The Comet Assay as a Sustainable Method for Evaluating the Genotoxicity Caused by the Soluble Fraction Derived from Sewage Sludge on Diverse Cell Types, Including Lymphocytes, Coelomocytes and Allium cepa L. Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Analysis of Sludge and Acquisition of the Soluble Fraction from Biosolids
2.2. Isolation of Lymphocytes and Treatment with SFS
2.3. Isolation of Coelomocytes and Treatment with SFS
2.4. Isolation of Allium cepa L. nuclei and Treatment with SFS

2.5. The Comet Assay
2.5.1. Slide Preparation
2.5.2. Lysis
2.5.3. Pre-Electrophoresis
2.5.4. Electrophoresis
2.6. Determining 3-Indole Acetic Acid (IAA)
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rusănescu, C.O.; Rusănescu, M.; Voicu, G.; Paraschiv, G.; Biriș, S.Ș.; Popescu, I.N. The Recovery of Vermicompost Sewage Sludge in Agriculture. Agronomy 2022, 12, 2653. [Google Scholar] [CrossRef]
- Arthur Andersen Environment Risk Consulting Department. Disposal and Recycling Routes for Sewage Sludge. In Part 3—Scientific and Technical Report; Office for Official Publications of the European Communities: Luxembourg, 2001; pp. 151–2001. [Google Scholar]
- Lima, M.; Mattos, C.; Vieira, P.; Almeida, L. Geração de Lodo de Esgoto e Seu Potencial Como Fonte de Matéria Orgânica Para a Agricultura. In Manual de uso Agrícola e Disposição do Lodo de Esgoto Para o Estado do Espirito Santo; INCAPER: Vitoria, Brazil, 2011. [Google Scholar]
- Singh, R.P.; Agrawal, M. Potential Benefits and Risks of Land Application of Sewage Sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Malara, A.; Jośko, I.; Lesiuk, A. The Phytotoxicity Changes of Sewage Sludge-Amended Soils. Water Air Soil. Pollut. 2012, 223, 4937–4948. [Google Scholar] [CrossRef] [PubMed]
- Yurievna Selivanovskaya, S.; Zinnatovna Latypova, V.; Aleksandrovna Artamonova, L. Use of Sewage Sludge Compost as the Restoration Agent on the Degraded Soil of Tatarstan. J. Environ. Sci. Health Part. A 2003, 38, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.W.A.; Barros, D.A.S.; Melo, E.E.C.; Oliveira, A.B. Alterações Químicas Em Solos e Crescimento de Milho e Feijoeiro Após Aplicação de Lodo de Esgoto. Rev. Bras. Ciênc. Solo 2004, 28, 385–392. [Google Scholar] [CrossRef][Green Version]
- de Lourdes Marzo Solano, M.; de Lima, P.L.; Luvizutto, J.F.; Silva, P.R.; de Aragão Umbuzeiro, G.; de Camargo, J.L. In Vivo Genotoxicity Evaluation of a Treated Urban Sewage Sludge Sample. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 676, 69–73. [Google Scholar] [CrossRef]
- Sabbahi, S.; Ben Ayed, L.; Trad, M.; Berndtsson, R.; Karanis, P. Parasitological Assessment of Sewage Sludge Samples for Potential Agricultural Reuse in Tunisia. Int. J. Environ. Res. Public. Health 2022, 19, 1657. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I. The Reuse of Biosolids on Agricultural Land: Critical Issues and Perspective. Water Environ. Res. 2020, 92, 11–25. [Google Scholar] [CrossRef]
- Chenon, P.; Gauthier, L.; Loubières, P.; Séverac, A.; Delpoux, M. Evaluation of the Genotoxic and Teratogenic Potential of a Municipal Sludge and Sludge-Amended Soil Using the Amphibian Xenopus laevis and the Tobacco: Nicotiana tabacum L. var. xanthi Dulieu. Sci. Total Environ. 2003, 301, 139–150. [Google Scholar] [CrossRef][Green Version]
- Rank, J.; Nielsen, M.H. Genotoxicity Testing of Wastewater Sludge Using the Allium cepa Anaphase-Telophase Chromosome Aberration Assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1998, 418, 113–119. [Google Scholar] [CrossRef]
- Grotto, D.; Carneiro, M.F.H.; Sauer, E.; Garcia, S.C.; De Melo, W.J.; Barbosa, F. Evaluation of Biochemical and Redox Parameters in Rats Fed with Corn Grown in Soil Amended with Urban Sewage Sludge. Ecotoxicol. Environ. Saf. 2013, 95, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef]
