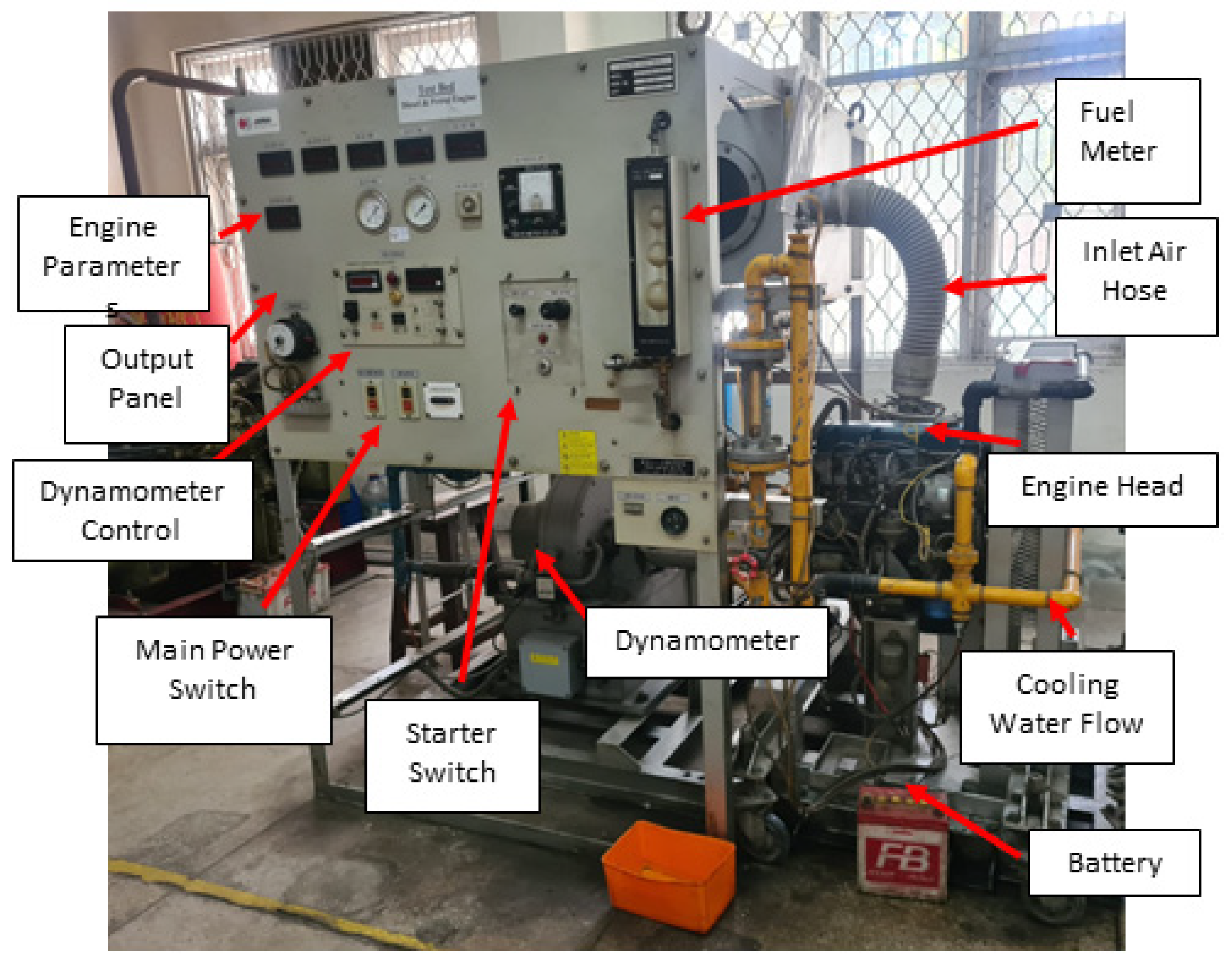
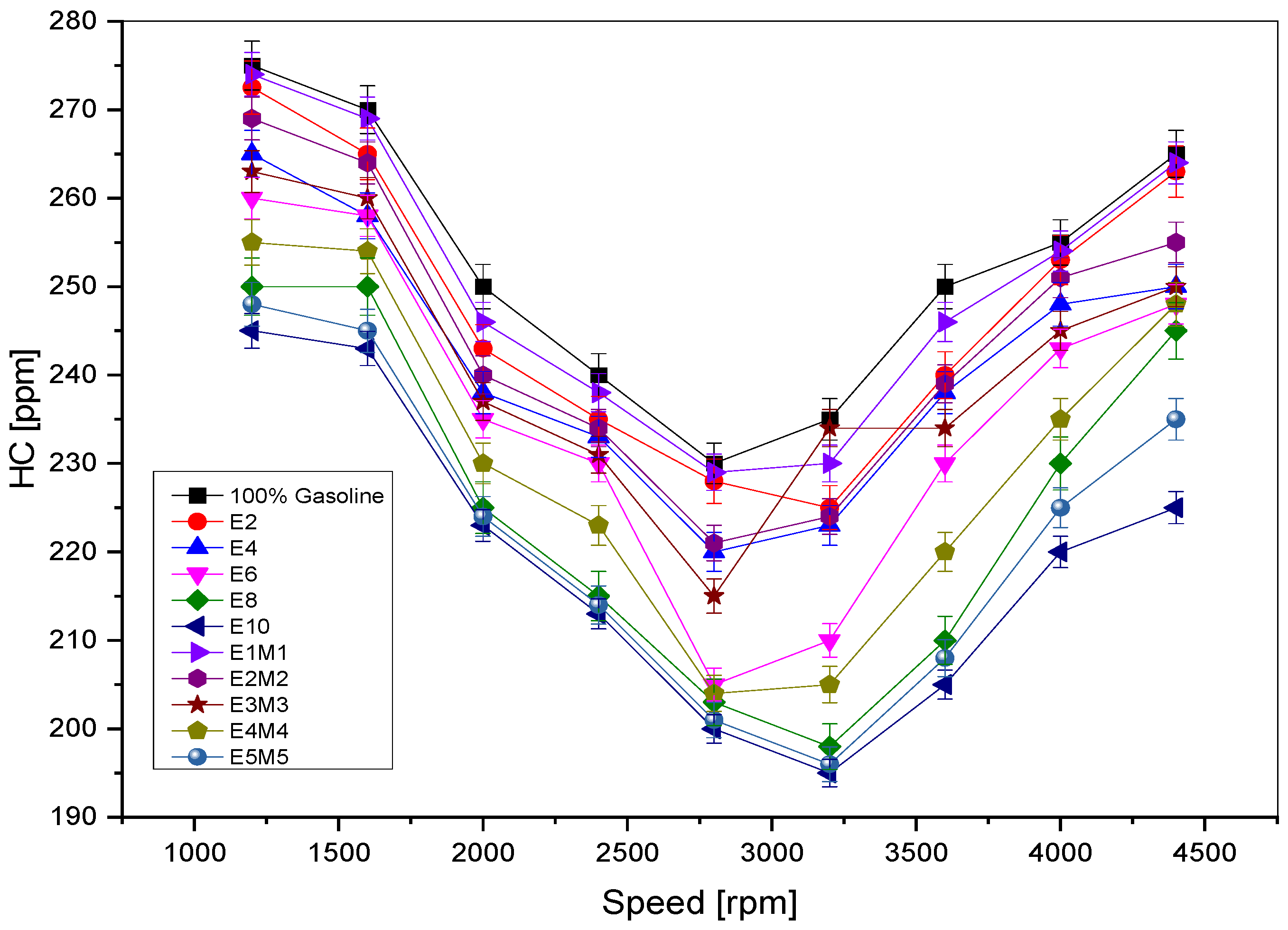
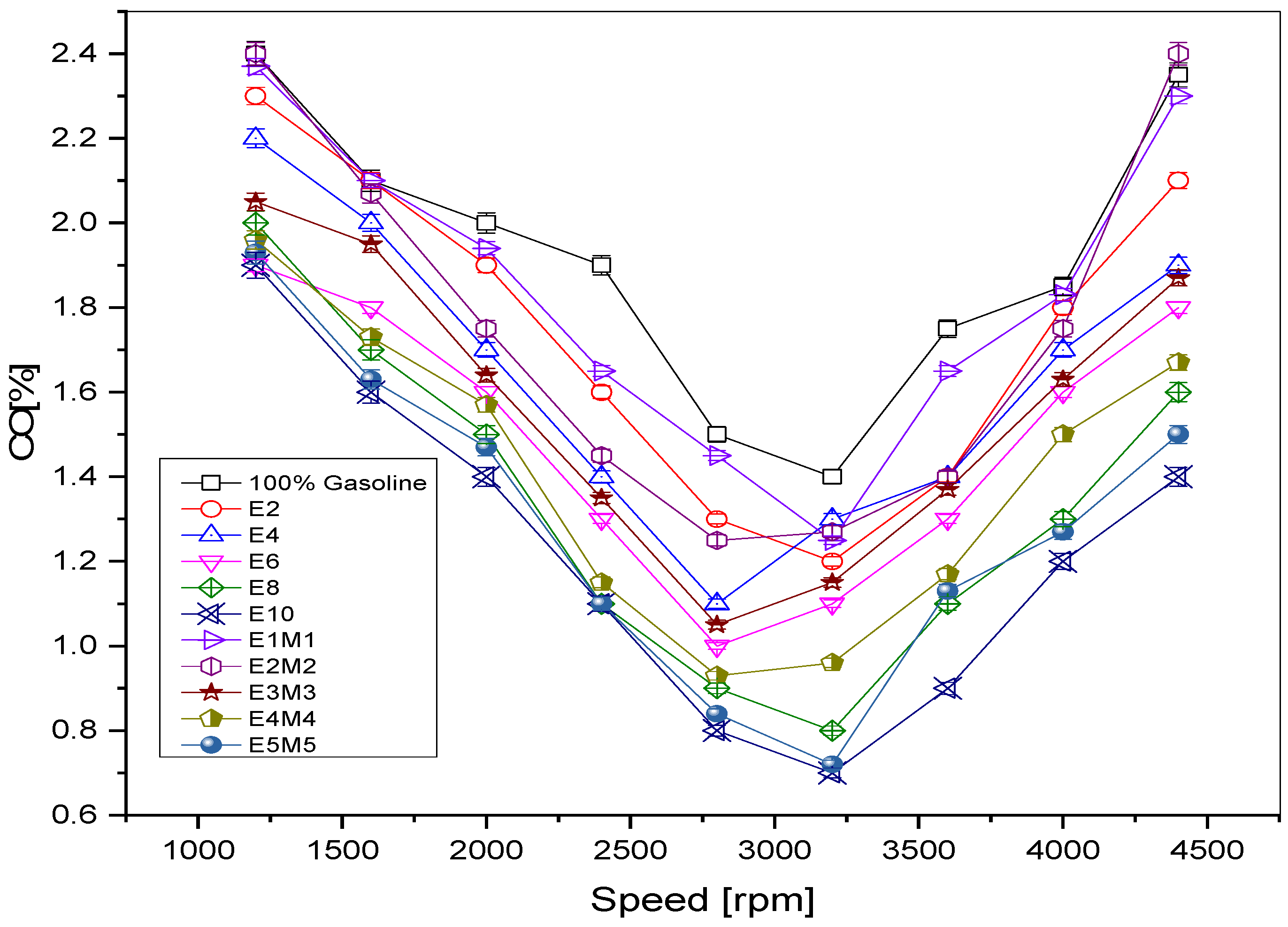
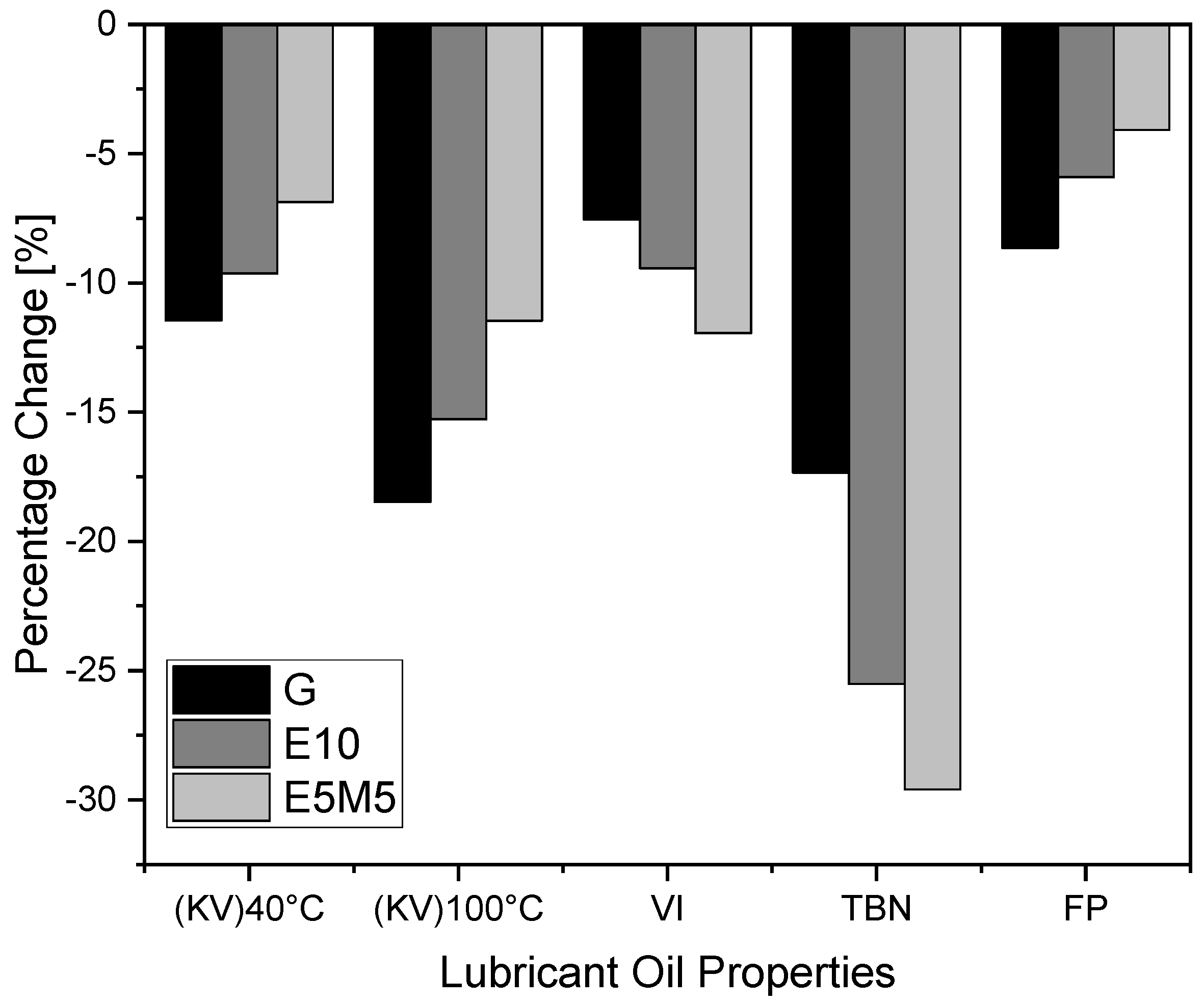
1. Introduction
The exploration of energy resources and their effective utilization constitute the prime focus of ongoing research. The major means of meeting energy requirements is fossil fuels [
1]. The use of fossil fuels is expanding on a daily basis with the growing energy needs of the population [
2]. One of the major downsides of these fuels is the fact that their emissions are accompanied by major environmental risks, i.e., global warming [
3,
4,
5]. The other problem is the depletion in resources of fossil fuels and, as per projections, these resources will be depleted within 40 years [
6]. Whilst the major part of today’s energy consumption comes from fossil fuels, in the future, it will become very difficult to fulfill our energy needs based on fossil fuels. Considering this situation, the need to use clean energy is increasing and the world is looking for cleaner fuels to use in automobiles and in other applications [
