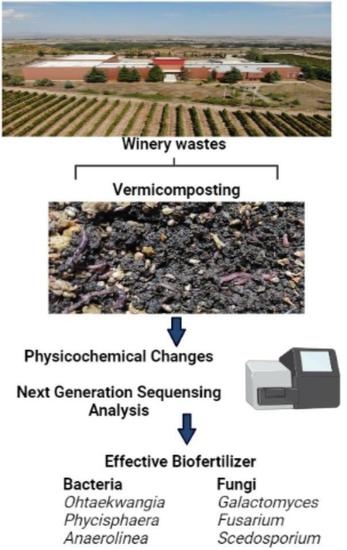
Physicochemical Changes and Microbiome Associations during Vermicomposting of Winery Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Vermireactor Setup and Sample Collection
2.2. Chemical Analysis
2.2.1. Moisture Content, pH, Conductivity, and Temperature
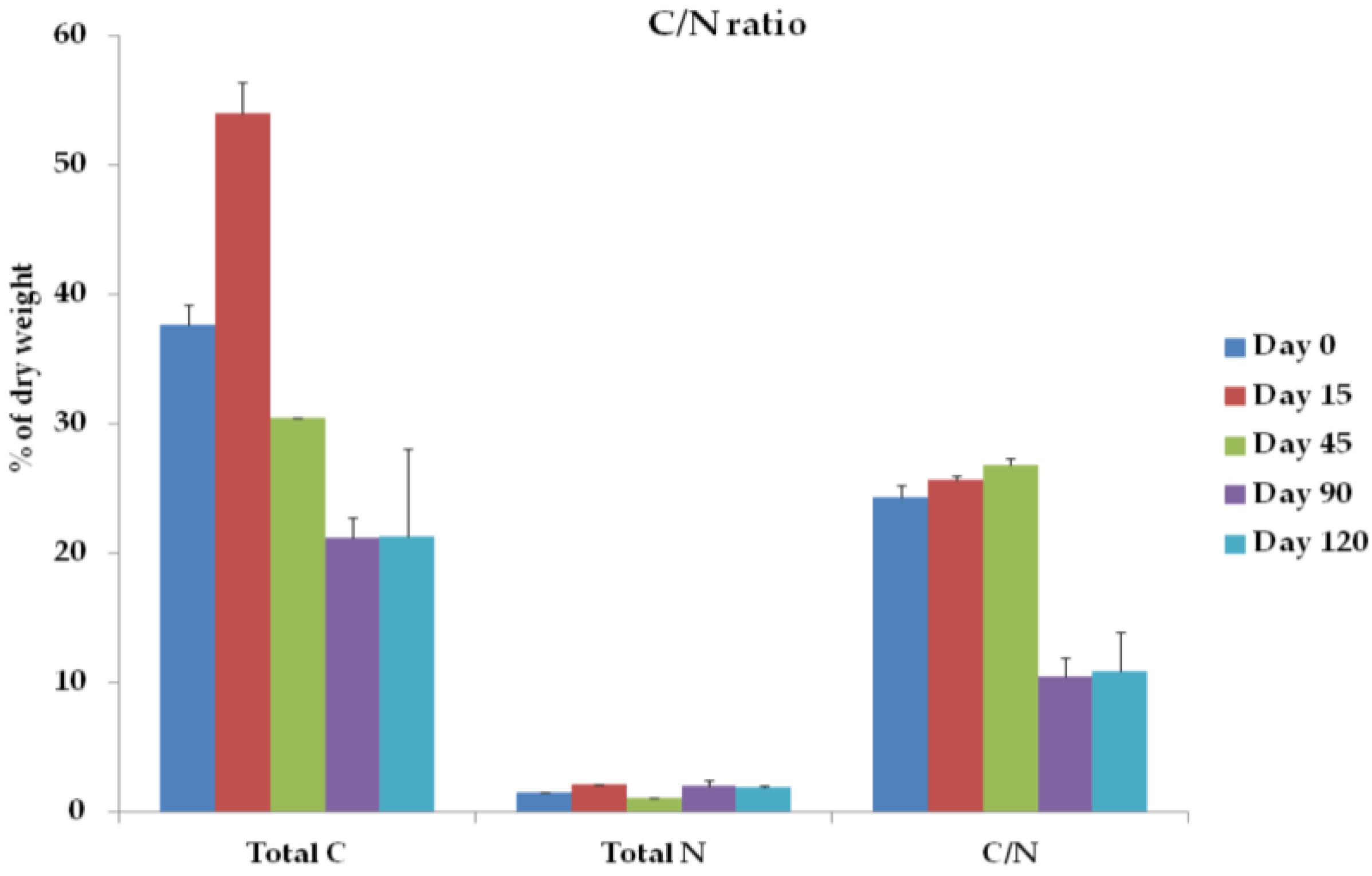