- Kasprzak, K.S. Possible Role of Oxidative Damage in Metal-Induced Carcinogenesis. Cancer Investig. 1995, 13, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.P. DNA Repair: Caretakers of the Genome? Curr. Biol. 1997, 7, R576–R579. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-X.; Chen, T.-B.; Huang, Z.-C.; Lei, M.; Liao, X.-Y. Effect of Arsenic on Chloroplast Ultrastructure and Calcium Distribution in Arsenic Hyperaccumulator Pteris vittata L. Chemosphere 2006, 62, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and Genotoxic Damages in Plants in Response to Heavy Metal Stress and Maintenance of Genome Stability. Plant Signal Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.W.; Sherif, F.K.; El-Atar, H.; Ez-Eldin, H. Residual Effect of Sewage Sludge on Soil and Several Yield Parameters of Zea mays. Res. J. Environ. Toxicol. 2009, 3, 86–93. [Google Scholar] [CrossRef]
- Marzougui, N.; Ounalli, N.; Sabbahi, S.; Fezzani, T.; Abidi, F.; Jebari, S.; Melki, S.; Berndtsson, R.; Oueslati, W. How Can Sewage Sludge Use in Sustainable Tunisian Agriculture Be Increased? Sustainability 2022, 14, 13722. [Google Scholar] [CrossRef]
- Kızılkaya, R.; Bayraklı, B. Effects of N-Enriched Sewage Sludge on Soil Enzyme Activities. Appl. Soil. Ecol. 2005, 30, 192–202. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, Y.-K.; Lin, S.-H.; Fang, Y.-Y.; Bai, J.-G. Hydrogen Peroxide Pretreatment Alters the Activity of Antioxidant Enzymes and Protects Chloroplast Ultrastructure in Heat-Stressed Cucumber Leaves. Sci. Hortic. 2010, 126, 20–26. [Google Scholar] [CrossRef]
- Gjorgieva, D.; Kadifkova Panovska, T.; Ruskovska, T.; Bačeva, K.; Stafilov, T. Influence of Heavy Metal Stress on Antioxidant Status and DNA Damage in Urtica Dioica. BioMed Res. Int. 2013, 2013, 276417. [Google Scholar] [CrossRef] [PubMed]
- Britt, A.B. Molecular Genetics of DNA Repair in Higher Plants. Trends Plant Sci. 1999, 4, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.-J.; Zhang, X.-H.; Chen, M.-M.; Cao, Q. Oxidative Stress and DNA Damages Induced by Cadmium Accumulation. J. Environ. Sci. 2007, 19, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S. DNA Damage in Cicer Plant Grown on Soil Polluted with Heavy Metals. J. King Saud. Univ. Sci. 2015, 27, 217–223. [Google Scholar] [CrossRef]
- Das, A.; Parbat, D.; Shome, A.; Manna, U. Sustainable Biomimicked Oil/Water Wettability That Performs Under Severe Challenges. ACS Sustain. Chem. Eng. 2019, 7, 11350–11359. [Google Scholar] [CrossRef]
- Das, A.; Naskar, S.; Dhar, M.; Manna, U. Rapid and Scalable Synthesis of a Vanillin-Based Organogelator and Its Durable Composite for a Comprehensive Remediation of Crude-Oil Spillages. ACS Appl. Mater. Interfaces 2021, 13, 46803–46812. [Google Scholar] [CrossRef]
- Jiang, N.; Naz, S.; Ma, Y.; Ullah, Q.; Khan, M.Z.; Wang, J.; Lu, X.; Luosang, D.-Z.; Tabassum, S.; Chatha, A.M.M.; et al. An Overview of Comet Assay Application for Detecting DNA Damage in Aquatic Animals. Agriculture 2023, 13, 623. [Google Scholar] [CrossRef]
- Amin, A.W. Evaluation of the Genotoxicity of Residual Repeated Applications of Sewage Sludge on M2 Meiocytes of Zea Plants. Res. J. Environ. Toxicol. 2011, 5, 235–250. [Google Scholar] [CrossRef]
- Gawdzik, J.; Gawdzik, B. Mobility of Heavy Metals in Municipal Sewage Sludge from Different Throughput Sewage Treatment Plants. Pol. J. Environ. Stud. 2012, 21, 1603–1611. [Google Scholar]
- McBride, M.B. Toxic Metal Accumulation from Agricultural Use of Sludge: Are USEPA Regulations Protective? J. Environ. Qual. 1995, 24, 5–18. [Google Scholar] [CrossRef]