7,
8]. The transportation sector is a major consumer of the world’s primary energy, representing approximately 18% [
4]. Consequently, today’s research is focused on the application of alternative and cleaner fuels which will improve engine performance and decrease emissions. Alcoholic fuels are one such alternative to gasoline. Ethanol and methanol comprise those physical properties that make them good alternatives to gasoline to be used in spark ignition (SI) engines [
9,
10,
11]. Additionally, these fusel oils (ethanol and methanol) do not require any significant modification in the SI engine, which makes them suitable alternative to gasoline. Both of these fuels are considered as among the best alcoholic fuels owing to their higher octane rating, higher flashpoint, higher volumetric efficiency and higher latent heat of vaporization (LHV) [
12,
13]. The ratio of density of the air–fuel mixture drawn into the engine cylinder at atmospheric pressure during intake stroke to the density of the same volume of air in the intake manifold is known as volumetric efficiency. This directly reflects the fuel consumption, as more volumetric efficiency means lower fuel utilization for generating the same power.
Fuel additives can play a significant role in optimizing engine performance by improving the fuel quality and ensuring that it burns more efficiently. Fuel additives can improve the fuel economy as fuel burns with higher efficiency, increasing engine power output and reducing emissions. Elfasakhany [
14] used methanol and ethanol–methanol additives in gasoline in order to investigate the exhaust emissions from an SI engine ran on pure gasoline and gasoline blended with additives. He found that, when added to gasoline, methanol enabled a 6% and 5.5% decline in