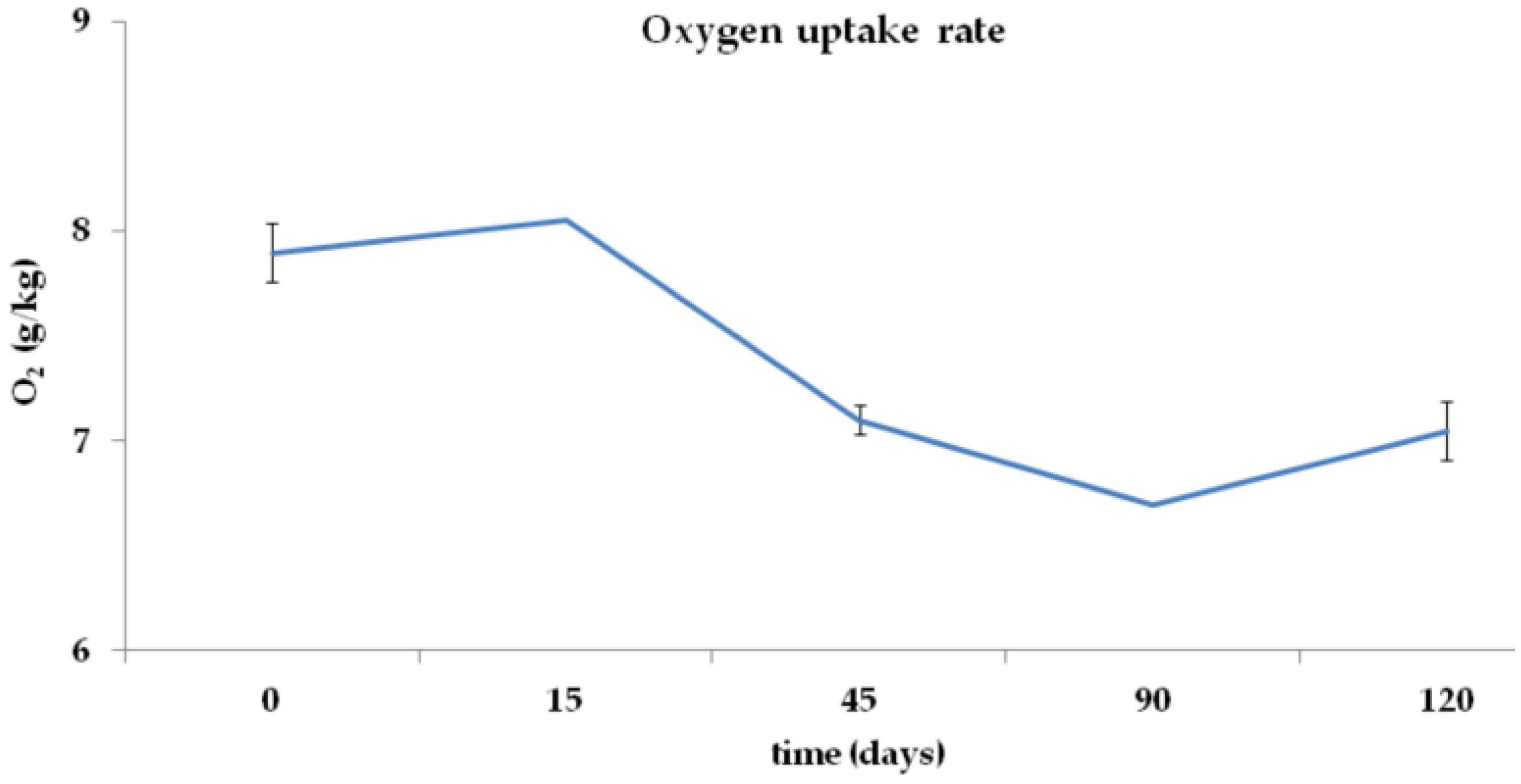
2.2.2. Total C, Total N, C/N Ratio, and O2 Uptake Rate
2.2.3. Macro- and Micronutrients
2.3. Enzymatic Activity
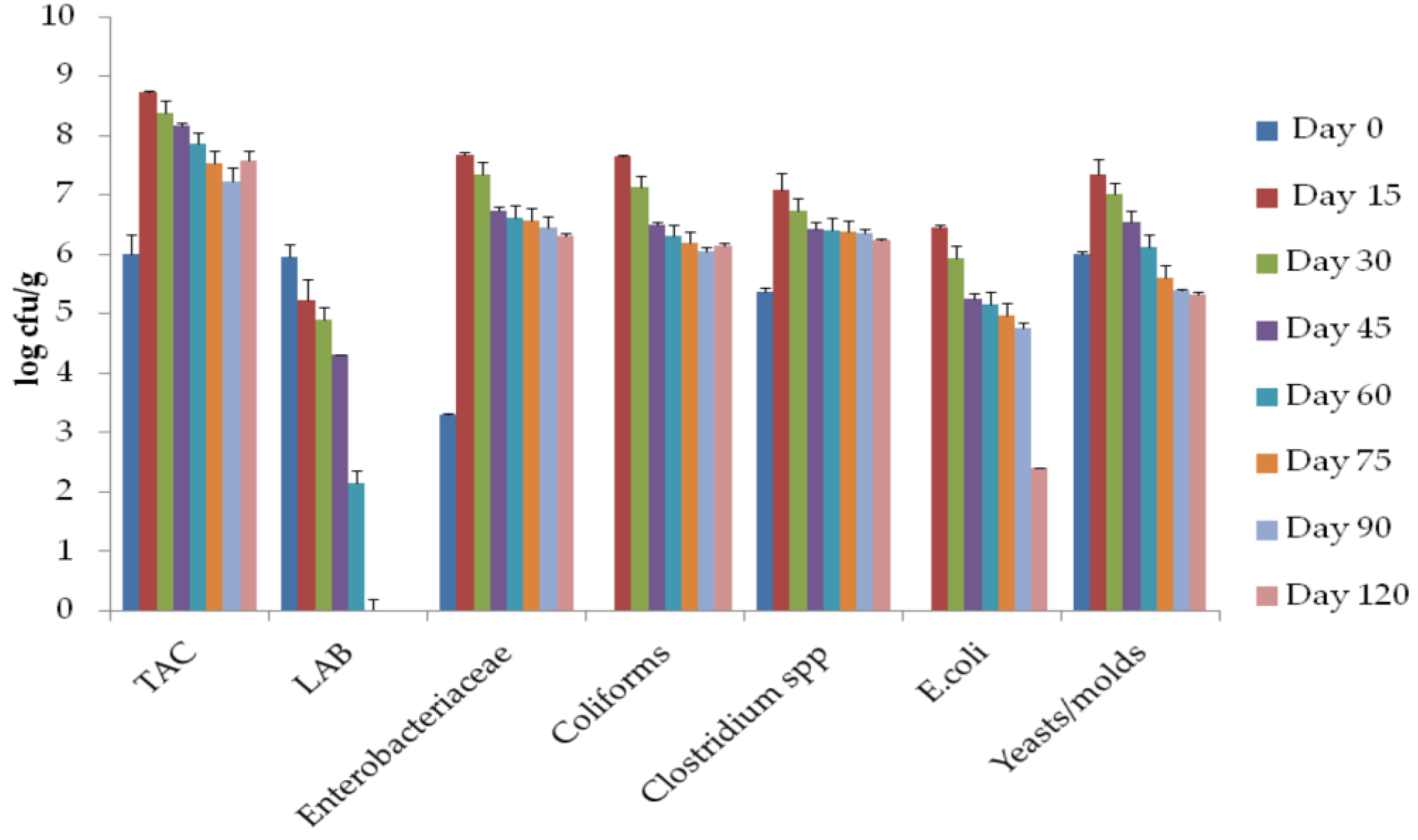
2.4. Microbiological Analysis
2.5. DNA Extraction, PCR Amplification, and 16S and ITS rRNA Sequencing
2.6. Germination Index
2.7. Statistical Analysis
3. Results and Discussion
