Electrochemical Application of Activated Carbon Derived from End-of-Life Tyres: A Technological Review
Abstract
1. Introduction
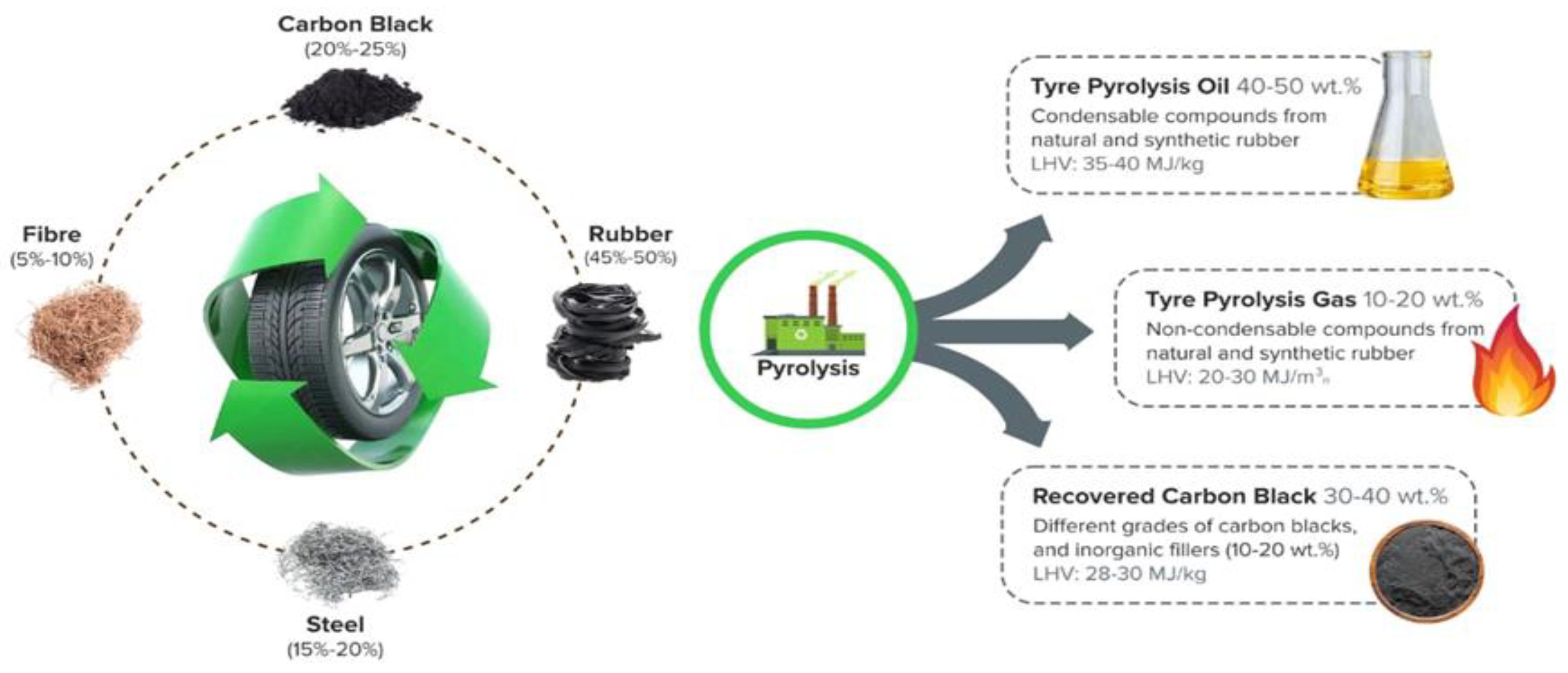
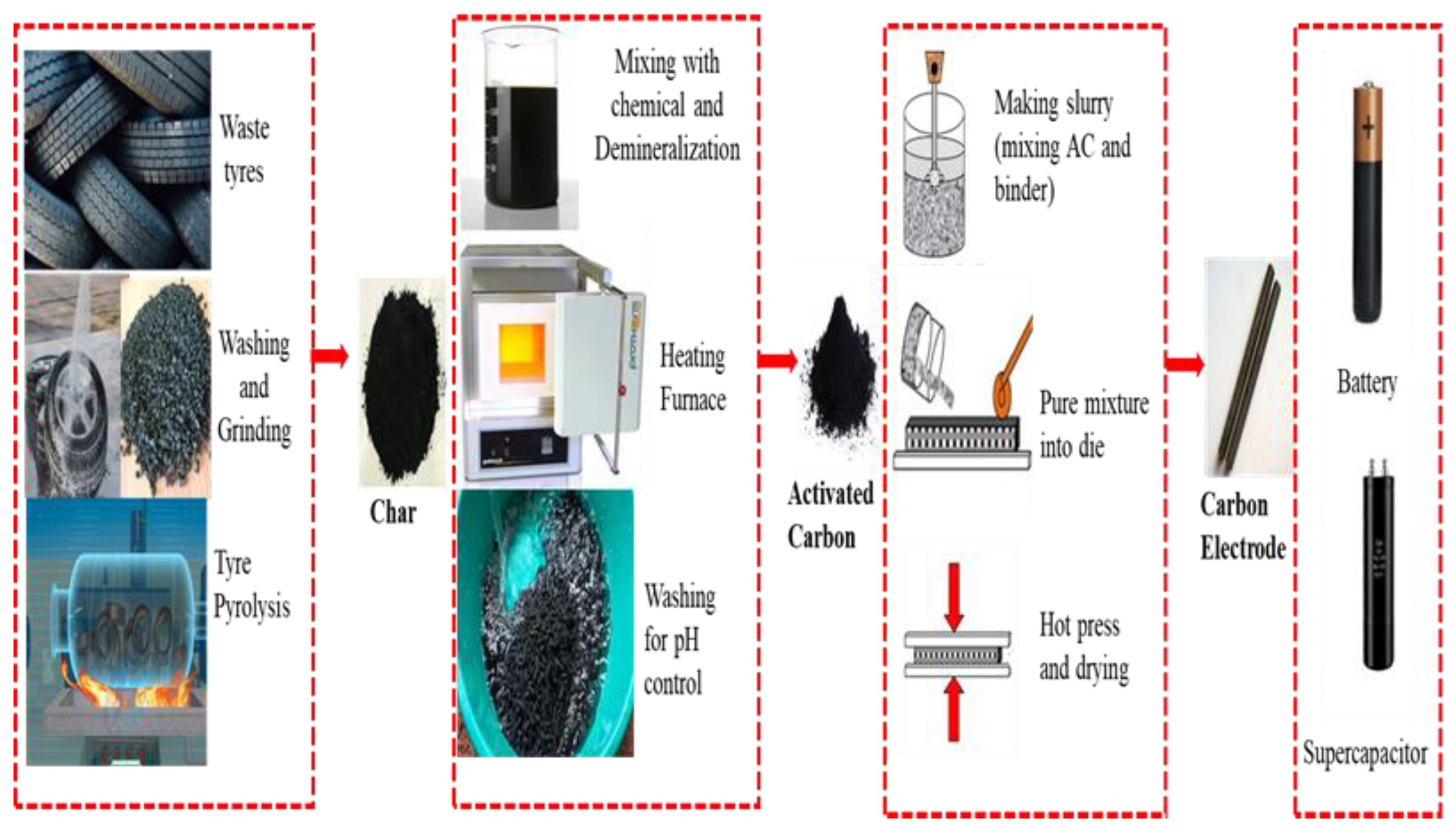
2. Pyrolysis Process
3. Carbon Activation
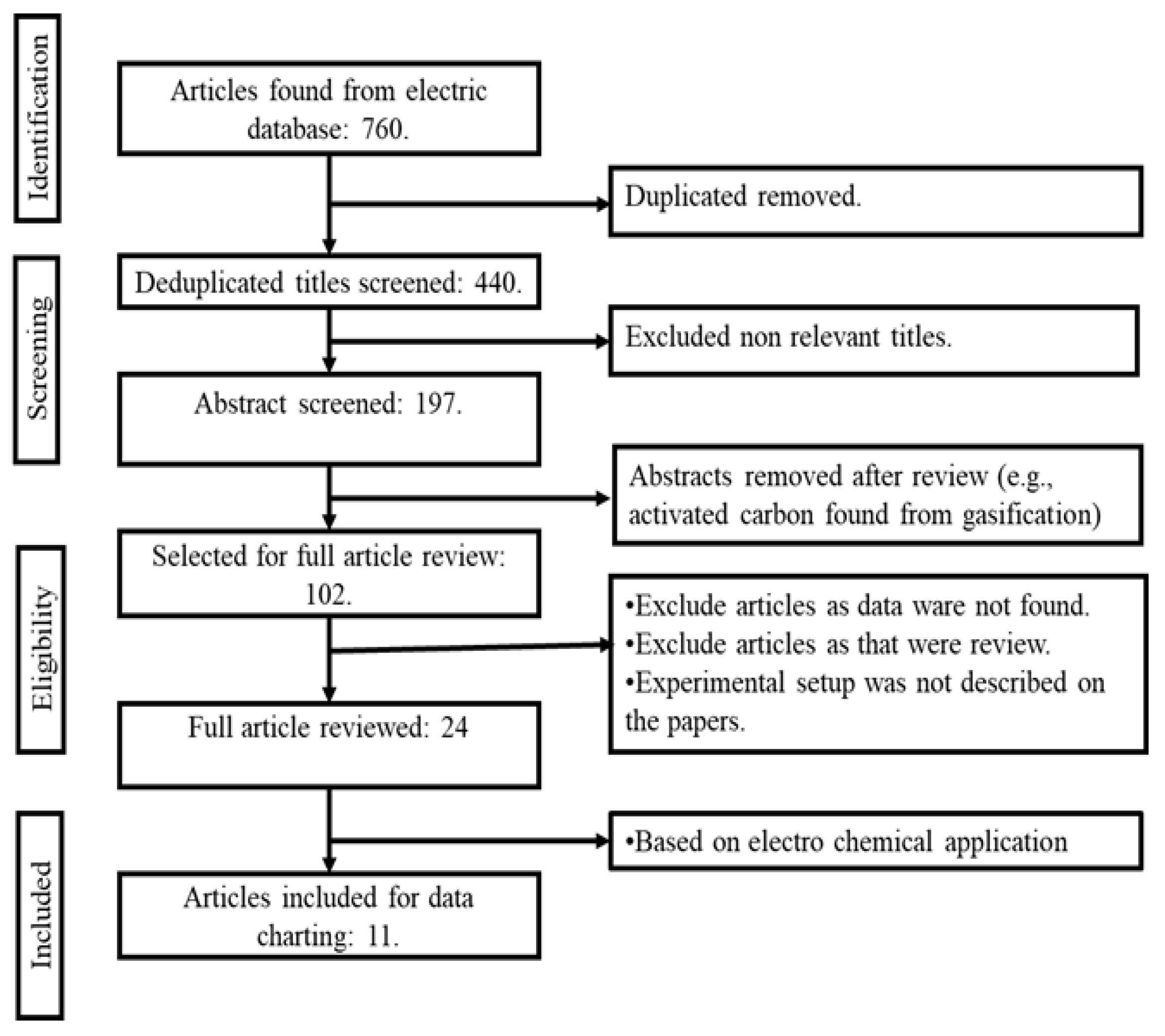
4. Bibliographical Search and Methods
5. Findings on Electrochemical Applications of TDAC
5.1. TDAC as Electrode Material
5.1.1. Crystal Structure of TDAC
5.1.2. Surface Morphology
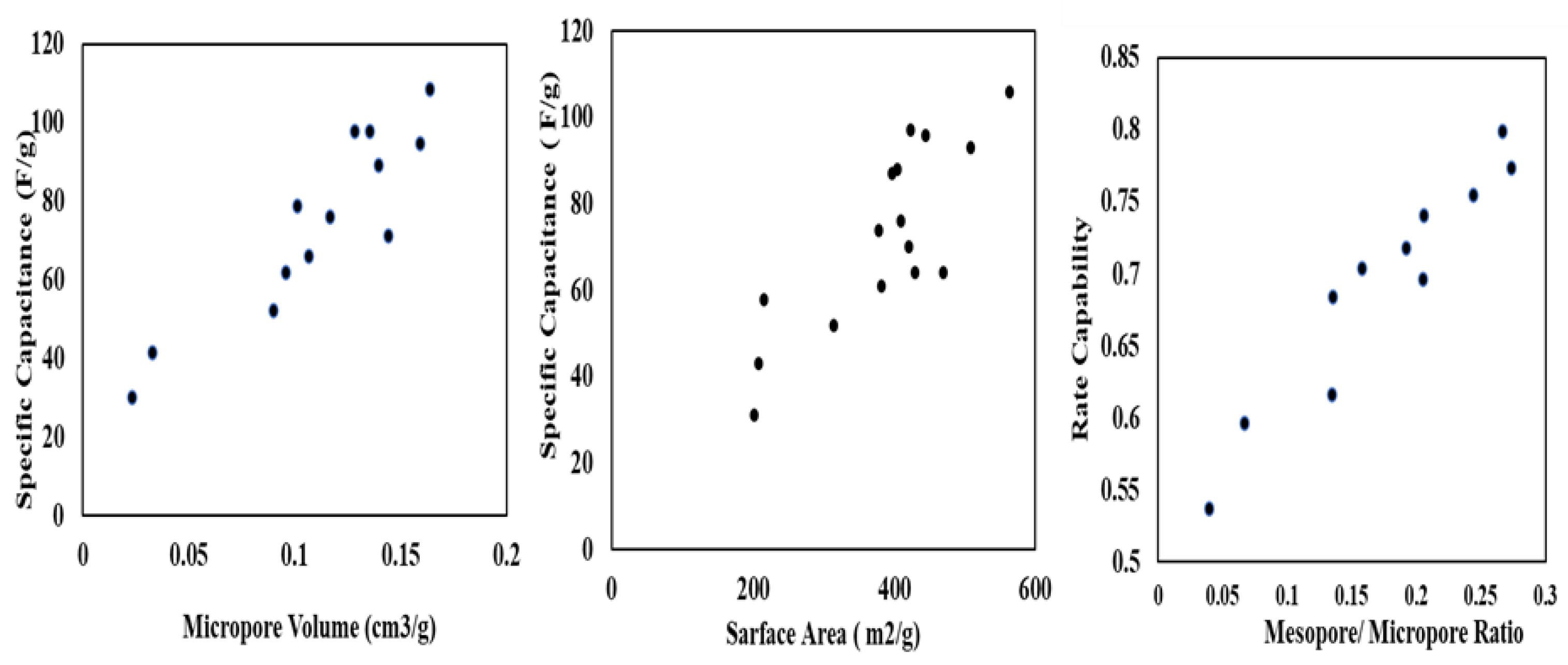
5.2. Electrochemical Performance of TDAC Electrode
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsoncheva, T.; Mileva, A.; Tsyntsarski, B.; Paneva, D.; Spassova, I.; Kovacheva, D.; Velinov, N.; Karashanova, D.; Georgieva, B.; Petrov, N. Activated carbon from Bulgarian peach stones as a support of catalysts for methanol decomposition. Biomass Bioenergy 2018, 109, 135–146. [Google Scholar] [CrossRef]
- Ahmed, M.J. Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption. J. Environ. Chem. Eng. 2016, 4, 89–99. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Hossain, F.M.; Rasul, M.G.; Chowdhury, A.A. A review on the thermochemical recycling of waste tyres to oil for automobile engine application. Energies 2021, 14, 3837. [Google Scholar] [CrossRef]
- Hasan, M.; Rasul, M.; Jahirul, M.; Khan, M. Characterization of pyrolysis oil produced from organic and plastic wastes using an auger reactor. Energy Convers. Manag. 2023, 278, 116723. [Google Scholar] [CrossRef]
- Faisal, F.; Rasul, M.; Jahirul, M.; Schaller, D. Pyrolytic conversion of waste plastics to energy products: A review on yields, properties, and production costs. Sci. Total Environ. 2023, 861, 160721. [Google Scholar] [CrossRef] [PubMed]