- Udom, B.E.; Mbagwu, J.S.C.; Adesodun, J.K.; Agbim, N.N. Distributions of Zinc, Copper, Cadmium and Lead in a Tropical Ultisol after Long-Term Disposal of Sewage Sludge. Environ. Int. 2004, 30, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Mateuca, R.; Lombaert, N.; Aka, P.V.; Decordier, I.; Kirsch-Volders, M. Chromosomal Changes: Induction, Detection Methods and Applicability in Human Biomonitoring. Biochimie 2006, 88, 1515–1531. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, L.; Zhao, J.; Ma, N. Characteristics of Sewage Sludge and Distribution of Heavy Metal in Plants with Amendment of Sewage Sludge. J. Environ. Sci. 2006, 18, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Henning, B.; Snyman, H.; Aveling, T. Plant-Soil Interactions of Sludge-Borne Heavy Metals and the Effect on Maize (Zea mays L.) Seedling Growth. WSA 2004, 27, 71–78. [Google Scholar] [CrossRef]
- Corrêa Martins, M.N.; de Souza, V.V.; Souza, T. da S. Genotoxic and Mutagenic Effects of Sewage Sludge on Higher Plants. Ecotoxicol. Environ. Saf. 2016, 124, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Pandey, A.K.; Gupta, P.K. Heavy Metals in Sludge Produced from UASB Treatment Plant at Mirzapur, India. Pollution 2021, 7, 607–616. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Sroka, A.; Jabłońska, K. The Impact of Auxins Used in Assisted Phytoextraction of Metals from the Contaminated Environment on the Alterations Caused by Lead(II) Ions in the Organization of Model Lipid Membranes. Colloids Surf. B Biointerfaces 2016, 143, 124–130. [Google Scholar] [CrossRef]
- Rodriguez, E.; Santos, C.; Azevedo, R.; Moutinho-Pereira, J.; Correia, C.; Dias, M.C. Chromium (VI) Induces Toxicity at Different Photosynthetic Levels in Pea. Plant Physiol. Biochem. 2012, 53, 94–100. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between Plant Hormones and Heavy Metals Responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Zhao, F.; Han, M.; Zhang, S.; Wang, K.; Zhang, C.; Liu, T.; Liu, W. Hydrogen Peroxide-Mediated Growth of the Root System Occurs via Auxin Signaling Modification and Variations in the Expression of Cell-Cycle Genes in Rice Seedlings Exposed to Cadmium Stress. J. Integr. Plant Biol. 2012, 54, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Schowanek, D.; Carr, R.; David, H.; Douben, P.; Hall, J.; Kirchmann, H.; Patria, L.; Sequi, P.; Smith, S.; Webb, S. A Risk-Based Methodology for Deriving Quality Standards for Organic Contaminants in Sewage Sludge for Use in Agriculture—Conceptual Framework. Regul. Toxicol. Pharmacol. 2004, 40, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Ladeira, C.; Giovannelli, L.; Boutet-Robinet, E.; Bonassi, S.; Neri, M.; Gajski, G.; Duthie, S.; Bo’, C.D.; Riso, P.; et al. Application of the Comet Assay in Human Biomonitoring: An hCOMET Perspective. Mutat. Res./Rev. Mutat. Res. 2020, 783, 108288. [Google Scholar] [CrossRef] [PubMed]
- Fomin, A.; Hafner, C. Evaluation of Genotoxicity of Emissions from Municipal Waste Incinerators with Tradescantia-Micronucleus Bioassay (Trad-MCN). Mutat. Res. 1998, 414, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.F. Higher Plant Assays for the Detection of Genotoxicity in Air Polluted Environments. Ecosyst. Health 1998, 4, 210–229. [Google Scholar] [CrossRef]
- Martins, M.; Costa, P.M. The Comet Assay in Environmental Risk Assessment of Marine Pollutants: Applications, Assets and Handicaps of Surveying Genotoxicity in Non-Model Organisms. Mutagenesis 2015, 30, 89–106. [Google Scholar] [CrossRef]
- Tondoh, J.E.; Monin, L.M.; Tiho, S.; Csuzdi, C. Can Earthworms Be Used as Bio-Indicators of Land-Use Perturbations in Semi-Deciduous Forest? Biol. Fertil. Soils 2007, 43, 585–592. [Google Scholar] [CrossRef]
- Peres, G.; Cluzeau, D.; Ferrand, C.; Peron, D. Earthworms Used as Indicators of Agricultural Managements. In BIO-BIO Project. Biodiversity-Bioindication to Evaluate Soil Health; Cenci, R.M., Sena, F., Eds.; 2006; Volume 22245, pp. 107–115. ISBN 92-79-02011-0. Available online: https://esdac.jrc.ec.europa.eu/ESDB_Archive/eusoils_docs/other/EUR22245.pdf (accessed on 7 December 2023).