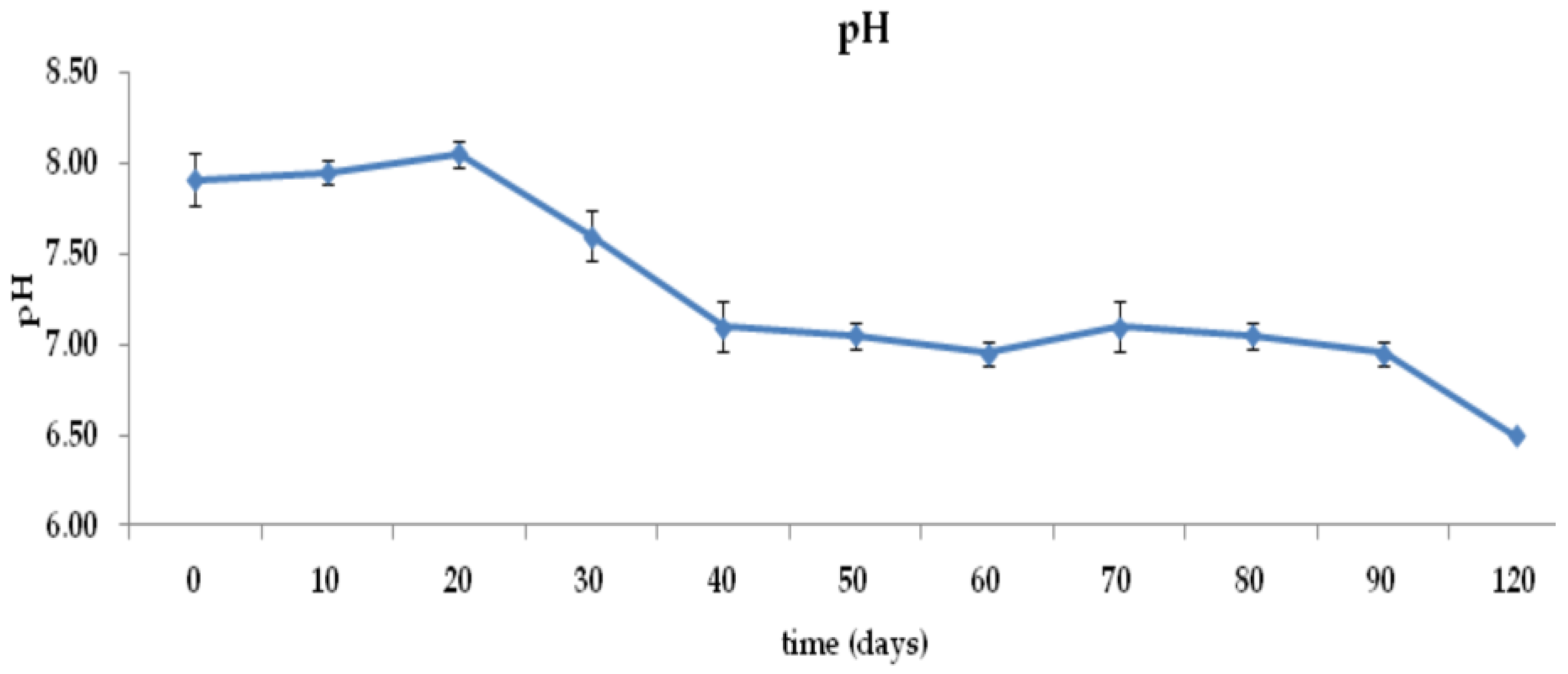
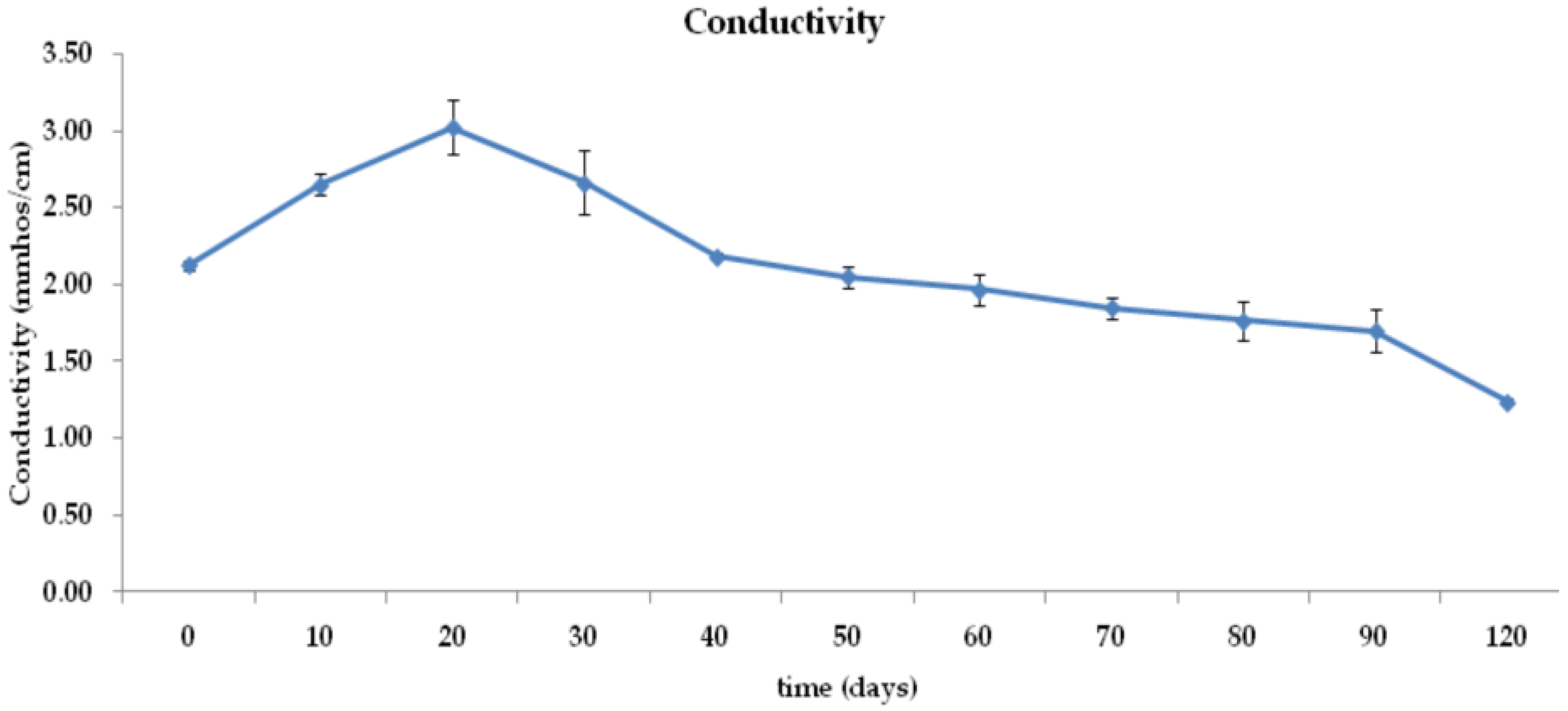
3.1. Moisture Content, pH, Conductivity, and Temperature
3.2. Total C, Total N, C/N Ratio, and O2 Concentration
3.3. Macro- and Micronutrients
3.4. Enzymatic Activity
3.5. Microbial Populations
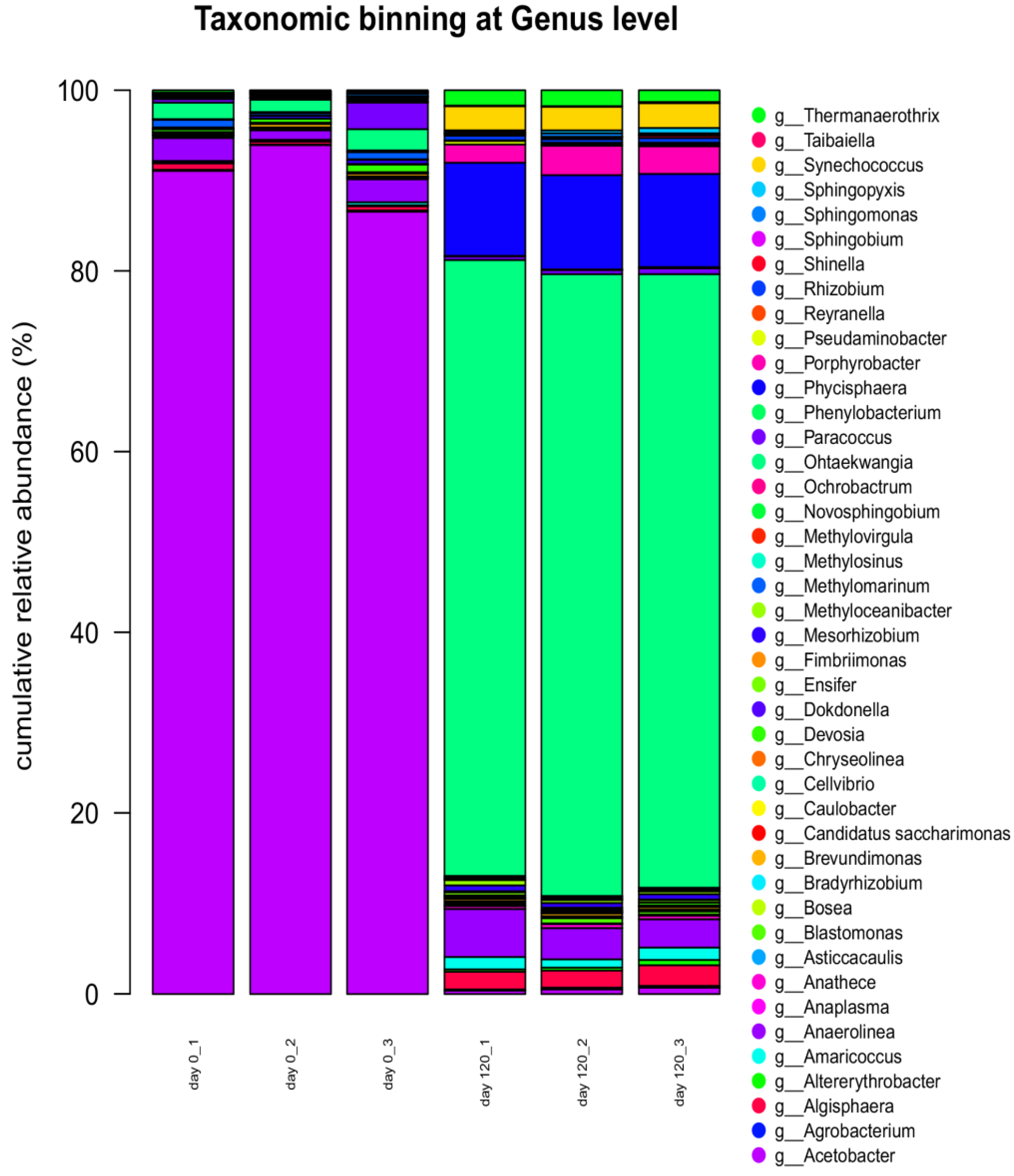
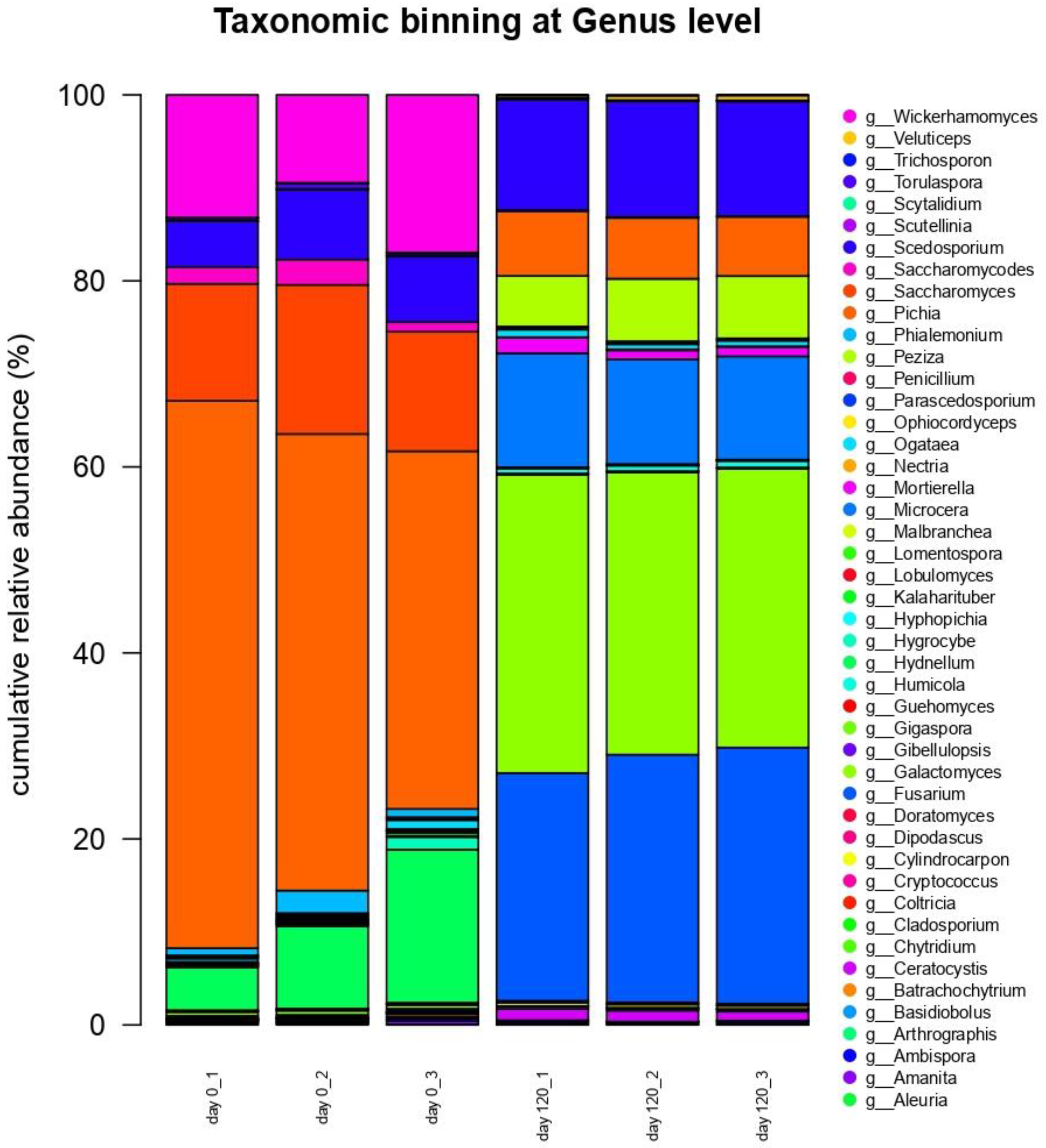
3.6. Next-Generation Sequencing DNA Analysis
3.7. Germination Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OIV Statistical Report on World Vitiviniculture, 2018 World Vitiviniculture Situation. Available online: http://www.oiv.int/public/medias/6371/oiv-statistical-report-on-world-vitiviniculture-2018.pdf (accessed on 19 November 2018).