HC and CO emissions, respectively, in comparison with the ethanol–methanol blend. However, when added to gasoline, ethanol produced 5% and 2% higher CO and HC emissions, respectively, in comparison to ethanol. The ethanol–gasoline fuel showed a decrease of 31% and 14% in CO and HC, respectively, in contrast to gasoline. For EM3 (3 vol.% ethanol and methanol in gasoline), CO and HC decreased by 17% and 10%, respectively; CO and HC decreased by 35% and 15% for EM7, respectively, and decreased by 46% and 23% for EM10 in comparison to gasoline.
Ors et al. [
15] executed an experiment on an SI engine by blending methanol and ethanol with gasoline. They found that the addition of methanol increased the brake-specific fuel consumption (BSFC) values by 10.3% in comparison to the addition of ethanol, while the brake thermal efficiency (BTE) was reduced by 6.12%. The addition of methanol decreased the HC, CO
2, NO
x and CO emissions by 4.75%, 6.48%, 9.16% and 26.6%, respectively, in comparison to ethanol. Usman et al. [
16] ascertained the performance of the SI engine when acetone was added to gasoline. A10 (10% acetone in 90% gasoline) produced 11.74% and 12.05% higher brake power (BP) and BTE, respectively, at a 6.72% lower BSFC. The CO, CO
2 and HC emissions declined by 56.54%, 33.67% and 50% in the case of the A10 fuel. However, it was concluded that acetone appeared to be more detrimental to lubricant oil for the existing metallurgy of the engine. In another study, Usman et al. [
17] recorded the impact of butanol–gasoline on the SI engine performance. They found 12.15%, 3.25% and 28% higher BP, BTE and exhaust gas temperature (EGT), respectively, in the case of B12 (12% butanol in 88% gasoline). However, the CO and HC emissions declined by 31% and 27% in the case of B12, respectively. They also highlighted that, as the concentration of butanol increased, the lubricant oil started deteriorating early.
