Application of Cotton Stalk as an Adsorbent for Copper(II) Ions in Sustainable Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Adsorbent Material
2.2.1. Elemental and Proximate Analysis
2.2.2. Zero Loading Point (pHPZC)
2.2.3. Acid and Basic Sites
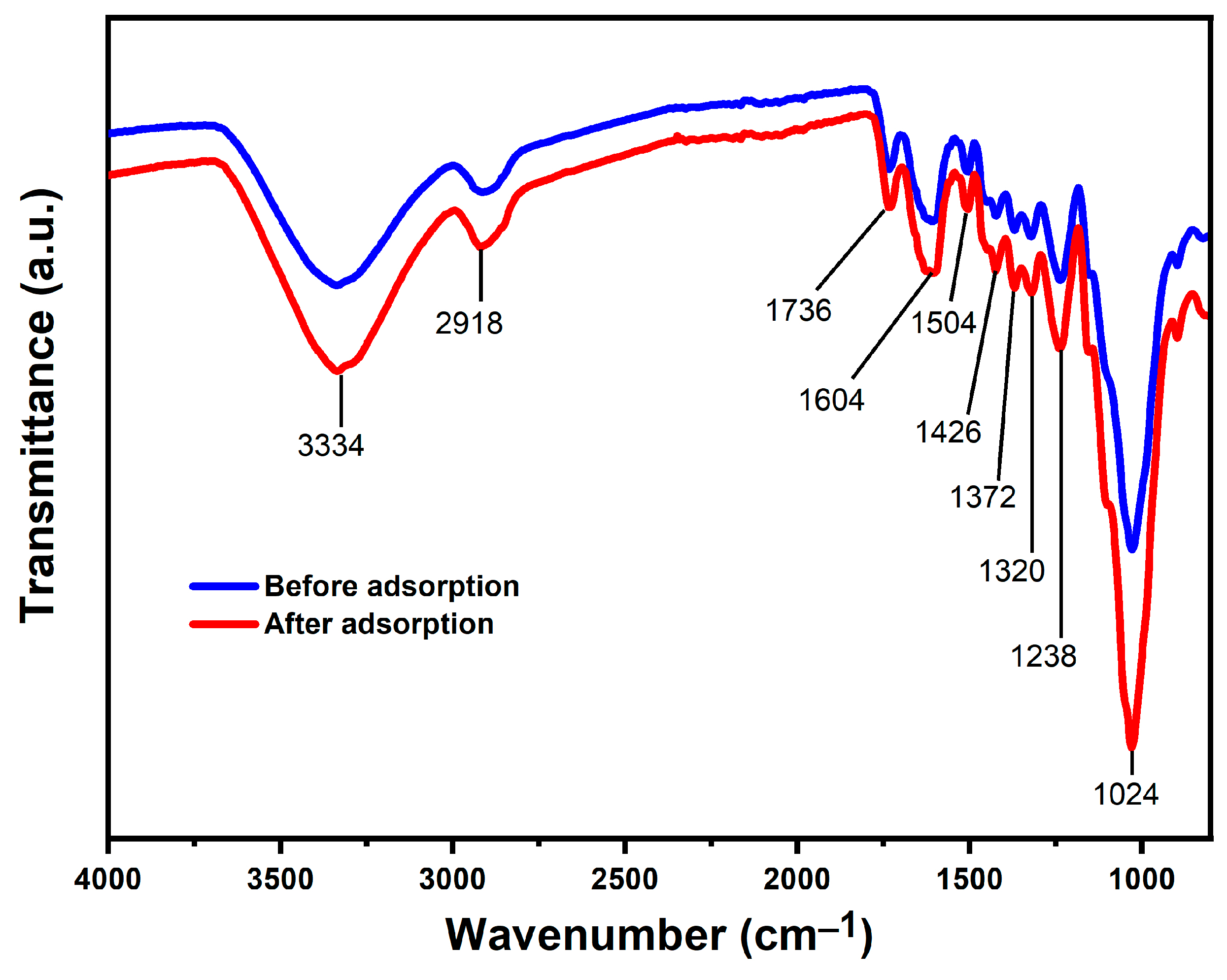
2.2.4. Fourier Transform Infrared Analysis (FTIR)
2.2.5. Energy Dispersive X-ray Fluorescence Analysis (EDX)
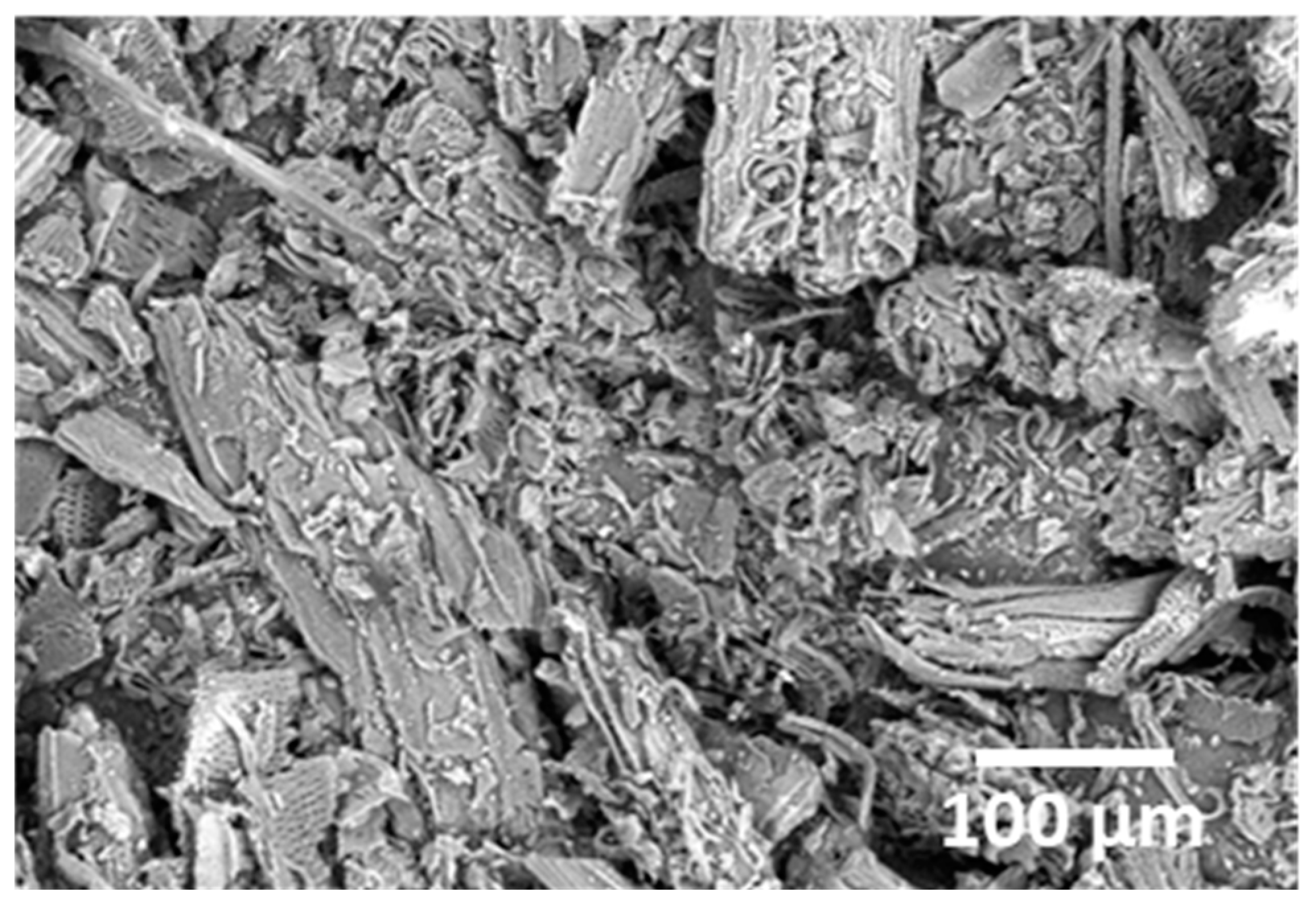
2.2.6. Microscopic Analysis
2.2.7. Thermogravimetric Analysis
2.3. Copper Concentration in Aqueous Solutions
2.4. Determination of Optimum Adsorption Conditions
2.4.1. Batch Adsorption Tests
2.4.2. pH Variation
2.4.3. Variation of Adsorbent Dosage
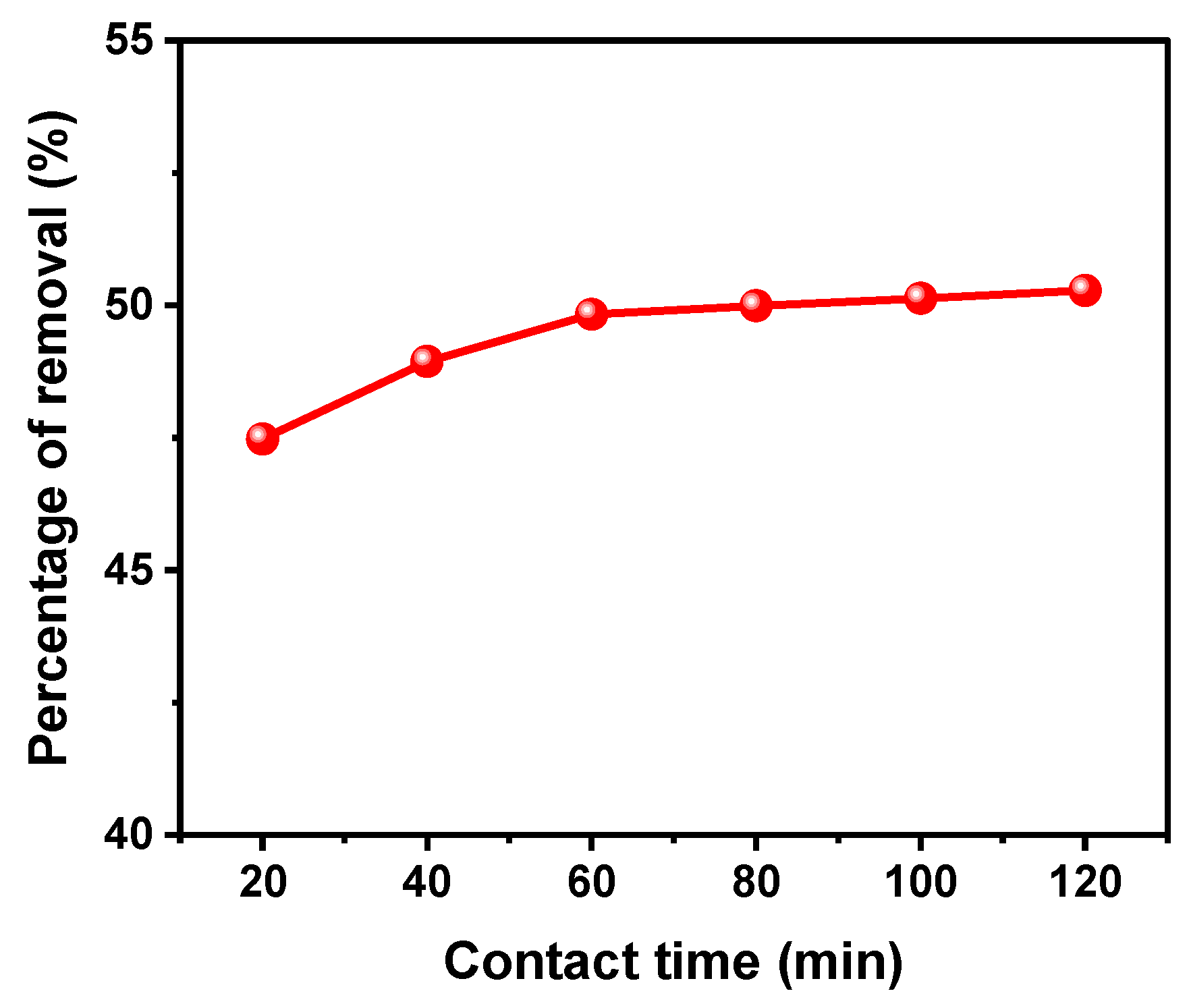
2.4.4. Variation of the Contact Time
2.5. Biosorbent Testing with Wastewater Samples
2.6. Adsorption Isotherms
2.6.1. Langmuir Model
2.6.2. Freundlich Model
2.7. Adsorption Kinetics
2.7.1. Pseudo-First-Order Model
2.7.2. Pseudo-Second-Order Model
3. Results and Discussion
3.1. Adsorbent Characterization