- Mustayen, A.; Rasul, M.; Wang, X.; Hazrat, M.; Jahirul, M.; Negnevitsky, M. Plastic-made diesel (PMD) from pyrolysis via vacuum distillation process-A waste recycling fuel to diesel engine performance and emissions improvement. J. Energy Inst. 2023, 107, 101198. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Production of activated carbons from waste tyres for low temperature NOx control. Waste Manag. 2016, 49, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Guo, J.; Sun, Y.; Liu, X.; Pan, J. Pyrolytic carbon black-derived porous carbon with spherical skeleton as recovered and enduring electrode material for supercapacitor. J. Energy Storage 2021, 44, 103372. [Google Scholar] [CrossRef]
- Özbaşa, E.E.; Balçıkb, B.; Ozcana, H. Preparation of activated carbon from waste tires, and its use for dye removal. Desalination Water Treat. 2019, 172, 78–85. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C. Texture and surface chemistry of activated carbons obtained from tyre wastes. Fuel Process. Technol. 2015, 134, 275–283. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; De Yuso, A.M.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Arias, A.M.; Moreno-Piraján, J.C.; Giraldo, L. Adsorption of Triton X-100 in aqueous solution on activated carbon obtained from waste tires for wastewater decontamination. Adsorption 2020, 26, 303–316. [Google Scholar] [CrossRef]
- Wang, C.; Li, D.; Zhai, T.; Wang, H.; Sun, Q.; Li, H. Direct conversion of waste tires into three-dimensional graphene. Energy Storage Mater. 2019, 23, 499–507. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, D.; Xie, Z.; Kang, Y.; Tang, Z.; Dai, Y.; Lei, Y.; Chen, J.; Liang, F. Activated carbon prepared from waste tire pyrolysis carbon black via CO2/KOH activation used as supercapacitor electrode. Sci. China Technol. Sci. 2022, 65, 2337–2347. [Google Scholar] [CrossRef]
- Rowhani, A.; Rainey, T.J. Scrap tyre management pathways and their use as a fuel—A review. Energies 2016, 9, 888. [Google Scholar] [CrossRef]
- Shulman, V. Tyre Recycling; iSmithers Rapra Publishing: Shawbury, UK, 2004; Volume 15. [Google Scholar]
- Mushunje, K.; Otieno, M.; Ballim, Y. A review of waste tyre rubber as an alternative concrete consituent material. MATEC Web Conf. 2018, 199, 11003. [Google Scholar] [CrossRef]