Veza et al. [
18] performed an experiment on the homogeneous combustion compression ignition (HCCI) engine fueled with an acetone, butanol and ethanol blend with diesel (ABE). They found that the oxygen content in ABE was primarily accountable for the improved combustion which ultimately reduced the CO, CO
2, NO
x and HC emissions. However, the soot and PM emissions remain unaffected by the ABE. However, a higher BTE and lower in-cylinder pressure, EGT and BSFC were observed for the ABE blended fuel. Yusuf et al. [
19] ran a diesel engine at 1800 rpm under 50% loading conditions fueled with cerium-dioxide (CeO
2) nanoparticles in waste cooking oil biodiesel. They found an upsurge in the cylinder pressure, heat release rate (HRR) and NO
x emissions, while the HC and CO emissions were decreased for the blended fuel. Soudagar et al. [
20] evaluated the impact of nanoparticles in diesel and biodiesel blends. They found that the addition of metal-based (manganese, nickel, magnesium, cobalt) additives and oxygenated additives to the biodiesel blend reduced the density, viscosity and flash point due to the increased oxygen content in the blend. These properties are responsible for improved combustion, better fuel spray characteristics, reduced emissions and ultimately, an improved engine performance. They also found that the presence of oxygenated additives (diethyl ether, isobutanol, ethanol) and metallic additives was responsible for reduced BSFC owing to their higher calorific value, catalytic oxidation enhancement and complete combustion of blended fuel.
Sivalakshmi et al. [
21] examined the performance of di-ethyl ether (DEE) in neem oil biodiesel in an engine operated at constant 1500 rpm under different loading conditions. They found an increase in NO
x emission, but CO, CO
2, HC and smoke emissions were considerably decreased. Akshatha et al. [
22] found that the mixing of DEE in neem oil significantly increased the BTE and reduced BSFC. Soudagar et al. [
23] used a strontium–zinc oxide additive in biodiesel (20% Ricinus communis and 80% diesel). They found 9.55%, 20.83% and 24.35% increases in in-cylinder pressure, BTE and HRR, respectively, while the ignition delay, BSFC, combustion duration as well as the CO
2, CO and HC emissions decreased by 20.64%, 20.07%, 14.5% as well as by 34.9%, 47.63% and 26.81%, respectively, with a minor rise in NO
x. In contrast with gasoline, the CO and HC emissions decreased by approximately 17% and 10% using EM3 (3 vol.% ethanol and methanol in gasoline), while they decreased by approximately 35% and 15% or 46% and 23%, respectively, when using EM7 or EM10. Malik et al. [
13] concluded with a comparison between M12 (12% methanol in 88% gasoline) and pure gasoline. The results depict the increase in brake power (BP) of 6.69% and a highest achieved efficiency of 23.69% for M12. However, the exhaust gas temperature (EGT), NO
x and CO
2 also increased by 16.91%, 27.58 and 2.61% for M12, respectively. The higher temperature was responsible for greater greenhouse gas emissions and the early degradation of the lubricant oil. The kinematic viscosity, total acid number (TAN) and ash content for the lubricant oil operated on M12 increased by 4.57%, 10.23% and 0.97%, respectively.