- Verschaeve, L.; Gilles, J. Single Cell Gel Electrophoresis Assay in the Earthworm for the Detection of Genotoxic Compounds in Soils. Bull. Environ. Contam. Toxicol. 1995, 54, 112–119. [Google Scholar] [CrossRef]
- Salagovic, J.; Gilles, J.; Verschaeve, L.; Kalina, I. The Comet Assay for the Detection of Genotoxic Damage in the Earthworms: A Promising Tool for Assessing the Biological Hazards of Polluted Sites. Folia Biol. 1996, 42, 17–21. [Google Scholar]
- Adamowicz, A. Morphology and Ultrastructure of the Earthworm Dendrobaena veneta (Lumbricidae) Coelomocytes. Tissue Cell 2005, 37, 125–133. [Google Scholar] [CrossRef]
- Liu, W.; Li, P.J.; Qi, X.M.; Zhou, Q.X.; Zheng, L.; Sun, T.H.; Yang, Y.S. DNA Changes in Barley (Hordeum vulgare) Seedlings Induced by Cadmium Pollution Using RAPD Analysis. Chemosphere 2005, 61, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Lemtiri, A.; Liénard, A.; Alabi, T.; Brostaux, Y.; Cluzeau, D.; Francis, F.; Colinet, G. Earthworms Eisenia fetida Affect the Uptake of Heavy Metals by Plants Vicia faba and Zea mays in Metal-Contaminated Soils. Appl. Soil. Ecol. 2016, 104, 67–78. [Google Scholar] [CrossRef]
- Karimi, F.; Rahimi, G.; Kolahchi, Z. Interaction Effects of Salinity, Sewage Sludge, and Earthworms on the Fractionations of Zn and Cu, and the Metals Uptake by the Earthworms in a Zn- and Cu-Contaminated Calcareous Soil. Environ. Sci. Pollut. Res. 2020, 27, 10565–10580. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Y.; Shen, M.; Zeng, G.; Zhou, M.; Li, M. Effect of Vermicomposting on Concentration and Speciation of Heavy Metals in Sewage Sludge with Additive Materials. Bioresour. Technol. 2016, 218, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.; Buschini, A.; Restivo, F.M.; Ficarelli, A.; Cassoni, F.; Ferrero, I.; Rossi, C. Comet Assay Application in Environmental Monitoring: DNA Damage in Human Leukocytes and Plant Cells in Comparison with Bacterial and Yeast Tests. Mutagenesis 1999, 14, 547–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ostling, O.; Johanson, K.J. Microelectrophoretic Study of Radiation-Induced DNA Damages in Individual Mammalian Cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Faust, F.; Kassie, F.; Knasmüller, S.; Boedecker, R.H.; Mann, M.; Mersch-Sundermann, V. The Use of the Alkaline Comet Assay with Lymphocytes in Human Biomonitoring Studies. Mutat. Res./Rev. Mutat. Res. 2004, 566, 209–229. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Azqueta, A.; Muruzabal, D.; Boutet-Robinet, E.; Milic, M.; Dusinska, M.; Brunborg, G.; Møller, P.; Collins, A.R. Technical Recommendations to Perform the Alkaline Standard and Enzyme-Modified Comet Assay in Human Biomonitoring Studies. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 843, 24–32. [Google Scholar] [CrossRef]
- Speit, G.; Vasquez, M.; Hartmann, A. The Comet Assay as an Indicator Test for Germ Cell Genotoxicity. Mutat. Res./Rev. Mutat. Res. 2009, 681, 3–12. [Google Scholar] [CrossRef]
- Cordelli, E.; Bignami, M.; Pacchierotti, F. Comet Assay: A Versatile but Complex Tool in Genotoxicity Testing. Toxicol. Res. 2021, 10, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Moya, C. Use of Comet Assay in Human Lymphocytes as a Molecular Biomarker for Simultaneous Monitoring of Genetic Damage and Genotoxicity in Residents Who Lived Nearby the Santiago River, Mexico, in 2012. Glob. J. Biotechnol. Biomater. Sci. 2015, 1, 4–8. [Google Scholar] [CrossRef]