- Jusli, E.; Nor, H.M.; Jaya, R.P.; Haron, Z.; Mohamed, A. A Review of Double Layer Rubberized Concrete Paving Blocks. J. Eng. Res. Technol. 2016, 2. [Google Scholar]
- Aziz, M.A.; Al-Khulaidi, R.A.; Rashid, M.; Islam, M.; Rashid, M. Design and fabrication of a fixed-bed batch type pyrolysis reactor for pilot scale pyrolytic oil production in Bangladesh. IOP Conf. Ser. Mater. Sci. Eng. 2017, 184, 012056. [Google Scholar] [CrossRef]
- Menares, T.; Herrera, J.; Romero, R.; Osorio, P.; Arteaga-Pérez, L.E. Waste tires pyrolysis kinetics and reaction mechanisms explained by TGA and Py-GC/MS under kinetically-controlled regime. Waste Manag. 2020, 102, 21–29. [Google Scholar] [CrossRef]
- Kordoghli, S.; Khiari, B.; Paraschiv, M.; Zagrouba, F.; Tazerout, M. Impact of different catalysis supported by oyster shells on the pyrolysis of tyre wastes in a single and a double fixed bed reactor. Waste Manag. 2017, 67, 288–297. [Google Scholar] [CrossRef]
- Oyedun, A.; Lam, K.-L.; Fittkau, M.; Hui, C.-W. Optimisation of particle size in waste tyre pyrolysis. Fuel 2012, 95, 417–424. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Czajka, K.; Krzyżyńska, R.; Jouhara, H. Waste tyre pyrolysis–Impact of the process and its products on the environment. Therm. Sci. Eng. Prog. 2020, 20, 100690. [Google Scholar] [CrossRef]
- Mkhize, N.M.; Danon, B.; Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M.; van der Gryp, P.; Görgens, J. Influence of reactor and condensation system design on tyre pyrolysis products yields. J. Anal. Appl. Pyrolysis 2019, 143, 104683. [Google Scholar] [CrossRef]
- Quek, A.; Balasubramanian, R. An algorithm for the kinetics of tire pyrolysis under different heating rates. J. Hazard. Mater. 2009, 166, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Jones, S.B.; Valkenburt, C.; Walton, C.W.; Elliott, D.C.; Holladay, J.E.; Stevens, D.J.; Kinchin, C.; Czernik, S. Production of Gasoline and Diesel from Biomass via Fast Pyrolysis, Hydrotreating and Hydrocracking: A Design Case; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2009.