Mallikarjun et al. [
24] observed the influence of 3–15% methanol blends for distinct load settings. They found higher BTE, CO
2 and NO
x and decreased HC and CO emissions. Under wide-open throttle (WOT) condition, Prasad et al. [
25] performed the experiment on a single-cylinder engine and fueled it with methanol-mixed fuel at sustained 14° BTDC ignition timing at three distinct compression ratios (8, 9 and 10). The combustion efficiency was improved for the methanol-blended fuel when the compression ratio increased, and the HRR and peak pressure also increased by 30% and 27.5%, respectively. BTE increased by 25% at the cost of 19% reduced BSFC. However, the reduction of 30–40% was found for HC, CO and NO
x emissions. Shayan et al. [
26] ascertained the exhaust emissions in the case of the methanol-blended gasoline fuel. They kept count of the average drop in HC emissions (24.9%) and CO emissions (23.7%). In contrast to petrol, increases of 7.5% and 17.5% were obtained for CO
2 and NO
x, respectively. Yontar AA [
27] used hydrogen to improve the qualities of gasoline, and after that, he contrasted the outcomes of ethanol and methyl tert butyl ether (MTBE) as well as pure gasoline and hydrogen–gasoline combination with these three fuels. Compared to gasoline, ethanol has a higher BSFC of 29.61%. G98H2 exhibited a BSFC which was approximately 9.24% lower than gasoline. However, ethanol exhibited a BSFC which was approximately 9.24% lower than gasoline. In contrast to gasoline, the ethanol generated 3.11% higher BTE and the G98H2 produced 1.42% higher BTE. For gasoline, MTBE, ethanol and G98H2, the HC formation ranges from 81 to 101 parts per million (ppm), from 80 ppm to 97 ppm and from 77 ppm to 93 ppm, respectively.
Radzali et al. [
28] investigated the effects of methanol–gasoline fuel mixes and ambient pressure on exhaust emissions and flame propagation. The flame propagation became wider when the percentage of methanol increased from 0% to 15% in gasoline, which further improved the burn rate. As a consequence of the improved combustion, the CO
2 emissions were increased, while the CO, NO
2 and HC emissions were lowered. Balki, Sayin and Canakci [
29] presented the comparative research between methanol and gasoline on a 196 cc gasoline engine. The achieved results exposed that the torque would rise by 4.7% and that the BSFC would rise by 84% with reference to gasoline.
Malik et al. [
1] also examined the behavior of M6 (6% methanol in 94% gasoline) on the SI engine performance. The obtained results revealed 3.72% and 1.38% higher brake power and BTE in the case of M6. However, M6 showed a 1.37% smaller brake specific energy consumption (BSEC) owing to the naturally lower calorific value of methanol. The lower calorific value is responsible for augmented fuel consumption and consequently, the combustion temperature rises. The EGT and NO emissions for M6 were 10.66% and 9.70% higher for the M6 fuel blend, respectively. The kinematic viscosity, total acid number (TAN), flashpoint and ash content of the lubricant oil operated on M6 were increased by 1.28%, 6.03%, 3.49% and 0.48%, respectively, in contrast to lubricant oil operated on gasoline. Ahmed et al. [
30] conducted experiments on various methanol blends with gasoline ranging from 0% to 18%. They found that the EGT was 3.38%, 11.43%, 19.48%, 24.65%, 25.53% and 27.38% higher for M3–M8 with an interval of 3% by volume methanol, respectively, whilst NO
x emissions increased by 5.49%, 12.54%, 19.19%, 28.73%, 35.81% and 41.13%, respectively. However, the HC emissions decreased by 2.12%, 4.97%, 7.92%, 11.05%, 14.14% and 17.04% for M3–M8. Thakur et al. [
31] deduced that BP increased by 2.31%, 2.77% and 4.16% for E5, E10 and E20, respectively, while maximum increases of 3.5%, 2.5% and 6% in BTE were found for E20, E10 and E40, respectively. Hsieh et al. [
32] found that CO and HC emissions declined by 10–90% and 20–80%, respectively, while CO
2 emissions increased by 5–25% depending on ethanol–gasoline blend ratio, varying from 5% to 30%. Masum et al. [
33] stated that a higher flame speed of methanol helped complete the combustion process, and as a consequence, a higher NO
x emission was achieved for the ethanol–gasoline fuel blend.
