Use of Continuous Stirred Tank Reactors for Anaerobic Co-Digestion of Dairy and Meat Industry By-Products for Biogas Production
Abstract
1. Introduction
2. Materials and Methods
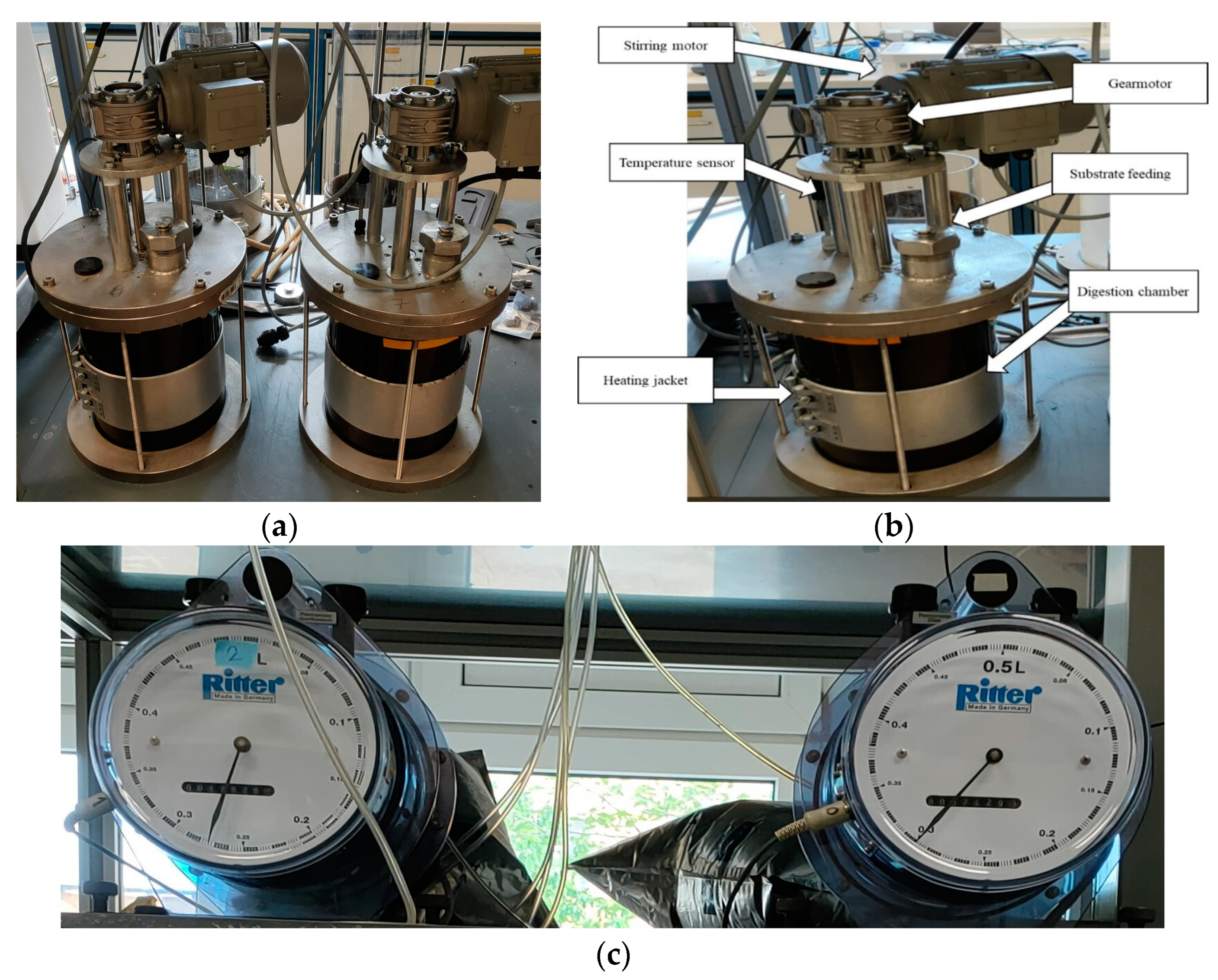
2.1. The CSTR Reactors
2.2. Experimental Set-Up
2.3. Physical–Chemical Characterisation of the Used Matrices
2.3.1. pH
2.3.2. Total Solids and Volatile Total Solids
2.3.3. Ammonium Nitrogen and Total Kjeldahl Nitrogen Determination
2.3.4. Chemical Oxygen Demand (COD) Determination
2.3.5. Determination of Total Volatile Fatty Acids (VFA) by HPLC
2.4. Data Analysis
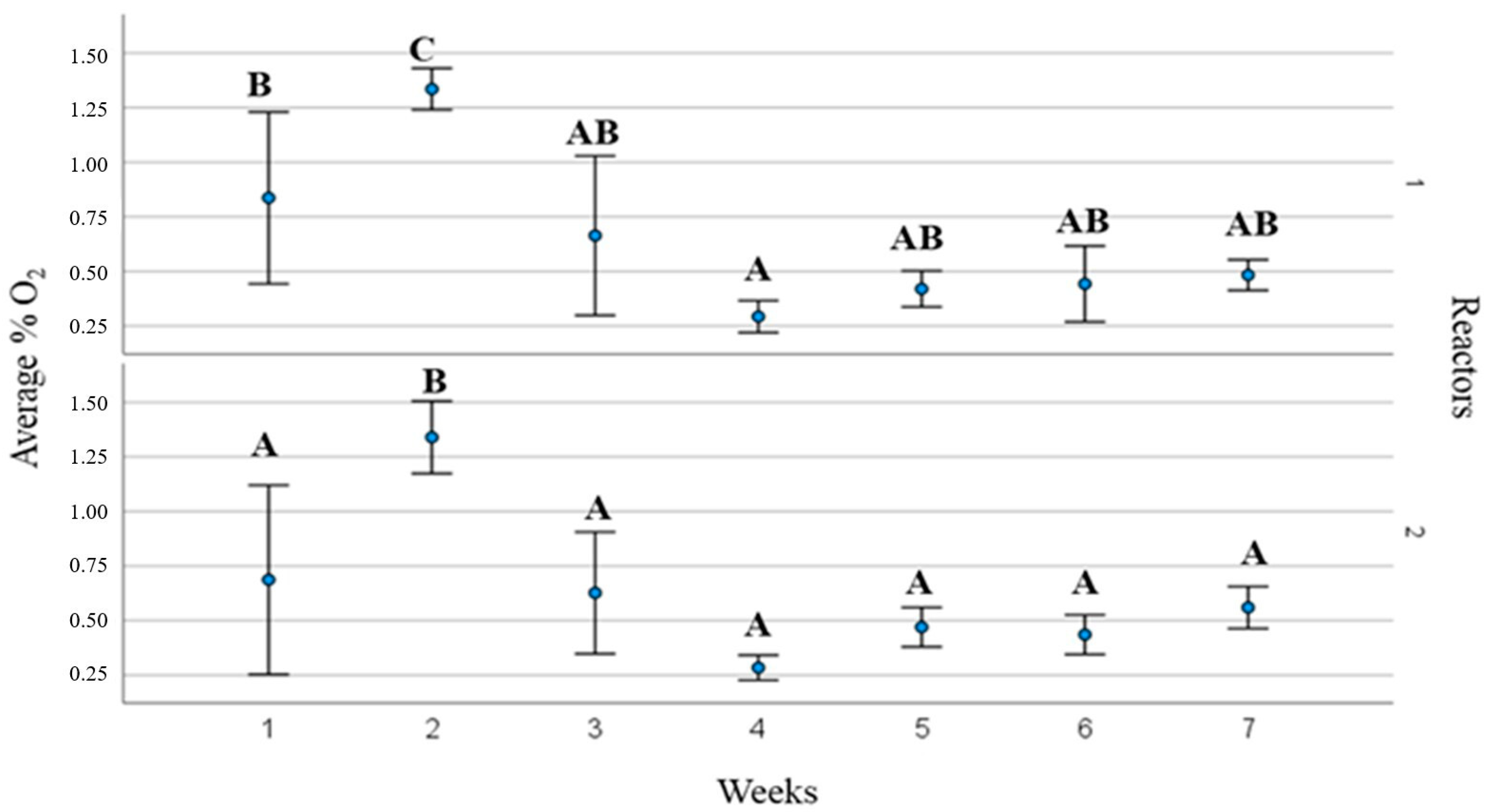
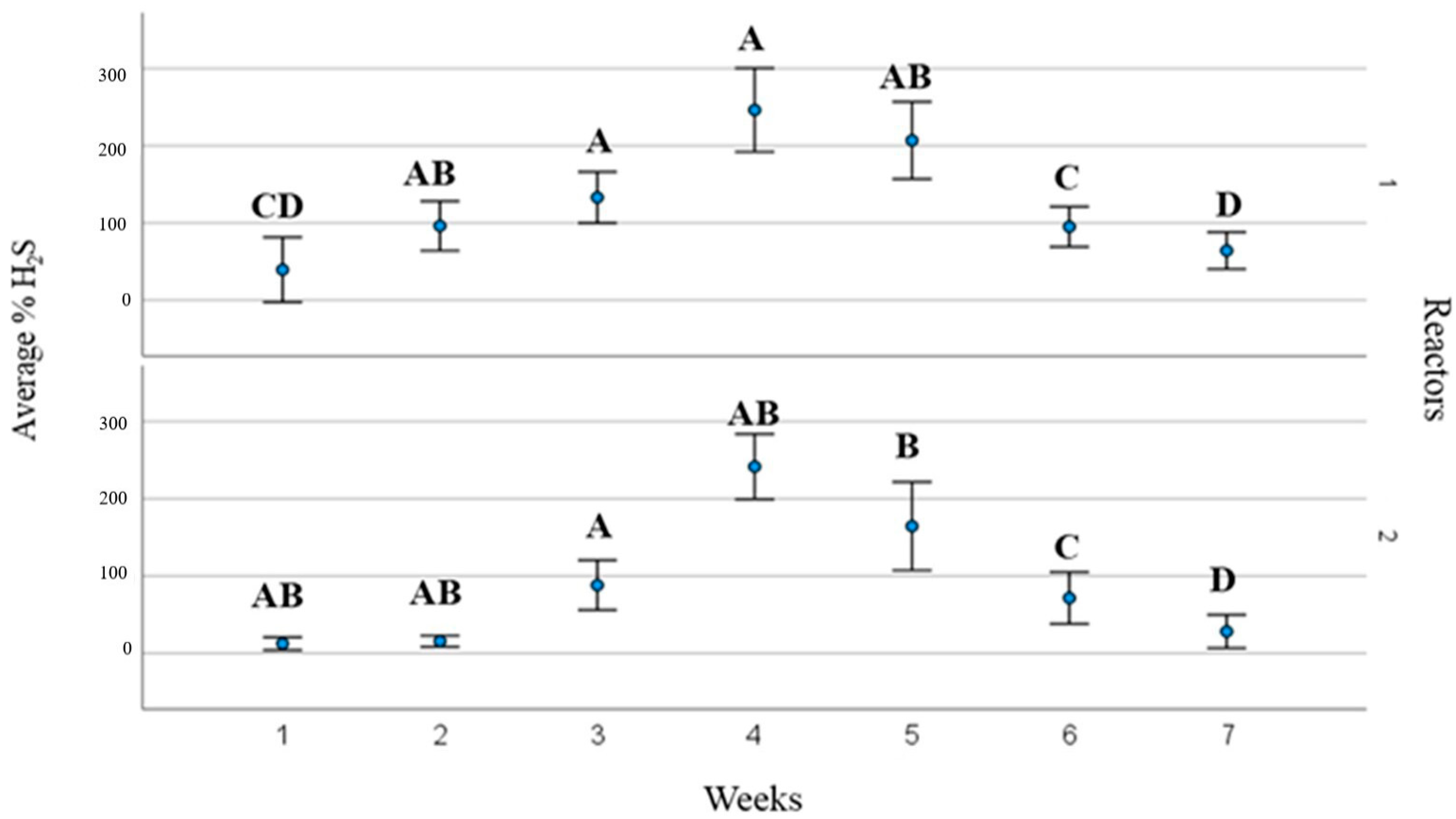
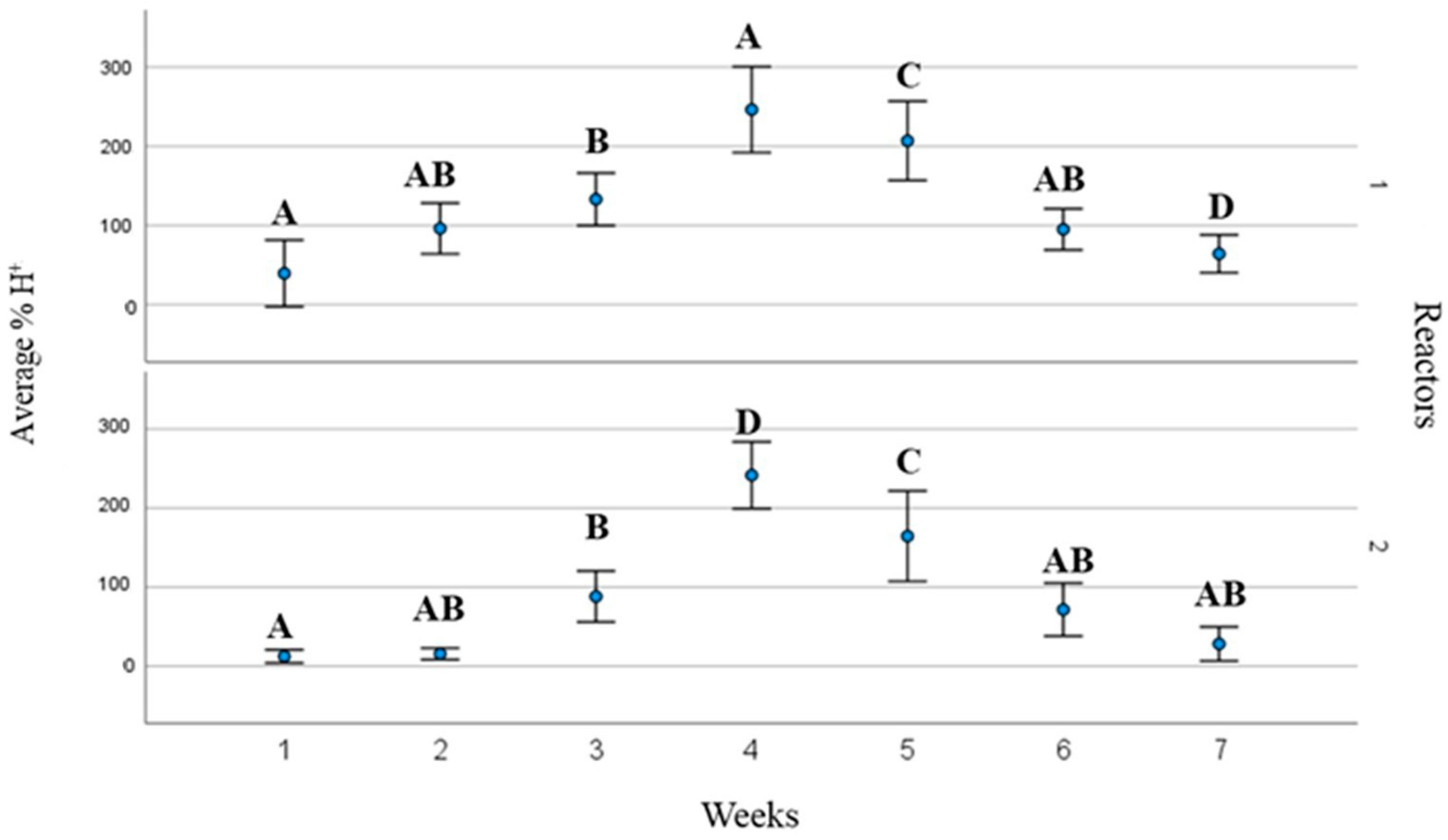
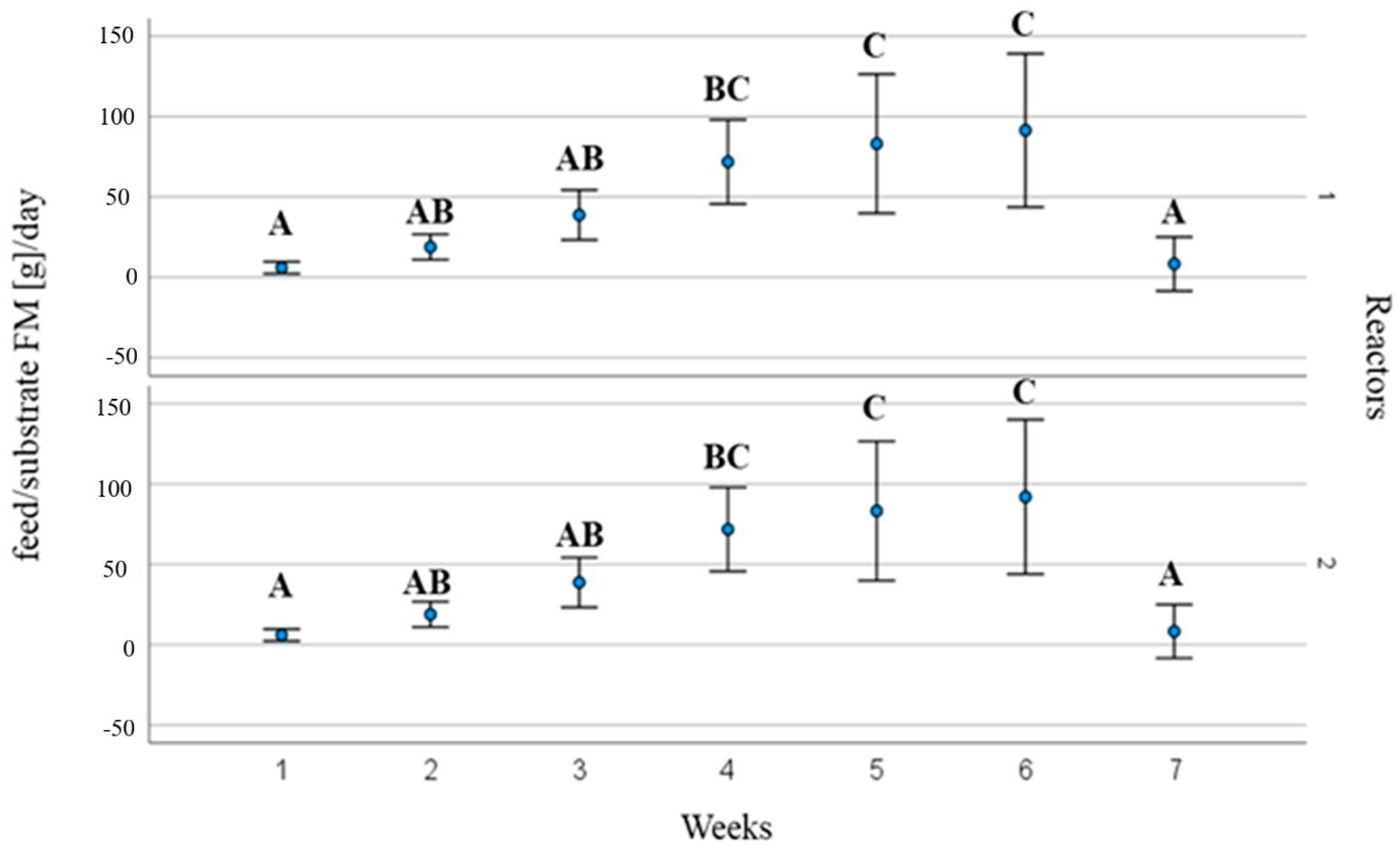
3. Results and Discussions
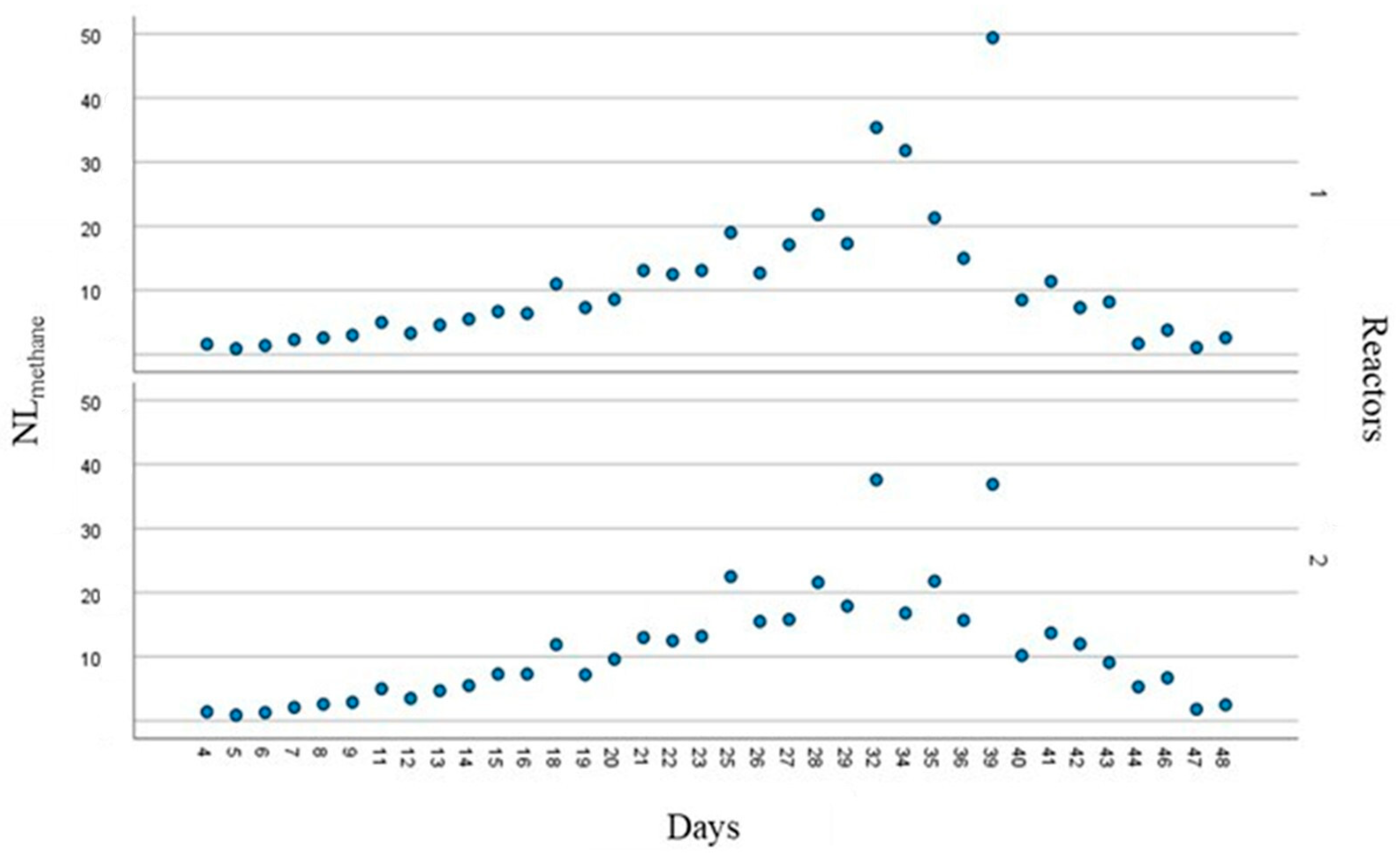
