Abstract
Nano zero-valent iron (nZVI) technologies have gained recognition for the remediation of heavily contaminated sites and reused as backfilling soil. The moisture environment at these sites not only impacts the reactions and reactivity of nZVI but also the dynamic responses of compacted backfilled soils. The research explored the effects of different nZVI dosages (0.2%, 0.5%, 1%, 2%, and 5%) on Lead-Zinc-Nickel ions contaminated soil under a controlled-moisture condition. Cyclic triaxial tests were performed to evaluate the dynamic responses of treated soil samples prepared using a consistent moisture compaction method. Particle size distribution and Atterberg limits tests assessed changes in particle size and plasticity. The study revealed a minor reduction in the particle size, liquid limit, plastic limit, and plasticity index of the contaminated soil. Notably, increasing nZVI dosages in treated soils led to growing Atterberg limits. An increase in the specific sand fraction of treated soils was observed with nZVI, suggesting nanoparticles–soil aggregations favoring existing larger particles. Stepwise loading cyclic triaxial tests indicated an optimal dynamic response of soil treated with 1% nZVI under the controlled-moisture condition, proven by notable enhancements in the maximum shear modulus, maximum shear stress, less shear strain, and higher damping ratio within the small strain range. It should be noted that moisture content in treated soils declined significantly with higher nZVI dosages during preparation, potentially impeding effective aggregation and the formation of a solid soil skeleton. These findings advance the importance of considering the balanced nZVI dosage and moisture content when employing the safety assessment of practical applications in both nano-remediation techniques and soil mechanics.
1. Introduction
The advancement of industrial technologies, encompassing sectors like mining, electronics, and smelting, has led to the consistent introduction of heavy metal ions (HMs) into the soil, posing significant environmental and engineering challenges and hindering the sustainable use of contaminated soil [1,2]. The sources of HMs are diverse and contaminated sites are often exposed to a variety of excess HMs. Notable examples included landfill leachate (Cd(II), Pb(II)) [3], soils near copper mines (Cu(II), Pb(II), Zn(II), Ni(II)) [4], sites near coal gasification plant (V(V), As(III)) [5], etc. These heavy metal ions, once present in the soil, could enter the human body, primarily through the food chain, posing serious health risks, including fatal diseases, damage the nervous and digestive systems and impair immune function [6,7,8,9]. Beyond health implications, the enrichment of heavy metal ions in soils also affects their physical, chemical, and mechanical properties, leading to potential engineering safety concerns. The impact of heavy metal ions on the engineering properties of soil not only leads to a reduction in shear strength but also causes a significant loss in deformation characteristics [10,11]. Therefore, the remediation technology that combines the treatment of heavy metal contaminants and the improvement of the engineering properties of contaminated soil could have high importance and should be a necessary requirement. In recent decades, nanotechnology-based remediation has emerged as a promising approach for the lab-scale, in situ and ex situ remediation of combined heavy metal-contaminated sites, offering high performance, lower prices and an environmentally friendly solution [12,13]. Nano-zero-valent iron (nZVI) has garnered significant attention due to its excellent efficacy in the remediation of combined heavy metals contamination and enhancement of the geotechnical engineering characteristics of treated soils [14,15,16,17].
The nanoscale zero-valent iron (nZVI) exhibits unique physical and chemical properties, making it highly effective in immobilizing a variety of heavy metals (HMs) in contaminated soils [18]. The mechanisms included reduction (e.g., Cu(II), Pb(II), Ni(II)), adsorption (e.g., Cr(VI), Pb(II), Cd(II)), precipitation (e.g., Cu(II), Pb(II), Zn(II)), and co-precipitation (e.g., Cr(VI), Ni(II)) [19,20]. Specifically, Cu(II) is reduced to Cu [21], Ni(II) is reduced to monomers after being adsorbed on the surface () [22,23], Zn(II) forms a Zn(OH)2 precipitate [24], and Pb(II) is adsorbed on the surface to form a surface complex () [25], etc. As a nano-material, nZVI physical properties, including electrostatic adsorption, magnetic attraction, aggregation, and bonding, significantly contribute to these chemical processes and particularly in a physical manner [26]. When nZVI is added into contaminated soils as a reagent, the degradation and immobilization of heavy metal contaminants can significantly improve the properties of the soil, indirectly. However, it is important to acknowledge that the interactions involving nZVI, water, oxygen and soil particles have the potential to modify the structure of the soil skeleton and further affect the engineering properties of the treated soil. These interactions included the formation of homo-aggregates (aggregation of nZVI particles and finer clay particles under strong van der Waals and magnetic forces), hetero-aggregates (nZVI and products adsorbed on the surface of electronegative soil aggregates), or reaction products of HM treatment [27,28]. The physical processes induced by nZVI could directly influence the contact and adhesion of soil particles and the formation of skeleton, hence resulting in uncertainty in mechanical properties of the soil [29,30,31]. Moreover, the abilities of nZVI were subject to environmental conditions. In aqueous environments, the oxide layer shell of nZVI could be corroded and destroyed, resulting in the release of the core Fe0 and facilitating the immobilization reaction. In contrast, in dry environments with limited water, the core-shell structure of nZVI remains intact, limiting its chemical effects. Consequently, the limitation emphasizes the importance of nZVI’s physical properties and water environment, such as aggregation and magnetic attraction, in the immobilization process under such conditions.
In geotechnical engineering, especially in projects like waste landfill sites, road and railway construction, where seismic requirements should be considered, it is essential to assess the cyclic shear characteristics of the treated soil. A few studies have been conducted in the role of nanomaterials in enhancing both the cyclic and static response of soils [32,33]. Chen et al. [34] observed that, as the nano-MgO, both the limiting cyclic stress and the maximum cyclic modulus of the soil increased accordingly. The significance observed a 16.5% increase in the maximum modulus of elasticity in hydromorphic soils after a 28-day curing period with nano-CaCO3 [35]. The mechanism for the observed improvements in dynamic shear characteristics of treated soil is that nanomaterials improve the aggregation and bonding between soil particles, as well as the pore-filling capabilities. Specifically focusing on nZVI, a few studies have reported an increase in static shear strength of contaminated soils following nZVI treatment. A laboratory application demonstrated that contaminated soil with 400 mg/kg of Pb(II) treated with nZVI increased its undrained shear strength from 25.83 kPa (with 0.2% nZVI) to 69.33 kPa (with 10% nZVI) [36]. It was reported that, after 5% nZVI treatment, the internal friction angle of the contaminated soil increased from 21.5° (contaminated soil) to 28.2° (5% nZVI treated soil), and the increasing moduli of treated soil was also observed [37]. Nasehi et al. [38] also noted improvements in Unconfined Compressive Strength (UCS) and undrained shear strength in diesel-contaminated soils after nZVI application. Given that each nanomaterial exhibits distinct physico-chemical properties, their effects on soil engineering properties could vary significantly. Unfortunately, few studies have pointed out the effect of water on the impacts of nanomaterials. Therefore, it is urgent to conduct a comprehensive study of the cyclic properties of combined heavy metal-contaminated soils treated with nZVI. Such cyclic research was particularly vital when considering the use of these treated soils as backfill material for roadbeds or embankments, where lab testing condition requirements, especially water content and compaction techniques, would be key considerations.
The objective of this study is to investigate the cyclic behavior of soil contaminated with a combination of three heavy metals and treated with nanoscale zero-valent iron (nZVI) underlying a limited water sample preparation. Three heavy metals were selected as contaminants based on the interaction mechanisms with nZVI: Zn(II) as a representative for adsorption, Pb(II) for precipitation, and Ni(II) for reduction. Subsequently, the contaminated soil samples underwent treatment with five different dosages of nZVI (i.e., 0.2%, 0.5%, 1%, 2%, 5%). To assess the geotechnical properties of the nZVI treated soil, a series of tests was designed, including compaction sample preparation, Atterberg limit tests, Particle size distribution (PSD) analysis, and cyclic triaxial tests. This study, by comprehensively exploring the application of nZVI in soil treatment, aims to broaden the scope and enhance the understanding of nZVI role in the cyclic shear properties of heavy metal-contaminated soils, providing insights for its application in geotechnical engineering.