- Nanni, A.; Parisi, M.; Colonna, M. Wine by-products as raw materials for the production of biopolymers and of natural reinforcing fillers: A critical review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and Distillery Industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the Council and the European Parliament—Towards a Sustainable European Wine Sector; 52006DC0319; Commision of the European Communities: Brussels, Belgium, 2006; pp. 1–13.
- Spigno, G.; De Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.; Barral, M.; Cruz, J.; Moldes, A. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Galanakis, C.M. Handbook of Grape Processing by-Products: Sustainable Solutions; Academic Press: San Diego, CA, USA, 2017. [Google Scholar]
- Vyas, P.; Sharma, S.; Gupta, J. Vermicomposting with Microbial Amendment: Implications for bioremediation of industrial and agricultural waste. BioTechnologia 2022, 103, 203–215. [Google Scholar] [CrossRef]
- Sinha, R.K.; Agarwal, S.; Chauhan, K.; Valani, D. The wonders of earthworms & its vermicompost in farm production: Charles Darwin’s ‘friends of farmers’, with potential to replace destructive chemical fertilizers. Agric. Sci. 2010, 1, 76–94. [Google Scholar]
- Ali, U.; Sajid, N.; Khalid, A.; Riaz, L.; Rabbani, M.M.; Syed, J.H.; Malik, R.N. A review on vermicomposting of Organic Wastes. Environ. Prog. Sustain. Energy 2015, 34, 1050–1062. [Google Scholar] [CrossRef]
- Ramnarain, Y.I.; Ansari, A.A.; Ori, L. Vermicomposting of different organic materials using the epigeic earthworm Eisenia foetida. Int. J. Recycl. Org. Waste Agric. 2018, 8, 23–36. [Google Scholar] [CrossRef]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. SpringerPlus 2012, 1, 26. [Google Scholar] [CrossRef]
- Domínguez, J.; Martínez-Cordeiro, H.; Álvarez-Casas, M.; Lores, M. Vermicomposting grape Marc yields high quality organic biofertiliser and bioactive polyphenols. Waste Manag. Res. J. A Sustain. Circ. Econ. 2014, 32, 1235–1240. [Google Scholar] [CrossRef]
- Kolbe, A.R.; Aira, M.; Gómez-Brandón, M.; Pérez-Losada, M.; Domínguez, J. Bacterial succession and functional diversity during vermicomposting of the white grape Marc Vitis vinifera v. Albariño. Sci. Rep. 2019, 9, 7472. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Pinter, I.F.; Fernández, A.S.; Martínez, L.E.; Riera, N.; Fernández, M.; Aguado, G.D.; Uliarte, E.M. Exhausted grape Marc and organic residues composting with polyethylene cover: Process and quality evaluation as plant substrate. J. Environ. Manag. 2019, 246, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Schinner, F. Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Li, Y.-T.; Rouland, C.; Benedetti, M.; Li, F.-bai; Pando, A.; Lavelle, P.; Dai, J. Microbial biomass, enzyme and mineralization activity in relation to soil organic C, N and P turnover influenced by acid metal stress. Soil Biol. Biochem. 2009, 41, 969–977. [Google Scholar] [CrossRef]
- Jackson, C.R.; Tyler, H.L.; Millar, J.J. Determination of microbial extracellular enzyme activity in waters, soils, and sediments using high throughput microplate assays. J. Vis. Exp. 2013, 80, 50399. [Google Scholar]
- Lagkouvardos, I.; Fischer, S.; Kumar, N.; Clavel, T. Rhea: A transparent and Modular R pipeline for microbial profiling based on 16S rrna gene amplicons. PeerJ 2017, 5, e2836. [Google Scholar] [CrossRef] [PubMed]
- Paradelo, R.; Moldes, A.B.; González, D.; Barral, M.T. Plant tests for determining the suitability of grape Marc composts as components of Plant Growth Media. Waste Manag. Res. J. A Sustain. Circ. Econ. 2012, 30, 1059–1065. [Google Scholar] [CrossRef]
- Singh, A.; Jain, A.; Sarma, B.K.; Abhilash, P.C.; Singh, H.B. Solid waste management of temple floral offerings by vermicomposting using Eisenia fetida. Waste Manag. 2013, 33, 1113–1118. [Google Scholar] [CrossRef]
- Santana, N.A.; Jacques, R.J.; Antoniolli, Z.I.; Martínez-Cordeiro, H.; Domínguez, J. Changes in the chemical and biological characteristics of grape Marc vermicompost during a two-year production period. Appl. Soil Ecol. 2020, 154, 103587. [Google Scholar] [CrossRef]
- Dume, B.; Hanc, A.; Svehla, P.; Michal, P.; Chane, A.D.; Nigussie, A. Vermicomposting technology as a process able to reduce the content of potentially toxic elements in sewage sludge. Agronomy 2022, 12, 2049. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Martínez-Cordeiro, H.; Domínguez, J. Changes in the nutrient dynamics and microbiological properties of grape Marc in a continuous-feeding vermicomposting system. Waste Manag. 2021, 135, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mago, M.; Gupta, R.; Yadav, A.; Kumar Garg, V. Sustainable treatment and nutrient recovery from leafy waste through vermicomposting. Bioresour. Technol. 2022, 347, 126390. [Google Scholar] [CrossRef]
- Khwairakpam, M.; Bhargava, R. Bioconversion of filter mud using vermicomposting employing two exotic and one local earthworm species. Bioresour. Technol. 2009, 100, 5846–5852. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2014, 95, 1143–1156. [Google Scholar] [CrossRef]