- Lira, C.S.; Berruti, F.M.; Palmisano, P.; Berruti, F.; Briens, C.; Pécora, A.A. Fast pyrolysis of Amazon tucumã (Astrocaryum aculeatum) seeds in a bubbling fluidized bed reactor. J. Anal. Appl. Pyrolysis 2013, 99, 23–31. [Google Scholar] [CrossRef]
- Iribarren, D.; Peters, J.F.; Dufour, J. Life cycle assessment of transportation fuels from biomass pyrolysis. Fuel 2012, 97, 812–821. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M. Biomass pyrolysis for liquid fuels and chemicals: A review. NIScPR Online Period. Repos. 2007, 66, 797–804. [Google Scholar]
- Taleb, D.A.; Abd Hamid, H.; Deris, R.R.R.; Zulkifli, M.; Khalil, N.A.; Yahaya, A.N.A. Insights into pyrolysis of waste tire in fixed bed reactor: Thermal behavior. Mater. Today Proc. 2020, 31, 178–186. [Google Scholar] [CrossRef]
- Undri, A.; Meini, S.; Rosi, L.; Frediani, M.; Frediani, P. Microwave pyrolysis of polymeric materials: Waste tires treatment and characterization of the value-added products. J. Anal. Appl. Pyrolysis 2013, 103, 149–158. [Google Scholar] [CrossRef]
- Lozhechnik, A.; Savchin, V. Pyrolysis of rubber in a screw reactor. J. Eng. Phys. Thermophys. 2016, 89, 1482–1486. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Mkhize, N.; Danon, B.; Van der Gryp, P.; Görgens, J.; Bilbao, J.; Olazar, M. Evaluation of the properties of tyre pyrolysis oils obtained in a conical spouted bed reactor. Energy 2017, 128, 463–474. [Google Scholar] [CrossRef]
- Galvagno, S.; Casu, S.; Casabianca, T.; Calabrese, A.; Cornacchia, G. Pyrolysis process for the treatment of scrap tyres: Preliminary experimental results. Waste Manag. 2002, 22, 917–923. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Jana, A.; Mondal, S.; Jana, B.; Sadhukhan, A.K.; Gupta, P. Pyrolysis of three different categories of automotive tyre wastes: Product yield analysis and characterization. J. Anal. Appl. Pyrolysis 2018, 135, 379–389. [Google Scholar] [CrossRef]
- Muenpol, S.; Jitkarnka, S. Effects of Fe supported on zeolites on structures of hydrocarbon compounds and petrochemicals in waste tire-derived pyrolysis oils. J. Anal. Appl. Pyrolysis 2016, 117, 147–156. [Google Scholar] [CrossRef]
- Ye, W.; Xu, X.; Zhan, M.; Huang, Q.; Li, X.; Jiao, W.; Yin, Y. Formation behavior of PAHs during pyrolysis of waste tires. J. Hazard. Mater. 2022, 435, 128997. [Google Scholar] [CrossRef]
- Choi, G.-G.; Jung, S.-H.; Oh, S.-J.; Kim, J.-S. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- de Marco Rodriguez, I.; Laresgoiti, M.; Cabrero, M.; Torres, A.; Chomon, M.; Caballero, B. Pyrolysis of scrap tyres. Fuel Process. Technol. 2001, 72, 9–22. [Google Scholar] [CrossRef]
- Ucar, S.; Karagoz, S.; Ozkan, A.R.; Yanik, J. Evaluation of two different scrap tires as hydrocarbon source by pyrolysis. Fuel 2005, 84, 1884–1892. [Google Scholar] [CrossRef]
- Ahoor, A.H.; Zandi-Atashbar, N. Fuel production based on catalytic pyrolysis of waste tires as an optimized model. Energy Convers. Manag. 2014, 87, 653–669. [Google Scholar] [CrossRef]
- Boxiong, S.; Chunfei, W.; Cai, L.; Binbin, G.; Rui, W. Pyrolysis of waste tyres: The influence of USY catalyst/tyre ratio on products. J. Anal. Appl. Pyrolysis 2007, 78, 243–249. [Google Scholar] [CrossRef]
- Witpathomwong, C.; Longloilert, R.; Wongkasemjit, S.; Jitkarnka, S. Improving light olefins and light oil production using Ru/MCM-48 in catalytic pyrolysis of waste tire. Energy Procedia 2011, 9, 245–251. [Google Scholar] [CrossRef]
- Dũng, N.A.; Klaewkla, R.; Wongkasemjit, S.; Jitkarnka, S. Light olefins and light oil production from catalytic pyrolysis of waste tire. J. Anal. Appl. Pyrolysis 2009, 86, 281–286. [Google Scholar] [CrossRef]
- Díez, C.; Sánchez, M.; Haxaire, P.; Martínez, O.; Moran, A. Pyrolysis of tyres: A comparison of the results from a fixed-bed laboratory reactor and a pilot plant (rotatory reactor). J. Anal. Appl. Pyrolysis 2005, 74, 254–258. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Mohamed, T.J.; Hussain, A.A.; Abas, F.O. Optimization of pyrolysis conditions of scrap tires under inert gas atmosphere. J. Anal. Appl. Pyrolysis 2004, 72, 165–170. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C.; Alvarez, R. Pyrolysis of blends of coal and tyre wastes in a fixed bed reactor and a rotary oven. Fuel 2013, 113, 817–825. [Google Scholar] [CrossRef]
- Williams, P.T.; Brindle, A.J. Aromatic chemicals from the catalytic pyrolysis of scrap tyres. J. Anal. Appl. Pyrolysis 2003, 67, 143–164. [Google Scholar] [CrossRef]
- Akkouche, N.; Balistrou, M.; Loubar, K.; Awad, S.; Tazerout, M. Heating rate effects on pyrolytic vapors from scrap truck tires. J. Anal. Appl. Pyrolysis 2017, 123, 419–429. [Google Scholar] [CrossRef]
- Islam, M.R.; Haniu, H.; Beg, M.R.A. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: Product yields, compositions and related properties. Fuel 2008, 87, 3112–3122. [Google Scholar]
- Raj, R.E.; Kennedy, Z.R.; Pillai, B. Optimization of process parameters in flash pyrolysis of waste tyres to liquid and gaseous fuel in a fluidized bed reactor. Energy Convers. Manag. 2013, 67, 145–151. [Google Scholar]
- Li, S.-Q.; Yao, Q.; Chi, Y.; Yan, J.-H.; Cen, K.-F. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Antoniou, N.; Zabaniotou, A. Experimental proof of concept for a sustainable End of Life Tyres pyrolysis with energy and porous materials production. J. Clean. Prod. 2015, 101, 323–336. [Google Scholar] [CrossRef]