2. Materials and Methods
The original soil used for this study was collected from a city lake of Haikou, Hainan Province, China. The liquid limit (LL), plastic limit (PL) and plastic index (PI) are 57.4%, 27.5% and 29.9, respectively, following the BS1377:1990 [39]. The particle size distribution (PSD) of original soil contains 10% clay, 50% silt and 40% sand. Original soil with a PI greater than 50 and below the “A line” (PI = 0.73 (LL-20)) were defined as MH or OH according to the Unified Soil Classification System (USCS, ASTM D2487-17, 2017) [40]. Three heavy metal nitrate contaminants (Zn(NO3)2·6H2O, Pb(NO3)2, Ni(NO3)2·6H2O, 99.8% AR) were selected because of their solubility and beneficial reactions of nZVI with heavy metals [41]. These contaminants were supplied by XiLong Chemical Co., Ltd., Shenzhen, China. The nZVI (purity 99.9% Fe0) was supplied by Xiang-Tian Co., Ltd. (Shanghai, China) as a treatment reagent and was kept under vacuum until used for experiments. The key parameters of nZVI particles are 50 nm diameter, with a surface area of 30 m2/g and a particle density of 1150–1250 kg/m3, as reported by the manufacturer.
2.1. Dosages
In order to ensure control over the variables and account for the competitive mechanism between heavy metals and nZVI reactions, the mass ratio of the three heavy metal contaminants (Zn (NO3)2·6H2O, Pb (NO3)2, Ni (NO3)2·6H2O) to the dry soil was determined at 200 mg/kg, respectively, (600 mg/kg divalent ions in total). The contamination level referred to the concentration of heavy metal pollution in the Yangtze River [42].
The statistical analysis of previous literature data was employed to determine the appropriate dosage of nZVI. Following the introduction of the three heavy metal contaminants into soil, the molar contents of Pb, Zn, Ni, and nitrate ions per kilogram of soil were 6 × 10−4, 1.1 × 10−3, 6.9 × 10−4, and 1.5 × 10−3, respectively. As reported by Li et al. [31], Li et al. [43] and Moazeni et al. [44], the optimal ratios of Ni(II), Pb(II), and Zn(II) to nZVI under solution conditions were determined to be 8/1, 10/1, and 9.5/1, respectively, in which that of total ionic quantities was approximately 9.2/1 in mass. Simultaneously, nZVI acts as a reducing agent and undergoes a reaction with NO3−, resulting in the reduction of nitrate ions to ammonia nitrogen and N2, which is not the focus of this paper. For this study, five different dosages of nZVI were determined to address heavy metal contaminates in soils. These dosages include 0.2% (Extra-Low level, 2.2/1), 0.5% (Low level, 5.5/1), 1% (Moderate level, 10.9/1), 2% (Extra-excessive level, 21.8/1), and 5% (Ultra-excessive level, 54.5/1), each based on the dry weight of the soil. It is worth noting the distinction between soil and aqueous environments in terms of complex soil mineral composition, which poses a challenge in transferring optimal nZVI amounts from an aqueous environment to soil. This disparity in comparison may potentially lead to an inaccurate estimation of the impact of nZVI in soil. All samples were categorized, namely Natural soil (NS), combined heavy metal contaminated soil (MS), contaminated soil treated with 0.2% nZVI (MNS0.2), 0.5% nZVI (MNS0.5), 1% nZVI (MNS1), 2% nZVI (MNS2), and 5% nZVI (MNS5).
2.2. Sample Preparation
To prepare the natural soil, the original soil was transformed into a slurry with a moisture content equivalent to 1.5 times the liquid limit (LL). Subsequently, the soil slurry underwent screening using a 1 mm mesh sieve. The sieved slurry was then dried in an oven at 105 °C for at least 24 h, followed by crushing and passing through a 1 mm sieve, namely Natural soil (NS). A compaction test was conducted on NS to determine the optimum moisture content (OMC), which was found to be 22%.
In the process of heavy metal-contaminated soil, the artificial contaminants, involving Zn(NO3)2·6H2O, Pb(NO3)2, Ni(NO3)2·6H2O, were dissolved in distilled water and added to NS for OMC. All metal contaminant ions were adjusted to achieve a metal mass ratio of 200 mg/kg, resulting in total heavy metal ions of 600 mg/kg of dry mass of soil. The mixture was manually stirred for approximately 10 min to ensure homogeneity. Subsequently, the contaminated soil was allowed to mature for one week to achieve equilibrium of adsorption and desorption before undergoing further treatment. As the nZVI-treated procedure, different dosages (i.e., 0.2%, 0.5%, 1%, 2%, and 5% by dry weight) of nZVI powder were evenly spread over the prepared contaminated soil. The soil was then hand-stirred for 5 min to ensure proper mixing. It is worth noting that no additional water was added beyond the initial OMC amount during the preparation, ensuring the consistency of the initial amount of water for all sequences. Afterward, the samples were compacted in six layers using a 100 mm high, 50 mm diameter mold to prepare the cyclic triaxial samples.
2.3. Particle Size Distribution (PSD)
The particle size distribution (PSD) of the soil samples was analyzed by the Malvern Particle Size Analyzer (Mastersizer 3000, Malvern Panalytical Ltd., Malvern, UK). The apparatus operates across a range spanning from 0.01 to 3500 μm and provides data for 118 size classes within this interval. Subsequently, the results were classified into three categories according to particle size following the USCS: clay (<1.5 μm), silt (1.5–75 μm), and sand (>75 μm).
2.4. Atterberg Limits
The Atterberg limits test was carried out on various types of soil accordance with the BS 1377:1990 [39]. A cone penetrometer (LP-100D, Glory Testing Ltd., Kunshan, China) was used to determine the Atterberg limits. The procedure involved measuring cone penetration at five different moisture contents and plotted the cone penetration moisture content line, taking the moisture contents corresponding to the cone penetration of 5 mm and 20 mm as the plastic limit (PL) and liquid limit (LL), respectively. In this case, the recommended Plastic Limit Rolling Procedure 1 (ASTM D4318-17) [45] was also used as a verification of plastic limits, and the results are consistent with that from the cone penetrometer.
2.5. Cyclic Triaxial
The cyclic triaxial test system (ELDYN, GDS Instruments, Hook, UK) was used to carry out tests to determine the cyclic properties of specimens with different treatments, according to ASTM D5311-2019 [46]. All the sensors were carefully calibrated by factory calibration and laboratory calibration. Vacuum and back pressure saturation were performed to ensure that the sample reaches a fully saturated state. The specimens were only considered completely saturated if the B-value > 0.95. During this study, all specimens achieved such full saturation condition (B-value > 0.96) for a back pressure of 200 kPa at least. Effective confining pressure of 100 kPa in isotropic consolidation stage was applied to each soil type until the excess pore pressure had completely dissipated (<5 kPa, >24 h). The cyclic shear stage utilized a 1 Hz sinusoidal wave, stress control, and a multi-step loading procedure. During the undrained cyclic shear stage, the cyclic load was started at 5 kPa and incrementally increased by the step 5 kPa per step for 5 cycles (first direction in compression, as shown in Figure 1), until the single amplitude strain in either extension or compression of 2.5% was achieved.
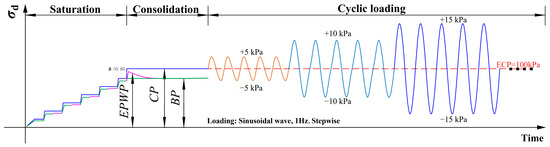
Figure 1.
Diagram of saturation, consolidation and stepwise cyclic loading of this study (CP: Cell Pressure; BP: Back Pressure EPWP: Excess Pore Water Pressure; ECP: Effective Confining Pressure).
2.6. Determination of Cyclic Parameters
Figure 2 shows the typical hysteresis loop (stress–strain curve) of soils subjected to cyclic loads. The backbone curve is defined as the line connecting peaks of the hysteresis curves for different load amplitudes, the dynamic elastic modulus (Ed) is equal to the slope of the line joining the vertices of the hysteresis curves, and the cyclic shear modulus (Gd) is obtained by indirect calculation of the elastic modulus:
where Ed is the dynamic elastic modulus, σdmax, σdmin and εdmax, εdmin are the pressure and strain at the top of the hysteresis loop, respectively, Gd is the shear modulus, and υ is Poisson’s ratio. For saturated undrained samples, Poisson’s ratio can be assumed to be 0.5 [34].