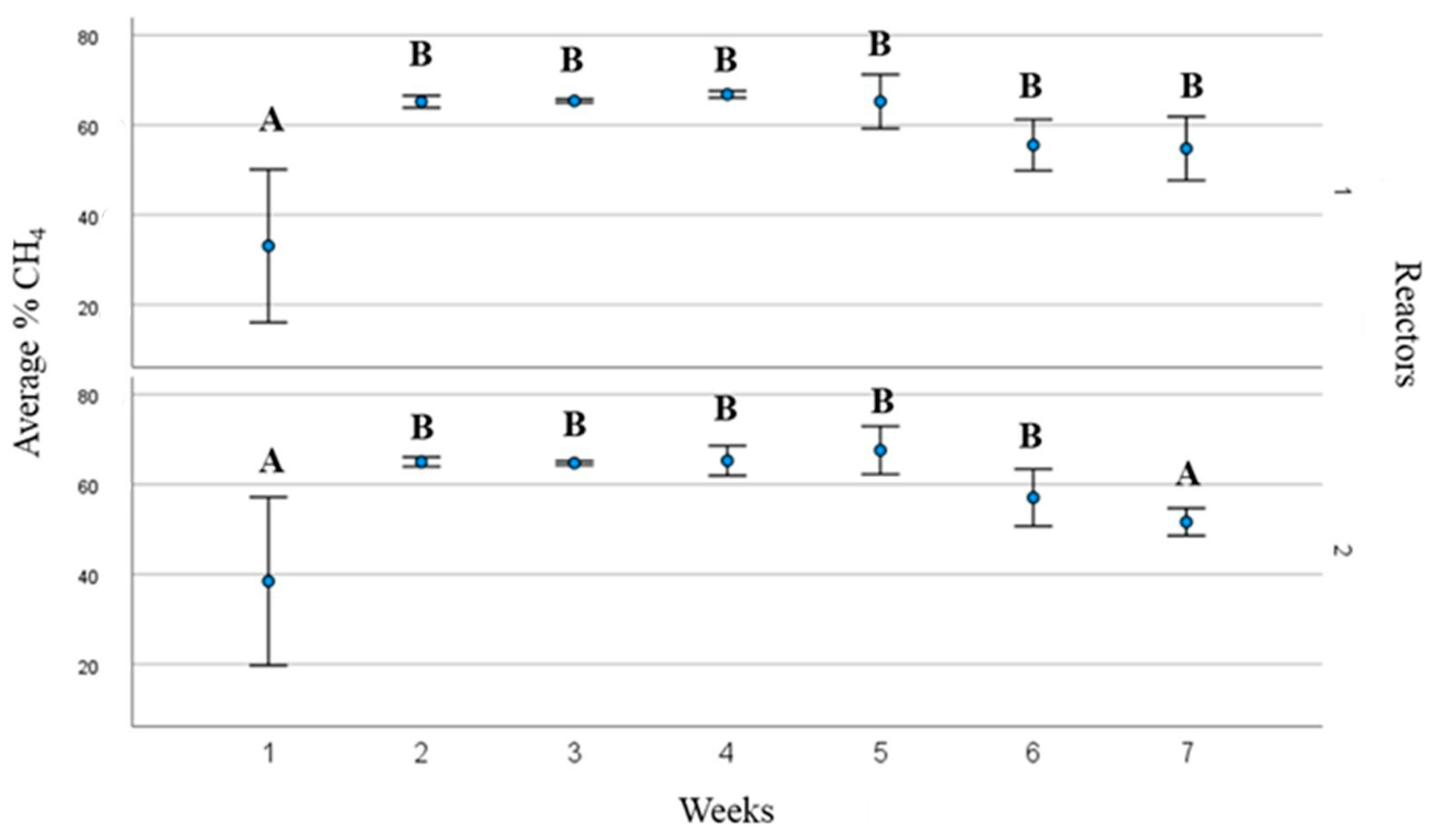
3.1. Biogas and Biomethane Production
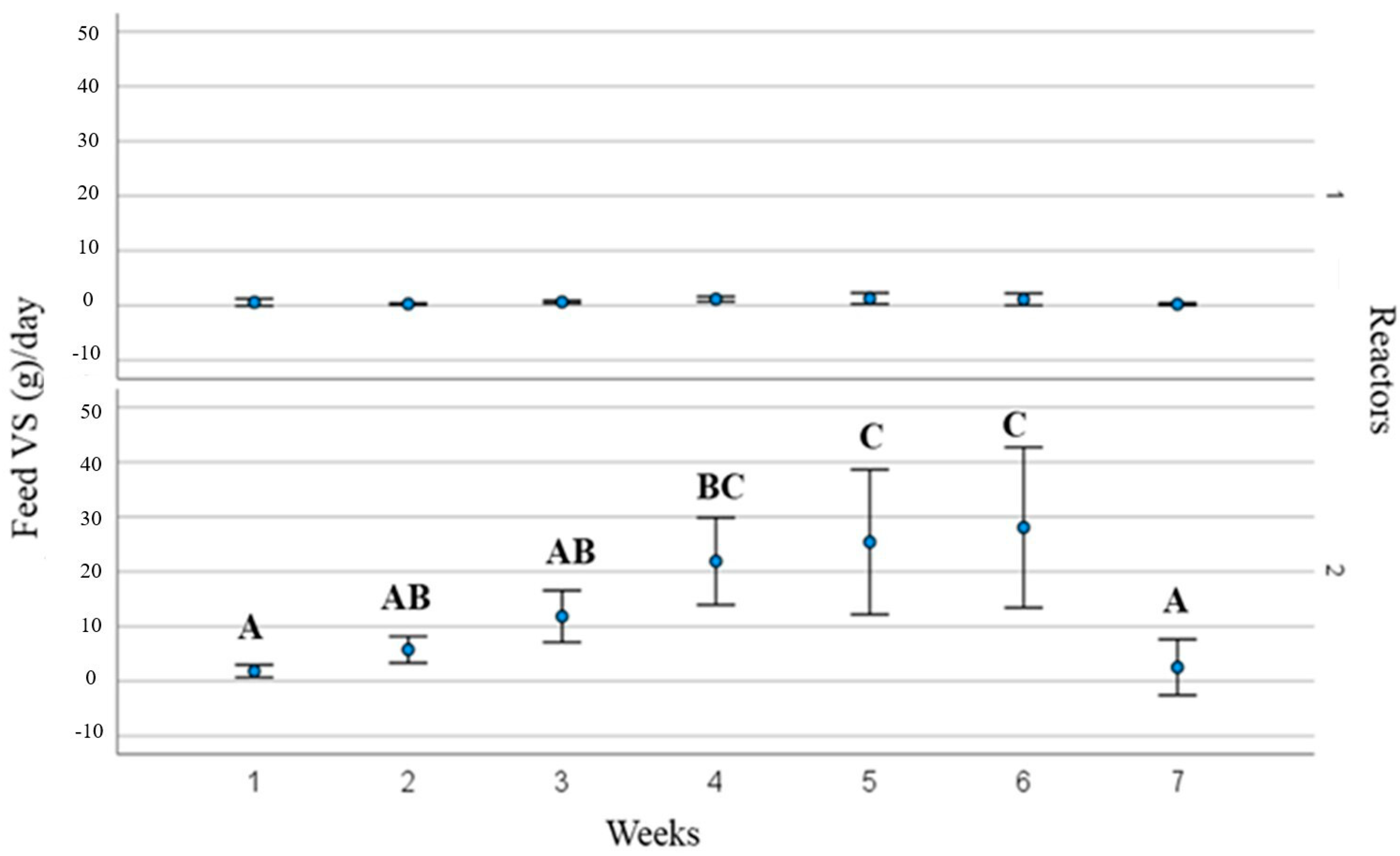
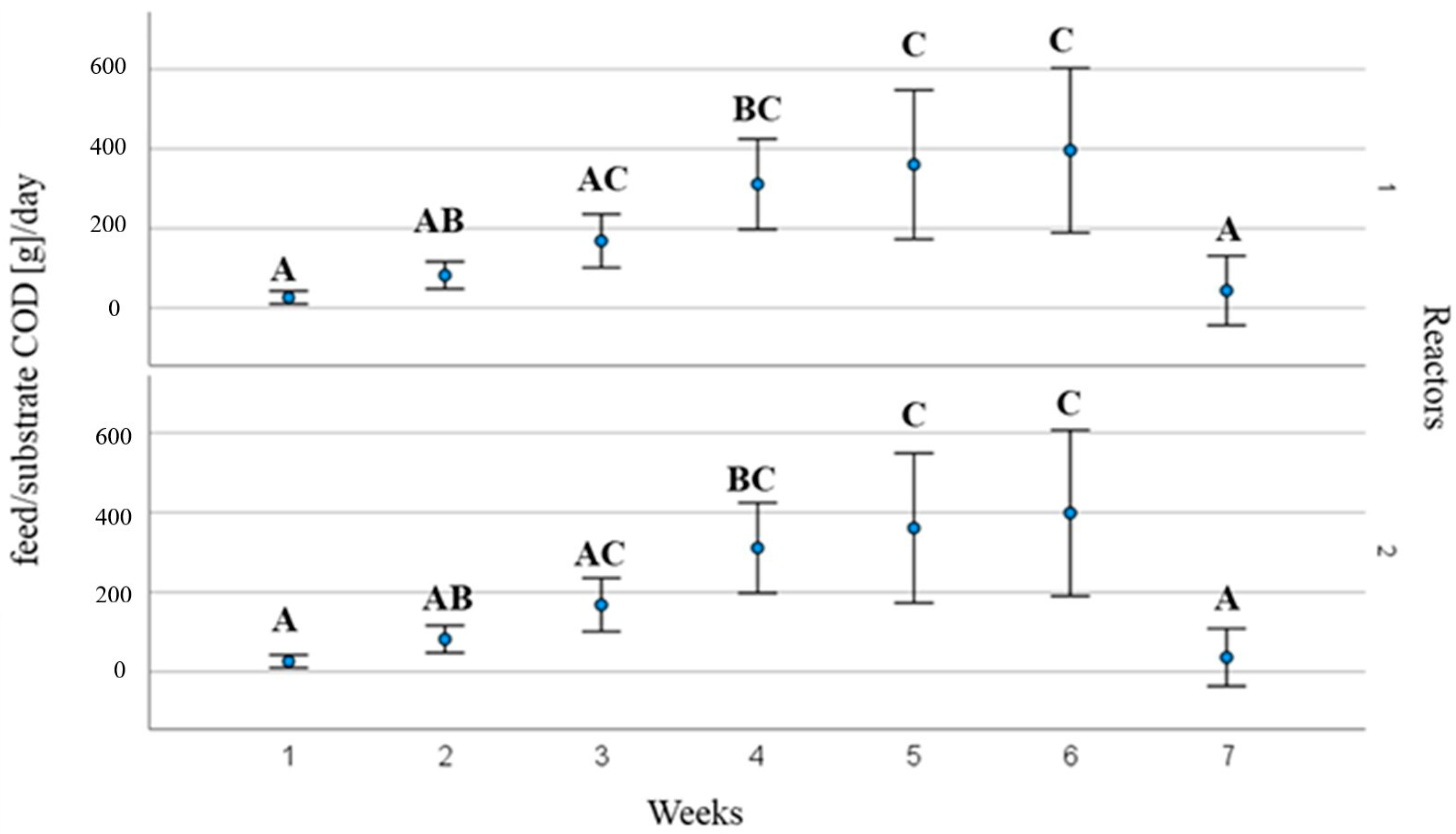
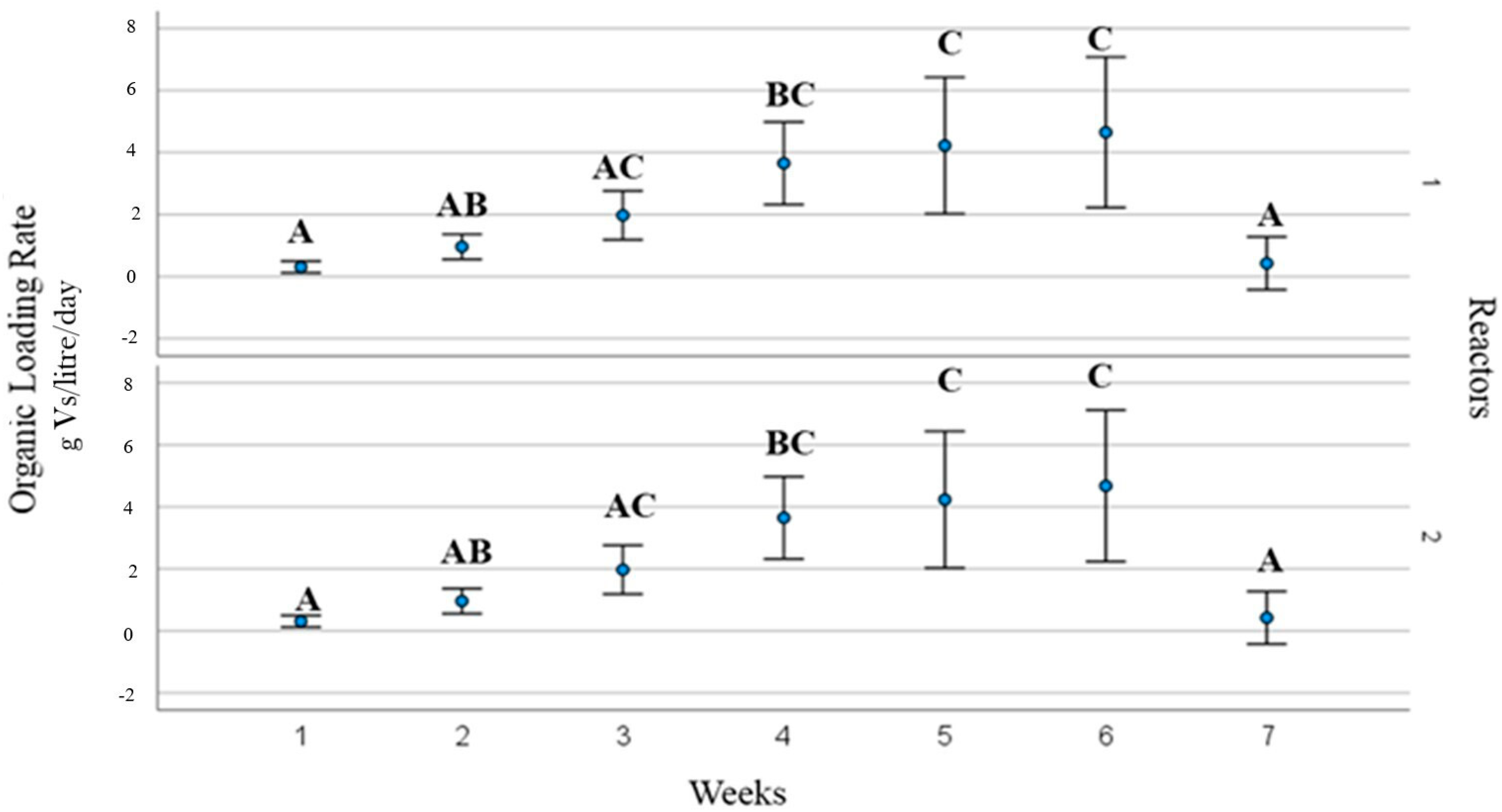
3.2. Process Operating Parameters and Substrate Behaviour
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obaideen, K.; Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Olabi, A.G. Biogas role in achievement of the sustainable development goals: Evaluation, Challenges, and Guidelines. J. Taiwan Inst. Chem. Eng. 2022, 131, 104207. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers In: Global Warming of 1.5 °C. In An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 3–24. [Google Scholar] [CrossRef]