- Li, W.; Huang, C.; Li, D.; Huo, P.; Wang, M.; Han, L.; Chen, G.; Li, H.; Li, X.; Wang, Y. Derived oil production by catalytic pyrolysis of scrap tires. Chin. J. Catal. 2016, 37, 526–532. [Google Scholar] [CrossRef]
- Martínez, J.D.; Murillo, R.; García, T.; Veses, A. Demonstration of the waste tire pyrolysis process on pilot scale in a continuous auger reactor. J. Hazard. Mater. 2013, 261, 637–645. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A.; Darmstadt, H. The vacuum pyrolysis of used tires: End-uses for oil and carbon black products. J. Anal. Appl. Pyrolysis 1999, 51, 201–221. [Google Scholar] [CrossRef]
- Kaminsky, W.; Mennerich, C. Pyrolysis of synthetic tire rubber in a fluidised-bed reactor to yield 1, 3-butadiene, styrene and carbon black. J. Anal. Appl. Pyrolysis 2001, 58, 803–811. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Lopez, G.; Alvarez, J.; Amutio, M.; Mkhize, N.; Danon, B.; Van der Gryp, P.; Görgens, J.; Bilbao, J.; Olazar, M. Waste truck-tyre processing by flash pyrolysis in a conical spouted bed reactor. Energy Convers. Manag. 2017, 142, 523–532. [Google Scholar] [CrossRef]
- Labaki, M.; Jeguirim, M. Thermochemical conversion of waste tyres—A review. Environ. Sci. Pollut. Res. 2017, 24, 9962–9992. [Google Scholar] [CrossRef]
- Suuberg, E.M.; Aarna, I. Porosity development in carbons derived from scrap automobile tires. Carbon 2007, 45, 1719–1726. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Processing methods, characteristics and adsorption behavior of tire derived carbons: A review. Adv. Colloid Interface Sci. 2014, 211, 93–101. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Atanes, E.; Morena, J.; Fernández-Martínez, F.; Valverde, J.L. Upgrading waste tires by chemical activation for the capture of SO2. Fuel Process. Technol. 2016, 144, 274–281. [Google Scholar] [CrossRef]
- Alexandre-Franco, M.; Fernández-González, C.; Alfaro-Domínguez, M.; Gómez-Serrano, V. Adsorption of cadmium on carbonaceous adsorbents developed from used tire rubber. J. Environ. Manag. 2011, 92, 2193–2200. [Google Scholar] [CrossRef]
- Sirimuangjinda, A.; Hemra, K.; Atong, D.; Pechyen, C. Comparison on pore development of activated carbon produced from scrap tire by potassium hydroxide and sodium hydroxide for active packaging materials. Key Eng. Mater. 2013, 545, 129–133. [Google Scholar] [CrossRef]
- Xu, J.; Yu, J.; Xu, J.; Sun, C.; He, W.; Huang, J.; Li, G. High-value utilization of waste tires: A review with focus on modified carbon black from pyrolysis. Sci. Total Environ. 2020, 742, 140235. [Google Scholar] [CrossRef]
- Zabaniotou, A.A.; Stavropoulos, G. Pyrolysis of used automobile tires and residual char utilization. J. Anal. Appl. Pyrolysis 2003, 70, 711–722. [Google Scholar] [CrossRef]
- Belgacem, A.; Rebiai, R.; Hadoun, H.; Khemaissia, S.; Belmedani, M. The removal of uranium (VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environ. Sci. Pollut. Res. 2014, 21, 684–694. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Pinedo-Flores, A.; Picasso, G.; Atanes, E.; Kou, R.S. Selective adsorption of Pb2+, Cr3+ and Cd2+ mixtures on activated carbons prepared from waste tires. J. Environ. Chem. Eng. 2017, 5, 1060–1067. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Z.; Zhang, Y.; Xu, Y.; Liu, H.; Wu, J.; Li, P. Activated Carbon Tailored by Potassium Hydroxide from Waste Tires as a Supercapacitor Electrode. ECS J. Solid State Sci. Technol. 2022, 11, 061004. [Google Scholar] [CrossRef]
- Veldevi, T.; Raghu, S.; Kalaivani, R.; Shanmugharaj, A. Waste tire derived carbon as potential anode for lithium-ion batteries. Chemosphere 2022, 288, 132438. [Google Scholar] [CrossRef]
- Naskar, A.K.; Bi, Z.; Li, Y.; Akato, S.K.; Saha, D.; Chi, M.; Bridges, C.A.; Paranthaman, M.P. Tailored recovery of carbons from waste tires for enhanced performance as anodes in lithium-ion batteries. Rsc Adv. 2014, 4, 38213–38221. [Google Scholar] [CrossRef]
- Zhao, P.; Han, Y.; Dong, X.; Zhang, C.; Liu, S. Application of activated carbons derived from scrap tires as electrode materials for supercapacitors. ECS J. Solid State Sci. Technol. 2015, 4, M35. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, F.; Meng, F.; Li, M.; Manivannan, A.; Wu, N. Effects of pore structure on performance of an activated-carbon supercapacitor electrode recycled from scrap waste tires. ACS Sustain. Chem. Eng. 2014, 2, 1592–1598. [Google Scholar] [CrossRef]
- Li, Y.; Adams, R.A.; Arora, A.; Pol, V.G.; Levine, A.M.; Lee, R.J.; Akato, K.; Naskar, A.K.; Paranthaman, M.P. Sustainable potassium-ion battery anodes derived from waste-tire rubber. J. Electrochem. Soc. 2017, 164, A1234. [Google Scholar] [CrossRef]
- Bello, A.; Momodu, D.Y.; Madito, M.; Makgopa, K.; Rambau, K.M.; Dangbegnon, J.K.; Musyoka, N.M.; Manyala, N. Influence of K3Fe (CN) 6 on the electrochemical performance of carbon derived from waste tyres by K2CO3 activation. Mater. Chem. Phys. 2018, 209, 262–270. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, P.-P.; Dong, X.-T.; Zhang, C.; Liu, S.-X. Improvement in electrochemical capacitance of activated carbon from scrap tires by nitric acid treatment. Front. Mater. Sci. 2014, 8, 391–398. [Google Scholar] [CrossRef]
- Gong, H.; Wang, D.; Jiang, Y.; Wang, L.; Zhang, K.; Qian, Y. Phosphorus-doped mesoporous carbon derived from waste tires as anode for K-ion batteries. Mater. Lett. 2021, 285, 128983. [Google Scholar] [CrossRef]