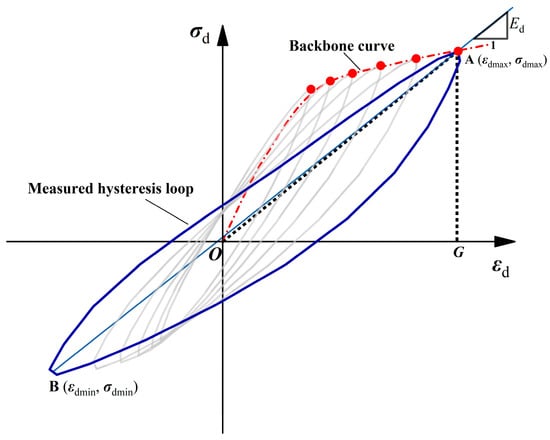
Figure 2.
Shear stress–strain curve and backbone curve.
Hardin and Drnevich [47] adopted a hyperbolic function to analyze the variation of dynamic shear stress and shear modulus with strain:
where a, b are fitting parameters, τd is the shear stress (τd = σd/2), and γd is the shear strain (γd = εd × (1 + υ)).
Thus, if γd is 0 or infinity, the maximum shear modulus Gmax = 1/a and the maximum shear stress τ.
Finally, the damping ratio is confirmed by the following equation:
where ΔW is the area of the hysteresis loop and W is the area of the triangle AOG.
3. Results and Discussion
3.1. Particle Size Distribution
Particle size distribution (PSD) is a basic property and classification of soils that significantly influences various engineering properties, including soil water retention, hydraulic conductivity, shear properties, and geo-mechanical properties. In this study, a laser particle size analyzer was employed to measure the changes in particle size. The obtained PSD results for each sample and variation in section ratio are plotted in Figure 3.
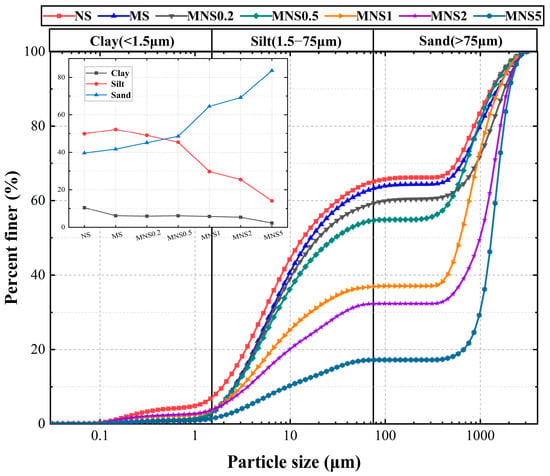
Figure 3.
Particle size distribution curves of each sample (NS: natural soil; MS: heavy metal contaminated soil; MNS0.2: soil treated with 0.2% nZVI).
The results indicated that the PSD of the contaminated soil and nZVI treated soils exhibited a higher proportion of coarse particles compared to the natural soil. As shown in Figure 3, the PSD curves for the contaminated and treated soils shift towards the right, with the degree of shifting becoming more prominent as the nZVI dosage increases. This shift implies the formation of larger particles and aggregates and reduction in the proportion of small particles with nZVI, especially in the increase to >300 μm. A gradual increase in Cu values occurred from 17.4 for NS to MS (for 18.1), MNS0.2 (for 65.9), MNS0.5 (for 185.8), MNS1 (for 262.8), and MNS2 (for 288.2), MNS5 (for 136.1). Notably, the PSD of nZVI treated soils could be divided into two distinct segments at a turning point of 1% according to the increment of Sand proportion, including Low-series (i.e., 0.2% and 0.5%) and Excess-series (i.e., 2% and 5%), by which the soil showed increasingly heterogeneous effects and aggregation with more introduction of nZVI, exhibiting a consistency with coarser increasing and finer decreasing. The MS shows a 4% decrease in clay and a corresponding 2% increase in sand and clay, respectively. Previous studies have shown that heavy metal cations can reduce the electrostatic repulsion between particles by compressing the thickness of the diffusion layer and reducing the surface charge number/density, which in turn induces agglomeration of soil particles. When the nZVI addition increased from 0.2% to 5%, the clay and silt content decreased by 4% and 35%, respectively, and the sand content increased by 39%. It is obviously noted that no significant increasing trend was found within the ranges of G-300 μm (i.e., no particle size in this interval), even with a 5% nZVI introduction. Hence, nZVI-soil products, including chemical and physical interactions, tended to aggregate or precipitate on existing large particle, rather than clumping themselves together. What can be confirmed is that the aggregation effect of nZVI has limitations [48,49]. The following reasons could be considered for this phenomenon. (1) Homogeneous aggregation of nZVI. nZVI exposed to air or water readily forms chain-like aggregates, and nZVI aggregation from the nanoscale to the micron scale is observed [50]. (2) Heterogeneous aggregation of nZVI with soil. Oxidized nZVI aggregates adsorb on and form complexes with soil particles through interfacial interactions of their surface functional groups, increasing the mean particle size due to complex formation [27,51]. (3) Agglomerates gel with agglomerates to form larger agglomerates. Aged nZVI becomes cemented to the surface of the soil aggregates and forms connecting structures between the aggregates [29]. Aggregates that do not break up under the influence of ultrasound are considered to be particles. Thus, the hetero-/homo-aggregation of nZVI with finer particles and on larger particles could be regarded as the main mechanism for the influence on the PSD of the treated soils. Overall, the application of nZVI resulted in an increase in the particle size of the treated soil due to the aggregation effect.
3.2. Atterberg Limits
The Atterberg Limits are empirical indices of the critical water contents defining the mechanical states of soil–water mixtures and are widely used for the identification, description and classification of soils, consisting of the Liquid Limit (LL), Plastic Limit (PL) and Plasticity Index (PI). The Atterberg limits are particularly critical in understanding the engineering behavior of fine-grained soils and diffusion double layer (e.g., water molecules and ions adsorption). Figure 4 highlights the effect of heavy metals and nZVI dosages on the Atterberg limits of the specimens.
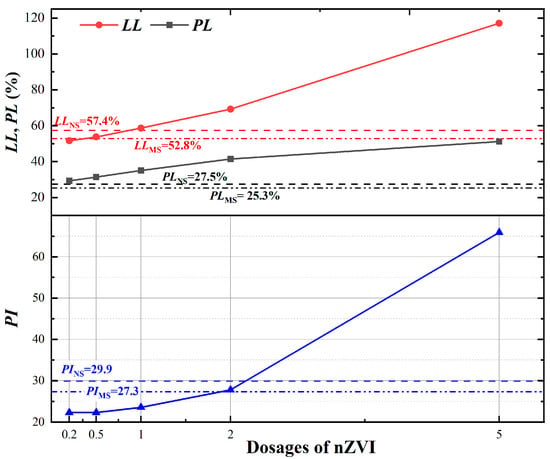
Figure 4.
Variations in Atterberg limits for each specimen (LLNS: indicates the LL of NS; PLMS: indicates the PL of MS).
The LL, PL and PI of MS slightly decreased from 57.4%, 27.5% and 29.9, respectively, to 52.8%, 25.3% and 27.3%. The changes could be explained by the interaction among replacement by heavy metal ions, changes in adsorbed water and free water, and soil particles with negative charge, affecting the diffusion double layer (DDL). Soil particles have an enormous surface area and negative surface charge, which enables them to adsorb polar water molecules by electrostatic forces or hydrogen bonding. The adsorbed water, tightly bound to the soil particle surface, is primarily responsible for the plastic properties of fine-grained soils [52]. Water away from the surface of the soil particles was easily moved by an external force, namely free water, which influenced the fluid behavior of the soil [53]. It can therefore be said that the Atterberg limits of the soil are a macroscopic representation of the thickness of the DDL. If the pore fluid comprises heavy metal cations with water, the heavy metal cations and polar water molecules will dominate the negatively charged sites on the clay particle surface, and hence the thickness of DDL will be reduced, and the soil particles will be able to slide at relatively lower water contents, reducing the LL and PL of the contaminated soil [10]. In general, the less adsorbed water the soil adsorbs, the thinner the DDL and the lower the PI value. Thus, a larger proportion of cations in contaminated soils adsorbed less bound water, thinner DDL, and led to the decline in PI.
