1 improves plant resistance to abiotic stress. World J. Microbiol. Biotechnol. 2010, 26, 719–733. [Google Scholar]
- Singh, R.P.; Yadav, V.B.; Yadav, N.S.; Dhyani, D. Plant-growth-promoting rhizobacteria: A potential tool for sustainable agriculture in drought-prone areas. Front. Microbiol. 2021, 12, 739395. [Google Scholar]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Yavuz, D.; Baştaş, K.K.; Seymen, M.; Yavuz, N.; Kurtar, E.S.; Süheri, S.; Türkmen, Ö.; Gür, A.; Kıymacı, G. Role of ACC deaminase-producing rhizobacteria in alleviation of water stress in watermelon. Sci. Hortic. 2023, 321, 112288. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]
- Santoyo, G.; Equihua, A.; Flores, A.; Sepulveda, E.; Valencia-Cantero, E.; Sanchez-Yañez, J.M.; Morales, L.R.; Govindappa, M.; de los Santos-Villalobos, S. Plant growth promotion by ACC deaminase-producing bacilli under salt stress conditions. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Springer: Cham, Switzerland, 2019; Volume 2, pp. 81–95. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Wang, L.; Yue, Y.; Wang, L.; Yang, X. The effects of plant growth-promoting rhizobacteria on plants under temperature stress: A meta-analysis. Rhizosphere 2023, 28, 100788. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Ashraf, M. Microbial ACC-deaminase: Prospects and applications for inducing salt tolerance in plants. Crit. Rev. Plant Sci. 2010, 29, 360–393. [Google Scholar] [CrossRef]
- Gholami, A.; Shahsavani, D.; Nezarat, S.; Shafiee, R. The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Res. J. Biol. Sci. 2009, 4, 670–677. [Google Scholar]
- Bharti, N.; Barnawal, D.; Awasthi, A.; Kalra, A. Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Jatropha curcas L. Bioresour. Technol. 2016, 215, 155–164. [Google Scholar]
- Ahanger, M.A.; Agarwal, R.M.; Tomar, N.S. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 2014, 252, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Yuwono, T.; Handayani, D.; Soedarsono, J. The potential of consortium Bacillus sp. as biofertilizer to increase nutrients uptake and yield of wheat (Triticum aestivum L.) under drought stress. Sustainability 2020, 12, 7952. [Google Scholar]
- Armada, E.; Probanza, A.; Roldán, A.; Azcón, R. Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J. Plant Physiol. 2015, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol. Biochem. 2014, 81, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gusain, Y.S.; Singh, U.S.; Sharma, A.K. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr. J. Biotechnol. 2015, 14, 918–928. [Google Scholar]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Come, D. Free radical scavenging as affected by accelerated aging and subsequent priming in sunflower seeds. Physiol. Plant. 2001, 111, 223–231. [Google Scholar]
- Barnawal, D.; Ojha, A.; Singh, R.; Garg, S.K. Combinatorial inoculation of plant growth-promoting bacteria and earthworms in the restoration of mine spoil: A field experiment. Ecol. Eng. 2017, 98, 183–191. [Google Scholar]
- Yadav, K.; Singh, J.; Giri, S.; Singh, G.; Tiwari, K.L.; Singh, K. Bio-priming with plant growth promoting rhizobacteria isolates ameliorates negative impact of drought in wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol. 2020, 28, 101703. [Google Scholar]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef] [PubMed]
- Mrkovacki, N.; Milic, V.; Bjelic, D.; Marinkovic, J. Quantitative effects of Zea mays L. inoculation with Bacillus amyloliquefaciens FZB42 on microbial community structure in rhizosphere soil under field conditions. Eur. J. Soil Biol. 2014, 60, 15–19. [Google Scholar]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.Y.; Lee, Y.H.; Cho, B.H.; Yang, K.Y.; Ryu, C.M. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef]


| PGPR Isolates | MMH 101 | MMH 102 | MMH 103 | MMH 104 |
|---|---|---|---|---|
| Siderophore | ++ | ++ | + | ++ |
| Nitrogen fixation | +++ | + | +++ | + |
| ACC deaminase | ++ | + | ++ | + |
| Phosphate solubilization | - | - | - | - |
| Antagonism | + | - | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, M.A.; Ismail, M.A.; Radwan, K.H.; Abd-Elhalim, H.M. Unlocking the Potential of Plant Growth-Promoting Rhizobacteria to Enhance Drought Tolerance in Egyptian Wheat (Triticum aestivum). Sustainability 2024, 16, 4605. https://doi.org/10.3390/su16114605
Salem MA, Ismail MA, Radwan KH, Abd-Elhalim HM. Unlocking the Potential of Plant Growth-Promoting Rhizobacteria to Enhance Drought Tolerance in Egyptian Wheat (Triticum aestivum). Sustainability. 2024; 16(11):4605. https://doi.org/10.3390/su16114605
Chicago/Turabian StyleSalem, Mahmoud A., Menattallah A. Ismail, Khaled H. Radwan, and Haytham M. Abd-Elhalim. 2024. "Unlocking the Potential of Plant Growth-Promoting Rhizobacteria to Enhance Drought Tolerance in Egyptian Wheat (Triticum aestivum)" Sustainability 16, no. 11: 4605. https://doi.org/10.3390/su16114605
APA StyleSalem, M. A., Ismail, M. A., Radwan, K. H., & Abd-Elhalim, H. M. (2024). Unlocking the Potential of Plant Growth-Promoting Rhizobacteria to Enhance Drought Tolerance in Egyptian Wheat (Triticum aestivum). Sustainability, 16(11), 4605. https://doi.org/10.3390/su16114605






