Changes in Soil Microbial Communities Associated with Pinus densiflora and Larix kaempferi Seedlings under Extreme Warming and Precipitation Manipulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Preparation
2.2. Soil Physicochemical Properties
2.3. Soil Enzyme Assay
2.4. Soil Microbial Biomass Assay
2.5. Analyses of Soil Microbial Community Composition
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Characteristics
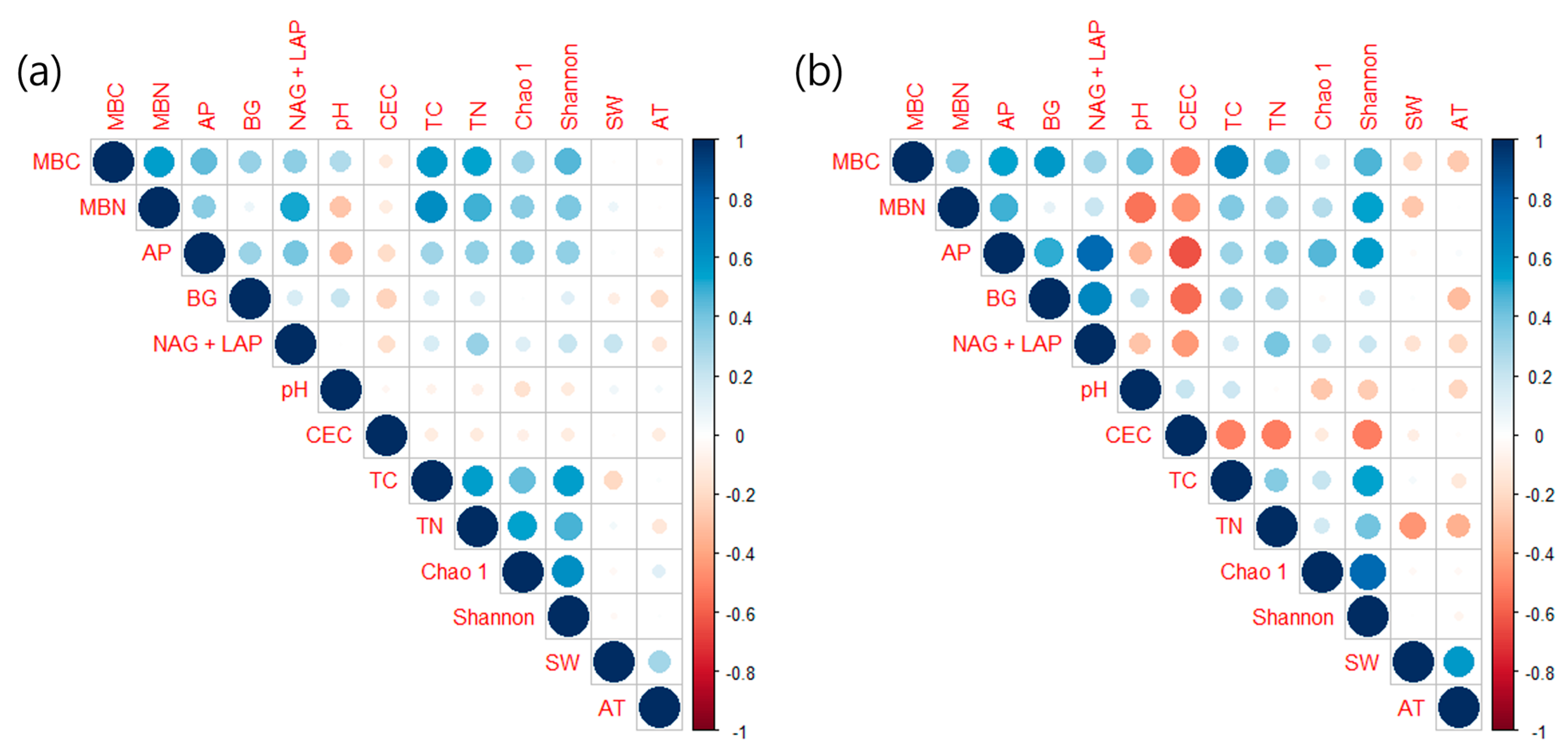
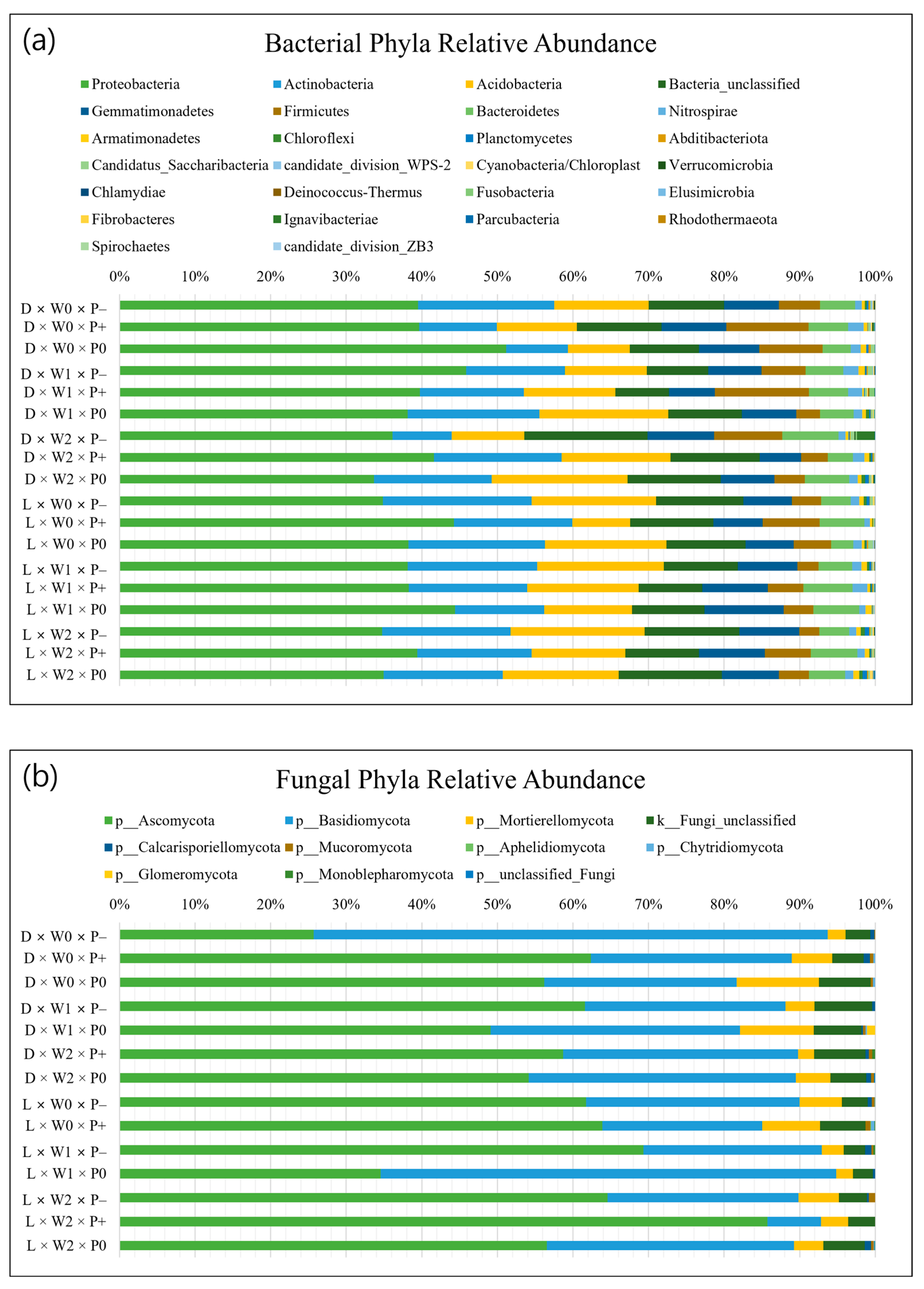
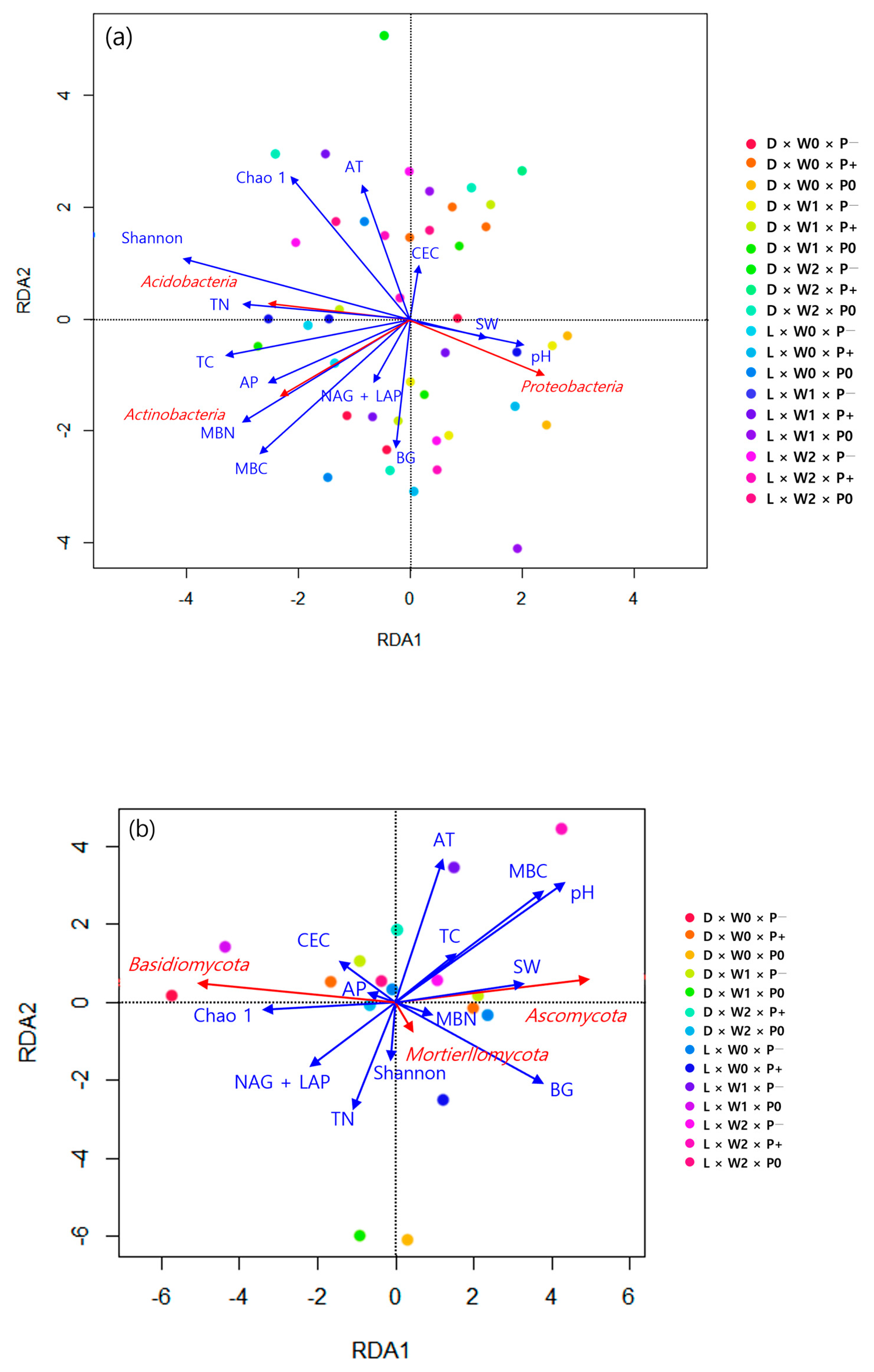
3.2. Soil Microbial Properties
4. Discussion
4.1. Responses of Soil Environment to Climatic Changes
4.2. Impact of Climatic Changes on Soil Microbial Dynamics
4.3. Effects of Different Tree Species on Soil Microbial Communities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cam-Bridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Naylor, D.; Sadler, N.; Bhattacharjee, A.; Graham, E.B.; Anderton, C.R.; McClure, R.; Lipton, M.; Hofmockel, K.S.; Jansson, J.K. Soil microbiomes under climate change and implications for carbon cycling. Annu. Rev. Environ. Resour. 2020, 45, 29–59. [Google Scholar] [CrossRef]
- Bhatia, C.R. Role of microbial diversity for soil, health and plant nutrition. In Molecular Mechanisms of Plant and Microbe Coex-Istence; Springer: Berlin/Heidelberg, Germany, 2008; pp. 53–74. [Google Scholar]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, S.; Chen, H.; Jin, K. Soil nitrogen availability drives the response of soil microbial biomass to warming. Sci. Total Environ. 2024, 917, 170505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, H.; Li, J.; Wang, H.; Liu, S.; Liu, X. Reduced precipitation neutralizes the positive impact of soil warming on soil microbial community in a temperate oak forest. Sci. Total Environ. 