- Eyambe, G.S.; Goven, A.J.; Fitzpatrick, L.C.; Venables, B.J.; Cooper, E.L. A Non-Invasive Technique for Sequential Collection of Earthworm (Lumbricus Terrestris) Leukocytes during Subchronic Immunotoxicity Studies. Lab. Anim. 1991, 25, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gichner, T.; Mühlfeldová, Z. Induced DNA Damage Measured by the Comet Assay in 10 Weed Species. Biol. Plant. 2002, 45, 509–516. [Google Scholar] [CrossRef]
- Singh, N.P. Microgel Electrophoresis of DNA from Individual Cells. In Technologies for Detection of DNA Damage and Mutations; Pfeifer, G.P., Ed.; Springer: Boston, MA, USA, 1996; pp. 3–24. ISBN 978-1-4899-0303-7. [Google Scholar]
- Juchimiuk, J.; Gnys, A.; Maluszynska, J. DNA Damage Induced by Mutagens in Plant and Human Cell Nuclei in Acellular Comet Assay. Folia Histochem. Cytobiol. 2006, 44, 127–131. [Google Scholar]
- Bandyopadhyay, A.; Mukherjee, A. Sensitivity of Allium and Nicotiana in Cellular and Acellular Comet Assays to Assess Differential Genotoxicity of Direct and Indirect Acting Mutagens. Ecotoxicol. Environ. Saf. 2011, 74, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Rajaguru, P.; Suba, S.; Palanivel, M.; Kalaiselvi, K. Genotoxicity of a Polluted River System Measured Using the Alkaline Comet Assay on Fish and Earthworm Tissues. Environ. Mol. Mutagen. 2003, 41, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, L.-S.; Wang, J.; Wang, J.-H.; Xie, H.; Song, Y. Assessment of the Genotoxicity of Endosulfan in Earthworm and White Clover Plants Using the Comet Assay. Arch. Environ. Contam. Toxicol. 2009, 56, 742–746. [Google Scholar] [CrossRef]
- Goldschmidt, E.E.; Goren, R.; Monselise, S.P. The IAA-Oxidase System of Citrus Roots. Planta 1966, 72, 213–222. [Google Scholar] [CrossRef]
- Júnior, E.O.D.C.; Pereira, B.B.; Morelli, S. Monitoring Genotoxicity Potential in the Mumbuca Stream, Minas Gerais, Brazil. J. Toxicol. Environ. Health Part. A 2015, 78, 1277–1287. [Google Scholar] [CrossRef]
- Fang, W.; Qi, G.; Wei, Y.; Kosson, D.S.; Van Der Sloot, H.A.; Liu, J. Leaching Characteristic of Toxic Trace Elements in Soils Amended by Sewage Sludge Compost: A Comparison of Field and Laboratory Investigations. Environ. Pollut. 2018, 237, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, W.N.D.; França, E.J.D.; Pereira, D.R.; Lima, M.D.V.; Silva, H.A.M.F.; Araújo, H.D.A.D.; Sá, J.L.F.; Melo, A.M.M.D.A. Study of Genotoxic and Cytotoxic Effects after Acute and Chronic Exposures to Industrial Sewage Sludge on Biomphalaria Glabrata Hemocytes. Chemosphere 2020, 249, 126218. [Google Scholar] [CrossRef]
- Navarrete, M.H.; Carrera, P.; de Miguel, M. Consuelo de la Torre A Fast Comet Assay Variant for Solid Tissue Cells. The Assessment of DNA Damage in Higher Plants. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1997, 389, 271–277. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.; Oliveira Feitosa, L.; Rodrigues Maruyama, C.; Abreu Barga, M.; Yamawaki, P.C.; Vieira, I.J.; Teixeira, E.M.; Corrêa, A.C.; Caparelli Mattoso, L.H.; Fernandes Fraceto, L. Evaluation of the Genotoxicity of Cellulose Nanofibers. Int. J. Nanomed. 