- Li, Y.; Paranthaman, M.P.; Akato, K.; Naskar, A.K.; Levine, A.M.; Lee, R.J.; Kim, S.-O.; Zhang, J.; Dai, S.; Manthiram, A. Tire-derived carbon composite anodes for sodium-ion batteries. J. Power Source 2016, 316, 232–238. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.; Simon, P.; Fauvarque, J.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Source 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Yeh, M.H.; Lin, L.Y.; Li, T.J.; Leu, Y.A.; Chen, G.L.; Tien, T.C.; Hsieh, C.Y.; Lo, S.C.; Huang, S.J.; Chiang, W.H. Synthesis of boron–doped multi–walled carbon nanotubes by an ammonia–assisted substitution reaction for applying in supercapacitors. Energy Procedia 2014, 61, 1764–1767. [Google Scholar] [CrossRef]
- Liao, M.D.; Peng, C.; Hou, S.P.; Chen, J.; Zeng, X.G.; Wang, H.-L.; Lin, J.-H. Large-Scale Synthesis of Nitrogen-Doped Activated Carbon Fibers with High Specific Surface Area for High-Performance Supercapacitors. Energy Technol. 2020, 8, 1901477. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Vellacheri, R.; Lei, Y. Recent advances in designing and fabricating self-supported nanoelectrodes for supercapacitors. Adv. Sci. 2017, 4, 1700188. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Ma, C.; Li, G.; Wang, Y.; Zhang, K.; Li, F.; Liu, C.; Cheng, H.M.; Du, Y. Nitrogen-superdoped 3D graphene networks for high-performance supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Parida, K. Montmorillonite supported metal nanoparticles: An update on syntheses and applications. Rsc Adv. 2013, 3, 13583–13593. [Google Scholar] [CrossRef]
- Karnan, M.; Subramani, K.; Sudhan, N.; Ilayaraja, N.; Sathish, M. Aloe vera derived activated high-surface-area carbon for flexible and high-energy supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. [Google Scholar] [CrossRef]
- Liang, Q.; Ye, L.; Huang, Z.-H.; Xu, Q.; Bai, Y.; Kang, F.; Yang, Q.-H. A honeycomb-like porous carbon derived from pomelo peel for use in high-performance supercapacitors. Nanoscale 2014, 6, 13831–13837. [Google Scholar] [CrossRef]
- Kim, S.-G.; Park, O.-K.; Lee, J.H.; Ku, B.-C. Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett. 2013, 14, 247–250. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, L.; Zhao, H.; Liang, F.; Chang, S.; Li, L.; Zhang, Y.; Lin, Z.; Kröger, J.; Lei, Y. Nanoelectrode design from microminiaturized honeycomb monolith with ultrathin and stiff nanoscaffold for high-energy micro-supercapacitors. Nat. Commun. 2020, 11, 299. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Lei, Y. Review on nanoarchitectured current collectors for pseudocapacitors. Small Methods 2019, 3, 1800341. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Charge storage mechanism in nanoporous carbons and its consequence for electrical double layer capacitors. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3457–3467. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J.; Peng, W.-J. Effect of activated carbon and electrolyte on properties of supercapacitor. Trans. Nonferrous Met. Soc. China 2007, 17, 1328–1333. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Jiang, L.; Wu, H.; Wu, C.; Su, J. Effect of aqueous electrolytes on the electrochemical behaviors of supercapacitors based on hierarchically porous carbons. J. Power Source 2012, 216, 290–296. [Google Scholar] [CrossRef]
- Boota, M.; Paranthaman, M.P.; Naskar, A.K.; Li, Y.; Akato, K.; Gogotsi, Y. Waste tire derived carbon–polymer composite paper as pseudocapacitive electrode with long cycle life. ChemSusChem 2015, 8, 3576–3581. [Google Scholar] [CrossRef]
- Hsiao, C.; Lee, C.; Tai, N. High retention supercapacitors using carbon nanomaterials/iron oxide/nickel-iron layered double hydroxides as electrodes. J. Energy Storage 2022, 46, 103805. [Google Scholar] [CrossRef]
- Zheng, H.; Guan, R.; Liu, Q.; Ou, K.; Li, D.-S.; Fang, J.; Fu, Q.; Sun, Y. A flexible supercapacitor with high capacitance retention at an ultra-low temperature of −65.0 °C. Electrochim. Acta 2022, 424, 140644. [Google Scholar] [CrossRef]
- Wang, K.-P.; Teng, H. Structural feature and double-layer capacitive performance of porous carbon powder derived from polyacrylonitrile-based carbon fiber. J. Electrochem. Soc. 2007, 154, A993. [Google Scholar] [CrossRef][Green Version]
- Yu, X.; Lu, J.; Zhan, C.; Lv, R.; Liang, Q.; Huang, Z.-H.; Shen, W.; Kang, F. Synthesis of activated carbon nanospheres with hierarchical porous structure for high volumetric performance supercapacitors. Electrochim. Acta 2015, 182, 908–916. [Google Scholar] [CrossRef]
- Shang, T.; Xu, Y.; Li, P.; Han, J.; Wu, Z.; Tao, Y.; Yang, Q.-H. A bio-derived sheet-like porous carbon with thin-layer pore walls for ultrahigh-power supercapacitors. Nano Energy 2020, 70, 104531. [Google Scholar] [CrossRef]

| Reactor | Temp | RT | HR | Oil % | Char% | Gas % | Ref. |
|---|---|---|---|---|---|---|---|
| Fix bed Reactor | 600 | 120 | 10 | 30.89 | 36.58 | 28.74 | [39] |
| 500 | 30 | 15 | 38 | 55.9 | 17.2 | [40] | |
| 650 | 60 | 7 | 48.4 | 41.7 | 7.6 | [41] | |
| 550 | 60 | 10 | 33 | 52 | 15 | [38] | |
| 475 | 30 | 13.1 | 65.7 | 21.2 | [42] | ||
| 430–500 | 185 | 49 | 38.3 | 12.7 | [19] | ||
| 500 | 30 | 10 | 45.9 | 37.59 | 16.5 | [43] | |
| 500 | 60 | 55.8 | 38.1 | 6.1 | [44] | ||