2022, 806, 150957. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ganjurjav, H.; Hu, G.; Wang, X.; Wan, Z.; Gao, Q. Seasonal patterns of soil microbial community response to warming and increased precipitation in a semiarid steppe. Appl. Soil. Ecol. 2023, 182, 104712. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.; Li, J.; Zhao, X.; Han, E.; Qu, H.; Ma, X.; Lian, J. Increasing precipitation weakened the negative effects of simulated warming on soil microbial community composition in a semi-arid sandy grassland. Front. Microbiol. 2023, 13, 1074841. [Google Scholar] [CrossRef]
- Ruan, Y.; Ling, N.; Jiang, S.; Jing, X.; He, J.S.; Shen, Q.; Nan, Z. Warming and altered precipitation independently and interactively suppress alpine soil microbial growth in a decadal-long experiment. eLife 2024, 12, RP89392. [Google Scholar] [CrossRef] [PubMed]
- A’Bear, A.D.; Jones, T.H.; Kandeler, E.; Boddy, L. Interactive effects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil. Biol. Biochem. 2014, 70, 151–158. [Google Scholar] [CrossRef]
- Henry, H.A. Reprint of “Soil extracellular enzyme dynamics in a changing climate”. Soil. Biol. Biochem. 2013, 56, 53–59. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic re-sponses to warming. Soil. Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Liu, W.; Allison, S.D.; Xia, J.; Liu, L.; Wan, S. Precipitation regime drives warming responses of microbial biomass and activity in temperate steppe soils. Biol. Fertil. Soils 2016, 52, 469–477. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.U.; Jing, X.; He, J.S.; Sun, R.; Yang, Y.; Shade, A.; Chu, H. Effects of short-term warming and altered precipitation on soil microbial communities in alpine grassland of the Tibetan Plateau. Front. Microbiol. 