2012, 7, 3555. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.; Littlewood, T. A Matter of Life and Cell Death. Science 1998, 281, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, M.; Devin, S.; Leyval, C.; Morel, J.-L.; Vasseur, P. The Influence of Thermal Desorption on Genotoxicity of Multipolluted Soil. Ecotoxicol. Environ. Saf. 2010, 73, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Kille, P.; Andre, J.; Anderson, C.; Ang, H.N.; Bruford, M.W.; Bundy, J.G.; Donnelly, R.; Hodson, M.E.; Juma, G.; Lahive, E.; et al. DNA Sequence Variation and Methylation in an Arsenic Tolerant Earthworm Population. Soil. Biol. Biochem. 2013, 57, 524–532. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic. Agronomy 2022, 12, 2366. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K.; Suprasanna, P.; D’Souza, S.F. Identification and Profiling of Arsenic Stress-Induced microRNAs in Brassica juncea. J. Exp. Bot. 2013, 64, 303–315. [Google Scholar] [CrossRef]
- Zelinová, V.; Alemayehu, A.; Bočová, B.; Huttová, J.; Tamás, L. Cadmium-Induced Reactive Oxygen Species Generation, Changes in Morphogenic Responses and Activity of Some Enzymes in Barley Root Tip Are Regulated by Auxin. Biologia 2015, 70, 356–364. [Google Scholar] [CrossRef]
- Bennett, T.; Sieberer, T.; Willett, B.; Booker, J.; Luschnig, C.; Leyser, O. The Arabidopsis MAX Pathway Controls Shoot Branching by Regulating Auxin Transport. Curr. Biol. 2006, 16, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ma, L.Q.; Vu, J.C.; Raj, A. Effects of Arsenic on Nitrate Metabolism in Arsenic Hyperaccumulating and Non-Hyperaccumulating Ferns. Environ. Pollut. 2009, 157, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Wang, X. Foliar Application of Silica Sol Alleviates Boron Toxicity in Rice (Oryza sativa) Seedlings. J. Hazard. Mater. 2022, 423, 127175. [Google Scholar] [CrossRef] [PubMed]
- Stobart, A.K.; Griffiths, W.T.; Ameen-Bukhari, I.; Sherwood, R.P. The Effect of Cd2+ on the Biosynthesis of Chlorophyll in Leaves of Barley. Physiol. Plant. 1985, 63, 293–298. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, C.; Zheng, Q.; Huang, Z.; Yu, C. Cadmium Stress Inhibits the Growth of Primary Roots by Interfering Auxin Homeostasis in Sorghum Bicolor Seedlings. J. Plant Biol. 2017, 60, 593–603. [Google Scholar] [CrossRef]
- Balestri, M.; Ceccarini, A.; Forino, L.M.C.; Zelko, I.; Martinka, M.; Lux, A.; Ruffini Castiglione, M. Cadmium Uptake, Localization and Stress-Induced Morphogenic Response in the Fern Pteris vittata. Planta 2014, 239, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Kumar, R.G.; Verma, S.; Dubey, R.S. Effect of Cadmium on Lipid Peroxidation, Superoxide Anion Generation and Activities of Antioxidant Enzymes in Growing Rice Seedlings. Plant Sci. 2001, 161, 1135–1144. [Google Scholar] [CrossRef]
- Verma, K.; Shekhawat, G.S.; Sharma, A.; Mehta, S.K.; Sharma, V. Cadmium Induced Oxidative Stress and Changes in Soluble and Ionically Bound Cell Wall Peroxidase Activities in Roots of Seedling and 3–4 Leaf Stage Plants of Brassica juncea (L.) Czern. Plant Cell Rep. 2008, 27, 1261–1269. [Google Scholar] [CrossRef]