| 500 | 60 | 10 | 45 | 47 | 11 | [45] | |
| 500 | 120 | 5 | 45 | 42 | 13 | [37] | |
| 550 | 30 | 15 | 34 | 66 | [46] | ||
| 430 | 36 | 46.8 | 17.1 | [47] | |||
| 850 | 5 | 58 | 38 | 4 | [48] | ||
| 500 | 60 | 10 | 57 | 38 | 6 | [49] | |
| 800 | 5 | 54.78 | 37.98 | 7.24 | [50] | ||
| 375 | 42 | 50 | 8 | [51] | |||
| 475 | 36.13 | 45.5 | 18.38 | [52] | |||
| Mechanical bed Reactor | 450 | 43 | 43.9 | 13.1 | [53] | ||
| 550 | 19.6 | 60 | 49.98 | 35.12 | 10.75 | [54] | |
| 500 | 30 | 15 | 55.12 | 38.71 | 6.17 | [55] | |
| 550 | 6000 | 42.6 | 40.5 | 16.9 | [56] | ||
| 485 | 10 | 43 | 39 | 5 | [57] | ||
| 500 | 143 | 65 | 31 | 5 | [58] | ||
| 450 | 20 | 32.9 | 51.7 | 15.4 | [59] | ||
| 425 | 50 | 58.4 | 37.9 | 2.7 | [34] | ||
| 475 | 50 | 58 | 36 | 4 | [60] |
| Application | Activation Condition | Electrode Preparation | Electrochemical Cell Preparation | Ref. |
|---|---|---|---|---|
| Capacitor | WTPC was mixed with KOH in three distinct proportions (1:3, 1:5, and 1:7) and then heated for one hour at 800 °C. The maximal surface area (524) was found at a mixing ratio of 1:7. | A suspension of 0.5 mg of activated carbon in 5 mL of DI water was made and dried at 25 °C. | Aqueous 6 M KOH solution was used as the electrolyte. Electrodes of Pt foil and calomel were used as counter and reference, respectively. | [72] |
| Capacitor | First WTPC was activated by CO2 for two hours at 1000 °C. Then, a blended a prepared sample with KOH (1:4) and heated at 850 °C for two hours. | Electrode was made by coating a 1 cm × 1 cm area of nickel foam with a blend of 80 wt% active ingredients, N-methyl-2-pyrrolidone (NMP) and 10 wt% conducting carbons black (Super P), 10 wt% PVDF, and the electrode was dried at 110 °C for twenty-four hours. | In a two-electrode system, 6 mol KOH electrolyte solution was used, and the counter electrode was a platinum sheet electrode. Base electrode in a 3-electrode setup was saturated Hg/HgO. | [14] |
| Battery | Tyre powder pyrolysed at 400 °C for two hours and subsequently activated by CO2 and HO2 for various temperatures (820, 1000, 1300, 1600) | Waste tyre, super P black, and poly vinylidene difluoride mixed in 8:1:1 ratio followed by drying at 120 °C for 10 h. | 1 M LiPF6 and DMC, used as electrolyte, and the counter electrode was made of lithium foil | [73] |
| Battery | At 1000 °C, the particulate rubber samples were heated, where the temperature of the furnace the temperature was elevated from room temperature to 1000 °C at a rate of 10 °C per minute; after the temperature attained 1000 °C, it was maintained for 15 min; the furnace then receded to room temperature. | By casting a slurry comprising 80% TDAC, 15% polyvinylidene difluoride (PVDF) binders, and 5% commercial carbon (super C45), an anode was created. | The electrolyte consists of 1.0 M LiPF6 mixed in 1:1:1 volumes of ethylene carbonate (EC), diethyl carbonate (DEC), and dimethyl carbonate (DMC). | [74] |
| Supper capacitor | WTPC was activated by steam for two to four hours at 700–900 °C | Pressing a mixture of ACs, acetylene black, and poly tetra fluoro ethylene (PTFE) having a 90:5:5 weight ratio, the electrode was made. | As a reference electrode, a Ni foil and a Hg/HgO electrode were used, respectively. A 6 M KOH aqueous solution was used as an electrolyte. | [75] |
| Electric Double Layer Capacitance (EDLC), | Tyre was pyrolysed at 600 °C for three hours. Combining the resulting char with H3PO4 powder and activated at varied temperatures and activation times. | Activated carbon and polyvinylidene fluoride (PVDF) binder were mixed 9:1 in N-methyl-2-pyrrolidone (NMP) to make a slurry. An electrode was formed by vacuum-drying the slurry on Ni foil at 120 °C. | As the electrolyte, an aqueous solution of 6 M KOH is utilised. Activated carbon was the working electrode, while a Pt foil was the counter electrode and Ag|AgCl was the reference electrode. | [76] |
| K-ion battery | Crumb tyre was pyrolysed from room temperature to 400 °C, followed by temperature increases to 1100 °C and 1600 °C. | Electrode was produced by distributing a slurry containing 80 percent active material. | Potassium foil was the counter electrode. The used electrolyte was 0.8 M KPF6 dissolved in diethyl carbonate (DEC) and ethylene carbonate (EC) in a volume ratio of 1:1. | [77] |
| Energy Storage Device | WTPC was combined with K2CO3 powder and activated at 800 °C with various mixing ratios. | Activated carbon, polyvinylidene fluoride (PVDF) binder, and acetylene black were mixed 80:10:10 to make a slurry and dried at 60 °C to form an electrode | Dissolving 6.5848 g of salt in 20 mL of deionized water produced 1 M K3[Fe(CN)]6, and 1 mL of the solution was mixed with 40 mL of 1 M HNO3 to prepare electrolyte | [78] |
| Super Capacitor | WTAC was activated at 800 °C for 4 h with HNO3 reagent | The electrodes were produced by incorporating 95 wt.% AC with 5 wt.% polytetrafluoroethylene (PTFE) binder in ethanol and then moulding the mixture into thick films. Then, electrodes with a diameter of 10 mm were cut and dried up in air at 60 °C for 12 h. | Utilized Ni foil as a counter electrode, Hg/HgO as a reference electrode, and a 6 mol/L KOH aqueous solution as the electrolyte. | [79] |