The LL, PL and LL of the nZVI-treated soil were significantly increased with higher nZVI dosages, after the dry mixing preparation process. Following the treatment with 0.2% nZVI, the LL and PI values of the MNS0.2 decreased to 51.6% and 22.3, respectively, while the PL values increased to 29.3%. The decrease in LL and PI values observed at Low- series of nZVI (i.e., 0.2% and 0.5%) could be attributed to the effective degradation of heavy metal ions. As reported by Zhou at al [36], as the heavy metal ions were immobilized, the thickness of the diffusion double layer (DDL) in the soil increased. Across the theoretical turning point—1%, the PL continually increased while LL increases even more, leading to a greater PI. As the nZVI increases to an Ultra excess dosage—5%, the LL, PL, and PI values continued to rise, reaching 117.1%, 51.2%, and 65.9, respectively. The adsorption and aggregated structures of hydroxides formed by the reaction of nZVI led to an overestimation of the water content present in the treated soil and resulted in an increase in the measured Atterberg limit during experimentation. Furthermore, residual nZVI in the soil could continue to react with the added water during the cone penetration experiment, contributing to an increase in the measured Atterberg limits. Significantly, the addition of nZVI resulted in a notable shift in the Atterberg limits of the soil, particularly for samples MNS1, MNS2, and MNS5. In these cases, the soil classification shifted from CH or OH (for MNS0.2 and MNS0.5) to GM, due to the presence of coarse grains exceeding 50% and fines exceeding 12%, according to the PSD analysis. Currently, for the complex mechanism for Atterberg limits, no consensus exists on the condition with specific nanomaterials [54]. It is important to acknowledge that the impact of heavy metal cations and nZVI on the Atterberg limits of soil is contingent upon the specific composition of minerals, pore fluids, products and microstructures in the soil. The water content would also be a key factor that cannot be ignored during the nZVI chemical and physical reaction.
3.3. Dynamic Responses
Dynamic loads, including traffic, seismic activity, and wave loads, have a significant impact on the shear and structural integrity of various infrastructure elements, such as roads, foundations, and landfill. Therefore, it is necessary to consider the effects of these dynamic loads during the design and construction phases. As important indicators of the dynamic strength of soils, the damping ratio and the dynamic modulus are used to describe the dynamic responses of soils treated with nano zero-valent iron (nZVI). The backbone curve and shear modulus curves were fitted using the Hardin and Drnevich model [55], and the strain-history curve and damping ratio relationship were plotted. Based on the results, it was observed that the MNS1 sample demonstrated optimal dynamic shear properties under controlled-moisture conditions.
3.3.1. Hysteresis Characteristics and Backbone Curve in Dynamic Shear Stress–Strain Relationship of Soil
The stress–strain curves (backbone curves) for soils treated with various nZVI dosages were constructed. The curves were selected from the peak points of the half-cycle compressive stress peak points at the fifth cycle under each load, with fitting using the Hardin–Drnevich model. The backbone curve reflected the dynamic stress–strain relationship of the soil at different dynamic stress amplitudes, and for the dynamic shear modulus of the treated soil.
From the hysteresis characteristics, the evolutionary patterns of all samples were meticulously delineated, as illustrated in Figure 5. The hysteresis curves of the samples uniformly exhibited non-linear behavior, describing a willow shape. Furthermore, with an increasing number of cycles, a notable enlargement in the area of each hysteresis loop was observed, accompanying a gradual flattening, along with accumulation and increase in irreversible plastic strain. Initially, the hysteresis curve of NS shifted from a dense and symmetrical feature with minimal cumulative strain. In the presence of contaminants, it a compactness feature at small strains was exhibited (i.e., Low cyclic loading level) and observed, whereas at large deformations (High cyclic loading level), a rapid expansion in the curve’s area was evident, alongside progressively increasing tensile strains along with each loading step. Upon addition of nZVI, a distinct transition point in hysteresis characteristics should be noted at a 1% dosage. For instance, an initial increase was followed by a decrease in shear strain along with the step loadings, with a reversed trend in tensile strain. Under higher cyclic loadings, the curve’s shape rapidly changed from shuttle-like to an elongated willow leaf shape. Notably, the initial rightward shift and subsequent re-stabilization of the curves at a 1% nZVI dosage (MNS1) indicated an internal particle rearrangement and compression process, which could lead to a stable and solid skeleton.
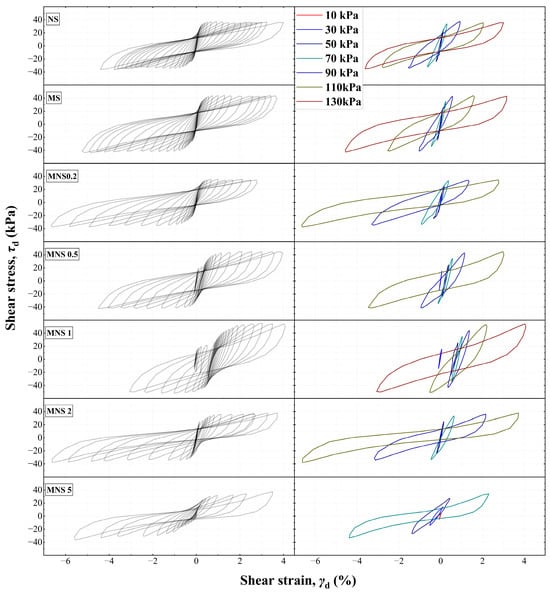
Figure 5.
The curves of shear stress–shear strain with different cyclic shear loading.
The results in Figure 6 indicated that the shear stress of the MNS1 samples exhibited a gradual increase along with increasing strain within the measured strain range, demonstrating a strain-hardening fitting, while the remaining samples showed an ideal elastic-plastic model. After contamination by three heavy metals, the maximum shear stress (τdmax) of the MS increased from 38.3 kPa of NS to 47.1 kPa. Furthermore, the introduction of nZVI showed a trend initial increase and then decrease in τdmax at 1% nZVI. For instance, τdmax increased from 37.9 kPa to 47.3 kPa and further to 64.4 kPa with 0.2%–0.5%–1% nZVI, while τdmax decreased to 38.3 kPa and 34.4 kPa for MNS2and MNS5, respectively. MNS1 exhibited the highest τdmax (64.4 kPa) and the lowest value of R2 (0.898), highlighting the 1% dosage of nZVI as a critical stage in inducing internal changes in the treated soil. The increase in the τdmax for MS could be attributed to key factors: a decrease in the PI, implying a thinner DDL thickness, and the coarsening of the soil particles. When nZVI is introduced into contaminated soils, its physical and chemical properties initiate rapid reactions within the soil-contaminants matrix, leading to several distinct processes. Based on the Particles size distribution and plasticity analysis, the following reasons could be induced: (1) Reaction with water on soil particles. By the oxide protective layer, nZVI typically exhibited a core-shell structure. The interaction of nZVI with on-particle water results in the erosion of its oxide shell, facilitating the release of the core active Fe0 and eventually forming iron-metal oxides, and adsorbed on particle surfaces. (2) Remediation with both heavy metals and water. nZVI acted to immobilize heavy metals through various mechanisms, including oxidation, reduction, adsorption, and precipitation around or on soil particles. During the process, water would be the indispensable reaction condition. (3) Adsorption and agglomeration on soil particles. nZVI, due to its charged and nanoscale nature, exhibited a propensity to adsorb onto the electronegative surfaces of soil particles. (4) Aggregation with soil particles. Aggregation of nZVI finer particles took place under strong van der Waals and magnetic forces on the larger particles (e.g., obviously in sand fractions, mentioned in the PSD section). It is noteworthy that these four processes occurred simultaneously in the nZVI-soil-contaminant system. Therefore, the decrease in τdmax observed in samples with 0.2% dosages of nZVI could be attributed to the effective degradation of heavy metal ions. The increase in adsorption and aggregation effects at the dosages of 0.5% and 1% significantly contributed to enhancing the agglomeration and aggregation between soil particles. This enhancement in cementation directly influenced the strength of the soil skeleton and the τdmax markedly increased. However, at higher nZVI dosages, (2% and 5%), the excess nZVI leads to excessive consumption of soil water. Importantly, the mentioned reaction, both in chemical or physical processes, could be dominated by water on soil particles and hence the products and aggregates would be precipitated on the soil surface. This overuse of soil water and excess coating nZVI particles could impede the ability of the treated soil to form a dense, cohesive and solid skeleton, by which it could have a negative effect on interlock capacity and cohesive force, resulting in a significant decrease in τdmax. In conclusion, the physico-chemical interactions of nZVI within the soil-heavy metals system, governed by various dosages of nZVI and water, exerted a significant enhancement to the dynamic shear stress of the soil.