- Gustavsson, L.; Engwall, M. Genotoxic Activity of Nitroarene-Contaminated Industrial Sludge Following Large-Scale Treatment in Aerated and Non-Aerated Sacs. Sci. Total Environ. 2006, 367, 694–703. [Google Scholar] [CrossRef]
- Srivastava, R.; Kumar, D.; Gupta, S.K. Bioremediation of Municipal Sludge by Vermitechnology and Toxicity Assessment by Allium cepa. Bioresour. Technol. 2005, 96, 1867–1871. [Google Scholar] [CrossRef]
- Silva, P.R.P.; Barbisan, L.F.; Dagli, M.L.Z.; Saldiva, P.H.N. Sewage Sludge Does Not Induce Genotoxicity and Carcinogenesis. Genet. Mol. Biol. 2012, 35, 657–663. [Google Scholar] [CrossRef]
- Basu, R.; Talapatra, S.N.; Mukhopadhyay, A.; Roy Goswami, M.; Ray, S.S.; Chakrabarti, P.; Ram, S.S.; Sudarshan, M.; Chakraborty, A.; Dasgupta, A.; et al. Genotoxicity Study with Special Reference to Comet Test in the Blood Cells of Workers Exposed to Sewage Water. Adv. Toxicol. 2014, 2014, 251812. [Google Scholar] [CrossRef][Green Version]
- Friis, L.; Vaghef, H.; Edling, C.; Hellman, B. No Increased DNA Damage in Peripheral Lymphocytes of Sewage Workers as Evaluated by Alkaline Single Cell Gel Electrophoresis. Occup. Environ. Med. 1997, 54, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Oreščanin, V.; Garaj-Vrhovac, V. Cytogenotoxicity of Sewage Sludge Leachate before and after Calcium Oxide-Based Solidification in Human Lymphocytes. Ecotoxicol. Environ. Saf. 2011, 74, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Tamae, K. Earthworms and Soil Pollutants. Sensors 2011, 11, 11157–11167. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Kashif Zahoor, M.; Riaz, D.; Arshad, M.; Yaqoob, R.; Ranian, K. Sewage Sludge-Induced Effect on Growth, Enzyme Inhibition, and Genotoxicity Can Be Ameliorated Using Wheat Straw and Biochar in Pheretima posthuma Earthworms. Front. Environ. Sci. 2022, 10, 888394. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Singh, K.; Vig, A.P. Genotoxicity Monitoring of Industrial Wastes Using Plant Bioassays and Management through Vermitechnology: A Review. Agric. Nat. Resour. 2017, 51, 325–337. [Google Scholar] [CrossRef]
- Corrêa Martins, M.N.; Souza, V.V.D.; Silva Souza, T.D. Cytotoxic, Genotoxic and Mutagenic Effects of Sewage Sludge on Allium cepa. Chemosphere 2016, 148, 481–486. [Google Scholar] [CrossRef]


| Elements Analyzed | u.m. * | Determined Values | Analysis Methods ** |
|---|---|---|---|
| pH (25 °C)-SFS L/S:10/1 | pH units | 6 | (1) |
| Conductivity (25 °C)-SFS L/S:10/1 | µS/cm | 1979 | (2) |
| Arsenic (As) | mg/kg | 1.18 | (3) |
| Barium (Ba) | mg/kg | 2.90 | (3) |
| Cadmium (Cd) | mg/kg | ˂0.5 | (3) |
| Chromium (Cr) | mg/kg | ˂0.5 | (3) |
| Copper (Cu) | mg/kg | 3.50 | (3) |
| Mercury (Hg) | mg/kg | 0.23 | (4) |
| Molybdenum (Mo) | mg/kg | ˂1 | (3) |
| Nickel (Ni) | mg/kg | ˂2 | (3) |
| Lead (Pb) | mg/kg | ˂2 | (3) |
| Selenium (Se) | mg/kg | ˂0.1 | (5) |
| Antimony (Sb) | mg/kg | ˂0.1 | (6) |
| Zinc (Zn) | mg/kg | 18.9 | (3) |
| Chloride | mg/kg | 482 | (7) |
| Fluoride | mg/kg | ˂100 | (7) |
| Sulphate | mg/kg | 109 | (7) |
| Total dissolved solids (TDS) | mg/kg | 16,560 | (8) |
| Dissolved organic carbon (DOC) | mg/kg | 620 | (9) |
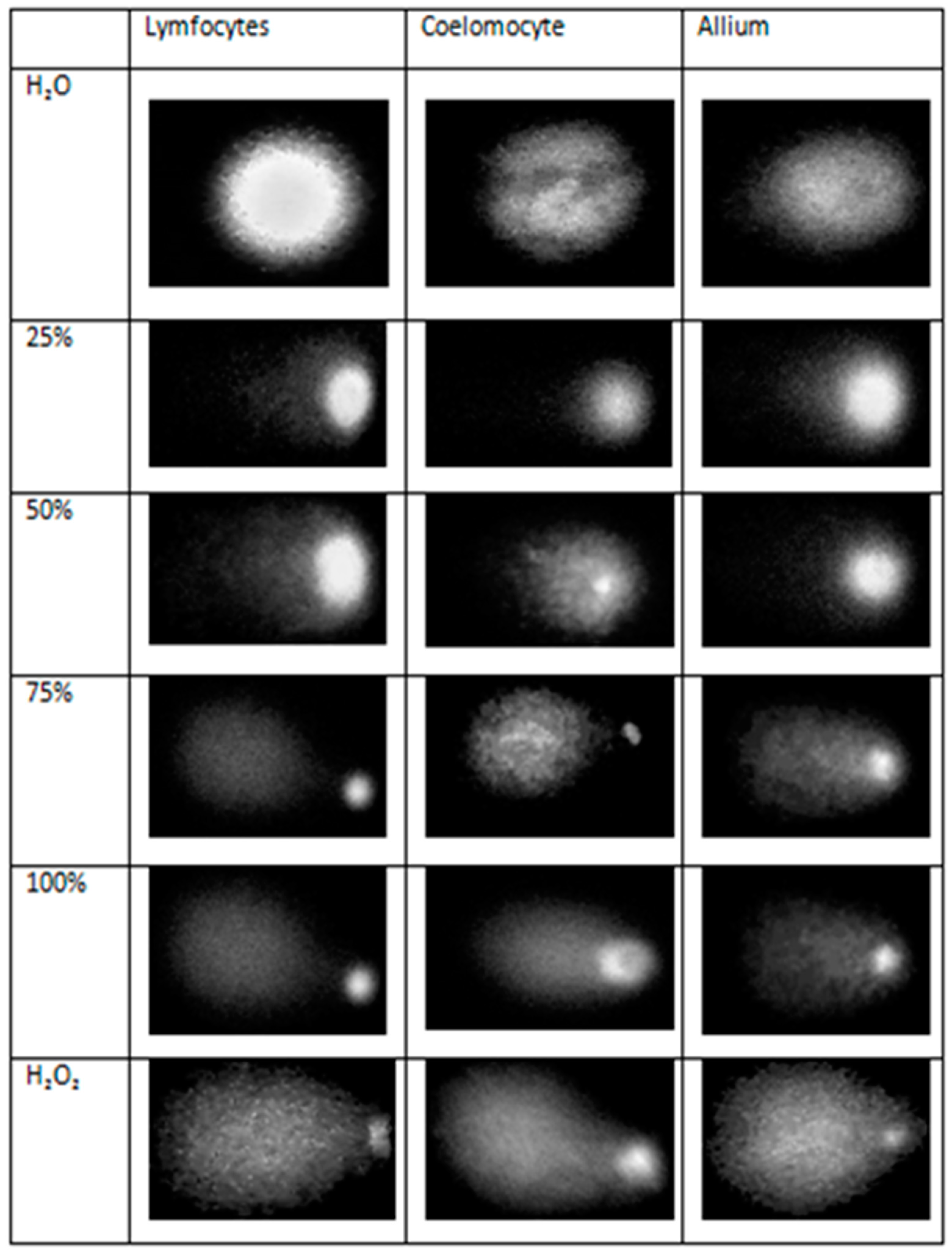
| Cell Bioindicators | Tail Length (µm) | Tail DNA % | OTM * (µm) |
|---|---|---|---|
| Lymphocytes | |||
| H2O | 2.34 ± 1.11 c | 10.95 ± 6.36 c | 0.41 ± 0.14 c |