3.1.1. Proximate and Elemental Analysis
3.1.2. pHPZC
3.1.3. Acidic–Basic Sites
3.1.4. FTIR Analysis
3.1.5. EDX Analysis
3.1.6. SEM Analysis
3.1.7. Thermogravimetric Analysis
3.2. Optimization of Factors Affecting Adsorption
3.2.1. Effect of pH
3.2.2. Effect of Adsorbent Dosage
3.2.3. Effect of Contact Time
3.3. Optimal Adsorption Conditions
3.4. Application of the Biosorbent on Wastewater Samples
3.5. Adsorption Isotherms
3.6. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelfatah, A.M.; El-Maghrabi, N.; Mahmoud, A.E.D.; Fawzy, M. Synergetic effect of green synthesized reduced graphene oxide and nano-zero valent iron composite for the removal of doxycycline antibiotic from water. Sci. Rep. 2022, 12, 19372. [Google Scholar] [CrossRef] [PubMed]
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Nagy, H.; Fawzy, M.; Hafez, E.; Mahmoud, A.E.D. Potentials of mono-and multi-metal ion removal from water with cotton stalks and date palm stone residuals. Environ. Sci. Pollut. Res. 2023, 1, 1–17. [Google Scholar] [CrossRef]
- Moosavi, S.G.; Seghatoleslami, M.J. Phytoremediation: A review. Adv. Agri. Biol. 2013, 1, 5–11. Available online: www.pscipub.com/AAB (accessed on 4 April 2024).
- Safardastgerdi, M.; Ardejani, F.D.; Mahmoodi, N.M. Lignocellulosic biomass functionalized with EDTA dianhydride for removing Cu (II) and dye from wastewater: Batch and fixed-bed column adsorption. Miner. Eng. 2023, 204, 108423. [Google Scholar] [CrossRef]
- NOM-001-SEMARNAT-1996; Que Establece los Límites Máximos Permisibles de Contaminantes en las Descargas de Aguas Residuales en Aguas y Bienes Nacionales. SEMARNAT, Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 1996.
- Semerjian, L. Removal of heavy metals (Cu, Pb) from aqueous solutions using pine (Pinus halepensis) sawdust: Equilibrium, kinetic, and thermodynamic studies. Environ. Technol. Innov. 2018, 12, 91–103. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Du, B.; Wei, Q.; Nguyen, P.D. Characterization of a multi-metal binding biosorbent: Chemical modification and desorption studies. Bioresour. Technol. 2015, 193, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Benaissa, H.; Elouchdi, M.A. Removal of copper ions from aqueous solutions by dried sunflower leaves. Chem. Eng. Process. 2007, 46, 614–622. [Google Scholar] [CrossRef]
- Phuengphai, P.; Singjanusong, T.; Kheangkhun, N.; Wattanakornsiri, A. Removal of copper(II) from aqueous solution using chemically modified fruit peels as efficient low-cost biosorbents. Appl. Water Sci. 2021, 14, 286–294. [Google Scholar] [CrossRef]
- Tong, K.S.; Kassim, M.J.; Azraa, A. Adsorption of copper ion from its aqueous solution by a novel biosorbent Uncaria gambir: Equilibrium, kinetics, and thermodynamic studies. J. Chem. Eng. 2011, 170, 145–153. [Google Scholar] [CrossRef]
- Rubio, D.I.C.; Calderón, R.A.M.; Gualtero, A.P.; Acosta, D.R.; Sandoval, J. Treatments for Removal of Heavy Metals Commonly Found in Industrial Wastewater. A Review. Ing. Y Región. 2015, 13, 73–90. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5432290 (accessed on 4 April 2024).