| K-ion Battery | In a furnace, phosphoric acid and tyre rubber were placed in a mass ratio of 1:3 for calcination for two hours at 800 °C in nitrogen flow | The composition of the electrode was (80:10:10) PMC, polyvinylidene fluoride, and super P. | Potassium sheets were utilized as the counter electrode. The solution of electrolyte comprised 1 M KPF6 dissolving in a 1:1 volume ratio of vinyl carbonate and diethyl carbonate (EC/DEC). | [80] |
| Na-ion Battery | From ambient temperature to 400 °C, tyre rubber was pyrolysed at 1 °C/min ramp rate, followed by 1400 °C and 1600 °C, respectively. | Active material, PVDF binder, and conductive carbon C45 were mixed in a slurry at the weight ratio of 80:10:10 to produce the electrode. | Electrolyte consisted of 1 M NaClO4 in diethyl carbonate (DEC) and ethylene carbonate (EC). | [81] |
| Deference | Graphite | TDAC | Ref |
|---|---|---|---|
| Surface area | 0.5 to 2.5 m2/g | 50 to 400 m2/g | [76,77,80,81] |
| Crystal Structure (Id/IG ratio/) | 0.05 to 0.3 | 0.6 to 1.2 | |
| Price | USD 3.5–9.90/Kg according to purity. | USD 1.50 to USD 2.50/Kg | |
| Environmental sustainability | Less | More environmental | |
| Performance in KIB | 197 mAhg−1 during 1st cycle, 10 mAhg−1 after 50 cycle, Rate C/2 | 155 mAhg−1 after 200 cycles | |
| Performance in NaIB | 290 mAhg−1 after 100 cycles (30 mAg−1), 83% | 203 mAhg−1 after 100 cycles (20 mAg−1), 66% |
| Activation Method | Physical Properties | Number of Electrodes | Electrolyte | Performance | Application | Ref. | |
|---|---|---|---|---|---|---|---|
| SA (m2/g) | TPV cm3/g | ||||||
| KOH | 524 | 1.236 | Three | 6 M KOH | Specific capacitance 408 Fg−1 (0.25 Ag−1); capacity retention 97% after 10,000 cycles | Supper capacitor | [72] |
| CO2 +KOH | 733 | 0.318 | Two/Three | 6 M KOH | The three-electrode system performed with 192 F/g specific capacitance at 0.5 A g−1 and 73% rate capability at 50 A g−1. A two-electrode system exhibited 106% capacitance retention after 10,000 cycles at 2 A g−1, 4.7 Whkg−1 energy density, and a maximal power density of 6362.6 W kg-1. | Electrical double-layer capacitor (EDLC) | [14] |
| Steam+ CO2 | 369 | Multiple | 1:1, 1 M LiPF6 and DMC | 350 mAhg−1 specific capacitance (at 300 mA g−1); capacity retention of 81% after 500 cycles; 99% coulombic efficiency (300 mAg−1) | Lithium-ion battery | [73] | |
| Thermal | Multiple | 1 M LiPF6 | Carbon derived from sulfonated tyre rubber had a 71% coulombic efficiency at the start. After 100 cycles, the cell with carbon from sulfonated tyre rubber as the anode had a reversible capacity of 390 mAh g−1, and it was nearly 100% efficient in terms of coulombic capacity. The first discharge capacity of sulfonated tire-rubber-derived carbon is approximately 545 mAhg−1, the reverse charge capacity is about 387 mAhg−1, and the capacity that cannot be changed is 158 mAhg−1. | Lithium-ion battery | [74] | ||
| Steam (850) | 981 | 2.07 | Multiple | 6 M KOH | The energy density of a supercapacitor is 13.9 Wh kg−1 at 500 W kg−1 of power and 13.3 Wh kg−1 at 4000 W kg−1 of power. | Supercapacitor | [75] |
| H3PO4 (900) | 563 | 0.201 | Three | 6 M KOH | EDLC was found to have a maximal specific capacitance of 106 F/g and a maximum rate capability of 0.723. The maximum specific surface area, 563 m2/g, was found for 0.201 cm3/g pore volume. | Supercapacitor | [76] |
| Thermal (1100) | 34.5 | 8 M KPF6 | Initial capacity of flow below 1.8 V is 192 mAh g−1 and capacity retention after 200 cycles is 80.7%, corresponding to a 155 mAh g−1 capacity. | K-Ion battery | [77] | ||
| K2CO3 | 385 | Three | 1 M K3[Fe(CN)]6 | After 1000 cycles, the battery has a discharge capacity of 50 mAh g−1 at 0.25 A g−1, a capacitance of 140 F g−1 at the identical specific current, and an energy efficiency of 70%. | Energy storage device | [78] | |
| HNO3 | 915 | 0.95 | Three | 6 M KOH | After 1000 cycles, the specific capacitance of the electrode decreases from an initial value of 190 F/g to 140 F/g. The retention rate is 72%. | supercapacitor | [79] |
| HNO3 | 241.5 | Multiple | 1 M KPF6 | At 100 mA/g, the reversible capacity was 181.8 mA h/g, and the rate performance was outstanding. It has a capacity of 128.5 mA h/g at 500 mA/g and at a current density of 50 mA/g. The capacity is 218.2 mA h/g. | K-ion battery | [80] | |
| Thermal | 148 | Multiple | 1 M NaClO4 | 203 mAh g−1 capacity after 100 cycles at 20 mA g−1; 66% cycle efficiency and 203 mAh g−1 capacity at the 100th cycle | Na-ion battery | [81] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerin, N.H.; Rasul, M.G.; Jahirul, M.I.; Sayem, A.S.M.; Haque, R. Electrochemical Application of Activated Carbon Derived from End-of-Life Tyres: A Technological Review. Sustainability 2024, 16, 47. https://doi.org/10.3390/su16010047
Zerin NH, Rasul MG, Jahirul MI, Sayem ASM, Haque R. Electrochemical Application of Activated Carbon Derived from End-of-Life Tyres: A Technological Review. Sustainability. 2024; 16(1):47. https://doi.org/10.3390/su16010047
Chicago/Turabian StyleZerin, Nusrat H., Mohammad G. Rasul, M. I. Jahirul, A.S.M. Sayem, and R. Haque. 2024. "Electrochemical Application of Activated Carbon Derived from End-of-Life Tyres: A Technological Review" Sustainability 16, no. 1: 47. https://doi.org/10.3390/su16010047
APA StyleZerin, N. H., Rasul, M. G., Jahirul, M. I., Sayem, A. S. M., & Haque, R. (2024). Electrochemical Application of Activated Carbon Derived from End-of-Life Tyres: A Technological Review. Sustainability, 16(1), 47. https://doi.org/10.3390/su16010047