2016, 7, 1032. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Niu, B.; Chen, Q.; Wang, J.; Zhao, J.; Luo, T.; Zhang, G. Effect of increasing precipitation and warming on microbial community in Tibetan alpine steppe. Environ. Res. 2020, 189, 109917. [Google Scholar] [CrossRef] [PubMed]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, V.K. Soil microbial biomass: A key soil driver in management of ecosystem functioning. Sci. Total Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef]
- Kim, G.J.; Jo, H.; Kim, H.; Cho, M.S.; Noh, N.J.; Chang, H.; Kim, H.; Son, Y. Experimental design of open-field temperature and precipitation manipulation system to simulate summer extreme climate events for plants and soils. Turk. J. Agric. For. 2023, 47, 132–142. [Google Scholar] [CrossRef]
- Brown, I.C. A rapid method of determining exchangeable hydrogen and total exchangeable bases of soils. Soil. Sci. 1943, 56, 353–358. [Google Scholar] [CrossRef]
- DeForest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil. Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Brookers, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil. Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil. Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil. Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L.; et al. The real-time polymerase chain reaction. Mol. Aspects Med. 2006, 27, 95–125. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P.; Rengel, Z. Nutrient availability in soils. In Marschner’s Mineral Nutrition of Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 499–522. [Google Scholar]