- Ghorbani, M.; Sabour, M.R. Global trends and characteristics of vermicompost research over the past 24 years. Environ. Sci. Pollut. Res. 2020, 28, 94–102. [Google Scholar] [CrossRef]
- Hait, S.; Tare, V. Vermistabilization of Primary Sewage Sludge. Bioresour. Technol. 2011, 102, 2812–2820. [Google Scholar] [CrossRef]
- Biruntha, M.; Karmegam, N.; Archana, J.; Karunai Selvi, B.; John Paul, J.A.; Balamuralikrishnan, B.; Chang, S.W.; Ravindran, B. Vermiconversion of biowastes with low-to-high C/N ratio into value added vermicompost. Bioresour. Technol. 2020, 297, 122398. [Google Scholar] [CrossRef]
- Boruah, T.; Barman, A.; Kalita, P.; Lahkar, J.; Deka, H. Vermicomposting of Citronella Bagasse and paper mill sludge mixture employing Eisenia fetida. Bioresour. Technol. 2019, 294, 122147. [Google Scholar] [CrossRef]
- Lv, B.; Zhang, D.; Cui, Y.; Yin, F. Effects of C/N ratio and earthworms on greenhouse gas emissions during vermicomposting of Sewage Sludge. Bioresour. Technol. 2018, 268, 408–414. [Google Scholar] [CrossRef]
- Suthar, S.; Singh, S. Feasibility of vermicomposting in biostabilization of sludge from a distillery industry. Sci. Total Environ. 2008, 394, 237–243. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R. Optimization of cow dung spiked pre-consumer processing vegetable waste for vermicomposting using Eisenia fetida. Ecotoxicol. Environ. Saf. 2011, 74, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Singh, J.; Vig, A.P. Instrumental characterization of organic wastes for evaluation of vermicompost maturity. J. Anal. Sci. Technol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Shak, K.P.; Wu, T.Y.; Lim, S.L.; Lee, C.A. Sustainable reuse of rice residues as feedstocks in vermicomposting for Organic Fertilizer Production. Environ. Sci. Pollut. Res. 2013, 21, 1349–1359. [Google Scholar] [CrossRef]
- Sonowal, P.; Khwairakpam, M.; Kalamdhad, A.S. Stability Analysis of dewatered sludge of pulp and Paper Mill during vermicomposting. Waste Biomass Valorization 2013, 5, 19–26. [Google Scholar] [CrossRef]
- Paul, S.; Kauser, H.; Jain, M.S.; Khwairakpam, M.; Kalamdhad, A.S. Biogenic stabilization and heavy metal immobilization during vermicomposting of vegetable waste with biochar amendment. J. Hazard. Mater. 2020, 390, 121366. [Google Scholar] [CrossRef]
- Ravindran, B.; Mnkeni, P.N. Bio-optimization of the carbon-to-nitrogen ratio for efficient vermicomposting of chicken manure and waste paper using Eisenia fetida. Environ. Sci. Pollut. Res. 2016, 23, 16965–16976. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Cui, X.; Jilani, G.; Lazzat, U.; Zehra, A.; Hamid, Y.; Hussain, B.; Tang, L.; Yang, X.; He, Z. Eisenia fetida and biochar synergistically alleviate the heavy metals content during valorization of biosolids via enhancing vermicompost quality. Sci. Total Environ. 2019, 684, 597–609. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/legal-content/EL/TXT/?uri=CELEX:32019R1009 (accessed on 5 June 2019).
- Nsiah-Gyambibi, R.; Essandoh, H.M.; Asiedu, N.Y.; Fei-Baffoe, B. Valorization of fecal sludge stabilization via vermicomposting in microcosm enriched substrates using organic soils for vermicompost production. Heliyon 2021, 7, e06422. [Google Scholar] [CrossRef]
- Hanc, A.; Dume, B.; Hrebeckova, T. Differences of enzymatic activity during composting and vermicomposting of sewage sludge mixed with straw pellets. Front. Microbiol. 2022, 12, 3892. [Google Scholar] [CrossRef] [PubMed]
- Karwal, M.; Kaushik, A. Co-composting and vermicomposting of coal fly-ash with press mud: Changes in nutrients, micro-nutrients and enzyme activities. Environ. Technol. Innov. 2020, 18, 100708. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, C.; Li, W.X.; Guo, L.; Cai, Z.J.; Wang, B.R.; Chen, J.; Shen, R.F. Changes of acid and alkaline phosphatase activities in long-term chemical fertilization are driven by the similar soil properties and associated microbial community composition in acidic soil. Eur. J. Soil Biol. 2021, 104, 103312. [Google Scholar] [CrossRef]
- Macci, C.; Masciandaro, G.; Ceccanti, B. Vermicomposting of olive oil Mill Wastewaters. Waste Manag. Res. J. A Sustain. Circ. Econ. 2009, 28, 738–747. [Google Scholar] [CrossRef]
- Busato, J.G.; Papa, G.; Canellas, L.P.; Adani, F.; de Oliveira, A.L.; Leão, T.P. Phosphatase activity and its relationship with physical and chemical parameters during vermicomposting of filter cake and cattle manure. J. Sci. Food Agric. 2015, 96, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Alidadi, H.; Hosseinzadeh, A.; Najafpoor, A.A.; Esmaili, H.; Zanganeh, J.; Dolatabadi Takabi, M.; Piranloo, F.G. Waste recycling by vermicomposting: Maturity and quality assessment via dehydrogenase enzyme activity, lignin, water soluble carbon, nitrogen, phosphorous and other indicators. J. Environ. Manag. 2016, 182, 134–140. [Google Scholar] [CrossRef]