The literature review showed that the performance characteristics of the gasoline engine, on average, increased using alcoholic fuels such as ethanol, methanol, etc. The brake power and torque are enhanced using ethanol blended with gasoline. On average, the maximum increase of 20% in the performance parameters was achieved with the ethanol blend, and then, these parameters started to decrease after attaining maximum values [
34,
35]. Similarly, the performance parameters were also found to be higher for methanol-blended gasoline compared to pure gasoline [
36]. The brake power was noticed to be higher for ethanol compared with methanol [
37]. The disadvantage of using the ethanol–gasoline blended fuel is that the BSFC increases with increasing concentrations of ethanol [
38]. The blend of methanol in gasoline also showed an increase in BSFC [
39,
40].
The assessment of ethanol and methanol blended with gasoline demonstrated that the BSFC for ethanol was lower than that for methanol [
41]. By employing alcoholic fuels, an improvement was observed in the BTE of the gasoline engine and it increased with increasing concentrations [
42,
43,
44]. When the BTEs of both ethanol and methanol were compared, that of ethanol was higher due to its better combustion compared to methanol [
45]. An imperative consideration to adjudicate the combustion completion is the exhaust gas temperature (EGT). Previous findings have shown that the addition of ethanol increased the exhaust temperature in comparison to gasoline, further resulting in more oxides of nitrogen (NO
x) emissions [
46,
47]. Similar results were obtained with methanol [
26,
47]. The comparison of the both alcoholic fuels showed a higher EGT for ethanol [
37]. Using alcoholic fuels such as ethanol, methanol, butanol, etc. with gasoline considerably reduced the emissions [
48]. The application of ethanol-blended gasoline also caused the emissions of unburnt hydrocarbon (HC) to drop significantly. It was also found that the emissions go on depleting with increasing concentrations of ethanol, which is due to the better combustion of ethanol-blended gasoline (E) [
42,
43,
49]. The same results were also found for methanol [
36]. Methanol generated greater HC contents when compared with ethanol [
37]. Carbon monoxide (CO) emissions were observed to reduce using the ethanol blend with gasoline [
50].
The use of methanol with gasoline revealed that the CO emissions decreased with increased proportions of methanol [
37]. The CO emissions for ethanol were found to be lower than those for methanol [
51]. The results showed a higher carbon dioxide (CO
2) content in the exhaust for ethanol and methanol compared with pure gasoline [
50,
52]. Comparing both of these alcoholic fuels, ethanol showed more CO
2 emissions than methanol [
37].
Nitrogen oxide (NO
x) emissions are related to the exhaust temperature and increase as the exhaust temperature increases. NO
x emissions are greater for ethanol–gasoline blends than they are for pure gasoline [
47]. The comparison of methanol blended with gasoline alone also showed an upsurge in the NO
x emissions in contrast with pure gasoline. The results also show that the NO
x emissions were found to be higher for ethanol compared with methanol [
37]. Tibaquira et al. opted for the eco-indicator-99 methodology to quantify the impact of the ethanol–gasoline blend on the environment. They obtained the results which declared that the effects on healths of humans, the environment and natural resources were decreased by 1.3%, 1.4% and 12.9%, respectively, when 20% ethanol in 80% gasoline (E20) was used [
53].
The studied literature reveals that there is an existing gap in analyzing the impact of the ethanol–methanol blend (EM) in gasoline on exhaust emissions and the performance characteristics of gasoline engine. In this very context, this study is focused on ascertaining the impact of ethanol–methanol as well as those of ethanol blends in gasoline on the performance and exhaust pollutants of a four-stroke SI engine. Additionally, the literature review reveals that CO2 and NOx emissions increase for alcoholic fuels. CO2 is major a contributor to the greenhouse effect and global warming, while NOx is an indicator of higher EGT. A higher temperature will evidence premature lubricant oil degradation. Therefore, attempts were made to develop such blends which not only reduced CO2 but also NOx and EGT in order to maintain optimum conditions for engine performance. This will surely be a valuable addition to research on alcoholic blends. In the current study, it was found that, despite their greater consumption due to their lower calorific value alcoholic fuels, these produce more brake power, EGT, CO2 and NOx emissions. The higher CO2, EGT and NOx emissions indicate better fuel combustion and ultimately improve the BTE and decrease the CO and HC emissions.