- Battistuzzi, F.U.; Hedges, S.B. A major clade of prokaryotes with ancient adaptations to life on land. Mol. Biol. Evol. 2009, 26, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Klenk, H.P.; Qin, S.; Hozzein, W.N.; Ahmed, I. Editorial: Actinobacteria in Special and Extreme Habitats: Diversity, Function Roles and Environmental Adaptations, Second Edition. Front. Microbiol. 2019, 10, 451595. [Google Scholar]
- Malchair, S.; Carnol, M. Microbial biomass and C and N transformations in forest floors under European beech, sessile oak, Norway spruce and Douglas-fir at four temperate forest sites. Soil. Biol. Biochem. 2009, 41, 831–839. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y.; Wu, J.; Fu, X.; Sun, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 319, 194–203. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, H.Y.; Kumar, P.; Chen, C.; Guan, Q. Multiple interactions between tree composition and diversity and microbial diversity underly litter decomposition. Geoderma 2019, 341, 161–171. [Google Scholar] [CrossRef]
- Awad, A.; Majcherczyk, A.; Schall, P.; Schröter, K.; Schöning, I.; Schrumpf, M.; Ehbrecht, M.; Boch, S.; Kahl, T.; Bauhus, J.; et al. Ectomycorrhizal and saprotrophic soil fungal biomass are driven by different factors and vary among broadleaf and coniferous temperate forests. Soil. Biol. Biochem. 2019, 131, 9–18. [Google Scholar] [CrossRef]
- Heděnec, P.; Nilsson, L.O.; Zheng, H.; Gundersen, P.; Schmidt, I.K.; Rousk, J.; Vesterdal, L. Mycorrhizal association of common European tree species shapes biomass and metabolic activity of bacterial and fungal communities in soil. Soil. Biol. Biochem. 2020, 149, 107933. [Google Scholar] [CrossRef]
- Fanin, N.; Hättenschwiler, S.; Fromin, N. Litter fingerprint on microbial biomass, activity, and community structure in the underlying soil. Plant Soil. 2014, 379, 79–91. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Jiang, H.; Hu, Y.; Lu, X. Influences of litter diversity and soil moisture on soil microbial communities in decomposing mixed litter of alpine steppe species. Geoderma 2020, 377, 114577. [Google Scholar] [CrossRef]