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification–A review. J. Chem. Eng. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Akperov, E.O.; Akperov, O.H. The wastage of the cotton stalks (Gossypium hirsutum L.) as low-cost adsorbent for removal of the Basic Green 5 dye from aqueous solutions. Appl. Water Sci. 2019, 9, 175–183. [Google Scholar] [CrossRef]
- Bohli, T.; Ouederni, A.; Fiol, N.; Villaescusa, I. Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. Comptes Rendus Chim. 2015, 18, 88–99. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Tasaso, P. Adsorption of copper using pomelo peel and depectinated pomelo peel. J. Clean. Energy Technol. 2014, 2, 154–157. [Google Scholar] [CrossRef][Green Version]
- Ozsoy, H.D.; Kumbur, H. Adsorption of Cu (II) ions on cotton boll. J. Hazard. Mater. 2006, 136, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, D.M.; Li, C.H.; Yu, B.Q. Adsorption Removal of Copper and Nickel Ions from Wastewater by Ammonia Modified Cotton Stalks. Adv. Mater. Res. 2014, 955, 2440–2443. [Google Scholar] [CrossRef]
- Kahraman, S.; Dogan, N.; Erdemoglu, S. Use of various agricultural wastes for the removal of heavy metal ions. IJEP 2008, 34, 275–284. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, B.; Diao, J. Synthesis of magnetic biochar derived from cotton stalks for the removal of Cr (VI) from aqueous solution. Water Sci. Technol. 2019, 79, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- SIAP, Servicio de Información Agroalimentaria y Pesquera. Producción Agrícola: Resumen Nacional por Estado. Available online: https://www.gob.mx/siap (accessed on 10 July 2023).
- Gallardo, T.M.F.; Cortez, L.A.A. Failed Water Governance Processes in the Mexicali Region. The Socio-environmental Conflict over the Constellation Brands Beer Plant. Norteamérica 2022, 17, 1–20. Available online: https://www.scielo.org.mx/scielo.php?pid=S1870-35502022000100011&script=sci_abstract&tlng=en (accessed on 4 April 2024).
- ASTM E777–08; Standard Test Method for Carbon and Hydrogen in the Analysis Sample of Refuse Derived Fuel. ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM E870-82; Standard Test Methods for Analysis of Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2006.
- ASTM E871-82; Standard Test Method for Moisture Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2006.
- Amaringo, V.F.A.; Hormaza, A.A. Determination of the point of zero charge and isoelectric point of two agricultural wastes and their application in the removal of colorants. Rev. De Investig. Agrar. Y Ambient. 2013, 4, 27–37. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5344979 (accessed on 4 April 2024).
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Method 3500 Cu; Standard Methods for the Examination of Water & Wastewater, 21st ed. APHA, AWWA, WPCF: Washington, DC, USA, 2005.
- Chaiyaraksa, C.; Jaipong, T.; Tamnao, P.; Imjai, A. Durian and mangosteen shell-derived biochar amendment on the removal of zinc, lead and cadmium. STA 2017, 22, 87–97. [Google Scholar] [CrossRef]
- Engates, K.E.; Shipley, H.J. Adsorption of Pb, Cd, Cu, Zn, and Ni to titanium dioxide nanoparticles: Effect of particle size, solid concentration, and exhaustion. Environ. Sci. Pollut. Res. 2011, 18, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. J. Chem. Eng. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Ueber Thixotropie. Colloid. Polym. Sci. 1928, 46, 289–299. [Google Scholar] [CrossRef]
- Benavente, M. Adsorption of Metallic Ions onto Chitosan: Equilibrium and Kinetic Studies. Ph.D. Dissertation, Royal Institute of Technology (KTH), Stockholm, Sweden, 2008. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A13755&dswid=-283 (accessed on 14 December 2023).
- Bozbaş, S.K.; Boz, Y. Low-cost biosorbent: Anadara inaequivalvis shells for removal of Pb(II) and Cu(II) from aqueous solution. Process Saf. Environ. Prot. 2016, 103, 144–152. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. Available online: https://sid.ir/paper/563615/en (accessed on 17 December 2023).