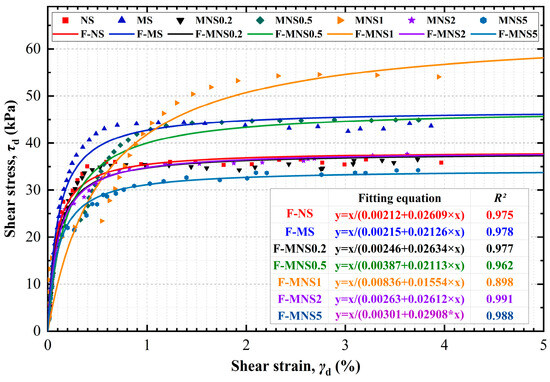
Figure 6.
Variations in backbone curves of all samples (F-NS: fitting curve representing Hardin–Drnevich model of NS).
3.3.2. Strain Time-History Characteristics
To better understand the effect of heavy metals and nZVI on axial deformation during stepwise loading, the strain time-history curve of different soil types under the same loading schedule were compared. The compressive and tensile stress peak points under each level load are selected and connected with a smoothed curve. The results and step loading schedule are plotted in Figure 7.
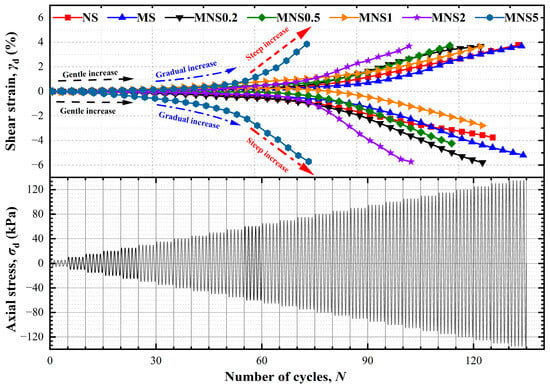
Figure 7.
Variation in strain time–history curve of samples treated with various dosages of nZVI.
The deformation process can be divided into three stages based on the growth rate of the curve. The gentle increase stage was defined as the early deformation at the initial cyclic loadings. During this, it exhibited a low strain range and a slower growth on or near the horizontal line. In the gradual increase stage, the strain rate gradually increases as the dynamic stress increases and this growth process includes the turning point from stable to rapid deformation for all specimens. From a microscopic view, internal rearrangement of the soil might occur, and the soil skeleton would be a broken and show a cracked form. The final stage was named the steep increase stage. Under dynamic loading with a continuous increase in dynamic shear strain, the failure surface gradually widens until fail in a short time. Compared to NS and MS, which withstood 138 and 140 cycles, respectively, reaching a critical axial strain of 2.5%, nZVI-treated soils showed varied durability under cyclic loading. Specifically, MNS0.2 and MNS1 endured 127 cycles each, MNS0.5 lasted for 118 cycles, MNS2 for 106 cycles, and MNS5 only 75 cycles. This indicated that the connection structures and skeleton within MNS0.5 and MNS1 soils were more easily damaged under identical cyclic loading, leading to a greater plastic deformation. For MNS2 and MNS5, the delicate nature of new-formed soil microstructure predisposed it to particle slippage during cyclic loading. The condition resulted in irreversible plastic deformation, even when subjected to smaller cyclic loads. In conclusion, NS and MS exhibited greater durability under cyclic loading compared to nZVI-treated soils. Despite the ability of the moderate nZVI dosage to form a connected structure between soil particles, these new-formed structures were found to be susceptible to fracturing and irreversible plastic deformation when subjected to cyclic loading cycles.
3.3.3. Dynamic Shear Modulus Characteristics
The cyclic shear modulus, derived from the slope of the line connecting two vertices of the hysteresis loop, serves as a crucial parameter for characterizing the cyclic properties of soils. Utilizing the Hardin–Drnevich model, the results of cyclic shear modulus were fitted and presented in Figure 8.
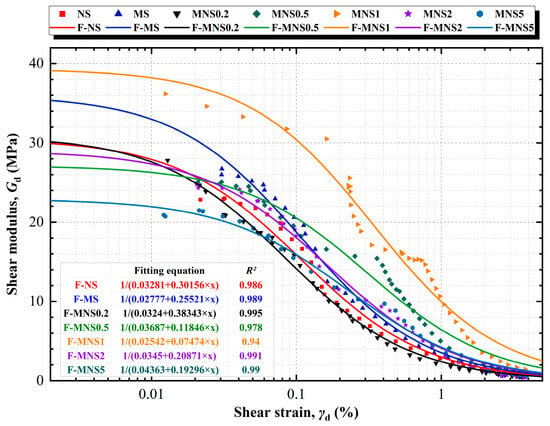
Figure 8.
Variations in dynamic shear modulus versus shear strain relationships.
A discernible threshold exists where the fitted curve for all samples nearly flattens, indicating that the Gdmax remains relatively constant over a range of small strains, numerically Gdmax = 1/a. The point was identified as the elastic strain threshold, a critical demarcation for distinguishing between elastic and plastic deformation in soils [56]. When deformation surpasses this elastic γd threshold (approximately 0.01%), a rapid decrease in Gd is observed with increasing γd. This decline is indicative of the soil undergoing irreversible plastic deformation, leading to a deterioration of its structural integrity [57]. Specifically, the introduction of heavy metals resulted in an increase in Gdmax of NS from 30.5 MPa to 36.1 MPa of MS. However, the addition of a 0.2% dosage of nZVI caused a decrease in Gdmax to a level similar to that of NS, measuring 30.9 MPa; as mentioned before, the reasons could include remediation and Extra-Low-level actions. As the quantity of nZVI added continued to increase, the Gdmax of soil treated with 0.5% nZVI decreased to 27.1 MPa. When the nZVI dosage reached the theoretical turning point of 1%, Gdmax reached its maximum value at 39.3 MPa, which noted a 45% increment. Subsequently, for MNS2, Gdmax decreased to 28.9 MPa, and for MNS5, it decreased further to 22.9 MPa. The fluctuation in Gdmax revealed that MNS1 exhibited the highest stiffness in the initial stages (within the 1% strain range). However, it does not adequately represent the alterations occurring in the sample throughout the entire shear process.