- van Lier, J.B.; Mahmoud, N.; Zeeman, G. Anaerobic wastewater treatment. In Biological Wastewater Treatment: Principles, Modeling and Design; IWA Publishing: London, UK, 2020; Volume 151, pp. 701–756. [Google Scholar] [CrossRef]
- Chiu, S.L.H.; Lo, I.M.C. Reviewing the anaerobic digestion and co-digestion process of food waste from the perspectives on biogas production performance and environmental impacts. Environ. Sci. Pollut. Res 2016, 23, 24435–24450. [Google Scholar] [CrossRef]
- Castellani, B.; Rinaldi, S.; Bonamente, E.; Nicolini, A.; Rossi, F.; Cotana, F. Carbon and energy footprint of the hydrate-based biogas upgrading process integrated with CO2 valorization. Stoten 2018, 615, 404–411. [Google Scholar] [CrossRef]
- European Union. Available online: https://energy.ec.europa.eu/system/files/2022-05/COM_2022_230_1_EN_ACT_part1_v5.pdf (accessed on 10 February 2024).
- Ware, A.; Power, N. Biogas from cattle slaughterhouse waste: Energy recovery towards an energy self-sufficient industry in Ireland. Renew. Energy 2016, 97, 541–549. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Effect of Digestate on Soil Organic Carbon and Plant-Available Nutrient Content Compared to Cattle Slurry and Mineral Fertilization. Agronomy 2020, 10, 379. [Google Scholar] [CrossRef]
- Lovato, G.; Albanez, R.; Triveloni, M.; Ratusznei, S.M.; Rodrigues, J.A.D. Methane production by co-digesting vinasse and whey in an AnSBBR: Effect of mixture ratio and feed strategy. Appl. Biochem. Biotechnol. 2019, 187, 28–46. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A. Enhancing the co-digestion efficiency of sewage sludge and cheese whey using brewery spent grain as an additional substrate. Bioresour. Technol. 2019, 291, 121863. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.J.; Puntano, N.F.; Mancilla-Leytón, J.M.; Borja, R. Batch mesophilic anaerobic co-digestion of spent goat batch mesophilic anaerobic co-digestion of spent goat straw bedding and goat cheese whey: Comparison with the mono-digestion of the two sole substrates. J. Environ. Manag. 2021, 280, 111733. [Google Scholar] [CrossRef]
- Sar, T.; Harirchi, S.; Ramezani, M.; Bulkan, G.; Akbas, M.Y.; Pandey, A.; Taherzadeh, M.J. Potential utilization of dairy industries by-products and wastes through microbial processes: A critical review. Sci. Total Environ. 2022, 810, 152253. [Google Scholar] [CrossRef]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef]
- Rincón-Catalán, N.I.; Pérez-Fabiel, S.; Mejía-González, G.; Herrera-López, D.; Castro-Chan, R.; Cruz-Salomón, A.; Sebastian, P.J. Power Generation from Cheese Whey Treatment by Anaerobic Digestion and Microbial Fuel Cell. Waste Biomass Valori. 2019, 13, 3221–3231. [Google Scholar] [CrossRef]
- Bella, P.; Rao, P.V. Anaerobic co-digestion of cheese whey and septage: Effect of substrate and inoculum on biogas production. J. Environ. Manag. 2022, 308, 114581. [Google Scholar] [CrossRef]
- Matjuda, D.S.; Tekere, M.; Thaela-Chimuka, M.J. Impact of Thermal Pretreatment on the Physicochemical Characteristics and Biomethane Yield Potential of Solid Slaughter Waste from High-Throughput Red Meat Abattoirs Valorized as a Potential Feedstock for Biogas Production. Sustainability 2024, 16, 2150. [Google Scholar] [CrossRef]
- Benalia, S.; Falcone, G.; Stillitano, T.; De Luca, A.I.; Strano, A.; Gulisano, G.; Zimbalatti, G.; Bernardi, B. Increasing the Content of Olive Mill Wastewater in Biogas Reactors for a Sustainable Recovery: Methane Productivity and Life Cycle Analyses of the Process. Foods 2021, 10, 1029. [Google Scholar] [CrossRef]
- Neri, A.; Bernardi, B.; Zimbalatti, G.; Benalia, S. An Overview of Anaerobic Digestion of Agricultural By-Products and Food Waste for Biomethane Production. Energies 2023, 16, 6851. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency—Office of Water. METHOD 1684 Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids; Draft January 2001 US Environmental Protection Agency Office of Water Office of Science and Technology Engineering and Analysis Division (4303) (January). Ertr, Maximale. n.d. “Bioextrusion®.”; U.S. Environmental Protection Agency: Washington, DC, USA, 2001; pp. 1–38.