| 25 | 3.45 ± 1.38 c | 16.42 ± 8.33 c | 0.62 ± 0.11 c |
| 50 | 37.96 ± 3.13 b | 43.77 ± 3.71 b | 5.18 ± 0.70 b |
| 75 | 40.32 ± 4.01 ab | 76.49 ± 7.81 a | 6.91 ± 1.36 b |
| 100 | 44.61 ± 0.54 a | 80.72 ± 2.69 a | 7.37 ± 1.33 b |
| H2O2 | 44.94 ± 2.08 a | 82.92 ± 2.85 a | 14.46 ± 0.82 a |
| Coelomocytes | |||
| H2O | 2.70 ± 0.39 c | 7.8562 ± 0.88 c | 0.2071 ± 0.04 c |
| 25% | 2.80 ± 0.22 c | 8.25 ± 0.30 c | 0.23 ± 0.02 c |
| 50% | 2.84 ± 0.15 c | 8.27 ± 0.08 c | 0.24 ± 0.01 c |
| 75% | 14.25 ± 1.48 b | 16.80 ± 1.23 b | 2.36 ± 0.25 b |
| 100% | 26.94 ± 4.53 a | 18.01 ± 2.60 ab | 5.22 ± 1.50 a |
| H2O2 | 30.08 ± 4.16 a | 20.98 ± 3.143a | 6.63 ± 1.51 a |
| Allium cepa L. | |||
| H2O | 0.18 ± 0.15 d | 7.75 ± 5.29 c | 0.79 ± 0.15 c |
| 25% | 30.55 ± 5.91 c | 72.34 ± 14.88 b | 4.67 ± 0.68 b |
| 50% | 39.59 ± 3.87 b | 75.02 ± 10.31 b | 5.31 ± 0.27 b |
| 75% | 43.00 ± 3.34 a | 77.91 ± 7.74 b | 5.31 ± 0.55 b |
| 100% | 44.61 ± 6.35 a | 90.39 ± 7.24 a | 5.64 ± 0.41 b |
| H2O2 | 45.11 ± 1.65 a | 98.39 ± 0.75 a | 6.45 ± 0.42 a |
| Cell Types | Test/Assay | Findings | Reference |
|---|---|---|---|
| Allium cepa L. | Chromosomal aberrations, Micronucleus test |
| [37] |
| Chromosomal aberrations, Micronucleus test |
| [37] | |
| Chromosomal aberrations |
| [94] | |
| Lymphocytes | Comet assay |
| [95] |
| Human Peripheral Blood | Comet assay |
| [96] |
| Lymphocytes | Comet assay |
| [97] |
| Lymphocytes | DNA diffusion assay, Micronucleus test, Comet assay |
| [98] |
| Eisenia fetida (coelomocytes) | Comet assay |
| [80] |
| Eisenia fetida | Comet assay |
| [99] |
| Pheretima posthuma (earthworms) | Comet assay RAPD-PCR * |
| [100] |
| Eisenia fetida | Allium bioassay, Chromosomal aberration, Micronucleus, Mitotic index |
| [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costea, M.A.; Rosan, C.A.; Laslo, V.; Agud, E.; Purcarea, C.; Vicas, S.I. The Comet Assay as a Sustainable Method for Evaluating the Genotoxicity Caused by the Soluble Fraction Derived from Sewage Sludge on Diverse Cell Types, Including Lymphocytes, Coelomocytes and Allium cepa L. Cells. Sustainability 2024, 16, 457. https://doi.org/10.3390/su16010457
Costea MA, Rosan CA, Laslo V, Agud E, Purcarea C, Vicas SI. The Comet Assay as a Sustainable Method for Evaluating the Genotoxicity Caused by the Soluble Fraction Derived from Sewage Sludge on Diverse Cell Types, Including Lymphocytes, Coelomocytes and Allium cepa L. Cells. Sustainability. 2024; 16(1):457. https://doi.org/10.3390/su16010457
Chicago/Turabian StyleCostea, Monica Adriana, Cristina Adriana Rosan, Vasile Laslo, Eliza Agud, Cornelia Purcarea, and Simona Ioana Vicas. 2024. "The Comet Assay as a Sustainable Method for Evaluating the Genotoxicity Caused by the Soluble Fraction Derived from Sewage Sludge on Diverse Cell Types, Including Lymphocytes, Coelomocytes and Allium cepa L. Cells" Sustainability 16, no. 1: 457. https://doi.org/10.3390/su16010457
APA StyleCostea, M. A., Rosan, C. A., Laslo, V., Agud, E., Purcarea, C., & Vicas, S. I. (2024). The Comet Assay as a Sustainable Method for Evaluating the Genotoxicity Caused by the Soluble Fraction Derived from Sewage Sludge on Diverse Cell Types, Including Lymphocytes, Coelomocytes and Allium cepa L. Cells. Sustainability, 16(1), 457. https://doi.org/10.3390/su16010457