To comprehensively depict the variations in shear modulus among different samples throughout the entire shear process, the results are graphically presented in Figure 9, considering two perspectives on the same stress (Figure 9a) and the same strain (Figure 9b). The data (Figure 9a) indicate that, at the τ of 5 kPa, the Gd values for all samples are as follows: 22.8 MPa (NS), 25.5 MPa (MS), 23.6 MPa (MNS0.2), 25.1 MPa (MNS0.5), 24.6 MPa (MNS1), 24.7 MPa (MNS2), and 10.4 MPa (MNS5). Notably, there are no significant differences in Gd among all samples, except for MNS5. However, when the τ is increased to 27.5 kPa, the Gd values for the same seven samples decrease to the following levels: 11.1 MPa, 16.9 MPa, 14.1 MPa, 15.7 MPa, 10.9 MPa, 2.1 MPa, and 0.4 MPa. These reductions represent percentage decreases of 51.1%, 33.6%, 62.1%, 43.8%, 36.4%, 56.1%, and 79.4%, respectively. Notably, MS, MNS0.5 and MNS1 demonstrate a lower attenuation trend in Gd. Hence, the same trend in stress-strain behavior proves the proposed mechanisms: (1) eaction with water on soil particles, (2) emediation with both heavy metals and water, (3) adsorption and agglomeration on soil particles, and (4) aggregation with soil particles. When considering the strain perspective, it became apparent that the various samples exhibited different relative attenuation magnitudes compared to the stress perspective. For instance, the normalized shear modulus decay curve of MNS5 was situated to the right of MS, which indicated that MS experiences a greater shear modulus decay magnitude and decay rate than MNS5, despite MNS5 having a lower Gdmax. One reason could be that the larger dosages of nZVI (i.e., Extra-excessive level) resulted in more water being consumed, so that the relatively dry nZVI particles adhere to the surface of the soil particles in a weakly bonded manner, especially under the controlled-moisture condition. It also significantly affected its interlocked force and cohesion. The disparity in the nonlinearity of soil stress–strain behavior accounts for this difference. However, irrespective of the perspective considered, it is evident that MNS1 demonstrates outstanding resistance to attenuation of Gdmax under step loadings, which was the key issues in seismic resistance and resistance to many types of dynamic loads in sites.
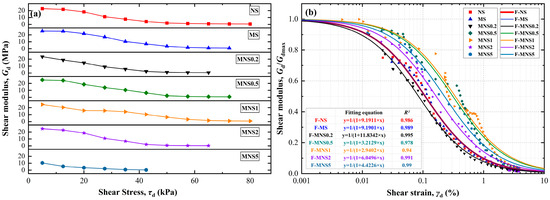
Figure 9.
Variations in (a) dynamic shear modulus versus shear stress and (b) normalized shear modulus decay curve.
3.3.4. Damping Ratio Characteristics
The damping ratio is the ratio of the energy consumed by the soil to produce damping in the next cycle of cyclic loading to the total elastic energy acting on the specimen, which reflects the degree of energy loss during soil deformation and is related to the area of the hysteresis circle and used for dynamic response analysis and seismic design.
Figure 10 illustrates that the damping ratio (λ) follows an “S” shaped trend with shear strain (γd) for all samples, except for the MNS1 sample, which exhibits a “V” shaped trend. The damping ratios for these samples predominantly ranged between 0 and 0.4. In the initial phases, a notably high λ was observed in the MNS1. This suggests that the presence of nZVI contributes to the formation of a more robustly connected structure amongst soil particles. In the shear strain range of 0.01% to 0.04% (and 0.01–1% for the MNS1, a long range with a slower decay), a gradual decrease in λ was recorded as the shear strain increased. It should be noted that a progressive decline in the soil’s ability to dissipate energy was observed. Under cyclic loading, larger soil particles were found to break down and rearrange, leading to a reconfiguration of the soil skeleton structure. The rearrangement effectively addressed deficiencies in the PSD curves, particularly in the 75–300 μm range, by which it highlighted enhancement of the soil skeleton’s strength and increasing λ. Notably, samples with a MNS0.2 exhibited a pronounced increase in λ, potentially attributable to the effective degradation of certain heavy metals within the soil matrix. However, after rearrangement as the cyclic loading continues, the emergence of a break-up of aggregate junctions within the soil samples could be deduced, culminating in failure. The development consequently led to a variation in the λ, manifesting either as a decrease or as a deceleration in the rate of increase across different samples. It is imperative to note that no further alterations in the damping ratio were detected within the specified strain range for MNS1. Thus, it could be inferred that the strength of inter-particle aggregation, in conjunction with changes in the particle size distribution (PSD) during cyclic loading, had significantly beneficial influences on the variations in λ significantly. Another promising mechanism was that, during the soil preparations under the controlled-moisture conditions (22%, OMC of NS), moisture used in this study should show a wider gap between PL with increasing nZVI effects. The larger gap was from 7.3 to 29.2 with nZVI from 0.2% to 5%, even with larger PI (usually related to the specific strength), which could suggest that the water content and its position in Atterberg limits should be considered as potential factors in the damping ratio and dynamic response with nZVI treatment [58].
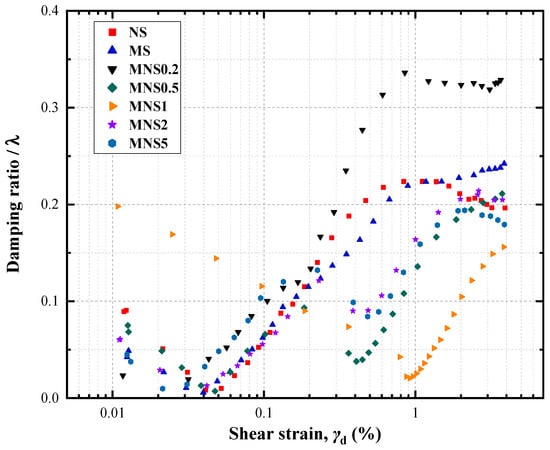
Figure 10.
Variations in damping ratio of all samples.
4. Conclusions
The study aimed to explore the dynamic responses of soils contaminated with combined heavy metals and treated with nZVI. For this purpose, natural soil was contaminated with three heavy-metal contaminants (Zn (NO3)2, Pb (NO3)2, Ni (NO3)2) at a specific water content (22%) and treated with five dosages of nZVI (0.2%, 0.5%, 1%, 2%, 5%). The compaction, PSD, Atterberg limits, and stepwise cyclic triaxial experiments were conducted on all samples to draw the following conclusions.
- (a)
- The introduction of nZVI into contaminated soil caused a rightward shift in the PSD curves of MS, and the increment was further accentuated with increasing dosages of nZVI. Notably, for MNS5, clay and silt fraction reduced significantly, with a marked transition (72.8% finer fraction) to sand fraction. It is suggested that nZVI treatment not only enhanced particle size but also selectively induced aggregation on larger soil particles, rather than forming independent clusters.
- (b)
- Upon nZVI treatment, a continuous increase in LL, PL, and PI values were observed, correlating with rising dosages. Heavy metal cations caused a decrease in that of MS. The increase was primarily due to the formation of hydroxide adsorption and aggregation structures as a result of the nZVI reactions. Additionally, residual nZVI presented in the soil continued to react with water added during the experimental process, further contributing to the increase in the measured Atterberg limits.
- (c)
- The backbone curve of the MNS1 exhibited a strain hardening behavior, in contrast to the other samples, which conformed to an ideal elastic-plastic model. Additionally, the maximum shear stress initially increased with the nZVI dosage but then decreased, with the 1% nZVI dosage marking the turning point. Specifically, the maximum shear stress of MNS1, was the highest among all treated soils, reaching 64.4 kPa. Furthermore, lower nZVI dosages (0.2% and 0.5%) primarily contributed to the immobilization of heavy metals in the soil. In contrast, excessive nZVI dosages (2% and 5%) led to the consumption of a significant amount of water on the soil and adsorption/aggregation coating on soil particles, which hindered the formation of dense structures.
- (d)
- The strain time-history curve for all the soil samples was divided into three distinct stages: initial gentle stage, gradual growth stage, and steeper growth stage. In terms of durability under stepwise cyclic loading, the NS reached failure at the 138th cycle when subjected to a 2.5% compression strain. In comparison, the MS exhibited slightly increased resilience, withstanding up to 140 cycles. However, the samples treated with varying dosages of nZVI demonstrated varying degrees of durability: MNS0.2 and MNS0.5withstood 127 cycles, MNS1 endured 118 cycles, MNS2 lasted for 106 cycles, and MNS5 sustained only 75 cycles. These findings indicated that, while moderate amounts of nZVI could facilitate the formation of a connective structure between soil particles, the new-formed structure tended to lead to weak connections and easy fracture under controlled-moisture and excess nZVI condition.