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia Inhibition and Toxicity in Anaerobic Digestion: A Critical Review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Morozova, I.; Nikulina, N.; Oechsner, H.; Krümpel, J.; Lemmer, A. Effects of Increasing Nitrogen Content on Process Stability and Reactor Performance in Anaerobic Digestion. Energies 2020, 13, 1139. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- De Zorzi, P.; Balzamo, S.; Barbizzi, S.; Calabretta, E.; Potalivo, M.; Rosamilia, S. Misurazione per La Determinazione Della Richiesta Chimica Di Ossigeno (COD) Mediante Test in Cuvetta: Metodo 5135; ISPRA: Clive, IA, USA, 2014. Available online: https://www.isprambiente.gov.it/files/pubblicazioni/manuali-lineeguida/MLG_117_14.pdf (accessed on 10 February 2024).
- Li, H.; Tian, Y.; Wang, H.; Simeonov, I.; Christov, N. A volatile fatty acids adaptive observer-based hierarchical optimal controller design to maximum gas production of two-stage anaerobic digestion process. Comp. Chem. Eng. 2024, 181, 108524. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Sun, H.; Ni, P.; Angelidaki, I.; Dong, R.; Wu, S. Exploring stability indicators for efficient monitoring of anaerobic digestion of pig manure under perturbations. Waste Manag. 2019, 91, 139–146. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Wang, Q.; Ngo, H.H.; Liu, Q.; Zhang, X.; Nghiem, L.D. Hydrogen sulphide management in anaerobic digestion: A critical review on input control, process regulation, and post-treatment. Bioresour. Technol. 2022, 346, 126634. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, C.M.; Pavlostathis, S.G. Hydrogen sulfide affects the performance of a methanogenic bioelectrochemical system used for biogas upgrading. Water Res. 2021, 200, 117268. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, S. Dissecting methanogenesis for temperature-phased anaerobic digestion: Impact of temperature on community structure, correlation, and fate of methanogens. Bioresour. Technol. 2020, 306, 123104. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Shelford, T.; Felton, G.; Gooch, C.; Lansing, S. Evaluation of Hydrogen Sulfide Scrubbing Systems for Anaerobic Digesters on Two U.S. Dairy Farms. Energies 2019, 12, 4605. [Google Scholar] [CrossRef]
- IEA Bioenergy. Available online: www.ieabioenergy.com/blog/publications/process-monitoring-in-biogas-plants/ (accessed on 20 January 2024).
- Hülsemann, B.; Zhou, L.; Merkle, W.; Hassa, J.; Müller, J.; Oechsner, H. Biomethane Potential Test: Influence of Inoculum and the Digestion System. Appl. Sci. 2020, 10, 2589. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kovalovszki, A.; Pan, J.; Lin, C.; Liu, H.; Duan, N.; Angelidaki, I. Early warning indicators for mesophilic anaerobic digestion of corn stalk: A combined experimental and simulation approach. Biotechnol. Biofuels 2019, 12, 106. [Google Scholar] [CrossRef]
- Kalamaras, S.D.; Vitoulis, G.; Christou, M.L.; Sfetsas, T.; Tziakas, S.; Fragos, V.; Samaras, P.; Kotsopoulos, T.A. The Effect of Ammonia Toxicity on Methane Production of a Full-Scale Biogas Plant—An Estimation Method. Energies 2021, 14, 5031. [Google Scholar] [CrossRef]
- Zema, D.A.; Zappia, G.; Benalia, S.; Zimbalatti, G.; Perri, E.; Urso, E.; Tamburino, V.; Bernardi, B. Limiting factors for anaerobic digestion of olive mill wastewater blends under mesophilic and thermophilic conditions. J. Agric. Eng. 2018, 49, 130–137. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, A.; Hummel, F.; Benalia, S.; Zimbalatti, G.; Gabauer, W.; Mihajlovic, I.; Bernardi, B. Use of Continuous Stirred Tank Reactors for Anaerobic Co-Digestion of Dairy and Meat Industry By-Products for Biogas Production. Sustainability 2024, 16, 4346. https://doi.org/10.3390/su16114346
Neri A, Hummel F, Benalia S, Zimbalatti G, Gabauer W, Mihajlovic I, Bernardi B. Use of Continuous Stirred Tank Reactors for Anaerobic Co-Digestion of Dairy and Meat Industry By-Products for Biogas Production. Sustainability. 2024; 16(11):4346. https://doi.org/10.3390/su16114346
Chicago/Turabian StyleNeri, Alessandro, Ferdinand Hummel, Souraya Benalia, Giuseppe Zimbalatti, Wolfgang Gabauer, Ivana Mihajlovic, and Bruno Bernardi. 2024. "Use of Continuous Stirred Tank Reactors for Anaerobic Co-Digestion of Dairy and Meat Industry By-Products for Biogas Production" Sustainability 16, no. 11: 4346. https://doi.org/10.3390/su16114346
APA StyleNeri, A., Hummel, F., Benalia, S., Zimbalatti, G., Gabauer, W., Mihajlovic, I., & Bernardi, B. (2024). Use of Continuous Stirred Tank Reactors for Anaerobic Co-Digestion of Dairy and Meat Industry By-Products for Biogas Production. Sustainability, 16(11), 4346. https://doi.org/10.3390/su16114346