- Ho, Y.S.; McKay, G.A. Comparaison of chemisorption kinetic models applied to pollutant removals on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Y.; Deng, J.; Kuang, J.; Zhang, Y. Pretreatment of biomass by torrefaction. Chin. Sci. Bull. 2011, 56, 1442–1448. [Google Scholar] [CrossRef]
- Chen, W.H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, J.Z.; Feng, X.X.; Chen, J.Y. Effect of high-temperature degumming on the constituents and structure of cotton stalk bark fibers. J. Appl. Polym. Sci. 2012, 125, 573–579. [Google Scholar] [CrossRef]
- Torres, R.; Valdez, B.; Beleño, M.T.; Coronado, M.A.; Stoytcheva, M.; García, C.; Rojano, B.A.; Montero, G. Char production with high-energy value and standardized properties from two types of biomass. Biomass Convers. Biorefin. 2021, 13, 4831–4847. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Shafee, N.M.; Abdullah, M.O.; Lim, L.L.P. Synthesis and characterization of biocoal from Cymbopogon citrates residue using microwave-induced torrefaction. Environ. Technol. Innov. 2017, 8, 431–440. [Google Scholar] [CrossRef]
- Nam, H.; Capareda, S. Experimental investigation of torrefaction of two agricultural wastes of different composition using RSM (response surface methodology). Energy 2015, 91, 507–516. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Yang, X. Evolution of chemical functional groups during torrefaction of rice straw. Bioresour. Technol. 2021, 320, 124328. [Google Scholar] [CrossRef] [PubMed]
- Manatura, K. Inert torrefaction of sugarcane bagasse to improve its fuel properties. Case Stud. Therm. Eng. 2020, 19, 100623. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Wang, Y.; Wang, X.; Liu, M.; Yin, D.; Zhang, Y. Adsorption of Copper (II) onto activated carbons from sewage sludge by microwave-induced phosphoric acid and zinc chloride activation. Desalination 2011, 278, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Altameemi, I.A.; Thuraya, M.A.; Tarikak, N. A new simple method for the treatment of wastewater containing Cu (II) and Zn (II) Ions using adsorption on dried Conocarpus erectus leaves. Basra J. Vet. Res. 2013, 39, 125–136. [Google Scholar]
- El-Ashtoukhy, E.S.; Amin, N.K.; Abdelwahab, O. Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination 2008, 223, 162–173. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb (II), Ni (II), Cu (II) and Cd (II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Aman, T.; Kazi, A.A.; Sabri, M.U.; Bano, Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf. B Biointerfaces 2008, 63, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Adsorption of heavy metals from water using banana and orange peels. Water Sci. Technol. 2003, 47, 185–190. [Google Scholar] [CrossRef]
- Grimm, A.; Zanzi, R.; Björnbom, E.; Cukierman, A.L. Comparison of different types of biomasses for copper biosorption. Bioresour. Technol. 2008, 99, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.K.; Arancibia, F.; Gutiérrez, C. Adsorption of copper onto agriculture waste materials. J. Hazard. Mater. 2010, 180, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Keawkim, K.; Khamthip, N. Removal of Pb2+ ion from industrial wastewater by new efficient biosorbents of Oyster plant (Tradescantia spathacea Steam) and Negkassar leaf (Mammea siamensis T. Anderson). Chiang Mai J. Sci. 2018, 45, 369–379. Available online: https://epg.science.cmu.ac.th/ejournal/search-journal.php?aid=1345 (accessed on 6 January 2024).