- (e)
- The fitting analysis of the Hardin–Drnevich model revealed that the variation in shear modulus for all soil samples remains relatively consistent pattern in the small strain range. Among these, the MNS1 possessed the maximum shear modulus of 39.3 MPa. This indicated a notably higher stiffness of MNS1 compared to that of other samples in this strain range. Further overall examination analysis highlighted that MNS1 exhibits superior resistance to attenuation of shear modulus, as observed in the strain–stress relationship.
- (f)
- For all samples except MNS1, the variation in the damping ratio exhibited an “S” shaped trend, while MNS1 demonstrated a “V” shaped trend. Specifically, in the small strain range, MNS1 displayed a larger damping ratio, attributable to the solid skeleton structure formed by nZVI between soil particles. Under cyclic loading conditions, the disintegration and rearrangement of larger soil particles occurred, leading to a reorganization of the soil skeleton structure. The phenomenon was recognized as an enhancement of the soil skeleton’s strength and an increase in the damping ratio.
Based on the discussions, it is noteworthy to explore in depth these potential micro mechanisms of nZVI-soil interactions closely related to dynamic responses, including (1) reaction with water on soil particles, (2) remediation with both heavy metals and water, (3) adsorption and agglomeration on soil particles, and (4) aggregation with soil particles. When nZVI is introduced into contaminated soil, these four reactions occur simultaneously in the nZVI-heavy metal-soil system. Due to electromagnetic forces, nZVI could adsorbed on the surface of electronegative soil particles, which will contribute to the connection structure between soil particles and the formation of larger aggregates. Under cyclic loadings, the fragile connective structures can effectively resist cyclic loading at the initial stage. As in a higher cyclic loading level, disintegration and rearrangement of particles and aggregates could occur, which led to variations in inter-particle interactions and soil skeleton structure. However, the uncertainty, between changes in macroscopic geotechnical properties and changes in microscopic geotechnical properties, needs to be demonstrated by more microscopic evidence. Therefore, investigating the microstructure of nZVI- or nanomaterial-treated soils, such as pore size distribution, particle characterization and soil skeleton structure, shows good prospects for future research in this field. Overall, it is important that every possible geological parameter should be studied in detail before nanomaterials’ application in soil.
Author Contributions
Conceptualization, Y.C.; writing—original draft preparation, J.W.; methodology, Y.C., J.W. and C.F.; formal analysis, Y.C. and J.W.; data preparation, J.W., C.F. and M.Z.; writing—review and editing, Q.D., Y.C. and J.W.; project administration, Q.D. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the special research fund of the Scientific Research Fund of Hainan University (grant number KYQD(ZR)-22038 and KYQD(ZR)-21067), The Innovation Platform for Academicians of Hainan Province (grant number YSPTZX202106), and the specific research fund of The Innovation Platform for Academicians of Hainan Province (grant number HD-YSZX-202106).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- He, Y.; Kasina, M. The Sequential Extraction of Municipal Solid Waste Incineration Bottom Ash: Heavy Metals Mobility and Sustainable Application of Ashes. Sustainability 2023, 15, 14638. [Google Scholar] [CrossRef]
- Aliyari Rad, S.; Nobaharan, K.; Pashapoor, N.; Pandey, J.; Dehghanian, Z.; Senapathi, V.; Minkina, T.; Ren, W.; Rajput, V.D.; Asgari Lajayer, B. Nano-Microbial Remediation of Polluted Soil: A Brief Insight. Sustainability 2023, 15, 876. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, K.; Zhang, J.; Ma, C.; Wang, Z.; Tian, X. Removal and Adsorption Mechanisms of Phosphorus, Cd and Pb from Wastewater Conferred by Landfill Leachate Sludge-Derived Biochar. Sustainability 2023, 15, 10045. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, M.; Wang, J.; Zhang, Z.; Duan, C.; Wang, X.; Zhao, S.; Bai, X.; Li, Z.; Li, Z. A global meta-analysis of heavy metal (loid) s pollution in soils near copper mines: Evaluation of pollution level and probabilistic health risks. Sci. Total Environ. 2022, 835, 155441. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Y.; Ma, Y.; Guo, C.; Jia, J. An Assessment Framework for Human Health Risk from Heavy Metals in Coal Chemical Industry Soils in Northwest China. Sustainability 2023, 15, 14768. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Zhang, Z.; Zhu, Y.; Hou, H.; Zhao, L.; Sun, Z.; Xue, W.; Shi, H. Linkage between human population and trace elements in soils of the Pearl River Delta: Implications for source identification and risk assessment. Sci. Total Environ. 2018, 610, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Chen, J.; Qian, H. Spatial characteristics of heavy metal contamination and potential human health risk assessment of urban soils: A case study from an urban region of South India. Ecotoxicol. Environ. Saf. 2020, 194, 110406. [Google Scholar] [CrossRef]
- Braun, J.M.; Hornung, R.; Chen, A.; Dietrich, K.N.; Jacobs, D.E.; Jones, R.; Khoury, J.C.; Liddy-Hicks, S.; Morgan, S.; Vanderbeek, S.B. Effect of residential lead-hazard interventions on childhood blood lead concentrations and neurobehavioral outcomes: A randomized clinical trial. JAMA Pediatr. 2018, 172, 934–942. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.-S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Zha, F.; Zhu, F.; Xu, L.; Kang, B.; Yang, C.; Zhang, W.; Zhang, J.; Liu, Z. Laboratory study of strength, leaching, and electrical resistivity characteristics of heavy-metal contaminated soil. Environ. Earth Sci. 2021, 80, 184. [Google Scholar] [CrossRef]
- Amiri, M.; Dehghani, M.; Javadzadeh, T.; Taheri, S. Effects of lead contaminants on engineering properties of Iranian marl soil from the microstructural perspective. Miner. Eng. 2022, 176, 107310. [Google Scholar] [CrossRef]
- Barbhuiya, G.H.; Hasan, S.D. Effect of nano-silica on physio-mechanical properties and microstructure of soil: A comprehensive review. Mater. Today Proc. 2021, 44, 217–221. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Akram, M.K.; Siddiqi, W.A.; Khan, A.; Al-Romaizan, A.N.; Hussein, M.A.; Puttegowda, M. Synthesis of atmospherically stable zero-valent iron nanoparticles (nZVI) for the efficient catalytic treatment of high-strength domestic wastewater. Catalysts 2021, 12, 26. [Google Scholar] [CrossRef]
- Liu, F.; Yi, S.; Zhou, W.H.; Chen, Y.Z.; Wong, M.H. Amendment additions and their potential effect on soil geotechnical properties: A perspective review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 535–576. [Google Scholar] [CrossRef]
- Grieger, K.D.; Fjordbøge, A.; Hartmann, N.B.; Eriksson, E.; Bjerg, P.L.; Baun, A. Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: Risk mitigation or trade-off? J. Contam. Hydrol. 2010, 118, 165–183. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd (II), Pb (II), and As (III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Lou, Y.; Cai, Y.; Tong, Y.; Hsieh, L.; Li, X.; Xu, W.; Shi, K.; Shen, C.; Xu, X.; Lou, L. Interaction between pollutants during the removal of polychlorinated biphenyl-heavy metal combined pollution by modified nanoscale zero-valent iron. Sci. Total Environ. 2019, 673, 120–127. [Google Scholar] [CrossRef]
- Radziemska, M.; Gusiatin, Z.M.; Holatko, J.; Hammerschmiedt, T.; Głuchowski, A.; Mizerski, A.; Jaskulska, I.; Baltazar, T.; Kintl, A.; Jaskulski, D. Nano Zero Valent Iron (nZVI) as an Amendment for Phytostabilization of Highly Multi-PTE Contaminated Soil. Materials 2021, 14, 2559. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Yang, J.; Tan, X.; Shaaban, M.; Cai, Y.; Wang, B.; Peng, Q. Remediation of Cr (VI)-Contaminated Soil by Biochar-Supported Nanoscale Zero-Valent Iron and the Consequences for Indigenous Microbial Communities. Nanomaterials 2022, 12, 3541. [Google Scholar] [CrossRef]