- Gusain, R.; Suthar, S. Vermicomposting of invasive weed ageratum conyzoids: Assessment of nutrient mineralization, enzymatic activities, and Microbial Properties. Bioresour. Technol. 2020, 312, 123537. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Guo, W.; Liu, H.; Cai, M. The synergistic effect between biofertility properties and biological activities in vermicomposting: A comparable study of pig manure. J. Environ. Manag. 2022, 324, 116280. [Google Scholar] [CrossRef]
- Aira, M.; Gómez-Brandón, M.; González-Porto, P.; Domínguez, J. Selective reduction of the pathogenic load of cow manure in an industrial-scale continuous-feeding vermireactor. Bioresour. Technol. 2011, 102, 9633–9637. [Google Scholar] [CrossRef]
- Roubalová, R.; Procházková, P.; Hanč, A.; Dvořák, J.; Bilej, M. Mutual interactions of E. andrei earthworm and pathogens during the process of vermicomposting. Environ. Sci. Pollut. Res. 2019, 27, 33429–33437. [Google Scholar] [CrossRef]
- Pahalagedara, A.S.; Flint, S.; Palmer, J.; Subbaraj, A.; Brightwell, G.; Gupta, T.B. Antimicrobial activity of soil clostridium enriched conditioned media against Bacillus mycoides, Bacillus cereus, and Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 608998. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. Molecular Detection of Human Bacterial Pathogens; Taylor & Francis CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Khyade, V.B. Bacterial diversity in the alimentary canal of earthworms. J. Bacteriol. Mycol. Open Access 2018, 6, 1. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; Wang, F.; Yang, W.; Zhou, J.; Muhoza, B.; Mugabowindekwe, M.; Yu, X. Harnessing the power of cellulolytic nitrogen-fixing bacteria for biovalorization of lignocellulosic biomass. Ind. Crops Prod. 2022, 186, 115235. [Google Scholar] [CrossRef]
- Monserrate, E.; Leschine, S.B.; Canale-Parola, E. Clostridium hungatei sp. nov., a mesophilic, N2-fixing cellulolytic bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2001, 51, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zou, W.; Zhang, K.; Ye, G.; Yang, J. Advances and applications of clostridium co-culture systems in biotechnology. Front. Microbiol. 2020, 11, 2842. [Google Scholar] [CrossRef] [PubMed]
- Yanni, A.E.; Mitropoulou, G.; Prapa, I.; Agrogiannis, G.; Kostomitsopoulos, N.; Bezirtzoglou, E.; Kourkoutas, Y.; Karathanos, V.T. Functional modulation of gut microbiota in diabetic rats following dietary intervention with pistachio nuts (Pistacia vera L.). Metab. Open 2020, 7, 100040. [Google Scholar] [CrossRef]
- Bach, H.-J.; Hartmann, A.; Schloter, M.; Munch, J.C. PCR primers and functional probes for amplification and detection of bacterial genes for extracellular peptidases in single strains and in Soil. J. Microbiol. Methods 2001, 44, 173–182. [Google Scholar] [CrossRef]
- Tabassum, S.; Wang, Y.; Zhang, X.; Zhang, Z. Novel mass bio system (MBS) and its potential application in advanced treatment of coal gasification wastewater. RSC Adv. 2015, 5, 88692–88702. [Google Scholar] [CrossRef]
- Okanya, P.W.; Mohr, K.I.; Gerth, K.; Jansen, R.; Müller, R. Marinoquinolines a−f, pyrroloquinolines from Ohtaekwangia kribbensis (bacteroidetes). J. Nat. Prod. 2011, 74, 603–608. [Google Scholar] [CrossRef]
- Wiegand, S.; Jogler, M.; Jogler, C. On the maverick planctomycetes. FEMS Microbiol. Rev. 2018, 42, 739–760. [Google Scholar] [CrossRef]
- Bienhold, C.; Pop Ristova, P.; Wenzhöfer, F.; Dittmar, T.; Boetius, A. How deep-sea wood falls sustain Chemosynthetic Life. PLoS ONE 2013, 8, e53590. [Google Scholar] [CrossRef]
- Wright, M.S.; Lima, I.M. Identification of microbial populations in blends of worm castings or sugarcane filter mud compost with biochar. Agronomy 2021, 11, 1671. [Google Scholar] [CrossRef]
- Astafyeva, Y.; Gurschke, M.; Qi, M.; Bergmann, L.; Indenbirken, D.; de Grahl, I.; Katzowitsch, E.; Reumann, S.; Hanelt, D.; Alawi, M.; et al. Microalgae and bacteria interaction—Evidence for division of diligence in the alga microbiota. Microbiol. Spectr. 2022, 10, e00633-22. [Google Scholar] [CrossRef] [PubMed]
- Kollmen, J.; Strieth, D. The beneficial effects of cyanobacterial co-culture on plant growth. Life 2022, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.; Dakora, F.D. Rhizobia as a source of plant growth-promoting molecules: Potential applications and possible operational mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676. [Google Scholar] [CrossRef]
- Cai, S.-T.; Chiu, M.-C.; Chou, J.-Y. Broad-Spectrum Activity of Volatile Organic Compounds from Three Yeast-like Fungi of the Galactomyces Genus Against Diverse Plant Pathogens. Mycobiology 2020, 49, 69–77. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Narayanan, D.B.; Kolbe, A.R.; Ramos-Tapia, I.; Castro-Nallar, E.; Crandall, K.A.; Domínguez, J. Comparative analysis of Metagenomics and Metataxonomics for the characterization of vermicompost microbiomes. Front. Microbiol. 2022, 13, 854423. [Google Scholar] [CrossRef]