| Analysis | Content | Weight (%) |
|---|---|---|
| Humidity | Water | 6.15 |
| Proximal | Fixed carbon | 22.88 |
| Volatile material | 72.35 | |
| Ash | 5.15 | |
| Elemental | Carbon (C) | 47.91 |
| Hydrogen (H) | 5.66 | |
| Oxygen (O) | 45.57 | |
| Nitrogen (N) | 0.75 | |
| Sulfur (S) | 0.11 |
| Natural Adsorbent Material | pH | (mg/g) | Ref. | Ref. |
|---|---|---|---|---|
| Potato peels | 6.0 | 0.38 | Aman et al. (2008) | [56] |
| Oak sawdust (Quercus coccifera) | 4 | 3.60 | Argun et al. (2007) | [17] |
| Orange peel | 5.9 | 3.65 | Annadurai et al. (2003) | [57] |
| Birchwood (Betula sp.) | 5.5 | 4.90 | Grimm et al. (2008) | [58] |
| Ammonia Modified Cotton Stalks | 5 | 1.88 | Li et al. (2014) | [20] |
| Cotton Stalk | 5.5 | 5.56 | This study | |
| Banana peel | 5.9 | 5.75 | Annadurai et al. (2003) | [57] |
| Uncaria gambir | 5.0 | 9.95 | Tong et al. (2011) | [12] |
| Peach stones/and pine sawdust | ˂7 | 10.0/15.0 | Hanse et al. (2010) | [59] |
| Pitahaya peel | 5.0 | 21.33 | Phuengphai et al. (2021) | [11] |
| Pomegranate peel | 5.8 | 30.12 | Ben-Ali et al. (2017) | [9] |
| Parameter | Agricultural Wastewater | Treated Livestock Wastewater | ||||
|---|---|---|---|---|---|---|
| Untreated | Treated | % Removed | Untreated | Treated | % Removed | |
| pH | 7.86 | 5.5 | - | 7.81 | 5.5 | - |
| C.E (dS/m) | 1.51 | 1.27 | - | 1.85 | 1.64 | - |
| TDS (mg/L) | 760 | 650 | 14.47 | 930 | 840 | 9.67 |
| Cu2+ (mg/L) * | 0.07 | - | - | 0.16 | - | - |
| Cu2+ (mg/L) ** | 25.97 | 3.22 | 87.60 | 25.16 | 3.76 | 85.05 |
| Isotherm Models | Isotherm Parameters | |||
|---|---|---|---|---|
| Langmuir Model | qm (mg/g) | KL (L/mg) | RL | R2 |
| Absorbent | 41.49 | 0.0039 | 0.83 | 0.5542 |
| Freundlich model | KF | nf | 1/nf | R2 |
| Absorbent | 0.1144 | 0.8771 | 1.1401 | 0.9910 |
| Kinetic Models | Kinetic Parameters | ||||
|---|---|---|---|---|---|
| Co (mg/L) | qe (mg/g) | k1 (min−1) | k2 (g/(mg∙min)) | R2 | |
| Pseudo-first order | 50 | 1.2319 | 0.9971 | - | 0.2549 |
| Pseudo-second order | 50 | 4.6210 | - | 0.3369 | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beleño Cabarcas, M.T.; Torres Ramos, R.; Valdez Salas, B.; González Mendoza, D.; Mendoza Gómez, A.; Curiel Álvarez, M.A.; Castillo Sáenz, J.R. Application of Cotton Stalk as an Adsorbent for Copper(II) Ions in Sustainable Wastewater Treatment. Sustainability 2024, 16, 4291. https://doi.org/10.3390/su16104291
Beleño Cabarcas MT, Torres Ramos R, Valdez Salas B, González Mendoza D, Mendoza Gómez A, Curiel Álvarez MA, Castillo Sáenz JR. Application of Cotton Stalk as an Adsorbent for Copper(II) Ions in Sustainable Wastewater Treatment. Sustainability. 2024; 16(10):4291. https://doi.org/10.3390/su16104291
Chicago/Turabian StyleBeleño Cabarcas, Mary Triny, Ricardo Torres Ramos, Benjamín Valdez Salas, Daniel González Mendoza, Aurelia Mendoza Gómez, Mario Alberto Curiel Álvarez, and Jonathan Rafael Castillo Sáenz. 2024. "Application of Cotton Stalk as an Adsorbent for Copper(II) Ions in Sustainable Wastewater Treatment" Sustainability 16, no. 10: 4291. https://doi.org/10.3390/su16104291
APA StyleBeleño Cabarcas, M. T., Torres Ramos, R., Valdez Salas, B., González Mendoza, D., Mendoza Gómez, A., Curiel Álvarez, M. A., & Castillo Sáenz, J. R. (2024). Application of Cotton Stalk as an Adsorbent for Copper(II) Ions in Sustainable Wastewater Treatment. Sustainability, 16(10), 4291. https://doi.org/10.3390/su16104291