- Hamdy, A. Experimental study of the relationship between dissolved iron, turbidity, and removal of Cu (II) ion from aqueous solutions using zero-valent iron nanoparticles. Arab. J. Sci. Eng. 2021, 46, 5543–5565. [Google Scholar] [CrossRef]
- Song, M.; Hu, X.; Gu, T.; Zhang, W.; Deng, Z. Nanocelluloses affixed nanoscale Zero-valent iron (nZVI) for nickel removal: Synthesis, characterization and mechanisms. J. Environ. Chem. Eng. 2022, 10, 107466. [Google Scholar] [CrossRef]
- Mahdy, A.M.; Zhang, T.; Lin, Z.Q.; Fathi, N.O.; Badr Eldin, R.M. Zero-valent iron nanoparticles remediate nickel-contaminated aqueous solutions and biosolids-amended agricultural soil. Materials 2021, 14, 2655. [Google Scholar] [CrossRef] [PubMed]
- Kržišnik, N.; Mladenovič, A.; Škapin, A.S.; Škrlep, L.; Ščančar, J.; Milačič, R. Nanoscale zero-valent iron for the removal of Zn2+, Zn (II)–EDTA and Zn (II)–citrate from aqueous solutions. Sci. Total Environ. 2014, 476, 20–28. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fang, T.; Wang, J.; Liu, X.; Yan, Z.; Lin, H.; Li, F.; Guo, G. Insight into the stabilization mechanism and long-term effect on As, Cd, and Pb in soil using zeolite-supported nanoscale zero-valent iron. J. Clean. Prod. 2022, 355, 131634. [Google Scholar] [CrossRef]
- Kumar, N.; Auffan, M.l.; Gattacceca, J.R.M.; Rose, J.R.M.; Olivi, L.; Borschneck, D.; Kvapil, P.; Jublot, M.; Kaifas, D.; Malleret, L. Molecular insights of oxidation process of iron nanoparticles: Spectroscopic, magnetic, and microscopic evidence. Environ. Sci. Technol. 2014, 48, 13888–13894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Su, G.; Yang, K.; Lin, D. Transformation and implication of nanoparticulate zero valent iron in soils. J. Hazard. Mater. 2021, 412, 125207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, K.; Chefetz, B.; Xing, B.; Lin, D. The pH and concentration dependent interfacial interaction and heteroaggregation between nanoparticulate zero-valent iron and clay mineral particles. Environ. Sci. Nano 2019, 6, 2129–2140. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, W.X. Sequestration of metal cations with zerovalent iron nanoparticles a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J. Phys. Chem. C 2007, 111, 6939–6946. [Google Scholar] [CrossRef]
- Yan, W.; Herzing, A.A.; Kiely, C.J.; Zhang, W.X. Nanoscale zero-valent iron (nZVI): Aspects of the core-shell structure and reactions with inorganic species in water. J. Contam. Hydrol. 2010, 118, 96–104. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, W.X. Iron nanoparticles: The core− shell structure and unique properties for Ni (II) sequestration. Langmuir 2006, 22, 4638–4642. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ni, P.; Sun, Z.; Yuan, K. Geotechnical Properties and Stabilization Mechanism of Nano-MgO Stabilized Loess. Sustainability 2023, 15, 4344. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Li, J.; Tao, F.; Li, C.; Qian, B.; Jiang, P. Influence of carbonization process on the mechanical properties of nano-MgO modified cement soil. Sustainability 2021, 13, 3558. [Google Scholar] [CrossRef]
- Chen, S.; Hou, X.; Luo, T.; Yu, Y.; Jin, L. Effects of MgO nanoparticles on dynamic shear modulus of loess subjected to freeze-thaw cycles. J. Mater. Res. Technol. 2022, 18, 5019–5031. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, G.; Zhuang, X.; Pain, A. Dynamic characteristics and microstructural study of nano calcium carbonate modified cemented soil under different salt water solutions. Transp. Geotech. 2022, 32, 100700. [Google Scholar] [CrossRef]
- Zhou, W.H.; Liu, F.; Yi, S.; Chen, Y.Z.; Geng, X.; Zheng, C. Simultaneous stabilization of Pb and improvement of soil strength using nZVI. Sci. Total Environ. 2019, 651, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Zhou, W.H.; Liu, F.; Yi, S. Exploring the effects of nanoscale zero-valent iron (nZVI) on the mechanical properties of lead-contaminated clay. Can. Geotech. J. 2019, 56, 1395–1405. [Google Scholar] [CrossRef]
- Nasehi, S.A.; Uromeihy, A.; Morsali, A.; Nikudel, M.R. Use of Nanoscale Zero-Valent Iron to Improve the Shear Strength Parameters of Gas Oil Contaminated Clay. Geopersia 2015, 5, 161–175. [Google Scholar]
- BS 1377; Methods of Test for Soils for Civil Engineering Purposes. British Standards Institute: London, United Kingdom, 1990.
- ASTM D2487-17; Standard Test Method for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017.
- Su, Y.; Adeleye, A.S.; Zhou, X.; Dai, C.; Zhang, W.; Keller, A.A.; Zhang, Y. Effects of nitrate on the treatment of lead contaminated groundwater by nanoscale zerovalent iron. J. Hazard. Mater. 2014, 280, 504–513. [Google Scholar] [CrossRef]
- Hu, B.; Shao, S.; Fu, Z.; Li, Y.; Ni, H.; Chen, S.; Zhou, Y.; Jin, B.; Shi, Z. Identifying heavy metal pollution hot spots in soil-rice systems: A case study in South of Yangtze River Delta, China. Sci. Total Environ. 2019, 658, 614–625. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Liang, F.; Zhang, W.X. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Moazeni, M.; Ebrahimi, A.; Rafiei, N.; Pourzamani, H.R. Removal of lead ions from aqueous solution by nano zero-valent iron (nZVI). Health Scope 2017, 6, e40240. [Google Scholar]
- ASTM D4318-17; Standard Test Method for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D5311; Standard Test Method for Load Controlled Cyclic Triaxial Strength of Soil. ASTM International: West Conshohocken, PA, USA, 2013.
- Kaya, Z.; Erken, A.; Cilsalar, H. Characterization of elastic and shear moduli of adapazari soils by dynamic triaxial tests and soil-structure interaction with site properties. Soil Dyn. Earthq. Eng. 2021, 151, 106966. [Google Scholar] [CrossRef]
- Hardin, B. Shear Modulus and Damping in Soils; Design Equations and Curves. J. Soil Mech. Found. Div. 1965, 91, 79–99. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhou, W.H.; Liu, F.; Yi, S.; Geng, X. Microstructure and morphological characterization of lead-contaminated clay with nanoscale zero-valent iron (nZVI) treatment. Eng. Geol. 2019, 256, 84–92. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, K.; Lin, D. Nanoparticulate zero valent iron interaction with dissolved organic matter impacts iron transformation and organic carbon stability. Environ. Sci. Nano 2020, 7, 1818–1830. [Google Scholar] [CrossRef]
- Li, X.Q.; Elliott, D.W.; Zhang, W.X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Nasehi, S.A.; Uromeihy, A.; Nikudel, M.R.; Morsali, A. Use of nanoscale zero-valent iron and nanoscale hydrated lime to improve geotechnical properties of gas oil contaminated clay: A comparative study. Environ. Earth Sci. 2016, 75, 733. [Google Scholar] [CrossRef]
- Das, B.M. Principles of Geotechnical Engineering; Cengage Learning: Boston, MA, USA, 2021. [Google Scholar]
- Norouzi, A.; Uygar, E.; Nalbantoglu, Z. A review on the effects of landfill leachate on the physical and mechanical properties of compacted clay liners for municipality landfills. Arab. J. Geosci. 2022, 15, 1174. [Google Scholar] [CrossRef]
- Yan, J.; Kong, L.; Wang, J. Evolution law of small strain shear modulus of expansive soil: From a damage perspective. Eng. Geol. 2023, 315, 107017. [Google Scholar] [CrossRef]
- Zou, Z.; Yan, J.; Tang, H.; Wang, S.; Xiong, C.; Hu, X. A shear constitutive model for describing the full process of the deformation and failure of slip zone soil. Eng. Geol. 2020, 276, 105766. [Google Scholar] [CrossRef]
- O’Kelly, B.C. Review of recent developments and understanding of Atterberg limits determinations. Geotechnics 2021, 1, 59–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).