- Bauhus, J.; Pare, D. Effects of tree species, stand age and soil type on soil microbial biomass and its activity in a southern boreal forest. Soil. Biol. Biochem. 1998, 30, 1077–1089. [Google Scholar] [CrossRef]
- Su, F.; Xu, S.; Sayer, E.J.; Chen, W.; Du, Y.; Lu, X. Distinct storage mechanisms of soil organic carbon in coniferous forest and evergreen broadleaf forest in tropical China. J. Environ. Manag. 2021, 295, 113142. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]


| Treatments | pH | CEC | TC | TN |
|---|---|---|---|---|
| T | 43.71 ** | 5.93 ** | 4.20 ** | 66.28 ** |
| T × W | 1.22 | 0.57 | 0.67 | 0.94 |
| T × P | 1.67 | 1.50 | 1.87 | 0.41 |
| T × S | 1.39 | 0.51 | 0.51 | 1.25 |
| T × W × P | 1.41 | 0.64 | 0.70 | 0.42 |
| T × W × S | 0.23 | 0.71 | 0.69 | 0.57 |
| T × P × S | 1.12 | 0.62 | 0.94 | 0.45 |
| T × W × P × S | 0.68 | 0.84 | 0.46 | 0.40 |
| Treatments | AP | BG | NAG + LAP | MBC | MBN |
|---|---|---|---|---|---|
| W | 0.43 | 1.02 | 0.63 | 1.08 | 0.02 |
| P | 1.14 | 1.59 | 0.80 | 0.71 | 0.66 |
| S | 0.18 | 2.75 | 1.48 | 5.04 * | 0.01 |
| W × P | 0.67 | 1.51 | 1.97 | 0.52 | 0.48 |
| W × S | 1.77 | 2.57 | 0.06 | 0.41 | 0.17 |
| P × S | 1.27 | 3.45 * | 0.41 | 0.22 | 0.13 |
| W × P × S | 1.22 | 1.28 | 1.08 | 0.32 | 0.27 |
| Treatments | Bacteria | Fungi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alpha Diversity | Dominant Phyla Relative Abundance | Alpha Diversity | Dominant Phyla Relative Abundance | |||||||
| Chao 1 | Shannon | Proteobacteria | Actinobacteria | Acidobacteria | Chao 1 | Shannon | Ascomycota | Basidiomycota | Mortierllomycota | |