- Xu, X.-L.; Zeng, Q.; Lv, Y.-C.; Jeewon, R.; Maharachchikumbura, S.S.; Wanasinghe, D.N.; Hyde, K.D.; Xiao, Q.-G.; Liu, Y.-G.; Yang, C.-L. Insight into the systematics of novel entomopathogenic fungi associated with armored scale insect, Kuwanaspis howardi (Hemiptera: Diaspididae) in China. J. Fungi 2021, 7, 628. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, S.; Chang, D.; Shimizu, K.-yoshi; Cao, W. Succession of fungal community and enzyme activity during the co-decomposition process of rice (Oryza sativa L.) straw and milk vetch (Astragalus sinicus L.). Waste Manag. 2021, 134, 1–10. [Google Scholar] [CrossRef]
- Ramya, P.; Gomathi, V.; Devi, R.P.; Balachandar, D. Pichia kudriavzevii—A potential soil yeast candidate for improving soil physical, chemical and biological properties. Arch. Microbiol. 2021, 203, 4619–4628. [Google Scholar] [CrossRef]
- Angelidaki, I.; Karakashev, D.; Batstone, D.J.; Plugge, C.M.; Stams, A.J.M. Biomethanation and its potential. In Methods in enzymology; Academic Press: Cambridge, MA, USA, 2011; Volume 494, pp. 327–351. [Google Scholar]
- Hu, X.; Zhang, T.; Tian, G.; Zhang, L.; Bian, B. Performance and mechanism of high-speed vermicomposting of dewatered sludge using a new type of laboratory earthworm reactor. Environ. Sci. Pollut. Res. 2021, 28, 26132–26144. [Google Scholar] [CrossRef]
- Alavi, N.; Daneshpajou, M.; Shirmardi, M.; Goudarzi, G.; Neisi, A.; Babaei, A.A. Investigating the efficiency of co-composting and vermicomposting of vinasse with the mixture of cow manure wastes, bagasse, and natural zeolite. Waste Manag. 2017, 69, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Niedzialkoski, R.K.; Marostica, R.; Damaceno, F.M.; Costa, L.A.; Costa, M.S. Combination of biological processes for agro-industrial Poultry Waste Management: Effects on vermicomposting and anaerobic digestion. J. Environ. Manag. 2021, 297, 113127. [Google Scholar] [CrossRef] [PubMed]
- Majlessi, M.; Eslami, A.; Najafi Saleh, H.; Mirshafieean, S.; Babaii, S. Vermicomposting of food waste: Assessing the stability and maturity. Iran. J. Environ. Health Sci. Eng. 2012, 9, 25. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | Day 0 | Day 15 | Day 45 | Day 90 | Day 120 |
|---|---|---|---|---|---|
| Total Ca (% Dry Weight) | 6.71 ± 0.86 | 9.77 ± 0.47 | 5.21 ± 0.08 | 0.95 ± 0.21 | 1.86 ± 0.21 |
| Active Ca (% Dry Weight) | 1.12 ± 0.03 | 6.46 ± 0.35 | 1.10 ± 0.04 | 0.31 ± 0.16 | 0.41 ± 0.01 |
| CaCO3 (% Dry Weight) | 7.83 ± 0.89 | 16.23 ± 0.81 | 6.31 ± 0.13 | 1.26 ± 0.06 | 1.45 ± 0.20 |
| P (ppm) | 0.28 ± 0.04 | 0.41 ± 0.06 | 0.21 ± 0.01 | 0.10 ± 0.01 | 0.24 ± 0.07 |
| K (ppm) | 4.23 ± 0.04 | 7.20 ± 0.14 | 3.78 ± 0.04 | 1.48 ± 0.04 | 0.35 ± 0.14 |
| Mg (ppm) | 1.60 ± 0.14 | 4.88 ± 0.32 | 1.42 ± 0.12 | 0.78 ± 0.04 | 2.48 ± 0.71 |
| Zn (ppm) | 35.80 ± 9.62 | 47.88 ± 0.46 | 37.25 ± 0.35 | 57.55 ± 3.46 | 55.25 ± 2.47 |
| Mn (ppm) | 60.86 ± 0.76 | 61.15 ± 1.2 | 54.48 ± 1.10 | 62.08 ± 3.46 | 29.90 ± 4.81 |
| Fe (ppm) | 49.30 ± 1.56 | 31.78 ± 2.3 | 45.75 ± 2.05 | 36.35 ± 1.06 | 18.00 ± 0.00 |
| Cu (ppm) | 11.25 ± 1.06 | 12.08 ± 1.52 | 10.50 ± 1.41 | 13.75 ± 0.35 | 25.5 ± 7.78 |
| Enzymatic Activity | Day 0 | Day 15 | Day 45 | Day 60 | Day 90 | Day 120 |
|---|---|---|---|---|---|---|
| Dehydrogenase (μg TPF/g dm/ 24 h) | 5.77 ± 0.9 | 26.1 ± 10.1 | 64.4 ± 9.3 | 42.3 ± 5.7 | 25.6 ± 1.2 | 27.0 ± 2.1 |
| Acid phosphatase (μmoles pNP/g dm/ h) | 8.2 ± 1.7 | 28.6 ± 11.3 | 22.9 ± 6.5 | 22.5 ± 4.1 | 22.2 ± 4.1 | 6.7 ± 0.6 |
| Urease (μg N/g dm/ 2 h) | 42.1 ± 22.0 | 1546.0 ± 138.6 | 2579.6 ± 80.3 | 2448.2 ± 85.6 | 2240.7 ± 160.9 | 2505.2 ± 88.4 |
| β-glycosidase (μmoles pNP/g dm/ h) | 4.4 ± 0.2 | 18.5 ± 8.7 | 14.5 ± 0.0 | 10.2 ± 0.5 | 7.7 ± 0.5 | 3.6 ± 0.2 |
| Bacterial Phyla | Day 0 | Day 120 | Fungal Phyla | Day 0 | Day 120 |
|---|---|---|---|---|---|
| Armatimonadetes | 0.02 | 0.06 | Ascomycota | 88.43 | 97.70 |
| Bacteroidetes | 1.93 | 68.44 | Basidiomycota | 11.00 | 0.62 |
| Candidatus saccharibacteria | 0.01 | 0.05 | Chytridiomycota | 0.21 | 0.24 |
| Chloroflexi | 2.21 | 5.53 | Entomophthoromycota | 0.1 | 0.01 |
| Cyanobacteria | 0.03 | 3.05 | Glomeromycota | 0.19 | 0.23 |
| Planctomycetes | 0.57 | 12.38 | Mucoromycota | 0.08 | 1.21 |
| Proteobacteria | 95.25 | 10.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karapantzou, I.; Mitropoulou, G.; Prapa, I.; Papanikolaou, D.; Charovas, V.; Kourkoutas, Y. Physicochemical Changes and Microbiome Associations during Vermicomposting of Winery Waste. Sustainability 2023, 15, 7484. https://doi.org/10.3390/su15097484
Karapantzou I, Mitropoulou G, Prapa I, Papanikolaou D, Charovas V, Kourkoutas Y. Physicochemical Changes and Microbiome Associations during Vermicomposting of Winery Waste. Sustainability. 2023; 15(9):7484. https://doi.org/10.3390/su15097484
Chicago/Turabian StyleKarapantzou, Ioanna, Gregoria Mitropoulou, Ioanna Prapa, Dimitra Papanikolaou, Vasileios Charovas, and Yiannis Kourkoutas. 2023. "Physicochemical Changes and Microbiome Associations during Vermicomposting of Winery Waste" Sustainability 15, no. 9: 7484. https://doi.org/10.3390/su15097484
APA StyleKarapantzou, I., Mitropoulou, G., Prapa, I., Papanikolaou, D., Charovas, V., & Kourkoutas, Y. (2023). Physicochemical Changes and Microbiome Associations during Vermicomposting of Winery Waste. Sustainability, 15(9), 7484. https://doi.org/10.3390/su15097484