| W | 0.41 | 0.40 | 1.04 | 0.97 | 0.19 | 5.58 | 4.96 | 9.51 | 0.14 | 8.83 |
| P | 0.14 | 0.86 | 0.11 | 0.36 | 0.55 | 26.26 * | 7.97 | 37.44 ** | 2.28 | 6.04 |
| S | 1.08 | 3.99 | 0.69 | 2.51 | 0.06 | 32.86 * | 25.88 * | 23.58 * | 1.30 | 12.73 * |
| W × P | 0.13 | 0.42 | 0.04 * | 1.68 | 0.45 | 2.15 | 3.48 | 5.94 | 0.99 | 2.38 |
| W × S | 0.43 | 0.11 | 2.12 | 1.20 | 2.20 | 0.87 | 3.63 | 8.97 | 0.29 | 4.44 |
| P × S | 0.40 | 1.51 | 6.03 | 5.60 ** | 0.44 | 7.49 | 2.86 | 7.03 | 0.31 | 2.32 |
| W × P × S | 0.50 | 0.46 | 2.03 | 1.54 | 1.07 | 29.17 * | 15.32 * | 69.67 ** | 4.26 | 10.75 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.; Li, G.; Jo, H.; Kim, G.-J.; Chung, H.; Son, Y. Changes in Soil Microbial Communities Associated with Pinus densiflora and Larix kaempferi Seedlings under Extreme Warming and Precipitation Manipulation. Sustainability 2024, 16, 4331. https://doi.org/10.3390/su16114331
Kwon M, Li G, Jo H, Kim G-J, Chung H, Son Y. Changes in Soil Microbial Communities Associated with Pinus densiflora and Larix kaempferi Seedlings under Extreme Warming and Precipitation Manipulation. Sustainability. 2024; 16(11):4331. https://doi.org/10.3390/su16114331
Chicago/Turabian StyleKwon, Minyoung, Guanlin Li, Heejae Jo, Gwang-Jung Kim, Haegeun Chung, and Yowhan Son. 2024. "Changes in Soil Microbial Communities Associated with Pinus densiflora and Larix kaempferi Seedlings under Extreme Warming and Precipitation Manipulation" Sustainability 16, no. 11: 4331. https://doi.org/10.3390/su16114331
APA StyleKwon, M., Li, G., Jo, H., Kim, G.-J., Chung, H., & Son, Y. (2024). Changes in Soil Microbial Communities Associated with Pinus densiflora and Larix kaempferi Seedlings under Extreme Warming and Precipitation Manipulation. Sustainability, 16(11), 4331. https://doi.org/10.3390/su16114331







